- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 48(2); 2024 > Article
-
ReviewOthers Risk Prediction and Management of Chronic Kidney Disease in People Living with Type 2 Diabetes Mellitus
-
Ying-Guat Ooi1
 , Tharsini Sarvanandan1, Nicholas Ken Yoong Hee1, Quan-Hziung Lim1, Sharmila S. Paramasivam1, Jeyakantha Ratnasingam1, Shireene R. Vethakkan1, Soo-Kun Lim1, Lee-Ling Lim1,2,3
, Tharsini Sarvanandan1, Nicholas Ken Yoong Hee1, Quan-Hziung Lim1, Sharmila S. Paramasivam1, Jeyakantha Ratnasingam1, Shireene R. Vethakkan1, Soo-Kun Lim1, Lee-Ling Lim1,2,3
-
Diabetes & Metabolism Journal 2024;48(2):196-207.
DOI: https://doi.org/10.4093/dmj.2023.0244
Published online: January 26, 2024
- 1,848 Views
- 350 Download
1Department of Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
2Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong SAR, China
3Asia Diabetes Foundation, Hong Kong SAR, China
-
Corresponding author: Lee-Ling Lim
 Department of Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur 50603, Malaysia E-mail: leeling.lim@ummc.edu.my
Department of Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur 50603, Malaysia E-mail: leeling.lim@ummc.edu.my
Copyright © 2024 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- People with type 2 diabetes mellitus have increased risk of chronic kidney disease and atherosclerotic cardiovascular disease. Improved care delivery and implementation of guideline-directed medical therapy have contributed to the declining incidence of atherosclerotic cardiovascular disease in high-income countries. By contrast, the global incidence of chronic kidney disease and associated mortality is either plateaued or increased, leading to escalating direct and indirect medical costs. Given limited resources, better risk stratification approaches to identify people at risk of rapid progression to end-stage kidney disease can reduce therapeutic inertia, facilitate timely interventions and identify the need for early nephrologist referral. Among people with chronic kidney disease G3a and beyond, the kidney failure risk equations (KFRE) have been externally validated and outperformed other risk prediction models. The KFRE can also guide the timing of preparation for kidney replacement therapy with improved healthcare resources planning and may prevent multiple complications and premature mortality among people with chronic kidney disease with and without type 2 diabetes mellitus. The present review summarizes the evidence of KFRE to date and call for future research to validate and evaluate its impact on cardiovascular and mortality outcomes, as well as healthcare resource utilization in multiethnic populations and different healthcare settings.
- • CKD is a complex condition with heterogeneous disease progression and health outcomes.
- • Residual CKD risk persists despite optimal risk factor control and RAS blockade.
- • New GDMTs (SGLT-2i, ns-MRAs, GLP-1 RAs) show cardiorenal benefits beyond RAS blockade.
- • Improved prediction of CKD progression aids prognostication and prioritization of GDMT.
- • KFRE is a well-validated prediction tool for CKD G3a–G5 with promising scalability.
Highlights
- Diabetes has emerged as one of the most pressing health emergencies of the 21st century, contributing to one death in every 5 seconds [1]. The number of people with diabetes worldwide is projected to reach over 1.3 billion by 2050 [2], and the total diabetes-related health expenditure will reach US$ 1 trillion by 2030 [1]. Of note, 80% of people with diabetes live in low- and middle-income countries [1].
- Type 2 diabetes mellitus (T2DM), especially when suboptimally controlled, can lead to the development and progression of chronic kidney disease (CKD) [3,4]. Studies have shown that 29% to 38% of people with T2DM develop CKD after a median follow-up of 15 years [5]. According to the Global Kidney Health Atlas 2023, upper-middle income countries (UMICs; 0.1%) and high-income countries (HICs; 0.2%) showed a greater kidney failure rate than low-income countries (LICs; 0.05%) or lower-middle income countries (LMICs; 0.07%). Interestingly, the prevalence of CKD increased with the national income level: LICs (3.6%), LMICs (7.5%), UMICs (10.7%), and HICs (11.1%) [6]. Nonetheless, inadequate access to kidney replacement therapy (KRT) is common in LICs and LMICs, leading to increased risk of premature deaths which are preventable if diagnosed and treated early. The proportion of people with end-stage kidney disease (ESKD) who are not receiving KRT is higher in LICs (98%) and LMICs (94%) than in UMICs (79%) and HICs (30%) [6].
- The natural history of CKD in T2DM has been proposed to involve glomerular hyperfiltration and progressive albuminuria, followed by kidney function decline with eventual kidney failure [7,8]. This concept was later complicated by the emergence of a phenotype called non-albuminuric CKD with differential clinical and molecular features [9], although its pathogenesis is not well understood. Although serum creatinine (and therefore, estimated glomerular filtration rate [eGFR]) and albuminuria have been regarded as the key parameters of CKD diagnosis and progression, these laboratory measures and the classification of CKD are not without caveats (see Section “Gaps in risk prediction and management of CKD in T2DM” below) [10]. Therefore, there is a crucial need for better risk stratification strategies to identify people with an increased risk of progressing to ESKD.
- In this present review, we aim to discuss (1) residual risk and care gaps in the management of people with CKD and T2DM, and (2) the potential utilization of a CKD risk prediction model to guide clinical decision-making.
INTRODUCTION
- Epidemiology
- CKD is defined as abnormalities of kidney structure or function for a minimum of 3 months with implications for health [11]. The Global Burden of Disease Study reported that the global prevalence of CKD had increased by 29.3% from 1990 and affected 9.1% of the global population in 2017 [12]. Globally, the mortality rate from CKD increased by 41.5% between 1990 and 2017, making CKD the 12th leading cause of death (from 17th in 1990) with the majority of the CKD burden occurring at LMICs [12].
