
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Current issue
- Page Path
- HOME > Browse > Current issue
Reviews
- Metabolic Risk/Epidemiology
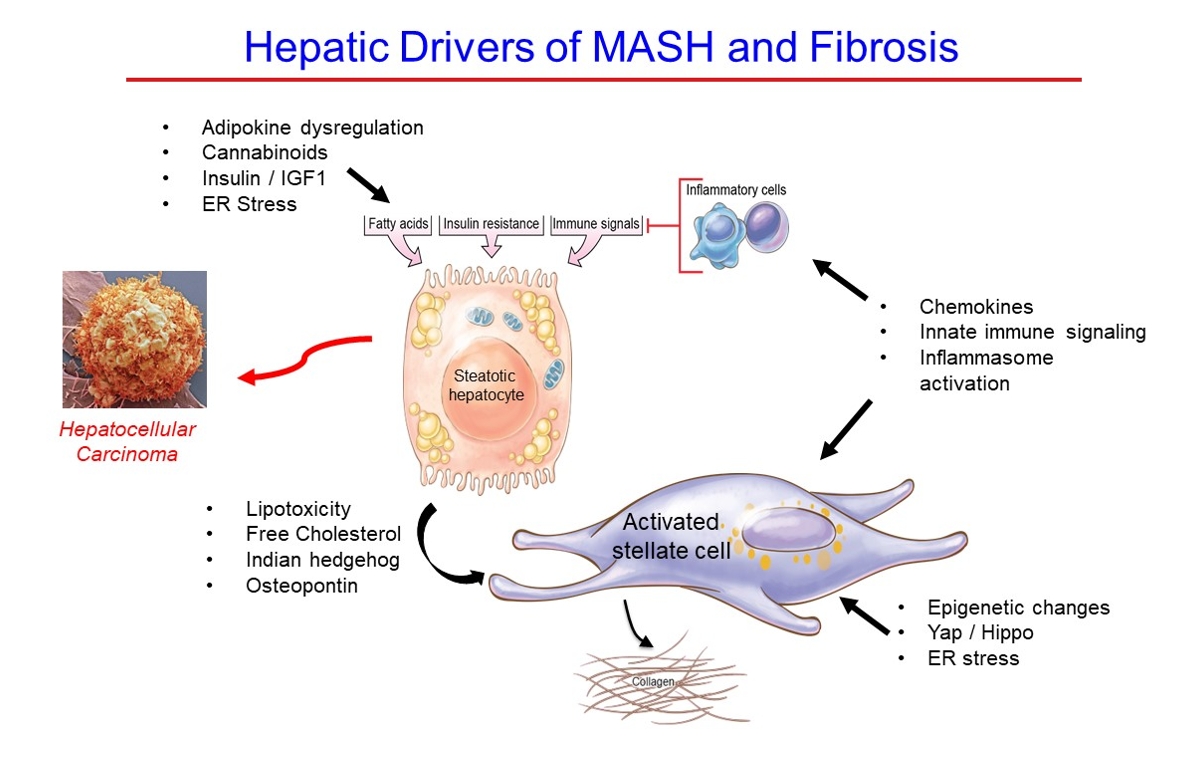
- Hepatic Fibrosis and Cancer: The Silent Threats of Metabolic Syndrome
- Scott L. Friedman
- Diabetes Metab J. 2024;48(2):161-169. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0240
- 2,391 View
- 290 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Metabolic dysfunction-associated steatotic (fatty) liver disease (MASLD), previously termed non-alcoholic fatty liver disease, is a worldwide epidemic that can lead to hepatic inflammation, fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). The disease is typically a component of the metabolic syndrome that accompanies obesity, and is often overlooked because the liver manifestations are clinically silent until late-stage disease is present (i.e., cirrhosis). Moreover, Asian populations, including Koreans, have a higher fraction of patients who are lean, yet their illness has the same prognosis or worse than those who are obese. Nonetheless, ongoing injury can lead to hepatic inflammation and ballooning of hepatocytes as classic features. Over time, fibrosis develops following activation of hepatic stellate cells, the liver’s main fibrogenic cell type. The disease is usually more advanced in patients with type 2 diabetes mellitus, indicating that all diabetic patients should be screened for liver disease. Although there has been substantial progress in clarifying pathways of injury and fibrosis, there no approved therapies yet, but current research seeks to uncover the pathways driving hepatic inflammation and fibrosis, in hopes of identifying new therapeutic targets. Emerging molecular methods, especially single cell sequencing technologies, are revolutionizing our ability to clarify mechanisms underlying MASLD-associated fibrosis and HCC.
- Metabolic Risk/Epidemiology
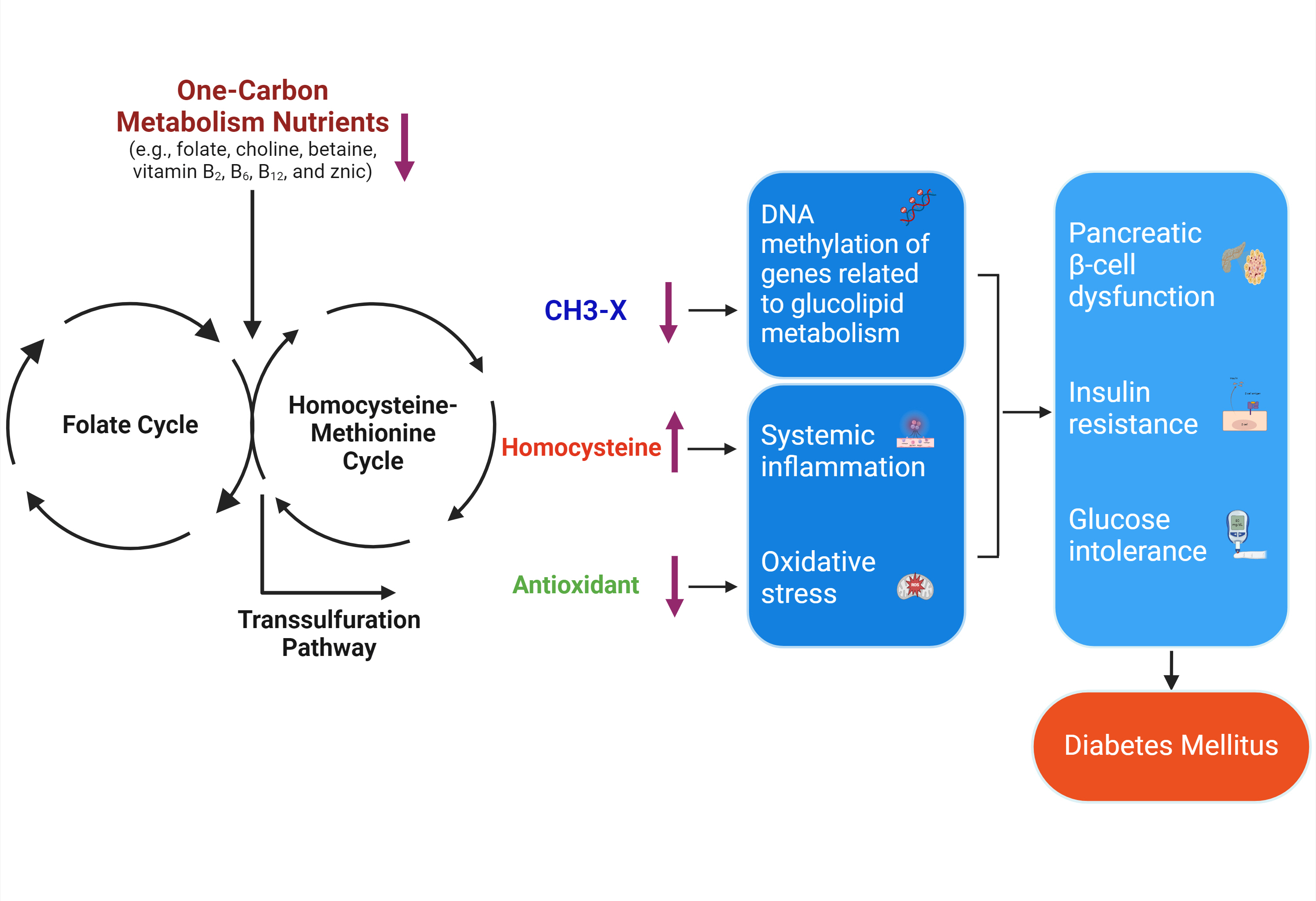
- One-Carbon Metabolism Nutrients, Genetic Variation, and Diabetes Mellitus
- Jie Zhu, Gunjana Saikia, Xiaotao Zhang, Xiaoxi Shen, Ka Kahe
- Diabetes Metab J. 2024;48(2):170-183. Published online March 12, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0272
- 1,298 View
- 167 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Diabetes mellitus (DM) affects about 9.3% of the population globally. Hyperhomocysteinemia (HHcy) has been implicated in the pathogenesis of DM, owing to its promotion of oxidative stress, β-cell dysfunction, and insulin resistance. HHcy can result from low status of one-carbon metabolism (OCM) nutrients (e.g., folate, choline, betaine, vitamin B6, B12), which work together to degrade homocysteine by methylation. The etiology of HHcy may also involve genetic variation encoding key enzymes in OCM. This review aimed to provide an overview of the existing literature assessing the link between OCM nutrients status, related genetic factors, and incident DM. We also discussed possible mechanisms underlying the role of OCM in DM development and provided recommendations for future research and practice. Even though the available evidence remains inconsistent, some studies support the potential beneficial effects of intakes or blood levels of OCM nutrients on DM development. Moreover, certain variants in OCM-related genes may influence metabolic handling of methyl-donors and presumably incidental DM. Future studies are warranted to establish the causal inference between OCM and DM and examine the interaction of OCM nutrients and genetic factors with DM development, which will inform the personalized recommendations for OCM nutrients intakes on DM prevention.
