
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Previous issues
- Page Path
- HOME > Browse > Previous issues
Reviews
- Basic Research
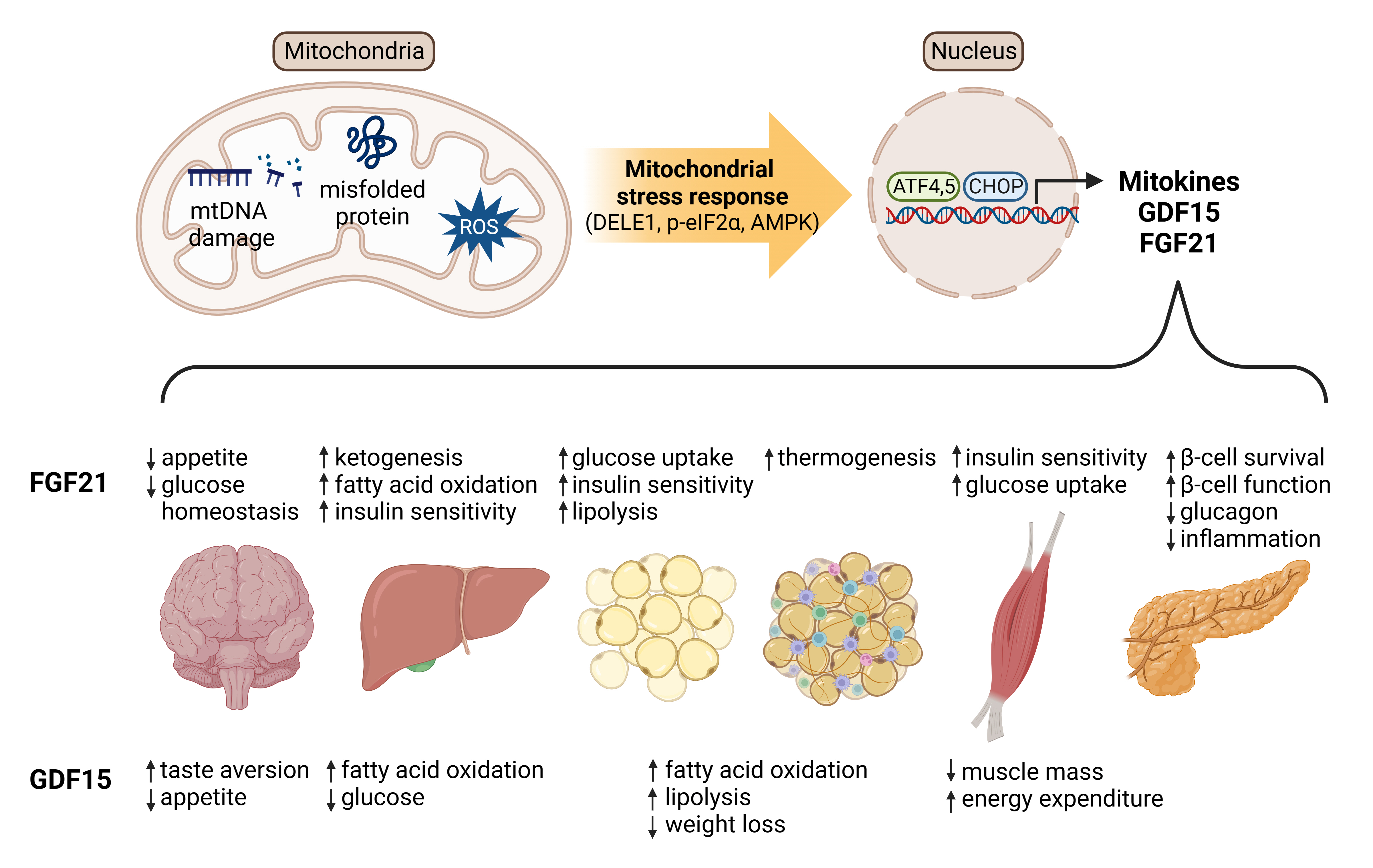
- Mitochondrial Stress and Mitokines: Therapeutic Perspectives for the Treatment of Metabolic Diseases
- Benyuan Zhang, Joon Young Chang, Min Hee Lee, Sang-Hyeon Ju, Hyon-Seung Yi, Minho Shong
- Diabetes Metab J. 2024;48(1):1-18. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0115
- 2,098 View
- 258 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Mitochondrial stress and the dysregulated mitochondrial unfolded protein response (UPRmt) are linked to various diseases, including metabolic disorders, neurodegenerative diseases, and cancer. Mitokines, signaling molecules released by mitochondrial stress response and UPRmt, are crucial mediators of inter-organ communication and influence systemic metabolic and physiological processes. In this review, we provide a comprehensive overview of mitokines, including their regulation by exercise and lifestyle interventions and their implications for various diseases. The endocrine actions of mitokines related to mitochondrial stress and adaptations are highlighted, specifically the broad functions of fibroblast growth factor 21 and growth differentiation factor 15, as well as their specific actions in regulating inter-tissue communication and metabolic homeostasis. Finally, we discuss the potential of physiological and genetic interventions to reduce the hazards associated with dysregulated mitokine signaling and preserve an equilibrium in mitochondrial stress-induced responses. This review provides valuable insights into the mechanisms underlying mitochondrial regulation of health and disease by exploring mitokine interactions and their regulation, which will facilitate the development of targeted therapies and personalized interventions to improve health outcomes and quality of life.