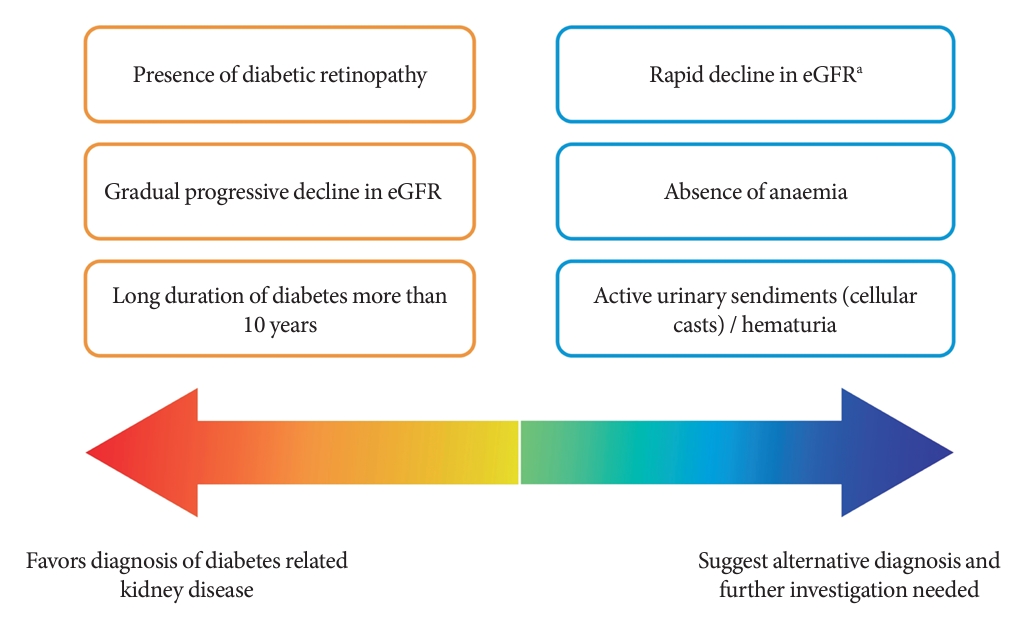
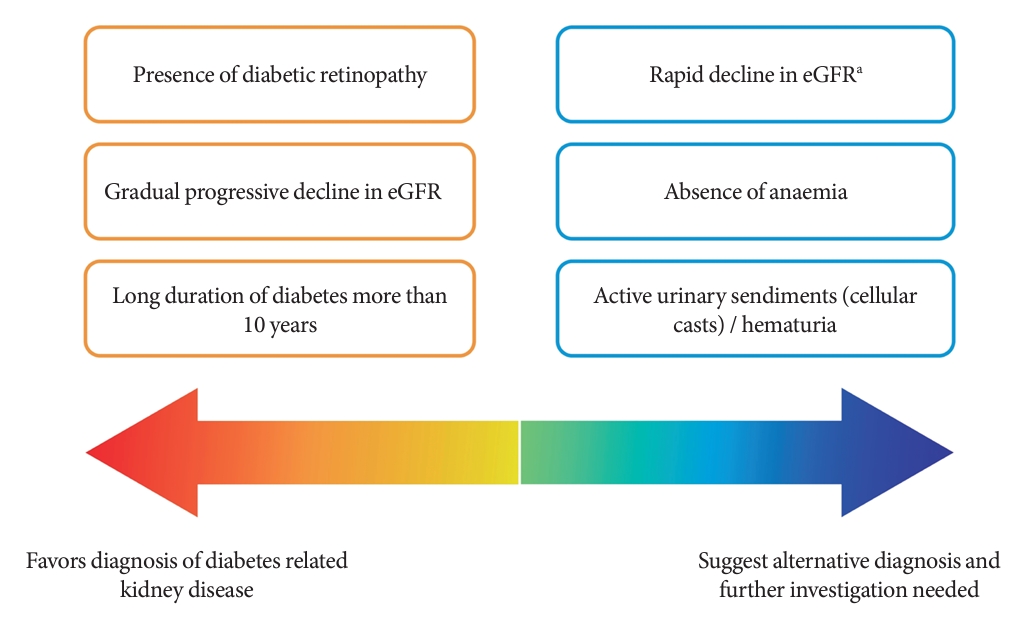
- Among people with T2DM, the annual incidence rate of eGFR below 60 mL/min/1.73 m2 and albuminuria are estimated at 2% and 8%, respectively [13]. The estimated incidence of ESKD among people with CKD G3a is 0.3 per 1,000 personyears, while it increases to 4 and 43 per 1,000 person-years in CKD G3b and G4, respectively [14]. Although kidney biopsy is the gold standard to ascertain the underlying etiologies of CKD, it is not routinely performed due to procedural risks, labor-intensity, and patients’ reluctance [15]. The clinical diagnosis of CKD in T2DM and the prediction of its progression are dependent on a person’s clinical profiles and laboratory measurements in routine clinical practice (Fig. 1) [16-18].
- Residual risk of CKD and cardiovascular disease
- Robust evidence has also shown a bidirectional relationship between CKD and atherosclerotic cardiovascular disease (ASCVD) among people with T2DM [3,4,19]. The incidence of ASCVD in people with T2DM has reduced in most HICs due to improved care delivery, treatment target attainment and use of guideline-directed medical therapy (GDMT) including renin-angiotensin system (RAS) inhibitors and statins [20,21]. However, a similar trend is yet to be reported in LMICs. Furthermore, a large gap remains in the incidence of ASCVD, CKD and mortality between people with and without T2DM [22,23]. Despite optimal management of diabetes, blood pressure and lipids, people with diabetes still have two to three times higher risk of morbidity and mortality than those without diabetes [24].
- Aging population and declining incidence of ASCVD have led to the emergence of heart failure and CKD with significant morbidity and mortality in people with T2DM [25,26]. While optimization of multiple cardiometabolic risk factors and use of RAS inhibitors and statins have reduced the incidence of CKD, residual risk persists which require additional treatment approaches [3,4,27].
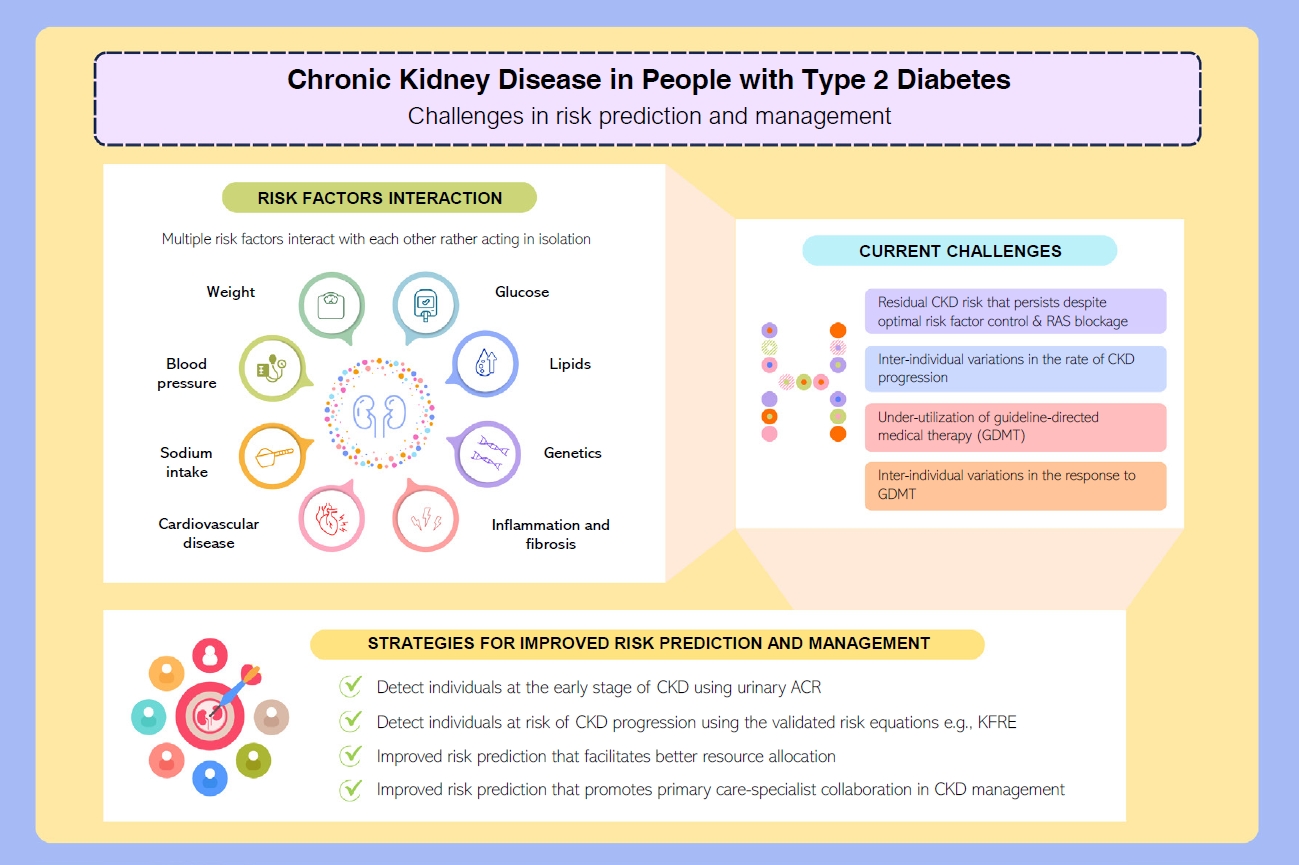
- Gaps in risk prediction and management of CKD in T2DM
- CKD is a complex condition with heterogenous disease progression and health outcomes [28,29]. A prospective cohort study involving 6,330 people with diabetic kidney disease identified four distinct patterns in eGFR trajectories: the slow decline (84.3%), the curvilinear decline (6.5%), the progressive decline (6.1%), and the accelerated decline (3.1%) [30]. Compared to the slow decline group, those with accelerated eGFR decline reported an odds ratio of 6.9 (95% confidence interval, 5.6 to 8.4) for all-cause mortality [30].