- Metabolic Risk/Epidemiology
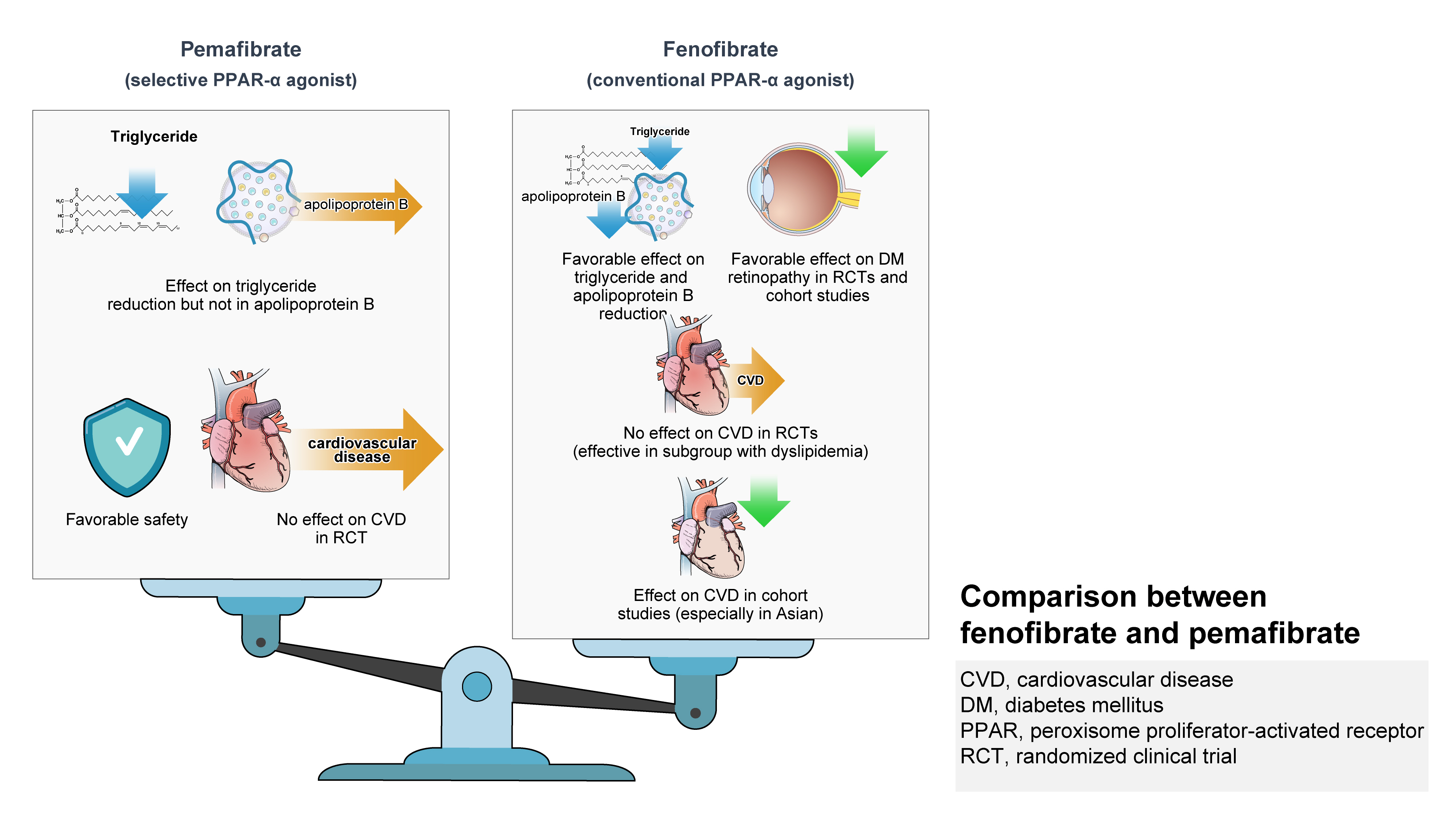
- Role of Fenofibrate Use in Dyslipidemia and Related Comorbidities in the Asian Population: A Narrative Review
- Chaicharn Deerochanawong, Sin Gon Kim, Yu-Cheng Chang
- Diabetes Metab J. 2024;48(2):184-195. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0168
- 2,367 View
- 347 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Hypertriglyceridemia and decreased high-density lipoprotein cholesterol (HDL-C) persist despite statin therapy, contributing to residual atherosclerotic cardiovascular disease (ASCVD) risk. Asian subjects are metabolically more susceptible to hypertriglyceridemia than other ethnicities. Fenofibrate regulates hypertriglyceridemia, raises HDL-C levels, and is a recommended treatment for dyslipidemia. However, data on fenofibrate use across different Asian regions are limited. This narrative review summarizes the efficacy and safety data of fenofibrate in Asian subjects with dyslipidemia and related comorbidities (diabetes, metabolic syndrome, diabetic retinopathy, and diabetic nephropathy). Long-term fenofibrate use resulted in fewer cardiovascular (CV) events and reduced the composite of heart failure hospitalizations or CV mortality in type 2 diabetes mellitus. Fenofibrate plays a significant role in improving irisin resistance and microalbuminuria, inhibiting inflammatory responses, and reducing retinopathy incidence. Fenofibrate plus statin combination significantly reduced composite CV events risk in patients with metabolic syndrome and demonstrated decreased triglyceride and increased HDL-C levels with an acceptable safety profile in those with high CV or ASCVD risk. Nevertheless, care is necessary with fenofibrate use due to possible hepatic and renal toxicities in vulnerable individuals. Long-term trials and real-world studies are needed to confirm the clinical benefits of fenofibrate in the heterogeneous Asian population with dyslipidemia.
