Role of Fenofibrate Use in Dyslipidemia and Related Comorbidities in the Asian Population: A Narrative Review
Article information
Abstract
Hypertriglyceridemia and decreased high-density lipoprotein cholesterol (HDL-C) persist despite statin therapy, contributing to residual atherosclerotic cardiovascular disease (ASCVD) risk. Asian subjects are metabolically more susceptible to hypertriglyceridemia than other ethnicities. Fenofibrate regulates hypertriglyceridemia, raises HDL-C levels, and is a recommended treatment for dyslipidemia. However, data on fenofibrate use across different Asian regions are limited. This narrative review summarizes the efficacy and safety data of fenofibrate in Asian subjects with dyslipidemia and related comorbidities (diabetes, metabolic syndrome, diabetic retinopathy, and diabetic nephropathy). Long-term fenofibrate use resulted in fewer cardiovascular (CV) events and reduced the composite of heart failure hospitalizations or CV mortality in type 2 diabetes mellitus. Fenofibrate plays a significant role in improving irisin resistance and microalbuminuria, inhibiting inflammatory responses, and reducing retinopathy incidence. Fenofibrate plus statin combination significantly reduced composite CV events risk in patients with metabolic syndrome and demonstrated decreased triglyceride and increased HDL-C levels with an acceptable safety profile in those with high CV or ASCVD risk. Nevertheless, care is necessary with fenofibrate use due to possible hepatic and renal toxicities in vulnerable individuals. Long-term trials and real-world studies are needed to confirm the clinical benefits of fenofibrate in the heterogeneous Asian population with dyslipidemia.
Highlights
· This review summarizes the efficacy and safety data of fenofibrate in Asian subjects.
· The cardiovascular benefit of fenofibrate has been proven in certain populations.
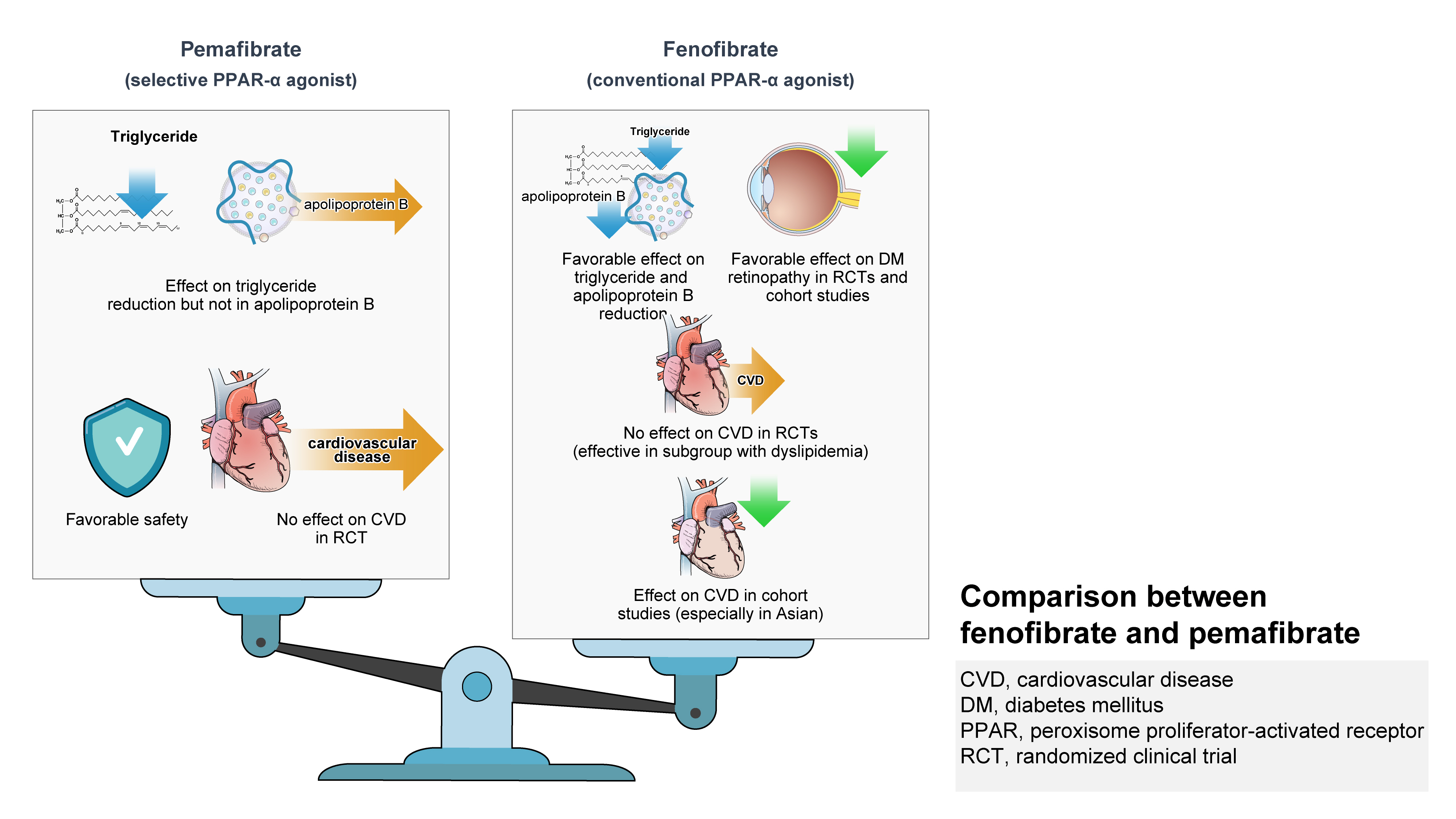
· On the contrary, pemafibrate didn’t show cardiovascular benefit in patients with diabetes.