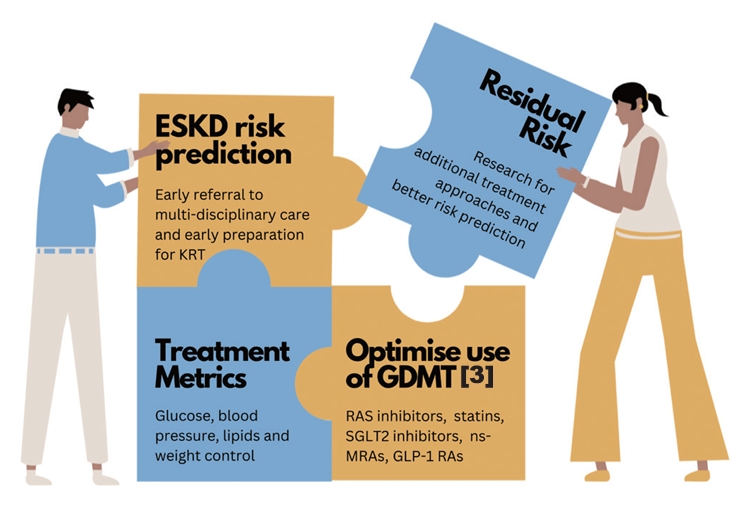
- Accurate prediction of the risk of ESKD is the cornerstone of optimal CKD management. It enables better management of people at high risk of ESKD in order to slow disease progression, improve prognostication and prioritize treatment pathways such as optimization of GDMT and early preparation for KRT, as summarized in (Fig. 2) [3,31].
- In 2002, National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) published the clinical practice guideline for evaluation and classification system for CKD based on eGFR [32]. Since 2009, the Kidney Disease: Improving Global Outcomes (KDIGO) group recommends risk stratification of CKD using eGFR and urinary albumin-creatinine ratio (ACR), as the latter has an independent effect on the progression of CKD [33]. The KDIGO CKD classification informs about the risk of progression to ESKD but lacks absolute risk quantification which is essential to facilitate clinical decision-making. When resources are scarce, especially in LMICs, lack of a reliable kidney failure risk prediction model delays treatment in those who are at high risk of progression to ESKD or unnecessarily offers treatment/referral to nephrologists among those who have low risk and stable CKD [34].
- These challenges call for a better CKD risk prediction model as the rates of CKD progression vary considerably between people with CKD. Cluster analysis has also suggested that the subclassification of T2DM may play a role in predicting risk of CKD. For example, the severe insulin-resistant diabetes, uric acid-related diabetes, and inheritance-related diabetes clusters were associated with an increased risk of CKD in Scandinavian populations [35,36].
- Developing clinical risk scores and prediction models have become increasingly popular. As of October 27, 2023 in the preparation of the present review, a PubMed search using the medical subject headings “risk prediction” AND “chronic kidney disease” revealed 437 clinical studies on CKD risk prediction. Notwithstanding a few notable examples like the kidney failure risk equation (KFRE) developed by Tangri et al. [34], most kidney failure prediction models were neither externally validated nor considered competing risk of death prior to ESKD [37,38].
- Of note, the emergence of biomarkers such as plasma soluble tumor necrosis factor receptors 1 (TNFR1), soluble tumor necrosis factor receptors 2 (TNFR2), and kidney injury molecule-1 (KIM-1) for early detection of people at high risk for CKD progression could help to guide management for better health outcomes [39]. On top of known clinical parameters included in the KFRE (eGFR, urinary ACR, and serum calcium), a machine-learning CKD risk prediction algorithm called KidneyIntelX has been developed by incorporating the aforementioned emerging biomarkers with glycosylated hemoglobin, systolic blood pressure, platelet count, and aspartate transferase [40]. The artificial intelligence-enabled KidneyIntelX algorithm stratifies people with T2DM and CKD G1–3b or albuminuria into three risk tiers namely low, intermediate, and high risk, with the corresponding recommendations on monitoring and treatment [40]. The KidneyIntelX algorithm has been externally validated in multinational cohorts from HICs [41-43] with proven clinical utility [44,45]. However, it is not widely available due to the high cost involved in testing the emerging biomarkers (TNFR1, TNFR2, and KIM-1) and has not been validated in LMICs. Nevertheless, there is an emergence of studies investigating the use of machine-learning algorithms to predict the risk of ESKD with good prediction ability, and algorithms incorporated into electronic health systems could potentially achieve large-scale population screening for CKD in regions with limited resources [46-49].
- Management of T2DM requires a holistic approach which includes attaining control of multiple cardiometabolic risk factors (glucose, blood pressure, lipids, and body weight) and cardiovascular risk reduction strategies to prevent complications and death [3,4,50]. This has been discussed extensively in a 2023 review that summarizes the latest evidence in cardiorenal risk reduction among people with and without T2DM [3]. Use of RAS inhibitors and statins have reduced the incidence of cardiovascular-renal events and related death, but the incidence remains higher than those without diabetes [3]. At a global level, there is also under-utilization of newer GDMT including glucagon-like peptide-1 receptor agonists (GLP-1 RA) and sodium-glucose co-transporter 2 (SGLT2) inhibitors at 9%–15% and 8%–15% among people with either T2DM or ASCVD, respectively [51].
- Although SGLT2 inhibitors are now included in the World Health Organization Essential Medications list, high acquisition cost, lack of reimbursement, insufficient risk assessment for timely intervention, and therapeutic inertia remain the major barriers to their implementation in clinical practice [52]. Nonetheless, SGLT2 inhibitors and non-steroidal mineralocorticoid receptor antagonists have shown positive cardiorenal benefits in the dedicated kidney end-points trials (Supplementary Table 1) [53-57]. Meanwhile, GLP-1 RA has proven benefits in cardiovascular outcome trials and recently, a dedicated kidney end-points trial (FLOW A Research Study to See How Semaglutide Works Compared to Placebo in People with Type 2 Diabetes and Chronic Kidney Disease; NCT03819153) is terminated early due to positive results [58].
- The 2023 Global Kidney Health Atlas reported significant gaps persist between HICs and LMICs in most key components of CKD care [6]. These disparities encompass areas such as the availability of public funding, screening practices, access to specialist care, essential medicines and technology, KRT and the establishment of CKD registries. Dialysis withdrawal due to cost contributed to mortality rates in 18% of LICs and 7% of LMICs, but none was reported in HICs.
- Taken together, early identification and risk stratification of people who have increased risk of rapid CKD progression will facilitate timely intervention to prevent ESKD and premature mortality. Significant disparities related to comprehensive CKD care in LMICs should be proactively addressed by policymakers by increasing health care financing for CKD care, addressing workforce shortages, developing a surveillance system and ensuring access to KRT [6].
CKD IN T2DM
- Development
- To address the need for improved prediction of 2- and 5-year risk of progression to ESKD, the KFRE has been developed in 2011 using a large Canadian cohort involving people with CKD G3a–5 [34]. There were four KFREs developed in the original cohort: the 3-variable (age, sex, and eGFR), the 4-variable (3-variable plus urinary ACR), the 6-variable (4-variable plus diabetes and hypertension), and the 8-variable equations (4-variable plus corrected serum calcium, phosphate, bicarbonate, and albumin). The 4-variable and 8-variable equations demonstrated the best performance in the original cohort [34].
- Validation
- To date, KFRE is the most well-validated kidney failure risk prediction tool with good discrimination for people with CKD stages 3a–5, taking into account the status of diabetes and the competing risk of death [34,37,38,59-71]. It has been externally validated in more than 30 countries, and recalibration factor was included for non-USA/Canada populations in 2016 and South East Asia in 2019 (Table 1).
- The KFRE was also validated among different populations including (1) kidney transplant recipients in Canada and USA with reduced eGFR after transplantation for 6 months to 1 year [64,66,69]; (2) elderly people [62]; and (3) across different etiologies of CKD such as diabetes, hypertension, glomerulonephritis, and autosomal dominant polycystic kidney disease (ADPKD) [67,68]. Of note, KFRE tends to underestimate the risk for CKD due to ADPKD [68]. This could be related to the unique pathophysiology of ADPKD in which its disease progression is generally associated with cyst growth and total kidney volume, rather than the KFRE variables such as degree of albuminuria [72]. It is also worth noting that the predictive performance of KFRE across different CKD etiologies were subjected to potential errors as these etiologies were not proven by kidney biopsy in most study populations, which was particularly challenging in LMICs and low-resource areas in HICs [67,68].