- Others
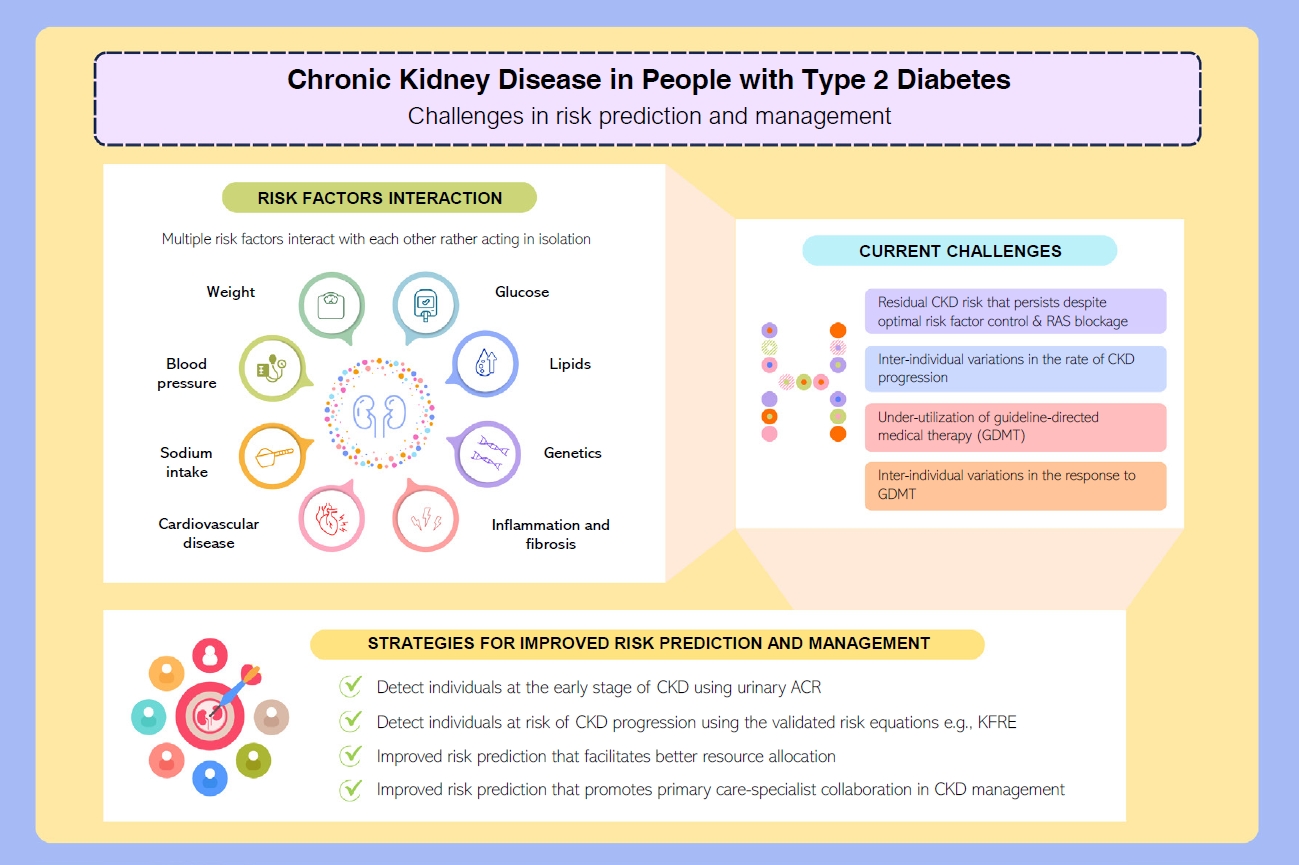
- Risk Prediction and Management of Chronic Kidney Disease in People Living with Type 2 Diabetes Mellitus
- Ying-Guat Ooi, Tharsini Sarvanandan, Nicholas Ken Yoong Hee, Quan-Hziung Lim, Sharmila S. Paramasivam, Jeyakantha Ratnasingam, Shireene R. Vethakkan, Soo-Kun Lim, Lee-Ling Lim
- Diabetes Metab J. 2024;48(2):196-207. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0244
- 1,952 View
- 366 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - People with type 2 diabetes mellitus have increased risk of chronic kidney disease and atherosclerotic cardiovascular disease. Improved care delivery and implementation of guideline-directed medical therapy have contributed to the declining incidence of atherosclerotic cardiovascular disease in high-income countries. By contrast, the global incidence of chronic kidney disease and associated mortality is either plateaued or increased, leading to escalating direct and indirect medical costs. Given limited resources, better risk stratification approaches to identify people at risk of rapid progression to end-stage kidney disease can reduce therapeutic inertia, facilitate timely interventions and identify the need for early nephrologist referral. Among people with chronic kidney disease G3a and beyond, the kidney failure risk equations (KFRE) have been externally validated and outperformed other risk prediction models. The KFRE can also guide the timing of preparation for kidney replacement therapy with improved healthcare resources planning and may prevent multiple complications and premature mortality among people with chronic kidney disease with and without type 2 diabetes mellitus. The present review summarizes the evidence of KFRE to date and call for future research to validate and evaluate its impact on cardiovascular and mortality outcomes, as well as healthcare resource utilization in multiethnic populations and different healthcare settings.
Editorials
- Enhancing Patient Outcomes: Prioritizing SGLT2is and GLP-1RAs in Diabetes with CVD
- Gwanpyo Koh
- Diabetes Metab J. 2024;48(2):208-212. Published online March 22, 2024
- DOI: https://doi.org/10.4093/dmj.2024.0096
- 777 View
- 119 Download
- SGLT2 Inhibitors and GLP-1 Agonists: A Beacon of Hope for Stroke Prevention in Diabetes
- Jae-Han Jeon
- Diabetes Metab J. 2024;48(2):213-214. Published online March 22, 2024
- DOI: https://doi.org/10.4093/dmj.2024.0079
- 971 View
- 142 Download
Original Articles
- Basic Research
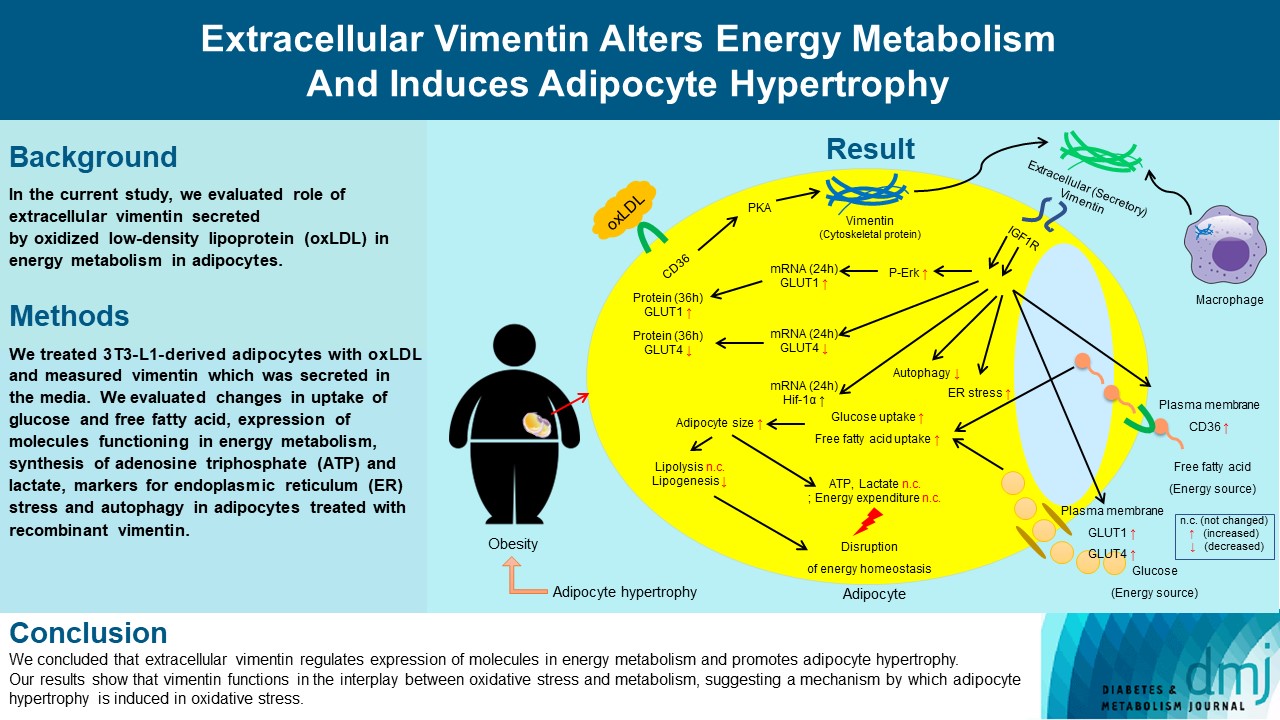
- Extracellular Vimentin Alters Energy Metabolism And Induces Adipocyte Hypertrophy
- Ji-Hae Park, Soyeon Kwon, Young Mi Park
- Diabetes Metab J. 2024;48(2):215-230. Published online September 26, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0332
- 2,373 View
- 201 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Previous studies have reported that oxidative stress contributes to obesity characterized by adipocyte hypertrophy. However, mechanism has not been studied extensively. In the current study, we evaluated role of extracellular vimentin secreted by oxidized low-density lipoprotein (oxLDL) in energy metabolism in adipocytes.
Methods
We treated 3T3-L1-derived adipocytes with oxLDL and measured vimentin which was secreted in the media. We evaluated changes in uptake of glucose and free fatty acid, expression of molecules functioning in energy metabolism, synthesis of adenosine triphosphate (ATP) and lactate, markers for endoplasmic reticulum (ER) stress and autophagy in adipocytes treated with recombinant vimentin.
Results
Adipocytes secreted vimentin in response to oxLDL. Microscopic evaluation revealed that vimentin treatment induced increase in adipocyte size and increase in sizes of intracellular lipid droplets with increased intracellular triglyceride. Adipocytes treated with vimentin showed increased uptake of glucose and free fatty acid with increased expression of plasma membrane glucose transporter type 1 (GLUT1), GLUT4, and CD36. Vimentin treatment increased transcription of GLUT1 and hypoxia-inducible factor 1α (Hif-1α) but decreased GLUT4 transcription. Adipose triglyceride lipase (ATGL), peroxisome proliferator-activated receptor γ (PPARγ), sterol regulatory element-binding protein 1 (SREBP1), diacylglycerol O-acyltransferase 1 (DGAT1) and 2 were decreased by vimentin treatment. Markers for ER stress were increased and autophagy was impaired in vimentin-treated adipocytes. No change was observed in synthesis of ATP and lactate in the adipocytes treated with vimentin.