· Both fibrates have distinct characteristics in reduction in LDL-C and apo-B levels.
INTRODUCTION
Epidemiology of dyslipidemia in the Asian region
Dyslipidemia refers to plasma lipid abnormalities including elevated levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C) or triglycerides (TG), and reduced levels of high-density lipoprotein cholesterol (HDL-C), or a combination of these attributes [1]. Hypertriglyceridemia is among the most common dyslipidemias observed in clinical practice and is characterized by increased plasma TG levels (fasting state, >150 mg/dL; fed state, >175 mg/dL) [2,3]. Primary causes of hypertriglyceridemia include congenital disorders while secondary causes include metabolic syndrome, type 2 diabetes mellitus (T2DM), central obesity, hypothyroidism, chronic kidney disease (CKD), and drug-induced dyslipidemia [4].
Elevated TG either independently or together with low levels of HDL-C and high levels of small, dense LDL poses a high cardiovascular (CV) risk as part of atherogenic dyslipidemia [5]. The Asia Pacific Cohort Studies Collaboration, reported serum TG as an independent predictor of CV risk. After adjustment for major CV risk factors, participants with TG levels in the highest fifth (≥168.3 mg/dL) had a 70% higher risk of coronary heart disease (CHD) death, whereas for fatal or nonfatal stroke and fatal or nonfatal CHD, an increased risk of 50% and 80%, respectively, was noted versus those in the lowest fifth (≤62.0 mg/dL) [6].
The prevalence of dyslipidemia differs substantially between the Asia-Pacific countries. Among the East and Southeast Asian countries, it ranged from 9.0% (Indonesia) to 46.9% (Philippines) for high TC, 7.8% (Taiwan) to 47.2% (Philippines) for high LDL-C, 10.1% (Taiwan) to 71.3% (Philippines) for low HDL-C, and 15.1% (China) to 38.6% (Philippines) for high TG [7]. The Responding to Increasing Cardiovascular Disease Prevalence (REDISCOVER) study of Malaysian adults reported the prevalence of raised TC (64.0%), LDL-C (56.7%), TG (37.4%), non-HDL-C (56.2%), and low HDL-C (36.2%) [8]. As per the ‘dyslipidemia fact sheets in Korea 2020,’ the prevalence of dyslipidemia in 2018 was 38.4% and increased TC, LDL-C, TG, and low HDL-C was 20.7% (crude), 19.2%, 16.1%, and 17.7%, respectively [9]. These disparities are mainly because of differences in types of the definition used for dyslipidemia in each country, lipid assays used, prescription rates of lipid-lowering drugs, year of investigation, age distribution of patients, ethnic differences, regional variations, and socioeconomic status [7]. Furthermore, a residual risk for atherosclerotic cardiovascular disease (ASCVD) persists when raised TG and lowered HDL-C levels are uncontrolled with statins [10]. Therefore, to improve the awareness and treatment rates and to reduce the prevalence of dyslipidemia and associated CV risk, country-specific epidemiological surveys and management guidelines in accordance with the national settings are recommended.
Fenofibrate: a peroxisome proliferator-activated receptor α agonist
Fibric acid derivatives or fibrates decrease TG or TG-rich lipoproteins and raise HDL levels through various peroxisome proliferator-activated receptor α (PPAR-α) actions [11,12]. Fenofibrate, the most commonly used PPAR-α agonist, exerts its lipid-lowering effects by activating the PPAR-α. Fig. 1 shows the mechanism of action of fenofibrate [13,14]. Additionally, fenofibrate maintains peroxisomal biogenesis and function, through PPAR-α pathway activation. It also attenuates liver injury and enhances hepatic fatty acid (FA) oxidation. The improved peroxisomal fitness induced by fenofibrate mediates protective effects against non-alcoholic fatty liver disease (NAFLD) as well [15].