KIDNEY FAILURE RISK EQUATIONS
- To date, the utilization of KFRE is limited by the access to and availability of urinary ACR measurement. In a large CKD cohort of over 60,000 people, it was found that only one-third of them had urinary ACR measurement performed [73]. To overcome this challenge, home-based albuminuria screening has shown promise with high participation rate, reliability, and accuracy [74]. Following that, an automated reporting of the validated KFRE in the presence of eGFR and urinary ACR measurements can increase physicians’ awareness, improve clinical decision-making, and reduce therapeutic inertia [73].
- Compared to those at lower risk, the direct medical costs associated with people with CKD G3 and G4 were 42% and 70% higher, respectively [64,75]. These were related to a high number of hospitalizations, outpatient specialist visits, and drug dispensaries [64,75]. Therefore, the KFRE has been examined to facilitate risk-based triage for facilitating referral to nephrology care and resource allocations [61,68,76,77]. In a 5-year retrospective cohort study involving 35,000 people managed in the UK primary care setting, utilization of the 4-variable KFRE with a triage threshold of ≥5% at 5-year reduced unnecessary referrals, shortened waiting time and prompted early referrals for those who went on to develop ESKD at a younger age, which could offer long-term cost-saving [61]. Another experimental study in tertiary care centers in Canada utilized ESKD risk of ≥3% at 5-year generated from the 4-variable KFRE as the triage threshold for referral to nephrology care. Although the median number of monthly referrals increased by 45%, the median waiting time was shortened by 172 days than the pretriage period [77]. Another study in China compared the performance of 3-variable KFRE (age, gender, and eGFR) with machine-learning algorithms applied in a resource-constraint setting wherein urinary ACR was not readily available. Both 3-variables KFRE and machine-learning algorithms showed similar predictive performance, although the KFRE which based on existing risk factors had better practicality among the LMICs [46].
- KFRE can provide accurate stratification of people at high risk of ESKD and guide dialysis planning [63]. Surveys are needed for evaluating healthcare providers and community satisfaction to risk-based CKD care before and after implementation of KFRE. Future research on the associations between these risk-based approaches to CKD care and a wide range of health outcomes including healthcare resource utilization, treatment metrics, cardiovascular and mortality outcomes, especially in resource-constrained settings, is very much in need [78].
CLINICAL AND RESEARCH UTILITY
- While there is robust evidence supporting cardiorenal risk reduction strategies among people with T2DM, the next essential step is to ensure their equitable and affordable access, especially in resource-constrained settings and under-privileged populations. The delivery of care needs to be optimized for reducing residual cardiorenal risks. Simple and inexpensive risk-based approaches such as KFRE can improve detection, risk stratification, patient empowerment and timely intervention to reduce therapeutic inertia and improve health outcomes. Although the KFRE has shown promising results in facilitating CKD risk stratification, extensive validations in multiethnic populations and different healthcare settings are needed to scale up its clinical utility.
CONCLUSIONS
SUPPLEMENTARY MATERIALS
Supplementary Table 1.
-
CONFLICTS OF INTEREST
Lee-Ling Lim has been international editorial board members of the Diabetes & Metabolism Journal since 2022. She was not involved in the review process of this article. Otherwise, there was no conflict of interest.
Ying-Guat Ooi and Shireene R. Vethakkan report no conflict of interest; Tharsini Sarvanandan reports receiving honoraria for giving lectures from Novo Nordisk; Nicholas Ken Yoong Hee reports receiving honoraria for giving lectures from AstraZeneca, Novo Nordisk and Zuellig Pharma; Quan-Hziung Lim reports receiving honoraria for giving lectures from AstraZeneca, Boehringer Ingelheim, Novo Nordisk and Zuellig Pharma; Sharmila S. Paramasivam reports receiving honoraria for consultancy or giving lectures from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk and Zuellig Pharma; Jeyakantha Ratnasingam reports receiving honoraria for consultancy or giving lectures from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Sanofi, Servier, Novartis, Ipsen and Zuellig Pharma; Soo-Kun Lim reports receiving grants and/or honoraria for consultancy or giving lectures from Abbott, AstraZeneca, Astellas, Baxter, Boehringer Ingelheim, Duopharma, Fresenius-Kabi, MSD, Novartis, Novo Nordisk, Roche, Sanofi and Taisho; Lee-Ling Lim reports receiving grants and/or honoraria for consultancy or giving lectures from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Novartis, Novo Nordisk, Roche, Sanofi, Servier and Zuellig Pharma.
-
FUNDING
This work was supported by the University of Malaya (UM) International Collaboration Grant (Grant number: ST048-2023). The funding source did not have any role in the design, interpretation of the study or the decision to publish the results.
NOTES
-
Acknowledgements
- None
| Study; Region | Study characteristics | Study design and aim | Key findings | KFRE, C statistic (95% CI) |
|---|---|---|---|---|
| Tangri et al. (2016) [59]; North America & Europe | a) 721,357 adults (40% had diabetes) | a) Meta-analysis | a) Excellent discrimination and appropriate cali-bration (death not treated as competing risk) | a) 4- and 8-variable 2-year score: 0.90 (0.89–0.92) |
| b) Multinational cohorts from Chronic Kidney Disease Prognosis Consortium (CKD-PC) | b) Validation and recalibration for non-USA/Canada cohorts | |||
| c) Mean±SD eGFR: 46±11 mL/min/1.73 m2 | b) Addition of a recalibration factor optimized performance in non-USA/Canada populations | b) 4- and 8-variable 5-year: 0.88 (0.86–0.90) | ||
| d) 40% has baseline urinary ACR of ≥30 mg/g | ||||
| e) Mean±SD age: 74±10 years | ||||
| f) Men 77% | ||||
| Lennartz et al. (2016) [60]; Germany | a) 403 adults | a) Prospective cohorts | a) Excellent discrimination and calibration | a) 4-variable 3-year score: 0.91 (0.83–0.99) |
| b) Referral to tertiary nephrology care center | b) Validation | b) Addition of ultrasound markers did not improve performance | ||
| c) Mean±SD eGFR: 55.7±32 mL/min/1.73 m2 | c) Addition of ultrasound markers: RRI or DI-RISK | |||
| d) Median urinary ACR: 44 mg/g (IQR, 15–204) | ||||
| e) Mean±SD age: 60±15 years | ||||
| f) Men 58% | ||||
| g) Mean±SD RRI: 72±9 | ||||
| h) Mean±SD DI-RISK: 8±5 | ||||
| Major et al. (2019) [61]; United Kingdom | a) 35,539 adults (31.5% had diabetes) | a) Retrospective cohort | a) Excellent discrimination (death not treated as competing risk) | a) 4-variable 2-year score: 0.93 (0.91–0.96) |
| b) Primary care CKD population | b) Validation in primary care setting and clinical utility | |||
| c) Median eGFR: 51 mL/min/1.73 m2 (IQR, 43–56) | b) Referral criteria based on 5-year risk of ≥5% and/or an urinary ACR of ≥70 mg/mmol is time and cost-effective | b) 4-variable 5-year score: 0.93 (0.91–0.94) | ||
| d) Median urinary ACR: 3.2 mg/mmol (IQR, 1.2–8.0) | ||||
| e) Mean±SD age: 76±10 years | ||||
| f) Men 43% | ||||
| Hallan et al. (2019) [62]; USA | a) 1,188 elderly (17% had diabetes) | a) Retrospective cohort | a) Well calibrated with excellent discrimination | a) 4-variable 5-year score: 0.88 (0.86–0.90) |
| b) General population-based data | b) Validation of both KFRE and MREK in elderly | b) Recommend using both MREK and KFRE to decide whether to prepare for RRT in elderly people with advanced CKD | ||
| c) Mean±SD eGFR: 36±8 mL/min/1.73 m2 | ||||
| d) Mean±SD urinary ACR: 7.1±18.7 mg/mmol | ||||
| e) Mean±SD age: 80±6.8 years | ||||
| f) Men 47% | ||||