- Pathophysiology
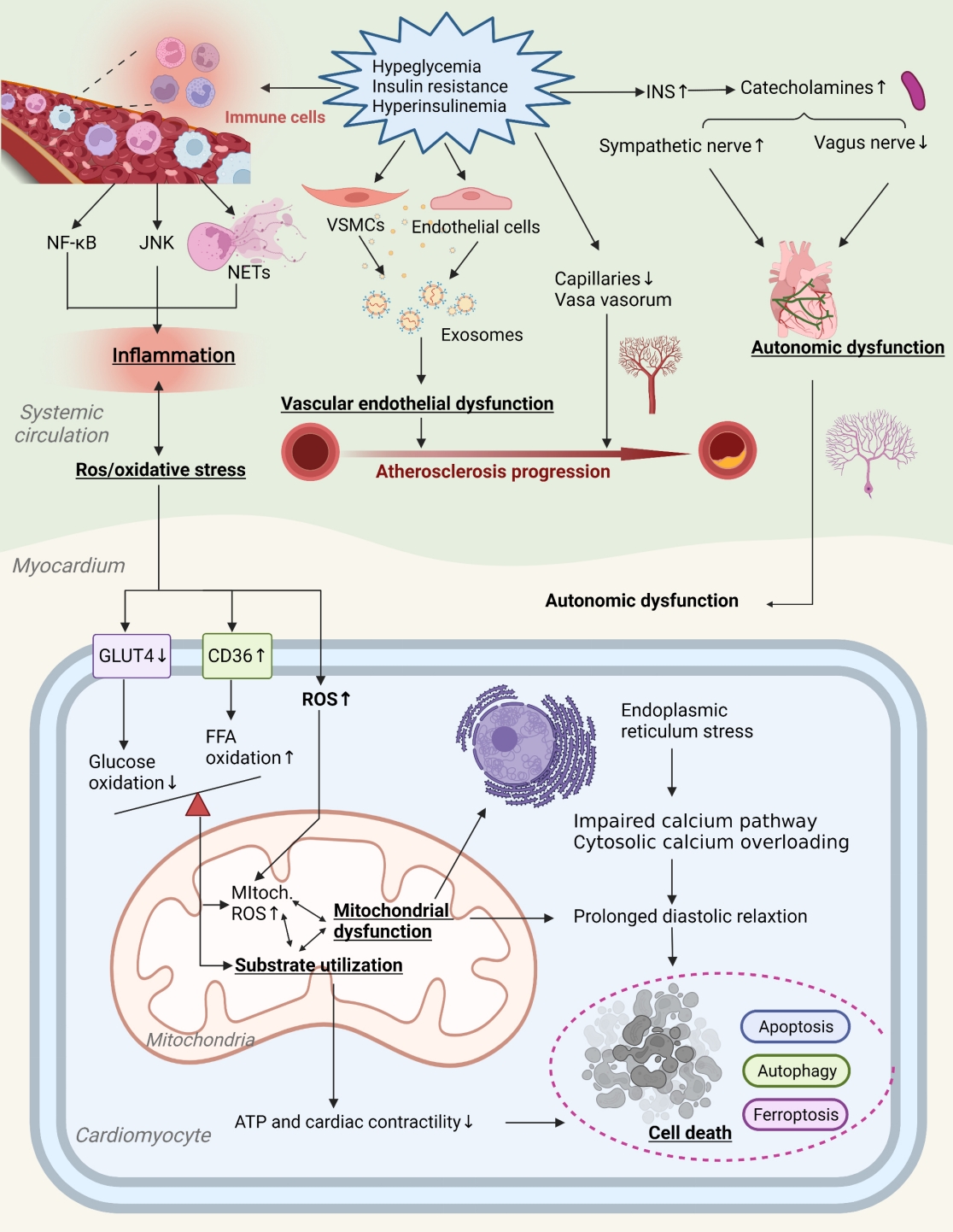
- Primordial Drivers of Diabetes Heart Disease: Comprehensive Insights into Insulin Resistance
- Yajie Fan, Zhipeng Yan, Tingting Li, Aolin Li, Xinbiao Fan, Zhongwen Qi, Junping Zhang
- Diabetes Metab J. 2024;48(1):19-36. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0110
- 2,213 View
- 185 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Insulin resistance has been regarded as a hallmark of diabetes heart disease (DHD). Numerous studies have shown that insulin resistance can affect blood circulation and myocardium, which indirectly cause cardiac hypertrophy and ventricular remodeling, participating in the pathogenesis of DHD. Meanwhile, hyperinsulinemia, hyperglycemia, and hyperlipidemia associated with insulin resistance can directly impair the metabolism and function of the heart. Targeting insulin resistance is a potential therapeutic strategy for the prevention of DHD. Currently, the role of insulin resistance in the pathogenic development of DHD is still under active research, as the pathological roles involved are complex and not yet fully understood, and the related therapeutic approaches are not well developed. In this review, we describe insulin resistance and add recent advances in the major pathological and physiological changes and underlying mechanisms by which insulin resistance leads to myocardial remodeling and dysfunction in the diabetic heart, including exosomal dysfunction, ferroptosis, and epigenetic factors. In addition, we discuss potential therapeutic approaches to improve insulin resistance and accelerate the development of cardiovascular protection drugs.
- Pathophysiology
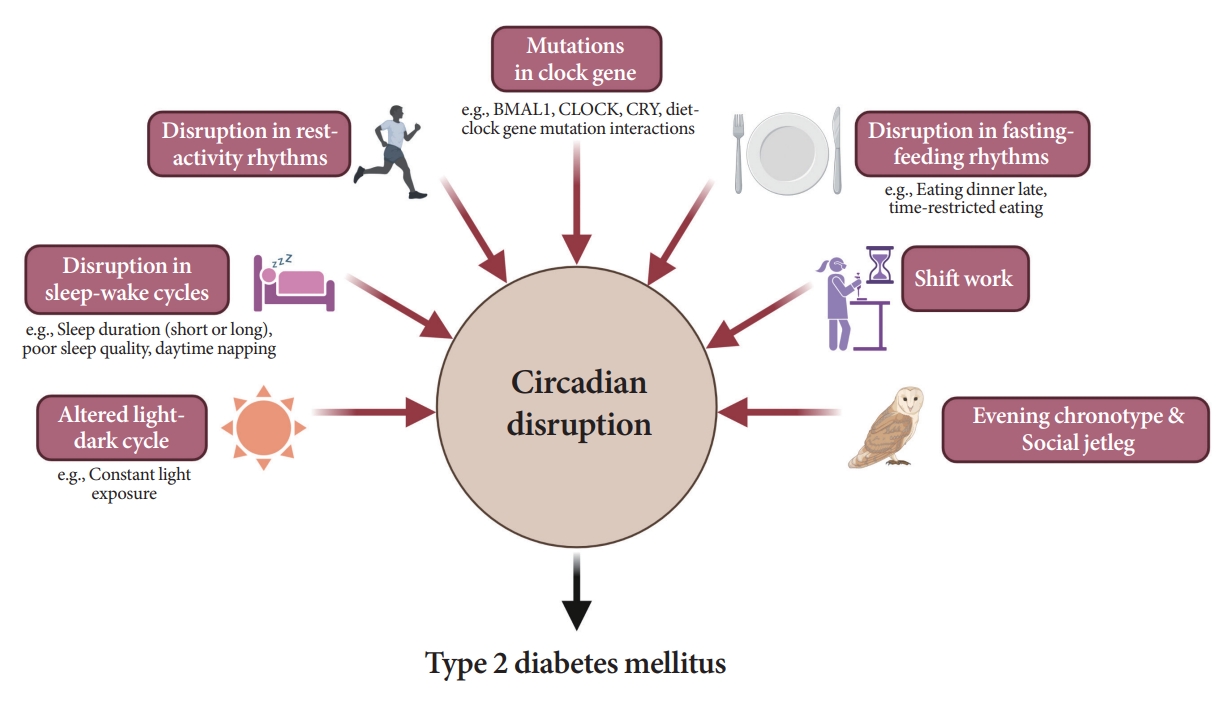
- Attention to Innate Circadian Rhythm and the Impact of Its Disruption on Diabetes
- Da Young Lee, Inha Jung, So Young Park, Ji Hee Yu, Ji A Seo, Kyeong Jin Kim, Nam Hoon Kim, Hye Jin Yoo, Sin Gon Kim, Kyung Mook Choi, Sei Hyun Baik, Nan Hee Kim
- Diabetes Metab J. 2024;48(1):37-52. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0193
- 2,258 View