Mechanism of action of fenofibrate. Fenofibrate mediates its lipid-lowering effects by activating the peroxisome proliferator-activated receptor-α (PPAR-α), which then forms a heterodimer with the nuclear receptor retinoid X receptor (RXR); and interacts with the peroxisome proliferator response element (PPRE) of target genes, including lipoprotein, fatty acid (FA), and cholesterol metabolism regulation genes. Fenofibrate enhances plasma clearance of atherogenic triglyceride (TG)-rich lipoproteins and lipolysis via activation of lipoprotein lipase and ApoAV, and reduced synthesis of the lipoprotein lipase inhibitor ApoCIII. It stimulates the production of high-density lipoprotein (HDL), ApoAI, and ApoAII, and reduces synthesis of very low-density lipoprotein (VLDL) and ApoB, while increasing the expression of adenosine triphosphate-binding cassette transporter A1 (ABCA1) and scavenger receptor B1 (SR-B1), leading to HDL-mediated cholesterol efflux from macrophages. Additionally, fenofibrate promotes β-oxidation, reduces the availability of free FAs, and thus inhibits TG synthesis; and inhibits the de novo FA synthesis by reducing the activities of acetyl-coenzyme A (CoA) carboxylase and FA synthase. Symbol ↑ indicates increase; ↓ indicates decrease;  indicates inhibition.
indicates inhibition.
Fibrates including fenofibrate, bezafibrate, gemfibrozil, ciprofibrate, and fenofibric acid [16-18] are recommended for the treatment of patients with dyslipidemia in Asia. Although treatment guidelines are available for each specific region, data on fenofibrate use across different ethnic regions of Asia are limited. It has been challenging to propose consensus management recommendations for dyslipidemia for these heterogeneous populations. In this review, we summarized the efficacy and safety data of fenofibrate in patients with dyslipidemia or hypertriglyceridemia and comorbid conditions, which may aid healthcare practitioners in informed clinical decision-making. The literature published in the English language in Embase and PubMed from 2012 to 2022 was reviewed. The keywords including fenofibrate, dyslipidemia, hypertriglyceridemia, T2DM, CV effect, and Asia were used as search strategy in different combinations to review the relevant publications.
POTENTIAL ROLE OF FENOFIBRATE
Dyslipidemia or hypertriglyceridemia with or without high risk of cardiovascular disease
Fenofibrate has demonstrated clinical benefits in patients with mixed or combined hyperlipidemia or hypertriglyceridemia with or without high CV risk (Supplementary Table 1). Fenofibrate significantly improved the coronary flow velocity reserve and arterial stiffness in Chinese patients with hypertriglyceridemia, demonstrating a potential protective role against atherosclerosis by normalizing endothelial disorders [19].
High risk of cardiovascular disease or ASCVD
Fenofibrate shows anti-inflammatory effects in selected high-risk patients with hypertriglyceridemia. Fenofibrate therapy decreased C-reactive protein (CRP) levels more in patients with high baseline CRP (≥1 mg/L) and without severe overweight (body mass index, ≤26 kg/m2; P=0.097) and/or in patients with low baseline HDL-C (<40 mg/dL) [20].
Statins plus fibrate combination could be beneficial in patients with high CV risk. However, the safety of this combination should be considered, and monitoring of aminotransferase levels is recommended to avoid hepatic complications [21,22]. Fenofibrate addition to statins improved lipid levels with an acceptable safety profile in Asian patients with mixed hyperlipidemia and high CV risk. The first randomized controlled trial (RCT) compared the non-lipid effects of rosuvastatin plus fenofibrate versus rosuvastatin monotherapy in this population. Combination therapy resulted in a significantly greater reduction in TG levels (after treatment, 143±81 mg/dL vs. 180±87 mg/dL, P=0.01) and a higher increase in HDL-C levels (after treatment, 53.7±12.8 mg/dL vs. 49.1±9.6 mg/dL. P=0.02) than the monotherapy [23]. In a phase 4 study of Chinese patients with dyslipidemia and high CV risk, fenofibrate addition to the existing statin treatment significantly decreased TG (by 38.1%) and increased HDL-C levels (by 17.4%) after 8 weeks of combination therapy (P<0.01 for both) [24]. A phase 4 RCT in Korean patients with elevated TG levels despite statin monotherapy, also reported significant improvements in the TG and HDL-C levels (P<0.05 for both) after 8 weeks of treatment with choline fenofibrate plus statin versus statin monotherapy [25]. Pitavastatin plus fenofibrate was associated with a greater reduction in non-HDL-C (between-group difference, −12.45%; P<0.001), and significant improvement in other lipid levels, apolipoproteins (Apos), and inflammatory markers (fibrinogen and high-sensitivity C-reactive protein [hsCRP]) with a safety profile similar to that of statin monotherapy in high-risk Korean patients with mixed dyslipidemia [10].