Conclusion
We concluded that extracellular vimentin regulates expression of molecules in energy metabolism and promotes adipocyte hypertrophy. Our results show that vimentin functions in the interplay between oxidative stress and metabolism, suggesting a mechanism by which adipocyte hypertrophy is induced in oxidative stress.
- Basic Research
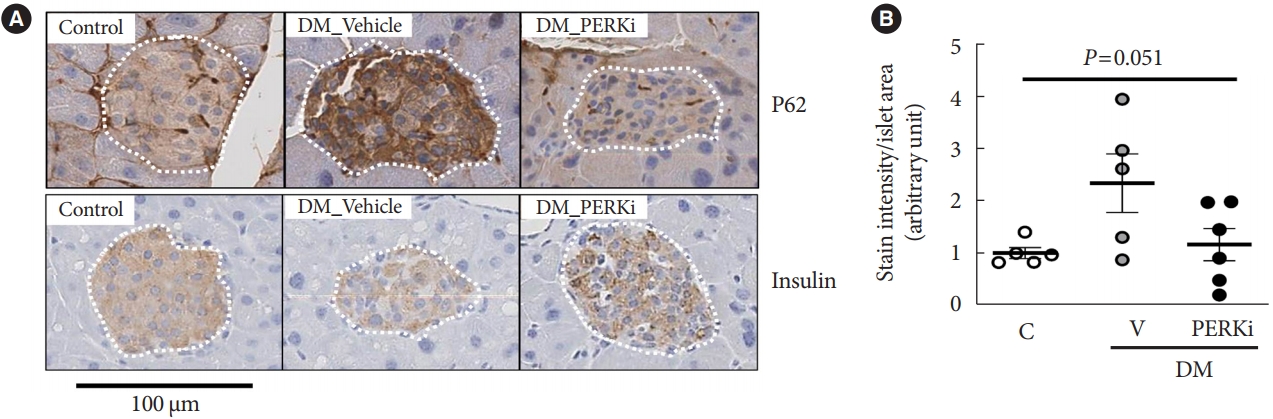
- Glucolipotoxicity Suppressed Autophagy and Insulin Contents in Human Islets, and Attenuation of PERK Activity Enhanced Them in an ATG7-Dependent Manner
- Seoil Moon, Ji Yoon Lim, Mirang Lee, Youngmin Han, Hongbeom Kim, Wooil Kwon, Jin-Young Jang, Mi Na Kim, Kyong Soo Park, Hye Seung Jung
- Diabetes Metab J. 2024;48(2):231-241. Published online September 6, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0366
- 1,772 View
- 164 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Administration of pancreatic endoplasmic reticulum kinase inhibitor (PERKi) improved insulin secretion and hyperglycemia in obese diabetic mice. In this study, autophagic balance was studied whether to mediate it.
Methods
Human islets were isolated from living patients without diabetes. PERKi GSK2606414 effects were evaluated in the islets under glucolipotoxicity by palmitate. Islet insulin contents and secretion were measured. Autophagic flux was assessed by microtubule associated protein 1 light chain 3 (LC3) conversion, a red fluorescent protein (RFP)-green fluorescent protein (GFP)- LC3 tandem assay, and P62 levels. For mechanical analyses, autophagy was suppressed using 3-methyladenine in mouse islets. Small interfering RNA for an autophagy-related gene autophagy related 7 (Atg7) was transfected to interfere autophagy.
Results
PERKi administration to mice decreased diabetes-induced P62 levels in the islets. Glucolipotoxicity significantly increased PERK phosphorylation by 70% and decreased insulin contents by 50% in human islets, and addition of PERKi (40 to 80 nM) recovered both. PERKi also enhanced glucose-stimulated insulin secretion (6-fold). PERKi up-regulated LC3 conversion suppressed by glucolipotoxicity, and down-regulated P62 contents without changes in P62 transcription, indicating enhanced autophagic flux. Increased autophagosome-lysosome fusion by PERKi was visualized in mouse islets, where PERKi enhanced ATG7 bound to LC3. Suppression of Atg7 eliminated PERKi-induced insulin contents and secretion.
Conclusion
This study provided functional changes of human islets with regard to autophagy under glucolipotoxicity, and suggested modulation of autophagy as an anti-diabetic mechanism of PERKi.
- Drug/Regimen
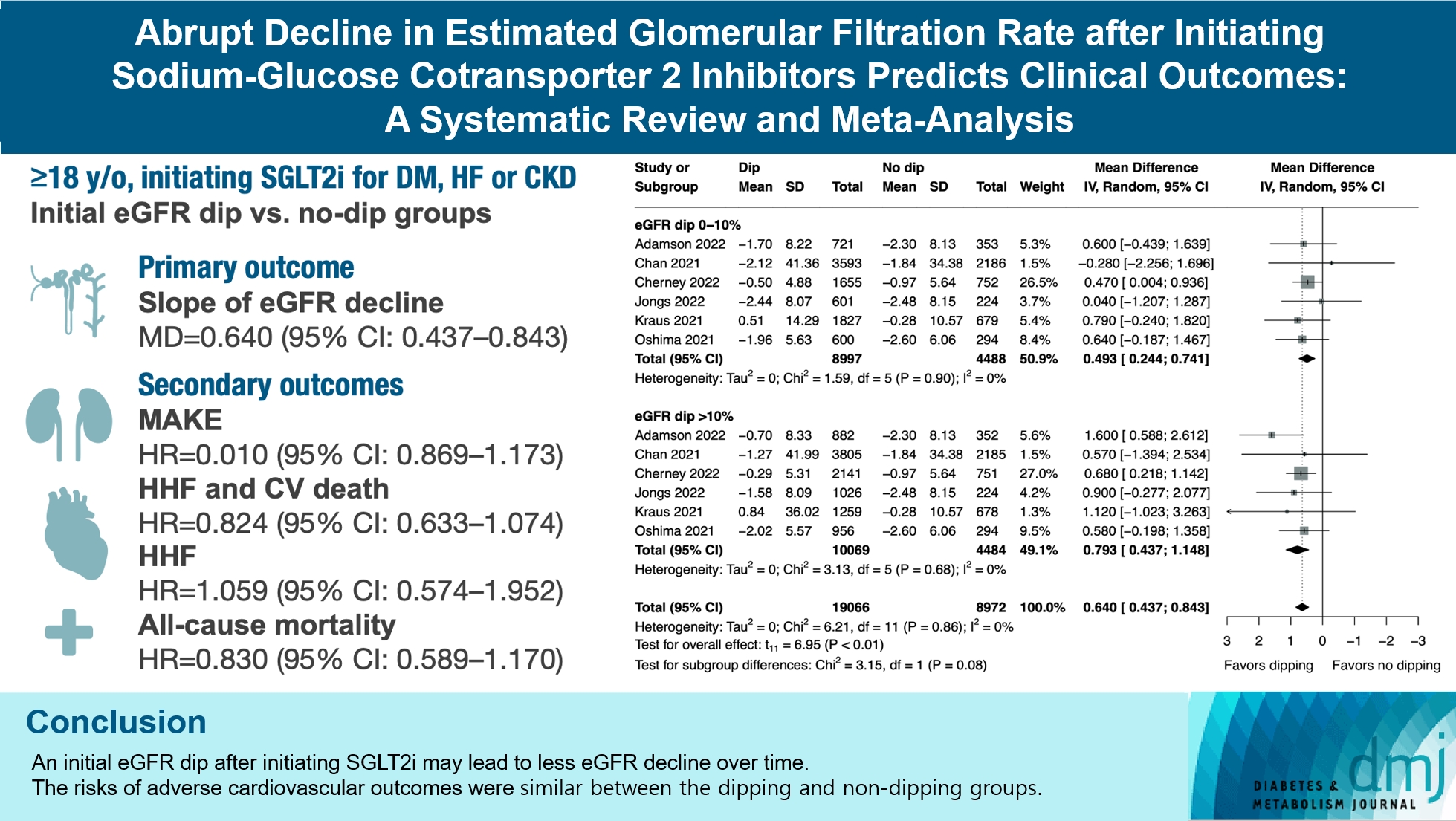
- Abrupt Decline in Estimated Glomerular Filtration Rate after Initiating Sodium-Glucose Cotransporter 2 Inhibitors Predicts Clinical Outcomes: A Systematic Review and Meta-Analysis
- Min-Hsiang Chuang, Yu-Shuo Tang, Jui-Yi Chen, Heng-Chih Pan, Hung-Wei Liao, Wen-Kai Chu, Chung-Yi Cheng, Vin-Cent Wu, Michael Heung
- Diabetes Metab J. 2024;48(2):242-252. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0201
- 1,617 View
- 214 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The initiation of sodium-glucose cotransporter-2 inhibitors (SGLT2i) typically leads to a reversible initial dip in estimated glomerular filtration rate (eGFR). The implications of this phenomenon on clinical outcomes are not well-defined.