- 222 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Novel strategies are required to reduce the risk of developing diabetes and/or clinical outcomes and complications of diabetes. In this regard, the role of the circadian system may be a potential candidate for the prevention of diabetes. We reviewed evidence from animal, clinical, and epidemiological studies linking the circadian system to various aspects of the pathophysiology and clinical outcomes of diabetes. The circadian clock governs genetic, metabolic, hormonal, and behavioral signals in anticipation of cyclic 24-hour events through interactions between a “central clock” in the suprachiasmatic nucleus and “peripheral clocks” in the whole body. Currently, circadian rhythmicity in humans can be subjectively or objectively assessed by measuring melatonin and glucocorticoid levels, core body temperature, peripheral blood, oral mucosa, hair follicles, rest-activity cycles, sleep diaries, and circadian chronotypes. In this review, we summarized various circadian misalignments, such as altered light-dark, sleep-wake, rest-activity, fasting-feeding, shift work, evening chronotype, and social jetlag, as well as mutations in clock genes that could contribute to the development of diabetes and poor glycemic status in patients with diabetes. Targeting critical components of the circadian system could deliver potential candidates for the treatment and prevention of type 2 diabetes mellitus in the future.
Special Editorial
- Diabetes & Metabolism Journal in 2024: We Will Keep Moving toward a Better Future
- Hyuk-Sang Kwon
- Diabetes Metab J. 2024;48(1):53-54. Published online January 29, 2024
- DOI: https://doi.org/10.4093/dmj.2024.0008
- 1,146 View
- 136 Download
Editorial
- Combining Ezetimibe and Rosuvastatin: Impacts on Insulin Sensitivity and Vascular Inflammation in Patients with Type 2 Diabetes Mellitus
- Eun Roh
- Diabetes Metab J. 2024;48(1):55-58. Published online January 29, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0442
- 1,253 View
- 164 Download
Original Articles
- Basic Research
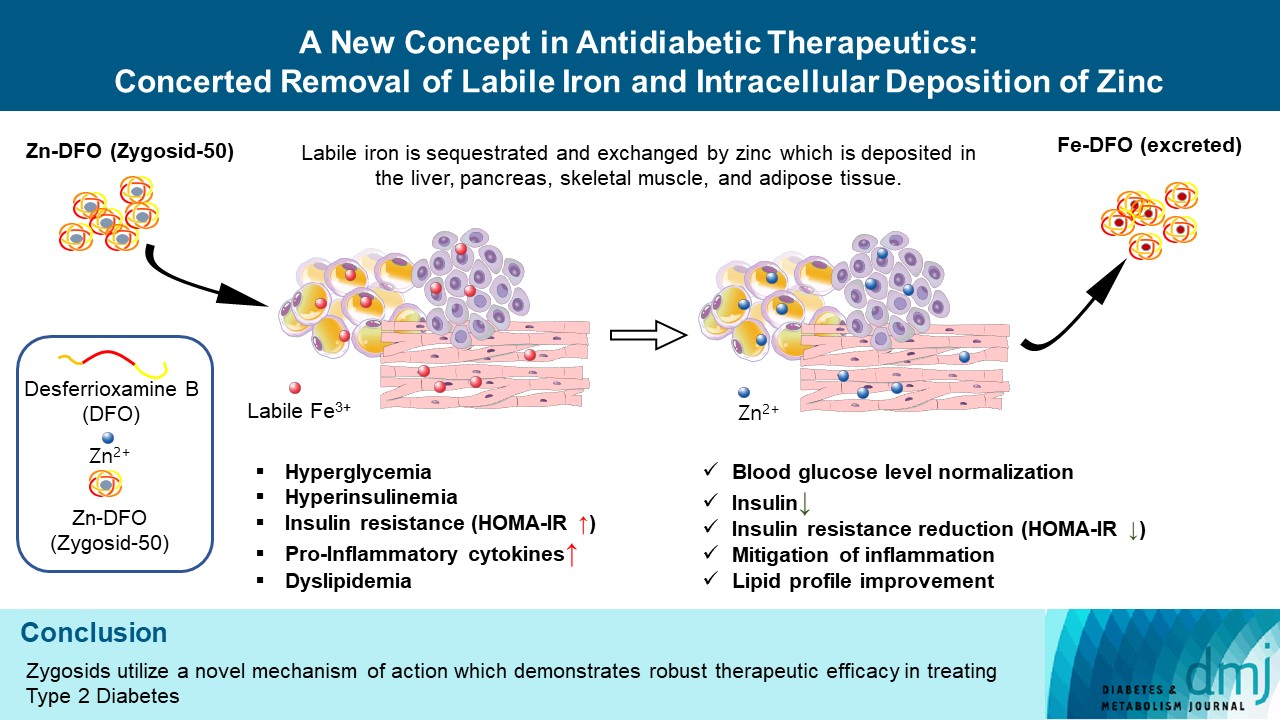
- A New Concept in Antidiabetic Therapeutics: A Concerted Removal of Labile Iron and Intracellular Deposition of Zinc
- Vladimir Vinokur, Eduard Berenshtein, Mordechai Chevion, Dror Chevion
- Diabetes Metab J. 2024;48(1):59-71. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0292
- Retraction in: Diabetes Metab J 2024;48(2):325
- 1,611 View
- 178 Download