Furthermore, a study in Korean patients with ≥1 CV risk factors, at low LDL-C goal but with high TG levels, receiving fenofibrate for the first time showed a median change in TG and HDL-C of −60.0% and 14.3%, respectively (P<0.001 for both). The study indicated that in real-world practice, only half of the patients receiving fenofibrate achieved optimal TG levels (<150 mg/dL), and more attention is required while treating males or patients with diabetes [26].
Dyslipidemia without high cardiovascular disease or AS-CVD risk
In Korean patients with hypertriglyceridemia, omega-3 FA and fenofibrate showed similar improvements in TG levels and endothelium-dependent dilation. However, fenofibrate had considerably better effects on lipoprotein and metabolic profiles [27]. Omega-3 FA plus fenofibrate significantly decreased TG and TG/HDL-C ratio compared with fenofibrate alone or placebo (P<0.001). Although significant compared with placebo, the decrease in ApoB, non-HDL-C, hsCRP, fibrinogen, insulin, and glucose, and an improvement in flow-mediated dilation and insulin sensitivity were comparable between the combination and fenofibrate alone groups [28]. In the Effect of Fenofibrate and Ezetimibe Combination Treatment on Lipid (EFECTL) study of Japanese patients with combined hyperlipidemia, long-term therapy of fenofibrate plus ezetimibe significantly decreased LDL-C (P<0.01 for each) compared with fenofibrate or ezetimibe monotherapy and improved TG (P<0.001 for each) versus ezetimibe alone in [29]. Policosanol plus fenofibrate significantly improved the lipid parameters, arterial stiffness, and quality of life, with good tolerability in elderly patients with mixed dyslipidemia [30].
In addition, severe hypertriglyceridemia (TG levels >1,000 mg/dL) may induce acute pancreatitis [31]. Fenofibrate plus octreotide acetate demonstrated a significantly better (P=0.037) and synergistic therapeutic effect in Chinese patients with acute hyperlipidemia pancreatitis. The combination provided a more significant anti-inflammatory effect and protected liver function leading to the improved prognosis of these patients; and has been recommended for patients with acute pancreatitis having diabetes and fatty liver simultaneously [32].
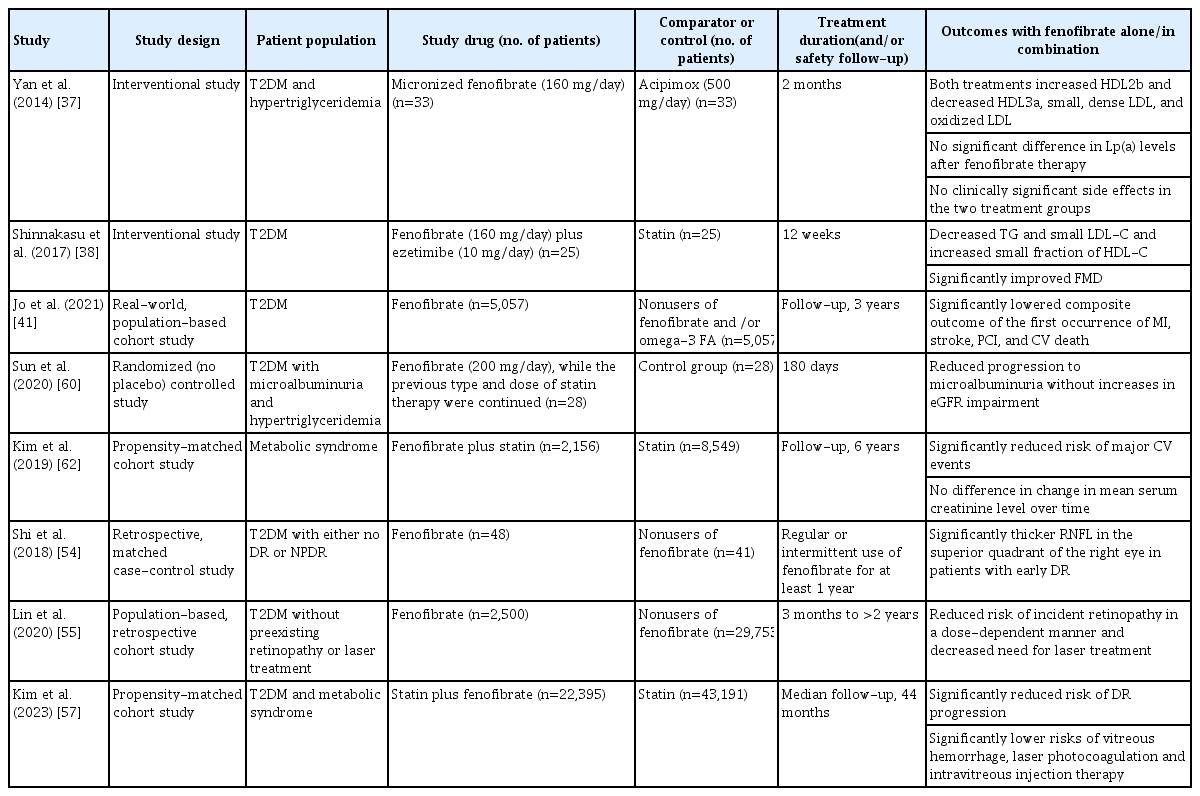
Beneficial effects of fenofibrate have also been demonstrated in patients with comorbidities like T2DM, diabetic retinopathy (DR), diabetic nephropathy (DN), and metabolic syndrome (Table 1).