| Wang et al. (2019) [63]; Singapore | a) 17,271 adults (59% had T2DM) | a) Retrospective cohort | a) The Recalibrated Pooled KFRE SEA has similar discrimination with slightly improved precision. | Original KFRE: |
| b) Primary care CKD population | b) Validation | a) 4-variable 2-year score: 0.96 (0.95–0.97) | ||
| c) 89% CKD stage 3 | c) Recalibration in Singapore population | b) The effect of competing mortality risk is unlikely to affect the ESKD prediction. | b) 4-variable 5-year score: 0.94 (0.93–0.95) | |
| d) Urinary ACR data not included | d) Clinical utility | Recalibrated Pooled KFRE SEA: | ||
| e) Mean±SD age: 75±9 years | c) Using calculated 5-year risk >10%–16% to guide dialysis planning and calculated 2-year risk >45% to guide nephrologist referral would facilitate more efficient care and accurate risk stratification. | a) 4-variable 2-year score: 0.96 (0.95–0.97) | ||
| f) Men 49% | b) 4-variable 5-year score: 0.94 (0.93–0.95) | |||
| Tangri et al. (2020) [64]; Canada | a) 3,659 kidney transplant recipients | a) Retrospective cohort | a) Accurately predicts graft failure in kidney transplant recipients with even better discrimination when eGFR is <45 mL/min/1.73 m2 (death was not treated as a competing risk). | a) 4-variable 2-year score: 0.81 (0.72–0.91) |
| b) Transplant database from multiple transplant centers in Canada | b) Validation among kidney transplant recipients 1 year post-transplant | |||
| c) 26% had eGFR <45 mL/min/1.73 m2 | b) 4-variable 5-year score: 0.73 (0.67–0.80) | |||
| d) Median urinary ACR: 2.2–9.8 mg/mmol across 4 cohorts | ||||
| e) Mean age: 47–53 years across 4 cohorts | ||||
| f) Men 60%–66% across 4 cohorts | ||||
| Kang et al. (2020) [65]; Korea | a) 13,244 adults (29% had diabetes) | a) Retrospective cohort | a) 28.0% subjects developed ESKD during the mean follow-up period of 4.1 years. | a) 4-variable 2-year score: 0.86 (0.86–0.87) |
| b) Nephrology clinic in 2 tertiary centers | b) Validation in Korean population and recalibration | b) 6-variable 2-year score: 0.87 (0.86–0.88) | ||
| c) Mean±SD eGFR: 36.1±17.1 mL/min/1.73 m2 | b) Excellent discrimination | c) 8-variable 2-year score: 0.88 (0.86–0.88) | ||
| d) Mean±SD urinary ACR: 992.9±2,325.6 mg/g | c) No need for recalibration | d) 4-variable 5-year score: 0.83 (0.82–0.85) | ||
| e) Mean±SD age: 60±14 years | e) 6-variable 5-year score: 0.83 (0.82–0.84) | |||
| f) Men 58% | f) 8-variable 5-year score: 0.83 (0.82–0.85) | |||
| Chu et al. (2020) [66]; USA | a) 2,889 kidney transplant recipients (43% living donor graft, 39.3% had diabetes) | a) Retrospective cohort | a) 2.4% developed graft loss by 2 years, 8.7% by 5 years | a) 4-variable 2-year score: 0.85 (0.81–0.88) |
| b) Multi-center cohort-majority from the USA, few from Canada and Brazil | b) Validation among kidney transplant recipients with eGFR ≤60 mL/min/1.73 m2 6 months post-transplant | b) Accurate calibration and discrimination (death treated as a competing risk) | b) 4-variable 5-year score: 0.81 (0.78–0.84) | |
| c) Mean±SD eGFR: 41±11 mL/min/1.73 m2 | c) Well calibrated for both deceased donor and living donor grafts | |||
| d) Median urinary ACR 28 mg/g (IQR, 10–119) | d) Poor calibration for subgroups of less than 2 years post-transplantation | |||
| e) Mean±SD age: 52±9 years | ||||
| f) Men 61% | ||||
| Hundemer et al. (2020) [67]; Canada | a) 1,293 adults (49% had diabetes) | a) Retrospective cohort | a) Excellent discrimination & adequate calibra-tion across CKD etiologies (death was not treated as a competing risk) | a) 4-variable 2-year score: 0.83 (0.81–0.85) |
| b) Specialty nephrology clinic at single academic tertiary center | b) Validation across different CKD etiologies | |||
| c) Median eGFR: 15 mL/min/1.73 m2 (IQR, 12–19) | b) Underestimation of risk for ADPKD subgroup | b) 4-variable 2-year score: 0.81 (0.77–0.84) | ||
| d) Median urinary ACR: 1,277 mg/g (IQR, 322–2,903) | ||||
| e) Median age: 68 years (IQR, 58–78) | ||||
| f) Men 61% | ||||
| Ali et al. (2021) [68]; United Kingdom | a) 743 adults (40% had diabetes) | a) Retrospective cohort | a) Good discrimination with similar predictive accuracy | a) 4- and 8-variable 2-year score: 0.79 (0.76–0.83) |
| b) Advanced kidney care service clinic at single tertiary care center | b) Validation & clinical utility across different etiologies of CKD | b) Underestimation of risk for ADPKD subgroup | ||
| c) Median eGFR: 16 mL/min/1.73 m2 (IQR, 13–18) | d) Better for guiding further intervention compared to using eGFR cut-offs | b) 4- and 8-variable 5-year score: 0.77 (0.74–0.81) | ||
| d) Median urinary ACR: 409 mg/g (IQR, 85–1,356) | ||||
| e) Median age: 69 years (IQR, 57–77) | ||||
| f) Men 62% | ||||
| Ramspek et al. (2021) [38]; Europe | a) 15,069 adults (21% had diabetes) | a) Retrospective cohorts | a) Excellent calibration and good discrimination | EQUAL cohort |
| b) Multi-center cohorts | b) Validation | b) 5-year KFRE overpredicted risk by 10%–18% (death as competing risk) | a) 4-variable 2-year score: 0.76 (0.72–0.80) | |
| c) Mean±SD eGFR: (EQUAL cohort) 18.5±4.7 mL/min/1.73 m2 (SRR cohort) 21.9±5.7 mL/min/1.73 m2 | b) 8-variable 2-year score: 0.78 (0.75–0.81) | |||
| c) 4-variable 5-year score: 0.75 (0.71–0.78) | ||||
| d) Median urinary ACR: (EQUAL cohort) 40 mg/mmol (IQR, 8–165) (SRR cohort) 36 mg/mmol (IQR, 7–155) | d) 8-variable 5-year score: 0.76 (0.73–0.79) | |||
| SRS cohort | ||||
| e) Mean age: 74–76 years across 2 cohorts | a) 4- and 8-variable 2-year score: 0.84 (0.83–0.85) | |||
| f) Men 61%–65% | b) 4- and 8-variable 5-year score: 0.81 (0.80–0.82) | |||
| Ali (2021) [69]; United Kingdom | a) 415 transplant recipients (18.9% living donor graft, 12% had diabetes) | a) Retrospective cohort | a) Adequate discrimination for predicting graft failure, especially in those with an eGFR <45 mL/min/1.73 m2 with poor calibration (likely due to lack of transplant related variables) | a) 4-variable 5-year score: 0.74 (0.61–0.88) |
| b) Single renal transplant center | b) Validation among kidney transplant recipients 1-year post-transplant | b) 8-variable 5-year score: 0.75 (0.63–0.87) | ||
| c) Median eGFR: 54.1 mL/min/1.73 m2 (IQR, 41.6–70.5) | ||||
| d) Median urinary ACR: 22.1 mg/g (IQR, 11.5–65.4) | ||||
| e) Median age: 50 years (IQR, 40–60) | ||||
| f) Men 59% | ||||
| Kwek et al. (2022) [70]; Singapore | a) 1,128 adults (64.6% had diabetes) | a) Retrospective cohort | a) Excellent discrimination (death not taken as a competing risk) | a) 4-variable 2-year score: 0.87 (0.85–0.90) |
| b) Nephrology clinic, single tertiary center | b) Validation | b) 8-variable 2-year score: 0.87 (0.85–0.89) | ||
| c) Median eGFR: 31.9 mL/min/1.73 m2 (IQR, 22.4–44.8) | ||||
| d) Median urinary ACR: 243 mg/g (range, 63–868) | ||||
| e) Mean±SD age: 67±12 years | ||||
| f) Men 58% | ||||
| Irish et al. (2023) [71]; Australia | a) 12,861 adults (54.9% had diabetes) | a) Retrospective cohort | a) Excellent discrimination and adequately calibrated (death not taken as a competing risk) | a) 4-variable 2-year score: 0.98 (0.97–0.99) |
| b) Multiple national registries | b) Validation among Australian cohort | b) 6-variable 2-year score: 0.97 (0.96–0.99) | ||
| c) Mean±SD eGFR: 50.3±9.3 mL/min/1.73 m2 | c) 8-variable 2-year score: 0.96 (0.94–0.98) | |||
| d) Median urinary ACR: 1.4 mg/g (IQR, 0.6–5.0) | d) 4-variable 5-year score: 0.96 (0.95–0.97) | |||
| e) Mean±SD age: 70±10 years | e) 6-variable 5-year score: 0.96 (0.95–0.97) | |||
| f) Men 48% | f) 8-variable 5-year score: 0.95 (0.93–0.96) |
KFRE, Kidney Failure Risk Equation; CI, confidence interval; SD, standard deviation; eGFR, estimated glomerular filtration rate; ACR, albumin-creatinine ratio; IQR, interquartile range; RRI, renal resistive index; DI-RISK, difference of resistive indices in spleen and kidney; CKD, chronic kidney disease; MREK, mortality risk equation for kidney disease; RRT, renal replacement therapy; T2DM, type 2 diabetes mellitus; SEA, South East Asia; ESKD, end-stage kidney disease; ADPKD, autosomal dominant polycystic kidney disease; EQUAL, European Quality Study; SRR, Swedish Renal Registry.