- Basic Research
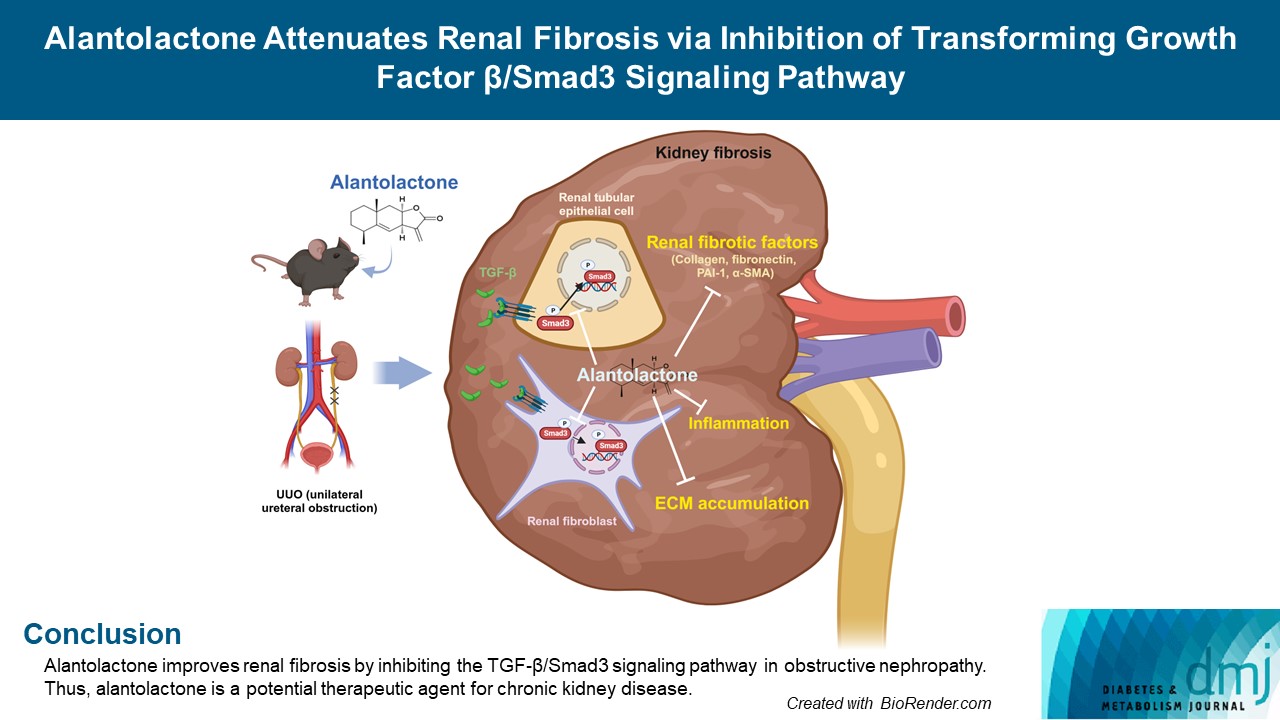
- Alantolactone Attenuates Renal Fibrosis via Inhibition of Transforming Growth Factor β/Smad3 Signaling Pathway
- Kyeong-Min Lee, Yeo Jin Hwang, Gwon-Soo Jung
- Diabetes Metab J. 2024;48(1):72-82. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0231
- 1,273 View
- 142 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Renal fibrosis is characterized by the accumulation of extracellular matrix proteins and interstitial fibrosis. Alantolactone is known to exert anticancer, anti-inflammatory, antimicrobial and antifungal effects; however, its effects on renal fibrosis remains unknown. Here, we investigated whether alantolactone attenuates renal fibrosis in mice unilateral ureteral obstruction (UUO) and evaluated the effect of alantolactone on transforming growth factor (TGF) signaling pathway in renal cells.
Methods
To evaluate the therapeutic effect of alantolactone, cell counting kit-8 (CCK-8) assay, histological staining, Western blot analysis, and real-time quantitative polymerase chain reaction were performed in UUO kidneys in vivo and in TGF-β-treated renal cells in vitro.
Results
Alantolactone (0.25 to 4 µM) did not affect the viability of renal cells. Mice orally administered 5 mg/kg of alantolactone daily for 15 days did not show mortality or liver toxicity. Alantolactone decreased UUO-induced blood urea nitrogen and serum creatinine levels. In addition, it significantly alleviated renal tubulointerstitial damage and fibrosis and decreased collagen type I, fibronectin, and α-smooth muscle actin (α-SMA) expression in UUO kidneys. In NRK-49F cells, alantolactone inhibited TGF-βstimulated expression of fibronectin, collagen type I, plasminogen activator inhibitor-1 (PAI-1), and α-SMA. In HK-2 cells, alantolactone inhibited TGF-β-stimulated expression of collagen type I and PAI-1. Alantolactone inhibited UUO-induced phosphorylation of Smad3 in UUO kidneys. In addition, it not only decreased TGF-β secretion but also Smad3 phosphorylation and translocation to nucleus in both kidney cell lines.
Conclusion
Alantolactone improves renal fibrosis by inhibiting the TGF-β/Smad3 signaling pathway in obstructive nephropathy. Thus, alantolactone is a potential therapeutic agent for chronic kidney disease.