Methods
We searched MEDLINE, Embase, and Cochrane Library from inception to March 23, 2023 to identify randomized controlled trials and cohort studies comparing kidney and cardiovascular outcomes in patients with and without initial eGFR dip after initiating SGLT2i. Pooled estimates were calculated using random-effect meta-analysis.
Results
We included seven studies in our analysis, which revealed that an initial eGFR dip following the initiation of SGLT2i was associated with less annual eGFR decline (mean difference, 0.64; 95% confidence interval [CI], 0.437 to 0.843) regardless of baseline eGFR. The risk of major adverse kidney events was similar between the non-dipping and dipping groups but reduced in patients with a ≤10% eGFR dip (hazard ratio [HR], 0.915; 95% CI, 0.865 to 0.967). No significant differences were observed in the composite of hospitalized heart failure and cardiovascular death (HR, 0.824; 95% CI, 0.633 to 1.074), hospitalized heart failure (HR, 1.059; 95% CI, 0.574 to 1.952), or all-cause mortality (HR, 0.83; 95% CI, 0.589 to 1.170). The risk of serious adverse events (AEs), discontinuation of SGLT2i due to AEs, kidney-related AEs, and volume depletion were similar between the two groups. Patients with >10% eGFR dip had increased risk of hyperkalemia compared to the non-dipping group.
Conclusion
Initial eGFR dip after initiating SGLT2i might be associated with less annual eGFR decline. There were no significant disparities in the risks of adverse cardiovascular outcomes between the dipping and non-dipping groups.
- Drug/Regimen
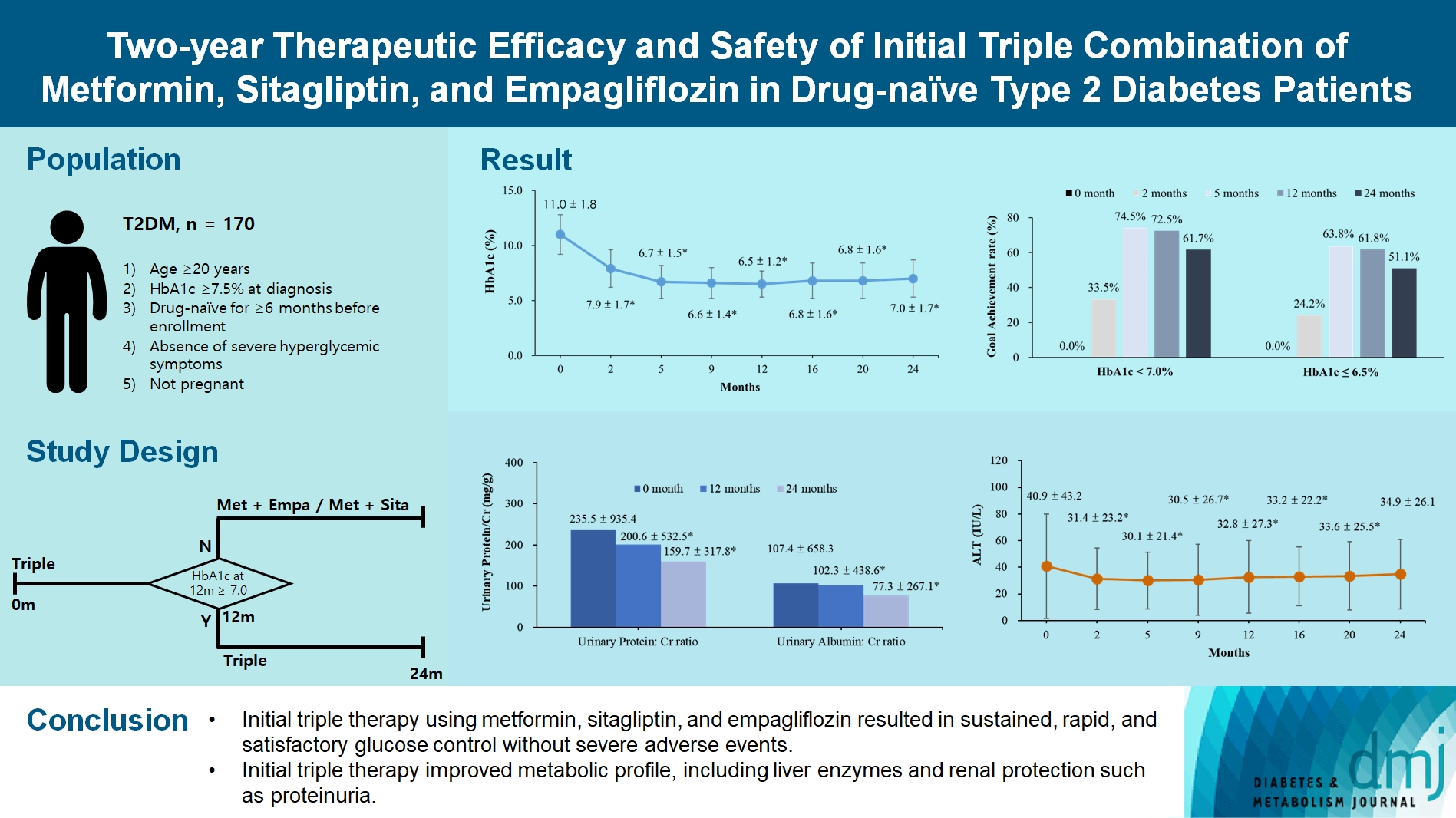
- Two-Year Therapeutic Efficacy and Safety of Initial Triple Combination of Metformin, Sitagliptin, and Empagliflozin in Drug-Naïve Type 2 Diabetes Mellitus Patients
- Young-Hwan Park, Minji Sohn, So Yeon Lee, Soo Lim
- Diabetes Metab J. 2024;48(2):253-264. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0128
- 1,814 View
- 290 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
We investigated the long-term efficacy and safety of initial triple therapy using metformin, a dipeptidyl peptidase-4 inhibitor, and a sodium-glucose cotransporter-2 inhibitor, in patients with type 2 diabetes mellitus.
Methods
We enrolled 170 drug-naïve patients with glycosylated hemoglobin (HbA1c) level >7.5% who had started triple therapy (metformin, sitagliptin, and empagliflozin). Glycemic, metabolic, and urinary parameters were measured for 24 months.
Results
After 24 months, HbA1c level decreased significantly from 11.0%±1.8% to 7.0%±1.7%. At 12 and 24 months, the rates of achievement of the glycemic target goal (HbA1c <7.0%) were 72.5% and 61.7%, respectively, and homeostasis model assessment of β-cell function and insulin resistance indices improved. Whole-body fat percentage decreased by 1.08%, and whole-body muscle percentage increased by 0.97% after 24 months. Fatty liver indices and albuminuria improved significantly. The concentration of ketone bodies was elevated at the baseline but decreased after 24 months. There were no serious adverse events, including ketoacidosis.
Conclusion
Initial triple combination therapy with metformin, sitagliptin, and empagliflozin led to achievement of the glycemic target goal, which was maintained for 24 months without severe hypoglycemia but with improved metabolic function and albuminuria. This combination therapy may be a good strategy for drug-naïve patients with type 2 diabetes mellitus.