- 1. International Diabetes Federation. IDF Diabetes Atlas. 10th ed. Brussels: IDF; 2021. p. 30-57.
- 2. GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023;402:203-34.PubMedPMC
- 3. Lim LL, Chow E, Chan JC. Cardiorenal diseases in type 2 diabetes mellitus: clinical trials and real-world practice. Nat Rev Endocrinol 2023;19:151-63.ArticlePubMedPDF
- 4. Chan JC, Lim LL, Wareham NJ, Shaw JE, Orchard TJ, Zhang P, et al. The Lancet Commission on diabetes: using data to transform diabetes care and patient lives. Lancet 2021;396:2019-82.PubMed
- 5. Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR; UKPDS Study Group. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006;55:1832-9.PubMed
- 6. Bello AK, Okpechi IG, Levin A, Ye F, Saad S, Zaidi D, et al. ISN– Global Kidney Health Atlas. A report by the International Society of Nephrology: an assessment of global kidney health care status focussing on capacity, availability, accessibility, affordability and outcomes of kidney disease. Brussels: International Society of Nephrology; 2023.
- 7. Mogensen CE. Glomerular hyperfiltration in human diabetes. Diabetes Care 1994;17:770-5.ArticlePubMedPDF
- 8. Tuttle KR, Stein JH, DeFronzo RA. The natural history of diabetic nephropathy. Semin Nephrol 1990;10:184-93.PubMed
- 9. Pugliese G, Penno G, Natali A, Barutta F, Di Paolo S, Reboldi G, et al. Diabetic kidney disease: new clinical and therapeutic issues. Joint position statement of the Italian Diabetes Society and the Italian Society of Nephrology on “The natural history of diabetic kidney disease and treatment of hyperglycemia in patients with type 2 diabetes and impaired renal function”. Nutr Metab Cardiovasc Dis 2019;29:1127-50.ArticlePubMedPMCPDF
- 10. Perez-Gomez MV, Bartsch LA, Castillo-Rodriguez E, Fernandez-Prado R, Fernandez-Fernandez B, Martin-Cleary C, et al. Clarifying the concept of chronic kidney disease for non-nephrologists. Clin Kidney J 2019;12:258-61.ArticlePubMedPMC
- 11. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020;98(4S):S1-115.ArticlePubMed
- 12. GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020;395:709-33.PubMedPMC
- 13. Koye DN, Magliano DJ, Nelson RG, Pavkov ME. The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis 2018;25:121-32.ArticlePubMedPMC
- 14. Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes: a collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011;80:93-104.ArticlePubMedPMC
- 15. Gonzalez Suarez ML, Thomas DB, Barisoni L, Fornoni A. Diabetic nephropathy: is it time yet for routine kidney biopsy? World J Diabetes 2013;4:245-55.ArticlePubMedPMC
- 16. Garcia-Carro C, Vergara A, Bermejo S, Azancot MA, Sanchez-Fructuoso AI, Sanchez de la Nieta MD, et al. How to assess diabetic kidney disease progression?: from albuminuria to GFR. J Clin Med 2021;10:2505.ArticlePubMedPMC
- 17. Liang S, Zhang XG, Cai GY, Zhu HY, Zhou JH, Wu J, et al. Identifying parameters to distinguish non-diabetic renal diseases from diabetic nephropathy in patients with type 2 diabetes mellitus: a meta-analysis. PLoS One 2013;8:e64184.ArticlePubMedPMC
- 18. American Diabetes Association. Standards of care in diabetes-2023 abridged for primary care providers. Clin Diabetes 2022;41:4-31.ArticlePubMedPDF
- 19. Ortiz A, Wanner C, Gansevoort R; ERA Council. Chronic kidney disease as cardiovascular risk factor in routine clinical practice: a position statement by the Council of the European Renal Association. Eur J Prev Cardiol 2022;29:2211-5.ArticlePubMedPDF
- 20. Ling W, Huang Y, Huang YM, Fan RR, Sui Y, Zhao HL. Global trend of diabetes mortality attributed to vascular complications, 2000-2016. Cardiovasc Diabetol 2020;19:182.ArticlePubMedPMCPDF
- 21. Ali MK, Pearson-Stuttard J, Selvin E, Gregg EW. Interpreting global trends in type 2 diabetes complications and mortality. Diabetologia 2022;65:3-13.ArticlePubMedPMCPDF
- 22. Ma CX, Ma XN, Guan CH, Li YD, Mauricio D, Fu SB. Cardiovascular disease in type 2 diabetes mellitus: progress toward personalized management. Cardiovasc Diabetol 2022;21:74.ArticlePubMedPMCPDF
- 23. Pearson-Stuttard J, Bennett J, Cheng YJ, Vamos EP, Cross AJ, Ezzati M, et al. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol 2021;9:165-73.ArticlePubMedPMC
- 24. Rawshani A, Rawshani A, Franzen S, Sattar N, Eliasson B, Svensson AM, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2018;379:633-44.ArticlePubMed
- 25. Norhammar A, Bodegard J, Eriksson JW, Haller H, Linssen GC, Banerjee A, et al. Cost of healthcare utilization associated with incident cardiovascular and renal disease in individuals with type 2 diabetes: a multinational, observational study across 12 countries. Diabetes Obes Metab 2022;24:1277-87.PubMedPMC
- 26. Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia 2019;62:3-16.ArticlePubMedPDF
- 27. Kovesdy C, Schmedt N, Folkerts K, Bowrin K, Raad H, Batech M, et al. Predictors of cardio-kidney complications and treatment failure in patients with chronic kidney disease and type 2 diabetes treated with SGLT2 inhibitors. BMC Med 2022;20:2.ArticlePubMedPMCPDF
- 28. Koye DN, Magliano DJ, Reid CM, Jepson C, Feldman HI, Herman WH, et al. Risk of progression of nonalbuminuric CKD to end-stage kidney disease in people with diabetes: the CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis 2018;72:653-61.ArticlePubMed
- 29. Yokoyama H, Araki SI, Kawai K, Yamazaki K, Shirabe SI, Sugimoto H, et al. The prognosis of patients with type 2 diabetes and nonalbuminuric diabetic kidney disease is not always poor: implication of the effects of coexisting macrovascular complications (JDDM 54). Diabetes Care 2020;43:1102-10.ArticlePubMedPDF