- Basic Research
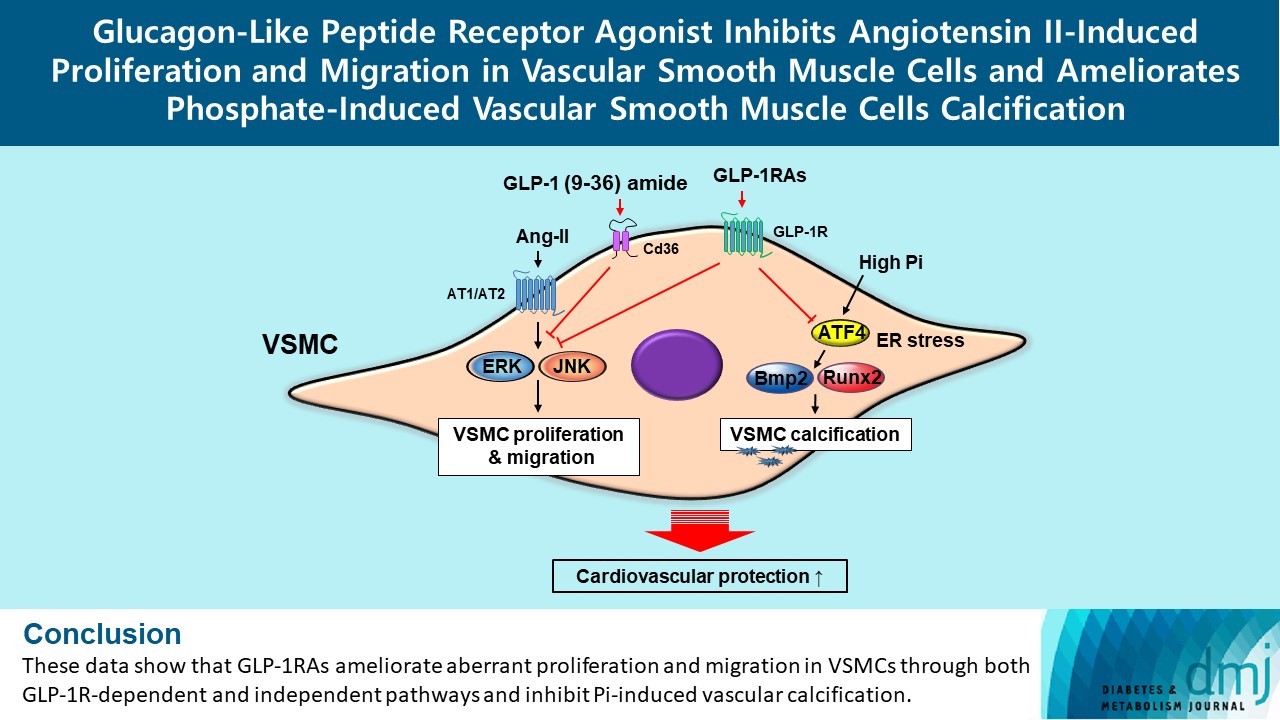
- Glucagon-Like Peptide Receptor Agonist Inhibits Angiotensin II-Induced Proliferation and Migration in Vascular Smooth Muscle Cells and Ameliorates Phosphate-Induced Vascular Smooth Muscle Cells Calcification
- Jinmi Lee, Seok-Woo Hong, Min-Jeong Kim, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
- Diabetes Metab J. 2024;48(1):83-96. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0363
- 1,745 View
- 168 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Glucagon-like peptide-1 receptor agonist (GLP-1RA), which is a therapeutic agent for the treatment of type 2 diabetes mellitus, has a beneficial effect on the cardiovascular system.
Methods
To examine the protective effects of GLP-1RAs on proliferation and migration of vascular smooth muscle cells (VSMCs), A-10 cells exposed to angiotensin II (Ang II) were treated with either exendin-4, liraglutide, or dulaglutide. To examine the effects of GLP-1RAs on vascular calcification, cells exposed to high concentration of inorganic phosphate (Pi) were treated with exendin-4, liraglutide, or dulaglutide.
Results
Ang II increased proliferation and migration of VSMCs, gene expression levels of Ang II receptors AT1 and AT2, proliferation marker of proliferation Ki-67 (Mki-67), proliferating cell nuclear antigen (Pcna), and cyclin D1 (Ccnd1), and the protein expression levels of phospho-extracellular signal-regulated kinase (p-Erk), phospho-c-JUN N-terminal kinase (p-JNK), and phospho-phosphatidylinositol 3-kinase (p-Pi3k). Exendin-4, liraglutide, and dulaglutide significantly decreased the proliferation and migration of VSMCs, the gene expression levels of Pcna, and the protein expression levels of p-Erk and p-JNK in the Ang II-treated VSMCs. Erk inhibitor PD98059 and JNK inhibitor SP600125 decreased the protein expression levels of Pcna and Ccnd1 and proliferation of VSMCs. Inhibition of GLP-1R by siRNA reversed the reduction of the protein expression levels of p-Erk and p-JNK by exendin-4, liraglutide, and dulaglutide in the Ang II-treated VSMCs. Moreover, GLP-1 (9-36) amide also decreased the proliferation and migration of the Ang II-treated VSMCs. In addition, these GLP-1RAs decreased calcium deposition by inhibiting activating transcription factor 4 (Atf4) in Pi-treated VSMCs.
Conclusion
These data show that GLP-1RAs ameliorate aberrant proliferation and migration in VSMCs through both GLP-1Rdependent and independent pathways and inhibit Pi-induced vascular calcification. -
Citations
Citations to this article as recorded by- Incretin Hormone Secretion in Women with Polycystic Ovary Syndrome: Roles of Obesity, Insulin Sensitivity and Treatment with Metformin and GLP-1s
Andrea Etrusco, Mislav Mikuš, Antonio D’Amato, Fabio Barra, Petar Planinić, Trpimir Goluža, Giovanni Buzzaccarini, Jelena Marušić, Mara Tešanović, Antonio Simone Laganà
Biomedicines.2024; 12(3): 653. CrossRef
- Incretin Hormone Secretion in Women with Polycystic Ovary Syndrome: Roles of Obesity, Insulin Sensitivity and Treatment with Metformin and GLP-1s
- Basic Research
- DWN12088, A Prolyl-tRNA Synthetase Inhibitor, Alleviates Hepatic Injury in Nonalcoholic Steatohepatitis
- Dong-Keon Lee, Su Ho Jo, Eun Soo Lee, Kyung Bong Ha, Na Won Park, Deok-Hoon Kong, Sang-In Park, Joon Seok Park, Choon Hee Chung
- Diabetes Metab J. 2024;48(1):97-111. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0367

- 1,794 View
- 181 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Nonalcoholic steatohepatitis (NASH) is a liver disease caused by obesity that leads to hepatic lipoapoptosis, resulting in fibrosis and cirrhosis. However, the mechanism underlying NASH is largely unknown, and there is currently no effective therapeutic agent against it. DWN12088, an agent used for treating idiopathic pulmonary fibrosis, is a selective prolyl-tRNA synthetase (PRS) inhibitor that suppresses the synthesis of collagen. However, the mechanism underlying the hepatoprotective effect of DWN12088 is not clear. Therefore, we investigated the role of DWN12088 in NASH progression.
Methods
Mice were fed a chow diet or methionine-choline deficient (MCD)-diet, which was administered with DWN12088 or saline by oral gavage for 6 weeks. The effects of DWN12088 on NASH were evaluated by pathophysiological examinations, such as real-time quantitative reverse transcription polymerase chain reaction, immunoblotting, biochemical analysis, and immunohistochemistry. Molecular and cellular mechanisms of hepatic injury were assessed by in vitro cell culture.