- Cardiovascular risk/Epidemiology
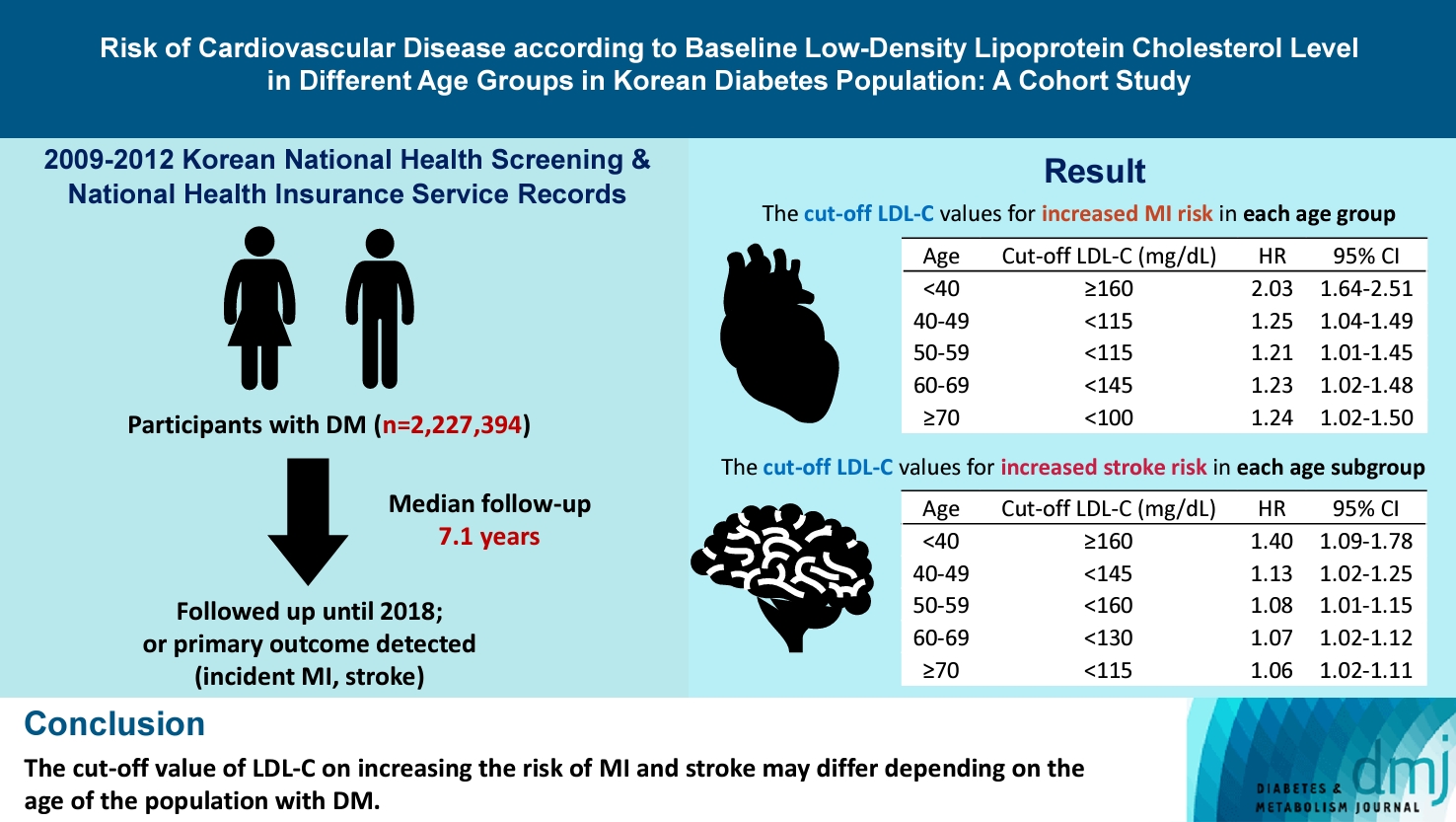
- Risk of Cardiovascular Disease according to Baseline Low-Density Lipoprotein Cholesterol Level in Different Age Groups in Korean Diabetes Population: A Cohort Study
- Tae Kyung Yoo, Kyung-Do Han, Eun-Jung Rhee, Won-Young Lee
- Diabetes Metab J. 2024;48(2):265-278. Published online February 26, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0443
- 755 View
- 149 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The association between low-density lipoprotein (LDL-C) levels and cardiovascular disease (CVD) risk in different age groups within the diabetes mellitus (DM) population remains unclear. The cohort study was conducted to investigate this relationship.
Methods
We assessed the 2009 to 2012 Korean National Health Screening and National Health Insurance Service records, with follow-up to the primary outcome (myocardial infarction [MI] or stroke) or December 2018. After excluding the participants with a history of MI or stroke, 2,227,394 participants with DM were included and categorized according to baseline LDL-C levels and age. Cox proportional hazards modeling was conducted. The CVD risk of age <40 years and LDL-C <70 mg/dL was set as the reference. In each age group, LDL-C <70 mg/dL was used as a reference for the subgroup analysis.
Results
The cut-off LDL-C value for increased MI risk in each age group varied (<40 years old, LDL-C ≥160 mg/dL: hazard ratios [HR], 2.03; 95% confidence interval [CI], 1.644 to 2.506) (40–49-year-old, LDL-C <115 mg/dL: HR, 1.245; 95% CI, 1.04 to 1.489) (50–59-year-old, LDL-C <115 mg/dL: HR, 1.21; 95% CI, 1.014 to 1.445) (60-69-year-old, LDL-C <145 mg/dL: HR, 1.229; 95% CI, 1.022 to 1.479) (≥70 years old group, LDL-C <100 mg/dL: HR, 1.238; 95% CI, 1.018 to 1.504). The cut-off LDL-C values for increased stroke risk varied in each age subgroup (<40 years old, LDL-C ≥160 mg/dL: HR, 1.395; 95% CI, 1.094 to 1.779) (40–49-year-old, LDL-C <145 mg/dL: HR, 1.13; 95% CI, 1.019 to 1.253) (50–59-year-old, LDL-C <160 mg/dL: HR, 1.079; 95% CI, 1.008 to 1.154) (60–69-year-old, LDL-C <130 mg/dL: HR, 1.07; 95% CI, 1.022 to 1.119) (≥70 years old, LDL-C <115 mg/dL: HR, 1.064; 95% CI, 1.019 to 1.112).
Conclusion
The effect of LDL-C on the risk of CVD differs depending on the age of the population with DM.
- Cardiovascular Risk/Epidemiology
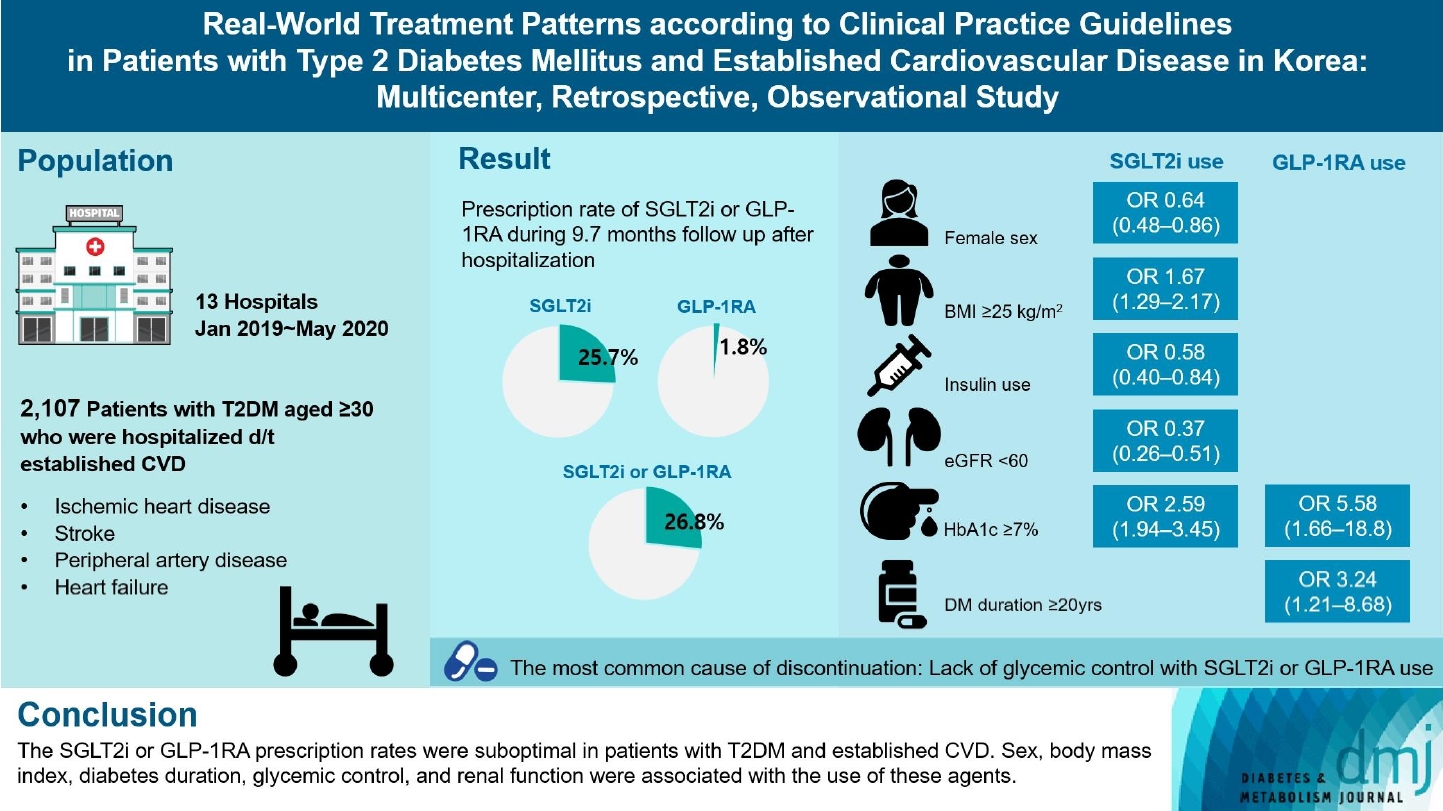
- Real-World Treatment Patterns according to Clinical Practice Guidelines in Patients with Type 2 Diabetes Mellitus and Established Cardiovascular Disease in Korea: Multicenter, Retrospective, Observational Study
- Ye Seul Yang, Nam Hoon Kim, Jong Ha Baek, Seung-Hyun Ko, Jang Won Son, Seung-Hwan Lee, Sang Youl Rhee, Soo-Kyung Kim, Tae Seo Sohn, Ji Eun Jun, In-Kyung Jeong, Chong Hwa Kim, Keeho Song, Eun-Jung Rhee, Junghyun Noh, Kyu Yeon Hur, Committee of Clinical Practice Guidelines, Korean Diabetes Association
- Diabetes Metab J. 2024;48(2):279-289. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0225
- 1,151 View
- 162 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Recent diabetes management guidelines recommend that sodium-glucose cotransporter 2 inhibitors (SGLT2is) or glucagon-like peptide 1 receptor agonists (GLP-1RAs) with proven cardiovascular benefits should be prioritized for combination therapy in patients with type 2 diabetes mellitus (T2DM) and established cardiovascular disease (CVD). This study was aimed at evaluating SGLT2i or GLP-1RA usage rates and various related factors in patients with T2DM and established CVD.