Type 2 diabetes mellitus
Hypertriglyceridemia is associated with insulin resistance and β-cell dysfunction that contribute to the incidence of T2DM [33,34]. Therefore, controlling hypertriglyceridemia and improving these two pathologic disturbances may prevent T2DM development. A retrospective cohort study showed that Taiwanese patients with dyslipidemia receiving fenofibrate (n=2,806) or gemfibrozil (n=1,009) were not associated with an increased risk of developing new-onset diabetes mellitus (NODM) and had a neutral risk for NODM [35]. Fenofibrate significantly decreased the homeostasis model assessment index 2 for β-cell function (HOMA2-%β) and insulin resistance (HOMA2-IR) and increased homeostasis model assessment index 2 for insulin sensitivity and McAuley index in impaired glucose tolerance patients with hypertriglyceridemia (P=0.018 for HOMA2-%β; P<0.001 for all other parameters) [36]. Similar benefits of significantly alleviated IR and decreased secreting load of β-cells with fenofibrate were observed in an interventional study conducted in Chinese patients with normal glucose tolerance [33].
An RCT conducted in Chinese patients with T2DM and hypertriglyceridemia showed that fenofibrate and acipimox were comparably efficacious in modifying the profile of atherogenic lipids and resulted in the metabolism of HDL subclasses maturation in these patients. TG and TC levels were significantly reduced, and HDL-C levels and ApoA-I/B-100 ratio were increased. Compared with the baseline, a significant increase in large-sized HDL2b, whereas a significant decrease in small-sized HDL3a, small, dense-LDL, and oxidized-LDL was noted in both treatment groups. However, the acipimox intensively decreased lipoprotein(a) levels [37]. In Japanese patients with T2DM, fenofibrate plus ezetimibe was effective in controlling the TG and LDL-C levels, by increasing the levels of HDL-C small fraction and decreasing the small LDL-C levels. Improvement of vascular function in these patients was significantly associated with an increase in the very-small fraction of HDL-C [38].
The two non-Asian RCTs, Fenofibrate Intervention and Event Lowering in Diabetes (FIELD; conducted in Australia, New Zealand, and Finland) [39] and Action to Control Cardiovascular Risk in Diabetes-Lipid (ACCORD-Lipid; conducted in the United States and Canada) trials [40] did not show the benefit of cardiovascular disease (CVD) prevention with fenofibrate in patients with T2DM. However, a real-world, population-based cohort study (n=63,727) using the South Korean National Health Insurance Service data demonstrated that the long-term usage of fenofibrate reduced the rate of total and cardiac mortality, and CV events in patients with T2DM compared with nonusers of fenofibrate and/or omega-3 FA. The primary endpoint (a composite of myocardial infarction, stroke, percutaneous coronary revascularization, and cardiac death) was significantly reduced in fenofibrate users versus those using neither fenofibrate nor omega-3 FA (13.4 vs. 15.5 per 1,000 person-years; hazard ratio [HR], 0.76; 95% confidence interval [CI], 0.62 to 0.94; P=0.010). In subgroup analysis, the beneficial effect of fenofibrate was maintained consistently across all subsets of patients, including those grouped by LDL-C, TG, and HDL-C levels [41]. A recent analysis (n=5,518; follow-up, 4.7 years) of the ACCORD-Lipid trial demonstrated that in patients with T2DM receiving simvastatin, fenofibrate reduced the composite outcome of heart failure (HF) hospitalizations or CV death compared with placebo (P=0.048). This beneficial effect was observed predominantly in patients receiving standard background glucose-lowering therapy (HR, 0.64; 95% CI, 0.48 to 0.85; Pinteraction=0.017). A similar pattern was noted for HF hospitalizations alone [42].
Furthermore, the myocyte hormone irisin is linked to insulin resistance and metabolic disorders and might have implications for reducing CV risk [43,44]. Regulated on activation, normal T-cell expressed and secreted (RANTES), a CC chemokine is suggested to play an important role in inflammatory processes, and increased RANTES levels may be associated with a high CV risk [45]. Cross-sectional studies conducted in China have investigated the influence of fenofibrate therapy on serum irisin and RANTES levels in patients with T2DM with hypertriglyceridemia [43,45]. Treatment with fenofibrate for 8 weeks significantly decreased the serum irisin (P=0.011) and serum RANTES levels (P=0.018) from baseline, suggesting that fenofibrate plays a significant role in protecting against metabolic disorders by improving irisin resistance and by inhibition of inflammatory responses and CVD [43,45].