Results
DWN12088 attenuated palmitic acid (PA)-induced lipid accumulation and lipoapoptosis by downregulating the Rho-kinase (ROCK)/AMP-activated protein kinase (AMPK)/sterol regulatory element-binding protein-1c (SREBP-1c) and protein kinase R-like endoplasmic reticulum kinase (PERK)/α subunit of eukaryotic initiation factor 2 (eIF2α)/activating transcription factor 4 (ATF4)/C/EBP-homologous protein (CHOP) signaling cascades. PA increased but DWN12088 inhibited the phosphorylation of nuclear factor-κB (NF-κB) p65 (Ser536, Ser276) and the expression of proinflammatory genes. Moreover, the DWN12088 inhibited transforming growth factor β (TGFβ)-induced pro-fibrotic gene expression by suppressing TGFβ receptor 1 (TGFβR1)/Smad2/3 and TGFβR1/glutamyl-prolyl-tRNA synthetase (EPRS)/signal transducer and activator of transcription 6 (STAT6) axis signaling. In the case of MCD-diet-induced NASH, DWN12088 reduced hepatic steatosis, inflammation, and lipoapoptosis and prevented the progression of fibrosis.
Conclusion
Our findings provide new insights about DWN12088, namely that it plays an important role in the overall improvement of NASH. Hence, DWN12088 shows great potential to be developed as a new integrated therapeutic agent for NASH.
- Drug/Regimen
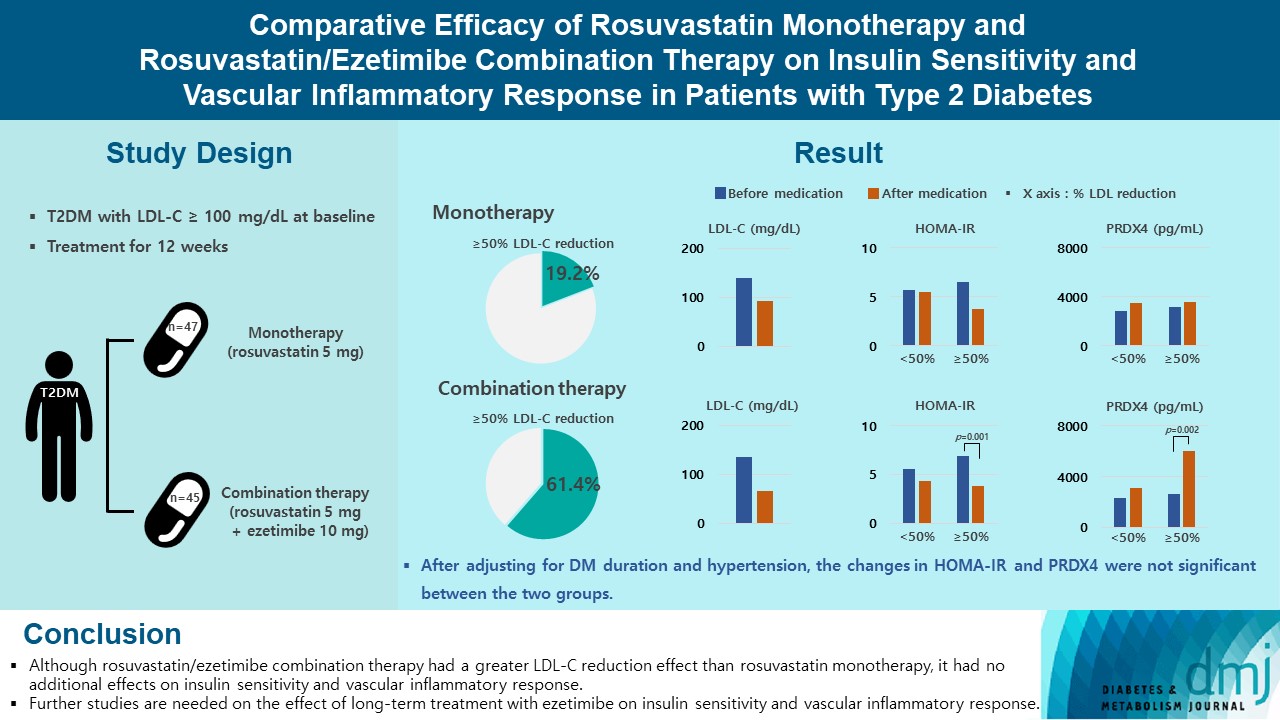
- Comparative Efficacy of Rosuvastatin Monotherapy and Rosuvastatin/Ezetimibe Combination Therapy on Insulin Sensitivity and Vascular Inflammatory Response in Patients with Type 2 Diabetes Mellitus
- Ji Hye Han, Kyong Hye Joung, Jun Choul Lee, Ok Soon Kim, Sorim Choung, Ji Min Kim, Yea Eun Kang, Hyon-Seung Yi, Ju Hee Lee, Bon Jeong Ku, Hyun Jin Kim
- Diabetes Metab J. 2024;48(1):112-121. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0402
- 2,113 View
- 224 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Type 2 diabetes mellitus (T2DM) induces endothelial dysfunction and inflammation, which are the main factors for atherosclerosis and cardiovascular disease. The present study aimed to compare the effects of rosuvastatin monotherapy and rosuvastatin/ezetimibe combination therapy on lipid profile, insulin sensitivity, and vascular inflammatory response in patients with T2DM.
Methods
A total of 101 patients with T2DM and dyslipidemia were randomized to either rosuvastatin monotherapy (5 mg/day, n=47) or rosuvastatin/ezetimibe combination therapy (5 mg/10 mg/day, n=45) and treated for 12 weeks. Serum lipids, glucose, insulin, soluble intercellular adhesion molecule-1 (sICAM-1), and peroxiredoxin 4 (PRDX4) levels were determined before and after 12 weeks of treatment.
Results
The reduction in low density lipoprotein cholesterol (LDL-C) by more than 50% from baseline after treatment was more in the combination therapy group. The serum sICAM-1 levels increased significantly in both groups, but there was no difference between the two groups. The significant changes in homeostasis model assessment of insulin resistance (HOMA-IR) and PRDX4 were confirmed only in the subgroup in which LDL-C was reduced by 50% or more in the combination therapy group. However, after adjusting for diabetes mellitus duration and hypertension, the changes in HOMA-IR and PRDX4 were not significant between the two groups.