Methods
We enrolled adults with T2DM aged ≥30 years who were hospitalized due to established CVD from January 2019 to May 2020 at 13 secondary and tertiary hospitals in Korea in this retrospective observational study.
Results
Overall, 2,050 patients were eligible for analysis among 2,107 enrolled patients. The mean patient age, diabetes duration, and glycosylated hemoglobin level were 70.0 years, 12.0 years, and 7.5%, respectively. During the mean follow-up duration of 9.7 months, 25.7% of the patients were prescribed SGLT2is after CVD events. However, only 1.8% were prescribed GLP-1RAs. Compared with SGLT2i non-users, SGLT2i users were more frequently male and obese. Furthermore, they had a shorter diabetes duration but showed worse glycemic control and better renal function at the time of the event. GLP-1RA users had a longer duration of diabetes and worse glycemic control at the time of the event than GLP-1RA non-users.
Conclusion
The SGLT2i or GLP-1RA prescription rates were suboptimal in patients with T2DM and established CVD. Sex, body mass index, diabetes duration, glycemic control, and renal function were associated with the use of these agents. -
Citations
Citations to this article as recorded by- Enhancing Patient Outcomes: Prioritizing SGLT2is and GLP-1RAs in Diabetes with CVD
Gwanpyo Koh
Diabetes & Metabolism Journal.2024; 48(2): 208. CrossRef
- Enhancing Patient Outcomes: Prioritizing SGLT2is and GLP-1RAs in Diabetes with CVD
- Complications
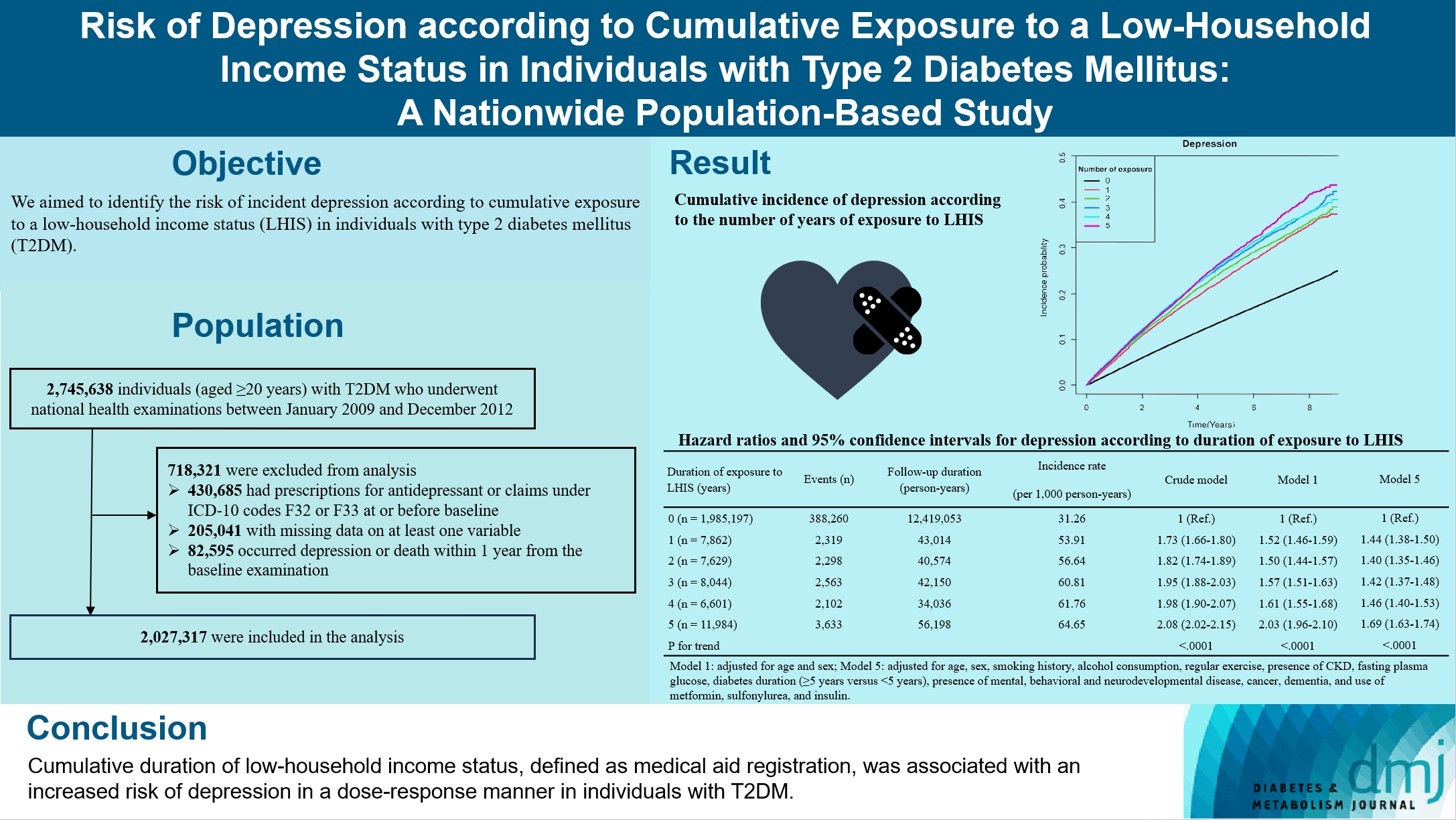
- Risk of Depression according to Cumulative Exposure to a Low-Household Income Status in Individuals with Type 2 Diabetes Mellitus: A Nationwide Population- Based Study
- So Hee Park, You-Bin Lee, Kyu-na Lee, Bongsung Kim, So Hyun Cho, So Yoon Kwon, Jiyun Park, Gyuri Kim, Sang-Man Jin, Kyu Yeon Hur, Kyungdo Han, Jae Hyeon Kim
- Diabetes Metab J. 2024;48(2):290-301. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0299
- 941 View
- 140 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
We aimed to identify the risk of incident depression according to cumulative exposure to a low-household income status in individuals with type 2 diabetes mellitus (T2DM).
Methods
For this retrospective longitudinal population-based cohort study, we used Korean National Health Insurance Service data from 2002 to 2018. Risk of depression was assessed according to cumulative exposure to low-household income status (defined as Medical Aid registration) during the previous 5 years among adults (aged ≥20 years) with T2DM and without baseline depression who underwent health examinations from 2009 to 2012 (n=2,027,317).
Results
During an average 6.23 years of follow-up, 401,175 incident depression cases occurred. Advance in cumulative number of years registered for medical aid during the previous 5 years from baseline was associated with an increased risk of depression in a dose-dependent manner (hazard ratio [HR], 1.44 [95% confidence interval (CI), 1.38 to 1.50]; HR, 1.40 [95% CI, 1.35 to 1.46]; HR, 1.42, [95% CI, 1.37 to 1.48]; HR, 1.46, [95% CI, 1.40 to 1.53]; HR, 1.69, [95% CI, 1.63 to 1.74] in groups with 1 to 5 exposed years, respectively). Insulin users exposed for 5 years to a low-household income state had the highest risk of depression among groups categorized by insulin use and duration of low-household income status.