Results of the Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) trial, a multinational (countries, 24; not limited to the Asian population), randomized, placebo-controlled trial that evaluated CV outcomes with pemafibrate treatment have been recently published. In patients with T2DM, mild-to-moderate hypertriglyceridemia, and HDL-C ≤40 mg/dL, pemafibrate treatment failed to prove CV benefit compared with placebo. Pemafibrate reduced the levels of TG (−26.2%), very LDL-C (−25.8%), remnant cholesterol (−25.6%), and ApoCIII (−27.6%), but increased LDL-C (12.3%) and ApoB (4.8%) levels, compared with placebo. The incidence of major adverse CV events (primary composite endpoint) per 100 person-years was not lower in the pemafibrate group than in the placebo group (P=0.67). Also, the number of patients with venous thromboembolism (HR, 2.05; 95% CI, 1.35 to 3.17; P<0.001), pulmonary embolism (HR, 2.13; 95% CI, 1.20 to 3.89; P=0.008), or deep-vein thrombosis (DVT) (HR, 2.39; 95% CI, 1.37 to 4.33; P=0.001) was higher in the pemafibrate group than in the placebo group. However, the trial reported a lower incidence of NAFLD with pemafibrate treatment [46]. In contrast, fenofibrate reduced LDL-C (women, –9.8%; men, –3.3%) and ApoB (women, –10.4%; men, –5.8%) compared with placebo [47] and demonstrated no difference in the proportion of patients with DVT (fenofibrate vs. placebo, 1% vs. 1%) or pulmonary embolism (fenofibrate vs. placebo, 1% vs. 0.7%) in the FIELD trial [39].
Diabetic retinopathy
Besides the established lipid-lowering potential, fenofibrate is effective in preventing DR. The FIELD study [48] and the ACCORD Eye study [49] demonstrated the benefits of fenofibrate in reducing the progression of DR, including DR requiring a laser treatment [48].
In an observational study of Chinese patients with T2DM, fenofibrate significantly reduced the inflammatory cytokine (including vascular endothelial growth factor, tumor necrosis factor-α, interleukin-1β, and lipoprotein-associated phospholipase A2) levels in the non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR) groups. This indicated that DR may be ameliorated by the improved inflammatory response [50]. A retrospective observational analysis using the Japan Medical Data Center health claims database showed that of the 69,070 individuals with T2DM at baseline (cohort 1; with neither DR nor diabetic macular edema [DME]), about 5,687 individuals developed DR during the 3-year follow-up period. The proportion of bezafibrate and fenofibrate use was 54.4% and 45.5%, respectively. In patients without DR at baseline, fibrates use reduced the risk of newly developed DR (odds ratio [OR], 0.780; 95% CI, 0.663 to 0.918; P=0.003), whereas in patients with DR at baseline (cohort 2; with or without DME and no prior history of DR-related treatment), fibrates reduced the risk of incidence of DME (OR, 0.286; 95% CI, 0.122 to 0.670; P=0.004) and any treatments for DR (OR, 0.407; 95% CI, 0.193 to 0.857; P=0.018), including laser photocoagulation and vitrectomy [51]. In the Japan Diabetes Complication and its Prevention prospective study baseline survey, about 27.6% of patients with T2DM had NPDR and reported that the use of lipid-lowering medications (statin or fibrate) was associated with decreased odds of having NPDR in these patients. This protective association was also confirmed by propensity score (PS) adjustment for receiving fibrate and/or statin (OR, 0.80; 95% CI, 0.70 to 0.92; P=0.002) [52]. In a population-based study conducted in Taiwanese patients with T2DM and dyslipidemia, statin use reduced the risk of DR and the need for invasive treatments for vision-threatening diabetic retinopathy (VTDR) [53]. However, the cross-sectional analysis conducted by Kawasaki et al. [52] does not completely support the efficacy of statin plus fibrate in a longitudinal study, and these findings need to be confirmed with an ongoing longitudinal follow-up study.