Conclusion
Although rosuvastatin/ezetimibe combination therapy had a greater LDL-C reduction effect than rosuvastatin monotherapy, it had no additional effects on insulin sensitivity and vascular inflammatory response. Further studies are needed on the effect of long-term treatment with ezetimibe on insulin sensitivity and vascular inflammatory response. -
Citations
Citations to this article as recorded by- Combining Ezetimibe and Rosuvastatin: Impacts on Insulin Sensitivity and Vascular Inflammation in Patients with Type 2 Diabetes Mellitus
Eun Roh
Diabetes & Metabolism Journal.2024; 48(1): 55. CrossRef
- Combining Ezetimibe and Rosuvastatin: Impacts on Insulin Sensitivity and Vascular Inflammation in Patients with Type 2 Diabetes Mellitus
- Cardiovascular Risk/Epidemiology
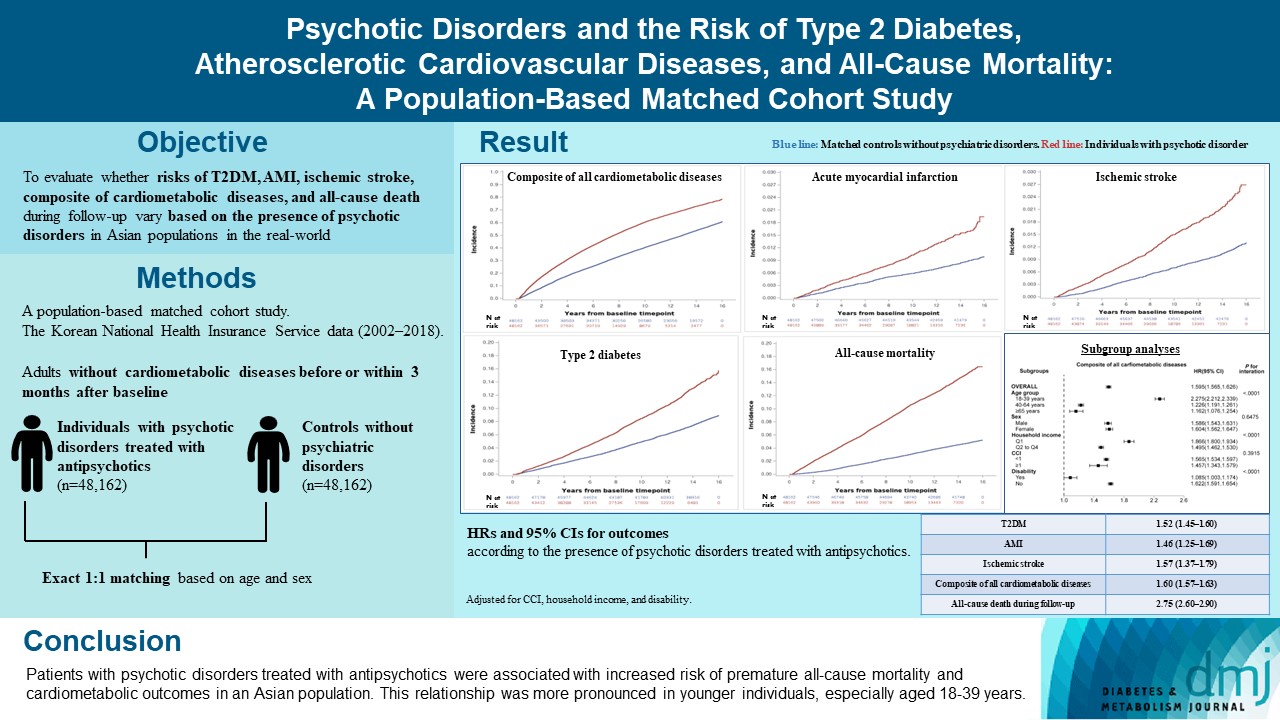
- Psychotic Disorders and the Risk of Type 2 Diabetes Mellitus, Atherosclerotic Cardiovascular Diseases, and All-Cause Mortality: A Population-Based Matched Cohort Study
- You-Bin Lee, Hyewon Kim, Jungkuk Lee, Dongwoo Kang, Gyuri Kim, Sang-Man Jin, Jae Hyeon Kim, Hong Jin Jeon, Kyu Yeon Hur
- Diabetes Metab J. 2024;48(1):122-133. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0431
- 1,124 View
- 144 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The effects of psychotic disorders on cardiometabolic diseases and premature death need to be determined in Asian populations.
Methods
In this population-based matched cohort study, the Korean National Health Insurance Service database (2002 to 2018) was used. The risk of type 2 diabetes mellitus (T2DM), acute myocardial infarction (AMI), ischemic stroke, composite of all cardiometabolic diseases, and all-cause death during follow-up was compared between individuals with psychotic disorders treated with antipsychotics (n=48,162) and 1:1 matched controls without psychiatric disorders among adults without cardiometabolic diseases before or within 3 months after baseline.
Results
In this cohort, 53,683 composite cases of all cardiometabolic diseases (during median 7.38 years), 899 AMI, and 1,216 ischemic stroke cases (during median 14.14 years), 7,686 T2DM cases (during median 13.26 years), and 7,092 deaths (during median 14.23 years) occurred. The risk of all outcomes was higher in subjects with psychotic disorders than matched controls (adjusted hazard ratios [95% confidence intervals]: 1.522 [1.446 to 1.602] for T2DM; 1.455 [1.251 to 1.693] for AMI; 1.568 [1.373 to 1.790] for ischemic stroke; 1.595 [1.565 to 1.626] for composite of all cardiometabolic diseases; and 2.747 [2.599 to 2.904] for all-cause mortality) during follow-up. Similar patterns of associations were maintained in subgroup analyses but more prominent in younger individuals (P for interaction <0.0001) when categorized as those aged 18–39, 40–64, or ≥65 years.
Conclusion
Patients with psychotic disorders treated with antipsychotics were associated with increased risk of premature allcause mortality and cardiometabolic outcomes in an Asian population. This relationship was more pronounced in younger individuals, especially aged 18 to 39 years.
- Metabolic Risk/Epidemiology
- Association of Measures of Glucose Metabolism with Colorectal Cancer Risk in Older Chinese: A 13-Year Follow-up of the Guangzhou Biobank Cohort Study-Cardiovascular Disease Substudy and Meta-Analysis
- Shu Yi Wang, Wei Sen Zhang, Chao Qiang Jiang, Ya Li Jin, Tong Zhu, Feng Zhu, Lin Xu
- Diabetes Metab J. 2024;48(1):134-145. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0383

- 1,154 View
- 141 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Abnormal glucose metabolism is a risk factor for colorectal cancer (CRC). However, association of glycosylated hemoglobin (HbA1c) with CRC risk remains under-reported. We examined the association between glycemic indicators (HbA1c, fasting plasma glucose, fasting insulin, 2-hour glucose, 2-hour insulin, and homeostasis model of risk assessment-insulin resistance index) and CRC risk using prospective analysis and meta-analysis.
Methods
Participants (n=1,915) from the Guangzhou Biobank Cohort Study-Cardiovascular Disease Substudy were included. CRC events were identified through record linkage. Cox regression was used to assess the associations of glycemic indicators with CRC risk. A meta-analysis was performed to investigate the association between HbA1c and CRC risk.