Conclusion
Cumulative duration of low-household income status, defined as medical aid registration, was associated with an increased risk of depression in a dose-response manner in individuals with T2DM.
- Complications
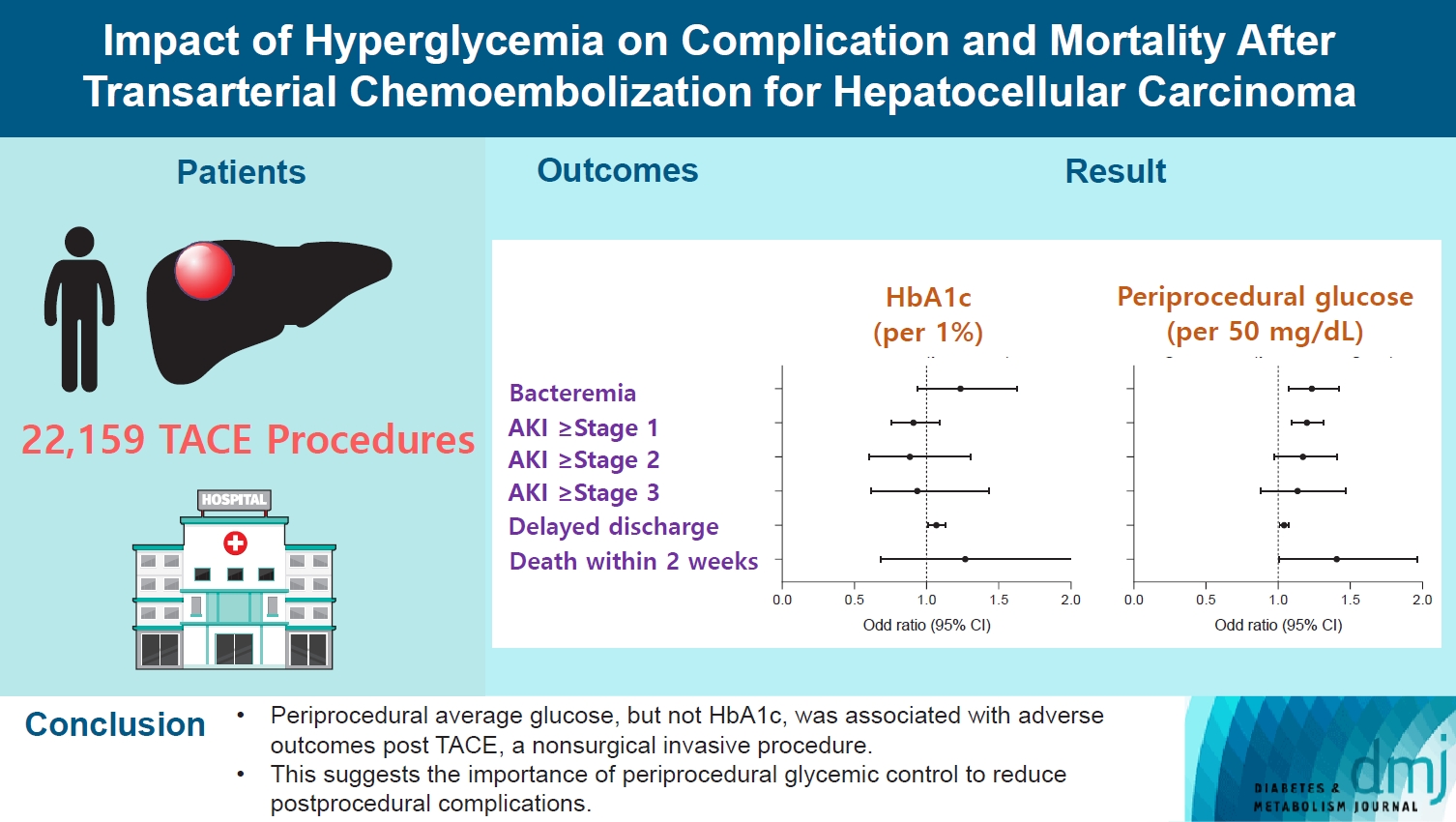
- Impact of Hyperglycemia on Complication and Mortality after Transarterial Chemoembolization for Hepatocellular Carcinoma
- Sun Joon Moon, Chang Ho Ahn, Yun Bin Lee, Young Min Cho
- Diabetes Metab J. 2024;48(2):302-311. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0255
- 790 View
- 118 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Current guidelines regarding periprocedural glycemic control to prevent complications after nonsurgical invasive procedures are insufficient. Transarterial chemoembolization (TACE) is a widely used treatment for unresectable hepatocellular carcinoma. We aimed to investigate the association between diabetes mellitus (DM) per se and the degree of hyperglycemia with postprocedural complications after TACE.
Methods
A total of 22,159 TACE procedures performed at Seoul National University Hospital from 2005 to 2018 were retrospectively analyzed. The associations between DM, preprocedural glycosylated hemoglobin (HbA1c), and periprocedural average glucose with postprocedural adverse outcomes were evaluated. The primary outcome was occurrence of postprocedural bacteremia. Secondary outcomes were acute kidney injury (AKI), delayed discharge and death within 14 days. Periprocedural glucose was averaged over 3 days: the day of, before, and after the TACE procedures. Propensity score matching was applied for procedures between patients with or without DM.
Results
Periprocedural average glucose was significantly associated with bacteremia (adjusted odds ratio per 50 mg/dL of glucose, 1.233; 95% confidence interval, 1.071 to 1.420; P=0.004), AKI, delayed discharge, and death within 14 days. DM per se was only associated with bacteremia and AKI. Preprocedural HbA1c was associated with delayed discharge. Average glucose levels above 202 and 181 mg/dL were associated with a significantly higher risk of bacteremia and AKI, respectively, than glucose levels of 126 mg/dL or lower.
Conclusion
Periprocedural average glucose, but not HbA1c, was associated with adverse outcomes after TACE, which is a nonsurgical invasive procedure. This suggests the importance of periprocedural glycemic control to reduce postprocedural complications.
- Others
- Comparative Effect of Glucose-Lowering Drugs for Type 2 Diabetes Mellitus on Stroke Prevention: A Systematic Review and Network Meta-Analysis
- Ji Soo Kim, Gyeongsil Lee, Kyung-Il Park, Seung-Won Oh
- Diabetes Metab J. 2024;48(2):312-320. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0421

- 1,276 View
- 212 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
There is still a lack of research on which diabetic drugs are more effective in preventing stroke. Our network metaanalysis aimed to compare cerebrovascular benefits among glucose-lowering treatments.
Methods
We searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, and the ClinicalTrials.gov registry for clinical trials from inception through May 25, 2021. We included both prespecified cerebrovascular outcomes and cerebrovascular events reported as severe adverse events. Subgroup analyses were conducted by stroke subtype, publication type, age of patients, baseline glycosylated hemoglobin (HbA1c), duration of type 2 diabetes mellitus, and cardiovascular risks.
Results
Of 2,861 reports and 1,779 trials screened, 79 randomized controlled trials comprising 206,387 patients fulfilled the inclusion criteria. In the pairwise meta-analysis, the use of glucagon-like peptide-1 (GLP-1) agonist was associated with a lower risk of total stroke compared with placebo (relative risk [RR], –0.17; 95% confidence interval [CI], –0.27 to –0.07). In the network meta- analysis, only the use of sodium-glucose cotransporter-2 (SGLT-2) inhibitor was associated with a reduction of total stroke, compared with placebo (RR, 0.81; 95% CI, 0.67 to 0.98). In the subgroup analyses, the use of SGLT-2 inhibitor and GLP-1 agonist was associated with a lower risk of stroke in those with high HbA1c (≥8.0) and low-risk of cardiovascular disease, respectively.
Conclusion
SGLT-2 inhibitors and GLP-1 agonists were shown to be beneficial for stroke prevention in patients with type 2 diabetes mellitus. -
Citations
Citations to this article as recorded by- SGLT2 Inhibitors and GLP-1 Agonists: A Beacon of Hope for Stroke Prevention in Diabetes
Jae-Han Jeon
Diabetes & Metabolism Journal.2024; 48(2): 213. CrossRef - Reply to comment on: Association of glucose-lowering drugs with incident stroke and transient ischaemic attacks in primary care patients with type 2 diabetes: disease analyser database
Wolfgang Rathmann, Karel Kostev
Acta Diabetologica.2024;[Epub] CrossRef
- SGLT2 Inhibitors and GLP-1 Agonists: A Beacon of Hope for Stroke Prevention in Diabetes

 KDA
KDA


 First
First Prev
Prev