In Chinese patients with T2DM with either no DR or NPDR (at an early stage of DR), the retinal nerve fiber layer (RNFL) of the superior quadrant of the right eye was significantly reduced in the non-fibrate users compared with the fenofibrate users (t=2.384, P=0.019). A positive association was noted between fenofibrate use and the RNFL thickness of the right eye (P=0.042) [54]. In Taiwanese patients with T2DM (without pre-existing retinopathy or laser treatment before recruitment), long-term and regular use of fenofibrate decreased the risk of incident retinopathy in a dose-dependent manner and the need for laser treatments (fenofibrate users vs. nonusers, 4.88% vs. 6.46%) [55]. A recent multicenter cohort study reported fenofibrate use to be associated with a decreased risk of PDR (HR, 0.76; 95% CI, 0.64 to 0.90; P=0.001) and VTDR (HR, 0.92; 95% CI, 0.87 to 0.98; P=0.01) but not DME alone (HR, 0.96; 95% CI, 0.90 to 1.03; P=0.27) [56].
Furthermore, the Effectiveness of Fenofibrate Therapy in Residual Cardiovascular Risk Reduction in the Real-World Setting (ECLIPSE-REAL) EYE study (conducted as part of the ECLIPSE-REAL study) aimed to determine if fenofibrate therapy is beneficial for preventing the progression of DR in patients with T2DM and metabolic syndrome (age, ≥30 years) receiving statin therapy and at risk of CVD in a real-world setting. Patients were matched 1:2 by PS into the statin plus fenofibrate group (n=22,395) and the statin-only group (n=43,191). The primary outcome was a composite of DR progression including vitreous hemorrhage, vitrectomy, laser photocoagulation, intravitreal injection therapy, and retinal detachment. In this propensity-matched cohort study, fenofibrate plus statin therapy reduced the risk of DR progression than statin therapy alone. The incident rate of treatment-mediated progression of DR per 1,000 person-years was lower in statin plus fenofibrate group (8.68) versus the statin-only group (9.66) (HR, 0.88; 95% CI, 0.81 to 0.96; P=0.005). Fenofibrate was associated with a 14% lower risk of vitreous hemorrhage, lower rates of undergoing laser photocoagulation (14%), and intravitreous injection therapy (27%). The magnitude of preventing the progression of DR was higher in patients with DR at baseline as in FIELD and ACCORD Eye studies. When combined with statin, fenofibrate is associated with a lower risk of DR progression in patients with T2DM having metabolic syndrome [57].
Diabetic nephropathy
Approximately 20% to 40% of patients with T2DM with microalbuminuria progress to evident nephropathy, and about 20% with evident nephropathy develop end-stage renal disease [58]. The FIELD study showed that fenofibrate could reduce albuminuria and prevent DN progression. In the sub-study, serum creatinine and estimated glomerular filtration rate (eGFR) measurements suggested a less significant loss of renal function in the fenofibrate group versus placebo for 5 years [59]. The ACCORD study demonstrated a reduction of microalbuminuria and macroalbuminuria in the fenofibrate group [40]. Similarly, in another RCT involving Chinese patients with T2DM and hypertriglyceridemia, fenofibrate improved microalbuminuria and did not increase the eGFR impairment. After 180 days of fenofibrate treatment, uric acid and TG levels, urinary microalbumin/creatinine ratio (UACR), and HOMA-IR were significantly reduced, whereas HDL-C levels significantly increased compared with the baseline (P<0.05 for all metabolic parameters). Also, the decrease in uric acid, TG, and UACR was greater at 180 days in the fenofibrate group than in the control group (P<0.05 for all). A decrease in UACR was positively associated with the decreases in TG (P=0.042) and uric acid (P=0.024) in the fenofibrate group [60]. Likewise, the recent ACCORD-Lipid trial analysis showed that the fenofibrate therapy caused more transitory worsening of eGFR events but slowed long-term eGFR decrease [42].
A retrospective Taiwanese national cohort study compared the outcomes of all-cause mortality, CV death, and incidence of permanent dialysis and major adverse cardiac cerebrovascular events (MACCEs) among patients with advanced CKD treated with fenofibrate, statins, a combination of both, and none of these. The prevalence of T2DM was >70% in all the treatment groups after the inverse probability of treatment weighting. Both fenofibrate and statin groups showed a lower risk of CV death than the nonuser group (fenofibrate vs. nonuser: HR, 0.84; 95% CI, 0.75 to 0.94 and statins vs. nonuser: HR, 0.94; 95% CI, 0.90 to 0.97). The fenofibrate group had the lowest incidence of permanent dialysis and fenofibrate plus high-intensity statins demonstrated a lower risk of MACCEs. This study suggested that continuing fenofibrate in patients with advanced CKD resulted in a protective effect on CV outcomes comparable with statins, and also delayed the requirement for permanent dialysis [61].