- 30. Jiang G, Luk AO, Tam CH, Xie F, Carstensen B, Lau ES, et al. Progression of diabetic kidney disease and trajectory of kidney function decline in Chinese patients with type 2 diabetes. Kidney Int 2019;95:178-87.PubMed
- 31. Grams ME, Coresh J. Predicting risk of RRT in patients with CKD. Clin J Am Soc Nephrol 2017;12:3-4.ArticlePubMedPMC
- 32. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(2 Suppl 1):S1-66.PubMed
- 33. Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011;80:17-28.ArticlePubMed
- 34. Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA 2011;305:1553-9.ArticlePubMed
- 35. Xiong XF, Yang Y, Wei L, Xiao Y, Li L, Sun L. Identification of two novel subgroups in patients with diabetes mellitus and their association with clinical outcomes: a two-step cluster analysis. J Diabetes Investig 2021;12:1346-58.ArticlePubMedPMCPDF
- 36. Ahlqvist E, Storm P, Karajamaki A, Martinell M, Dorkhan M, Carlsson A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018;6:361-9.ArticlePubMed
- 37. Dekker FW, Ramspek CL, van Diepen M. Con: most clinical risk scores are useless. Nephrol Dial Transplant 2017;32:752-5.ArticlePubMed
- 38. Ramspek CL, Evans M, Wanner C, Drechsler C, Chesnaye NC, Szymczak M, et al. Kidney failure prediction models: a comprehensive external validation study in patients with advanced CKD. J Am Soc Nephrol 2021;32:1174-86.ArticlePubMedPMC
- 39. Chen Y, Lee K, Ni Z, He JC. Diabetic kidney disease: challenges, advances, and opportunities. Kidney Dis (Basel) 2020;6:215-25.ArticlePubMedPMCPDF
- 40. Chan L, Nadkarni GN, Fleming F, McCullough JR, Connolly P, Mosoyan G, et al. Derivation and validation of a machine learning risk score using biomarker and electronic patient data to predict progression of diabetic kidney disease. Diabetologia 2021;64:1504-15.ArticlePubMedPMCPDF
- 41. Lam D, Nadkarni GN, Mosoyan G, Neal B, Mahaffey KW, Rosenthal N, et al. Clinical utility of KidneyIntelX in early stages of diabetic kidney disease in the CANVAS trial. Am J Nephrol 2022;53:21-31.ArticlePubMedPDF
- 42. Connolly P, Stapleton S, Mosoyan G, Fligelman I, Tonar YC, Fleming F, et al. Analytical validation of a multi-biomarker algorithmic test for prediction of progressive kidney function decline in patients with early-stage kidney disease. Clin Proteomics 2021;18:26.ArticlePubMedPMCPDF
- 43. Nadkarni GN, Takale D, Neal B, Mahaffey KW, Yavin Y, Hansen MK, et al. A post hoc analysis of KidneyIntelX and cardiorenal outcomes in diabetic kidney disease. Kidney360 2022;3:1599-602.ArticlePubMedPMC
- 44. Tokita J, Vega A, Sinfield C, Naik N, Rathi S, Martin S, et al. Real world evidence and clinical utility of KidneyIntelX on patients with early-stage diabetic kidney disease: interim results on decision impact and outcomes. J Prim Care Community Health 2022;13:21501319221138196.ArticlePubMedPMCPDF
- 45. Datar M, Ramakrishnan S, Chong J, Montgomery E, Goss TF, Coca SG, et al. A kidney diagnostic’s impact on physician decision-making in diabetic kidney disease. Am J Manag Care 2022;28:654-61.ArticlePubMed
- 46. Bai Q, Su C, Tang W, Li Y. Machine learning to predict end stage kidney disease in chronic kidney disease. Sci Rep 2022;12:8377.ArticlePubMedPMCPDF
- 47. Zou Y, Zhao L, Zhang J, Wang Y, Wu Y, Ren H, et al. Development and internal validation of machine learning algorithms for end-stage renal disease risk prediction model of people with type 2 diabetes mellitus and diabetic kidney disease. Ren Fail 2022;44:562-70.ArticlePubMedPMC
- 48. Belur Nagaraj S, Pena MJ, Ju W, Heerspink HL; BEAt-DKD Consortium. Machine-learning-based early prediction of end-stage renal disease in patients with diabetic kidney disease using clinical trials data. Diabetes Obes Metab 2020;22:2479-86.ArticlePubMedPMCPDF
- 49. Yao L, Zhang H, Zhang M, Chen X, Zhang J, Huang J, et al. Application of artificial intelligence in renal disease. Clin eHealth 2021;4:54-61.Article
- 50. Gregg EW, Buckley J, Ali MK, Davies J, Flood D, Mehta R, et al. Improving health outcomes of people with diabetes: target setting for the WHO Global Diabetes Compact. Lancet 2023;401:1302-12.ArticlePubMedPMC
- 51. Mosenzon O, Alguwaihes A, Leon JL, Bayram F, Darmon P, Davis TM, et al. CAPTURE: a multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc Diabetol 2021;20:154.ArticlePubMedPMCPDF
- 52. Van Spall HG, Fonarow GC, Mamas MA. Underutilization of guideline-directed medical therapy in heart failure: can digital health technologies PROMPT change? J Am Coll Cardiol 2022;79:2214-8.PubMed
- 53. Heerspink HJ, Parving HH, Andress DL, Bakris G, Correa-Rotter R, Hou FF, et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet 2019;393:1937-47.PubMed
- 54. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJ, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295-306.ArticlePubMed
- 55. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347-57.ArticlePubMed
- 56. The EMPA-KIDNEY Collaborative Group, Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023;388:117-27.ArticlePubMedPMC
- 57. Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020;383:2219-29.ArticlePubMed
- 58. Novo Nordisk. Novo Nordisk will stop the once-weekly injectable semaglutide kidney outcomes trial, FLOW, based on interim analysis [Internet]. Available from: https://www.novonordisk.com/content/nncorp/global/en/news-and-media/news-and-ir-materials/news-details.html?id=166327 (cited 2023 Dec 15).