Results
During an average of 12.9 years follow-up (standard deviation, 2.8), 42 incident CRC cases occurred. After adjusting for potential confounders, the hazard ratio (95% confidence interval [CI]) of CRC for per % increment in HbA1c was 1.28 (95% CI, 1.01 to 1.63) in overall population, 1.51 (95% CI, 1.13 to 2.02) in women and 1.06 (95% CI, 0.68 to 1.68) in men. No significant association of other measures of glycemic indicators and baseline diabetes with CRC risk was found. Meta-analyses of 523,857 participants including our results showed that per % increment of HbA1c was associated with 13% higher risk of CRC, with the pooled risk ratio being 1.13 (95% CI, 1.01 to 1.27). Subgroupanalyses found stronger associations in women, colon cancer, Asians, and case-control studies.
Conclusion
Higher HbA1c was a significant predictor of CRC in the general population. Our findings shed light on the pathology of glucose metabolism and CRC, which warrants more in-depth investigation.
- Complications
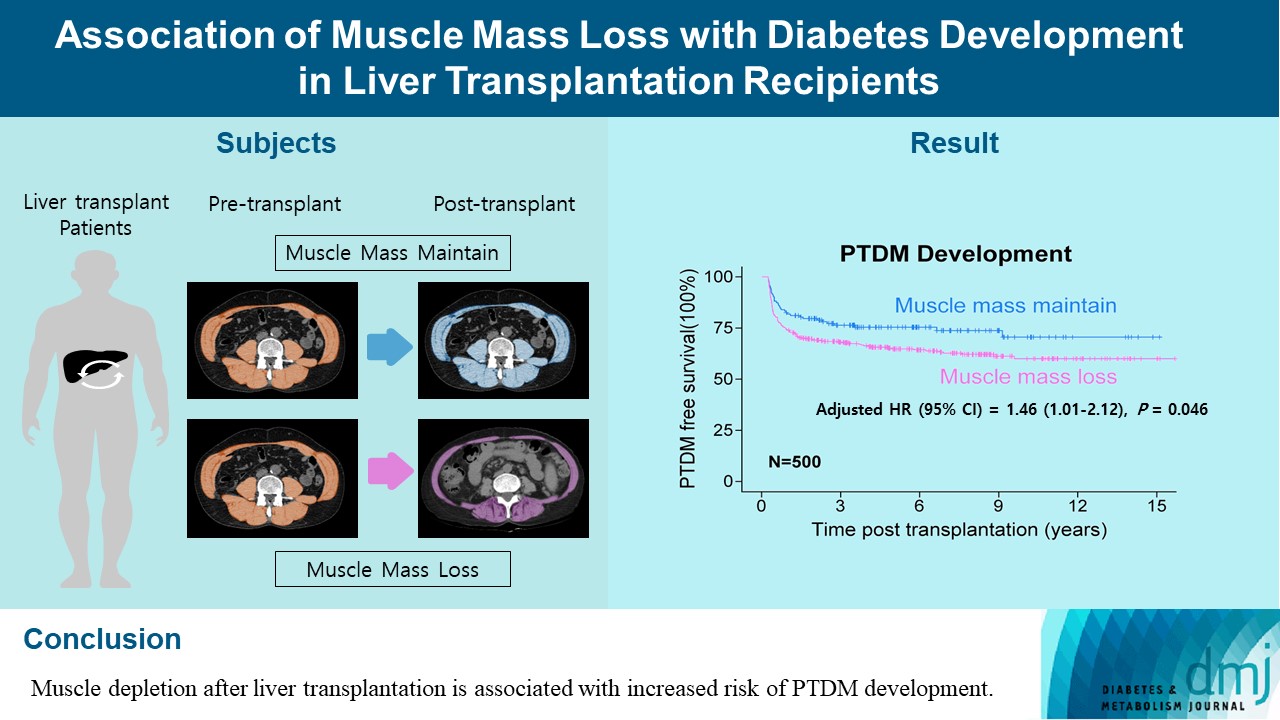
- Association of Muscle Mass Loss with Diabetes Development in Liver Transplantation Recipients
- Sejeong Lee, Minyoung Lee, Young-Eun Kim, Hae Kyung Kim, Sook Jung Lee, Jiwon Kim, Yurim Yang, Chul Hoon Kim, Hyangkyu Lee, Dong Jin Joo, Myoung Soo Kim, Eun Seok Kang
- Diabetes Metab J. 2024;48(1):146-156. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0100
- 963 View
- 120 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Post-transplant diabetes mellitus (PTDM) is one of the most significant complications after transplantation. Patients with end-stage liver diseases requiring transplantation are prone to sarcopenia, but the association between sarcopenia and PTDM remains to be elucidated. We aimed to investigate the effect of postoperative muscle mass loss on PTDM development.
Methods
A total of 500 patients who underwent liver transplantation at a tertiary care hospital between 2005 and 2020 were included. Skeletal muscle area at the level of the L3–L5 vertebrae was measured using computed tomography scans performed before and 1 year after the transplantation. The associations between the change in the muscle area after the transplantation and the incidence of PTDM was investigated using a Cox proportional hazard model.
Results
During the follow-up period (median, 4.9 years), PTDM occurred in 165 patients (33%). The muscle mass loss was greater in patients who developed PTDM than in those without PTDM. Muscle depletion significantly increased risk of developing PTDM after adjustment for other confounding factors (hazard ratio, 1.50; 95% confidence interval, 1.23 to 1.84; P=0.001). Of the 357 subjects who had muscle mass loss, 124 (34.7%) developed PTDM, whereas of the 143 patients in the muscle mass maintenance group, 41 (28.7%) developed PTDM. The cumulative incidence of PTDM was significantly higher in patients with muscle loss than in patients without muscle loss (P=0.034).
Conclusion
Muscle depletion after liver transplantation is associated with increased risk of PTDM development.
Letter
- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
- Jae Hyun Bae
- Diabetes Metab J. 2024;48(1):157-158. Published online January 29, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0430
- 669 View
- 114 Download
Response
- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
- Ho Gyun Lee, Il Hyeon Jung, Byong Seo Park, Hye Rim Yang, Kwang Kon Kim, Thai Hien Tu, Jung-Yong Yeh, Sewon Lee, Sunggu Yang, Byung Ju Lee, Jae Geun Kim, Il Seong Nam-Goong
- Diabetes Metab J. 2024;48(1):159-160. Published online January 29, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0458
- 662 View
- 93 Download

 KDA
KDA


 First
First Prev
Prev