Metabolic syndrome
Individuals of Asian origin are metabolically more susceptible to metabolic syndrome than those from another origin [62]. In the FIELD and ACCORD-Lipid trials, there was no decrease in major CV events in patients with diabetes. However, a statistically significant decrease in CV risk was noted in a subgroup of patients with atherogenic dyslipidemia [59,63,64]. Hence, studies investigating fenofibrate efficacy in CV risk reduction in this patient population are necessary. A 1:5 PS-weighted cohort study (ECLIPSE-REAL; fenofibrate plus statin treatment, n=2,156; statin monotherapy, n=8,549) evaluated the efficacy of fenofibrate as an add-on to statin treatment in reducing the risk of major CV events in Korean adults (≥40 years) with metabolic syndrome. Patients from the National Health Insurance ServiceHealth Screening Cohort database who had used statins for at least 3 months were included (pre-existing CVD, 9.2%; T2DM, 37.8%). The risk of composite CV events was significantly reduced with the combination treatment compared with statin monotherapy during 6 years of follow-up (adjusted HR, 0.74; 95% CI, 0.58 to 0.93; P=0.01). Significance was retained in the on-treatment analysis as well (HR, 0.63; 95% CI, 0.44 to 0.92; P=0.02). Safety data reported an increase in mean serum creatinine level within 6 months of fenofibrate plus statin therapy use, followed by a gradual decline (mean change from baseline, 2.4%). However, change over time was not significantly different compared with statin monotherapy. Also, the proportions of participants with liver enzyme levels more than twice the upper limit of normal were similar between the two groups [62].
Furthermore, the ECLIPSE-REAL 2 study conducted in subjects with metabolic syndrome compared fenofibrate plus statin with omega-3 FA plus statin combination therapy based on 1:1 PS matching, and the results would be soon published. The primary endpoint of the study was 3-point major adverse CV events, comprising ischemic heart disease, ischemic stroke, and CV death. Incidence of all-cause mortality, hospitalization for HF, and adverse events of interest such as atrial fibrillation, hemorrhagic stroke, doubling of serum creatinine levels, and end-stage kidney disease were also evaluated.
GUIDELINES ON THE MANAGEMENT OF DYSLIPIDEMIA IN THE ASIAN REGION
A number of Asian countries have developed, or are in the process of developing clinical practice guidelines to improve the management of dyslipidemia with fibrate use:
(1) In individuals with a TG ≥500 mg/dL, immediate initiation of fibrates has been recommended to prevent acute pancreatitis [16,17].
(2) If TG levels persist at >200 mg/dL even after achieving the LDL-C target goal through therapeutic lifestyle modification and statin treatment, therapy with fibrate and omega-3 FAs can be considered [65].
(3) Statin plus gemfibrozil combination is not recommended over statin and fenofibrate combination therapy [17,18,66] due to the highest risk of myopathy with gemfibrozil among fibrates [18].
The recent Consensus recommendations by the Asian Pacific Society of Cardiology on dyslipidemia address the need for a unified management approach and are intended to guide clinicians and facilitate screening, early diagnosis, and treatment in this region [67].
However, dyslipidemia management should be patient-specific, considering the distinct baseline risk, clinical characteristics, comorbidities, as well as the individual concerns and preferences [67]. Clinicians should be aware of the factors that may restrict the uniform applicability of these consensus recommendations across all countries of the Asia Pacific region. The limiting factors may include accessibility and affordability of certain drugs, interventions, and other expertise, disparities in healthcare resources between countries; and currently recognized standards of care along with cultural aspects in respective countries [67].
CONCLUSIONS
Overall data suggest that fenofibrate treatment is effective and well-tolerated among Asian patients with dyslipidemia or hypertriglyceridemia and associated comorbidities. Recent evidence supports the potential role of fenofibrate in residual CV risk management, especially in patients with hypertriglyceridemia and/or low HDL-C level, despite appropriate statin therapy. However, the combination therapy with statins has potential risk of hepatic or renal side effects in vulnerable individuals that necessitate careful monitoring. Also, fenofibrate protects against prediabetes and diabetes-related CV complications by improving insulin sensitivity and β-cell function and has beneficial effects on microvascular complications (retinopathy and nephropathy). Furthermore, well-designed RCTs and real-world studies in Asian subjects are needed to confirm the benefit of fenofibrate focusing on metabolic syndrome or atherogenic dyslipidemia.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2023.0168.
Main characteristics of the studies in patients with dyslipidemia or hypertriglyceridemia with or without a high risk of CV disease
Notes
CONFLICTS OF INTEREST
Chaicharn Deerochanawong has received honoraria for lectures from Sanofi Aventis, Bayer, Novo Nordisk, AstraZeneca, Boehringer Ingelheim, Abbott, and Celltrion. Sin Gon Kim has received research grants and honoraria for lectures from Abbott. Yu-Cheng Chang has no competing interests to declare.
FUNDING
This narrative review was funded by Abbott Laboratories Ltd., Bangkok, Thailand.
Acknowledgements
Medical writing and editorial support were provided by Neetu Menghani, Ph.D. and Bhavani Yamsani, M.Pharm., MBA, Indegene Pvt. Ltd., Bangalore, India. This assistance was funded by Abbott Laboratories Ltd., Bangkok, Thailand.