- 59. Tangri N, Grams ME, Levey AS, Coresh J, Appel LJ, Astor BC, et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA 2016;315:164-74.ArticlePubMedPMC
- 60. Lennartz CS, Pickering JW, Seiler-MuBler S, Bauer L, Untersteller K, Emrich IE, et al. External validation of the kidney failure risk equation and re-calibration with addition of ultrasound parameters. Clin J Am Soc Nephrol 2016;11:609-15.ArticlePubMedPMC
- 61. Major RW, Shepherd D, Medcalf JF, Xu G, Gray LJ, Brunskill NJ. The Kidney Failure Risk Equation for prediction of end stage renal disease in UK primary care: an external validation and clinical impact projection cohort study. PLoS Med 2019;16:e1002955.ArticlePubMedPMC
- 62. Hallan SI, Rifkin DE, Potok OA, Katz R, Langlo KA, Bansal N, et al. Implementing the European Renal Best Practice Guidelines suggests that prediction equations work well to differentiate risk of end-stage renal disease vs. death in older patients with low estimated glomerular filtration rate. Kidney Int 2019;96:728-37.ArticlePubMed
- 63. Wang Y, Nguyen FN, Allen JC, Lew JQ, Tan NC, Jafar TH. Validation of the kidney failure risk equation for end-stage kidney disease in Southeast Asia. BMC Nephrol 2019;20:451.ArticlePubMedPMCPDF
- 64. Tangri N, Ferguson TW, Wiebe C, Eng F, Nash M, Astor BC, et al. Validation of the kidney failure risk equation in kidney transplant recipients. Can J Kidney Health Dis 2020;7:2054358120922627.ArticlePubMedPMCPDF
- 65. Kang MW, Tangri N, Kim YC, An JN, Lee J, Li L, et al. An independent validation of the kidney failure risk equation in an Asian population. Sci Rep 2020;10:12920.ArticlePubMedPMCPDF
- 66. Chu CD, Ku E, Fallahzadeh MK, McCulloch CE, Tuot DS. The kidney failure risk equation for prediction of allograft loss in kidney transplant recipients. Kidney Med 2020;2:753-61.ArticlePubMedPMC
- 67. Hundemer GL, Tangri N, Sood MM, Ramsay T, Bugeja A, Brown PA, et al. Performance of the kidney failure risk equation by disease etiology in advanced CKD. Clin J Am Soc Nephrol 2020;15:1424-32.ArticlePubMedPMC
- 68. Ali I, Donne RL, Kalra PA. A validation study of the kidney failure risk equation in advanced chronic kidney disease according to disease aetiology with evaluation of discrimination, calibration and clinical utility. BMC Nephrol 2021;22:194.ArticlePubMedPMCPDF
- 69. Ali I, Kalra PA. A validation study of the 4-variable and 8-variable kidney failure risk equation in transplant recipients in the United Kingdom. BMC Nephrol 2021;22:57.ArticlePubMedPMCPDF
- 70. Kwek JL, Pang HQ, Li H, Lim WW, Choo JC, Choong HL, et al. Validation of the kidney failure risk equation in predicting the risk of progression to kidney failure in a multi-ethnic Singapore chronic kidney disease cohort. Singapore Med J 2022;63:313-8.ArticlePubMedPMC
- 71. Irish GL, Cuthbertson L, Kitsos A, Saunder T, Clayton PA, Jose MD. The kidney failure risk equation predicts kidney failure: validation in an Australian cohort. Nephrology (Carlton) 2023;28:328-35.ArticlePubMedPMC
- 72. Cunningham A, Benediktsson H, Muruve DA, Hildebrand AM, Ravani P. Trends in biopsy-based diagnosis of kidney disease: a population study. Can J Kidney Health Dis 2018;5:2054358118799690.ArticlePubMedPMCPDF
- 73. Ahmed S, Mothi SS, Sequist T, Tangri N, Khinkar RM, Mendu ML. The kidney failure risk equation score and CKD care delivery measures: a cross-sectional study. Kidney Med 2021;4:100375.ArticlePubMedPMC
- 74. van Mil D, Kieneker LM, Evers-Roeten B, Thelen MH, de Vries H, Hemmelder MH, et al. Participation rate and yield of two home-based screening methods to detect increased albuminuria in the general population in the Netherlands (THOMAS): a prospective, randomised, open-label implementation study. Lancet 2023;402:1052-64.ArticlePubMed
- 75. Prasad B, Osman M, Jafari M, Gordon L, Tangri N, Ferguson TW, et al. Kidney failure risk equation and cost of care in patients with chronic kidney disease. Clin J Am Soc Nephrol 2022;17:17-26.ArticlePubMedPMC
- 76. Bhachu HK, Fenton A, Cockwell P, Aiyegbusi O, Kyte D, Calvert M. Use of the kidney failure risk equation to inform clinical care of patients with chronic kidney disease: a mixed-methods systematic review. BMJ Open 2022;12:e055572.ArticlePubMedPMC
- 77. Hingwala J, Wojciechowski P, Hiebert B, Bueti J, Rigatto C, Komenda P, et al. Risk-based triage for nephrology referrals using the kidney failure risk equation. Can J Kidney Health Dis 2017;4:2054358117722782.ArticlePubMedPMCPDF
- 78. Harasemiw O, Drummond N, Singer A, Bello A, Komenda P, Rigatto C, et al. Integrating risk-based care for patients with chronic kidney disease in the community: study protocol for a cluster randomized trial. Can J Kidney Health Dis 2019;6:2054358119841611.ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations

- Figure
- Related articles
-
- Low Household Income Status and Death from Pneumonia in People with Type 2 Diabetes Mellitus: A Nationwide Study
- Glycemic Control and Adverse Clinical Outcomes in Patients with Chronic Kidney Disease and Type 2 Diabetes Mellitus: Results from KNOW-CKD
- Cardiovascular Outcomes according to Comorbidities and Low-Density Lipoprotein Cholesterol in Korean People with Type 2 Diabetes Mellitus
- Implication of Sex Differences in Visceral Fat for the Assessment of Incidence Risk of Type 2 Diabetes Mellitus
- Advanced Liver Fibrosis Is Associated with Chronic Kidney Disease in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease

 KDA
KDA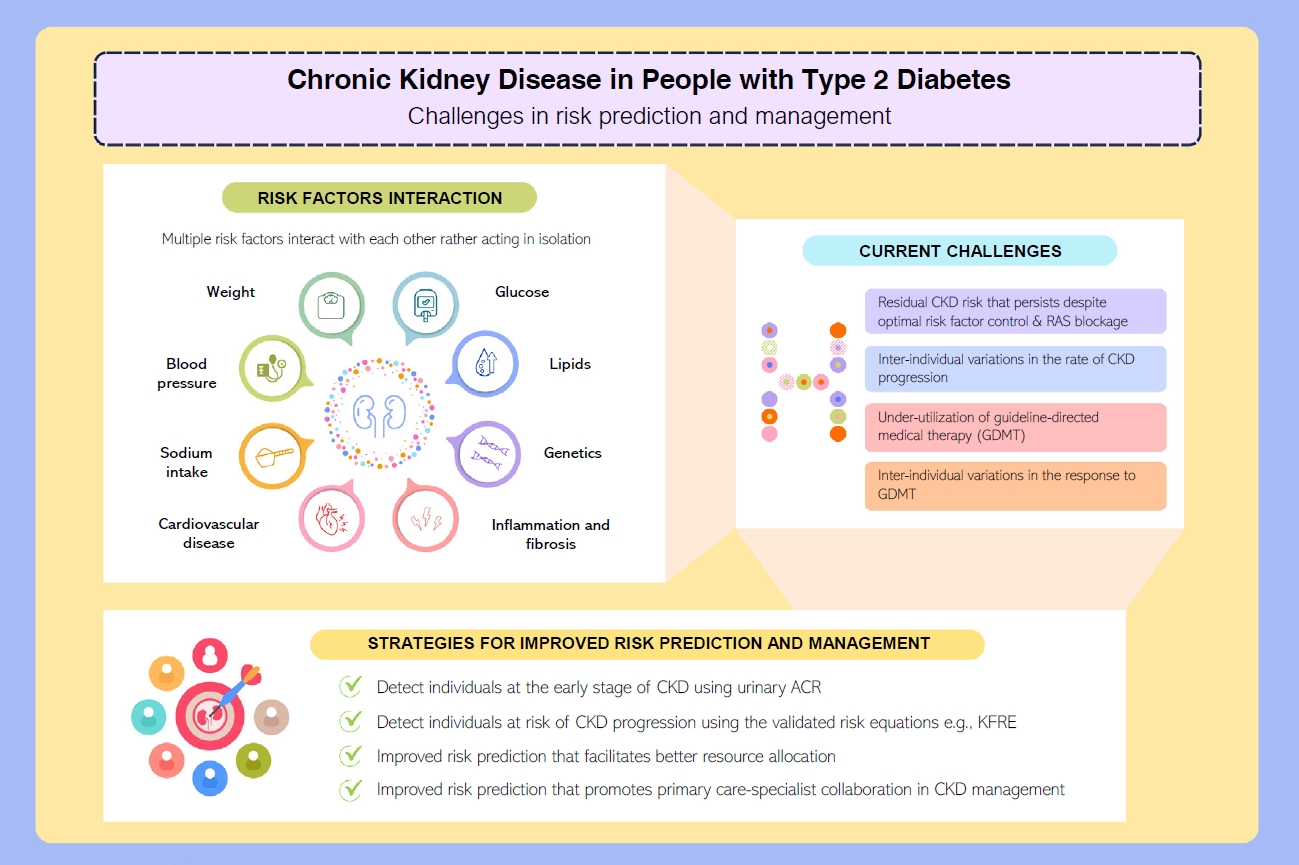
 PubReader
PubReader ePub Link
ePub Link Cite
Cite