Risk Prediction and Management of Chronic Kidney Disease in People Living with Type 2 Diabetes Mellitus
Article information
Abstract
People with type 2 diabetes mellitus have increased risk of chronic kidney disease and atherosclerotic cardiovascular disease. Improved care delivery and implementation of guideline-directed medical therapy have contributed to the declining incidence of atherosclerotic cardiovascular disease in high-income countries. By contrast, the global incidence of chronic kidney disease and associated mortality is either plateaued or increased, leading to escalating direct and indirect medical costs. Given limited resources, better risk stratification approaches to identify people at risk of rapid progression to end-stage kidney disease can reduce therapeutic inertia, facilitate timely interventions and identify the need for early nephrologist referral. Among people with chronic kidney disease G3a and beyond, the kidney failure risk equations (KFRE) have been externally validated and outperformed other risk prediction models. The KFRE can also guide the timing of preparation for kidney replacement therapy with improved healthcare resources planning and may prevent multiple complications and premature mortality among people with chronic kidney disease with and without type 2 diabetes mellitus. The present review summarizes the evidence of KFRE to date and call for future research to validate and evaluate its impact on cardiovascular and mortality outcomes, as well as healthcare resource utilization in multiethnic populations and different healthcare settings.
Highlights
• CKD is a complex condition with heterogeneous disease progression and health outcomes.
• Residual CKD risk persists despite optimal risk factor control and RAS blockade.
• New GDMTs (SGLT-2i, ns-MRAs, GLP-1 RAs) show cardiorenal benefits beyond RAS blockade.
• Improved prediction of CKD progression aids prognostication and prioritization of GDMT.
• KFRE is a well-validated prediction tool for CKD G3a–G5 with promising scalability.
INTRODUCTION
Diabetes has emerged as one of the most pressing health emergencies of the 21st century, contributing to one death in every 5 seconds [1]. The number of people with diabetes worldwide is projected to reach over 1.3 billion by 2050 [2], and the total diabetes-related health expenditure will reach US$ 1 trillion by 2030 [1]. Of note, 80% of people with diabetes live in low- and middle-income countries [1].
Type 2 diabetes mellitus (T2DM), especially when suboptimally controlled, can lead to the development and progression of chronic kidney disease (CKD) [3,4]. Studies have shown that 29% to 38% of people with T2DM develop CKD after a median follow-up of 15 years [5]. According to the Global Kidney Health Atlas 2023, upper-middle income countries (UMICs; 0.1%) and high-income countries (HICs; 0.2%) showed a greater kidney failure rate than low-income countries (LICs; 0.05%) or lower-middle income countries (LMICs; 0.07%). Interestingly, the prevalence of CKD increased with the national income level: LICs (3.6%), LMICs (7.5%), UMICs (10.7%), and HICs (11.1%) [6]. Nonetheless, inadequate access to kidney replacement therapy (KRT) is common in LICs and LMICs, leading to increased risk of premature deaths which are preventable if diagnosed and treated early. The proportion of people with end-stage kidney disease (ESKD) who are not receiving KRT is higher in LICs (98%) and LMICs (94%) than in UMICs (79%) and HICs (30%) [6].
The natural history of CKD in T2DM has been proposed to involve glomerular hyperfiltration and progressive albuminuria, followed by kidney function decline with eventual kidney failure [7,8]. This concept was later complicated by the emergence of a phenotype called non-albuminuric CKD with differential clinical and molecular features [9], although its pathogenesis is not well understood. Although serum creatinine (and therefore, estimated glomerular filtration rate [eGFR]) and albuminuria have been regarded as the key parameters of CKD diagnosis and progression, these laboratory measures and the classification of CKD are not without caveats (see Section “Gaps in risk prediction and management of CKD in T2DM” below) [10]. Therefore, there is a crucial need for better risk stratification strategies to identify people with an increased risk of progressing to ESKD.
In this present review, we aim to discuss (1) residual risk and care gaps in the management of people with CKD and T2DM, and (2) the potential utilization of a CKD risk prediction model to guide clinical decision-making.
CKD IN T2DM
Epidemiology
CKD is defined as abnormalities of kidney structure or function for a minimum of 3 months with implications for health [11]. The Global Burden of Disease Study reported that the global prevalence of CKD had increased by 29.3% from 1990 and affected 9.1% of the global population in 2017 [12]. Globally, the mortality rate from CKD increased by 41.5% between 1990 and 2017, making CKD the 12th leading cause of death (from 17th in 1990) with the majority of the CKD burden occurring at LMICs [12].
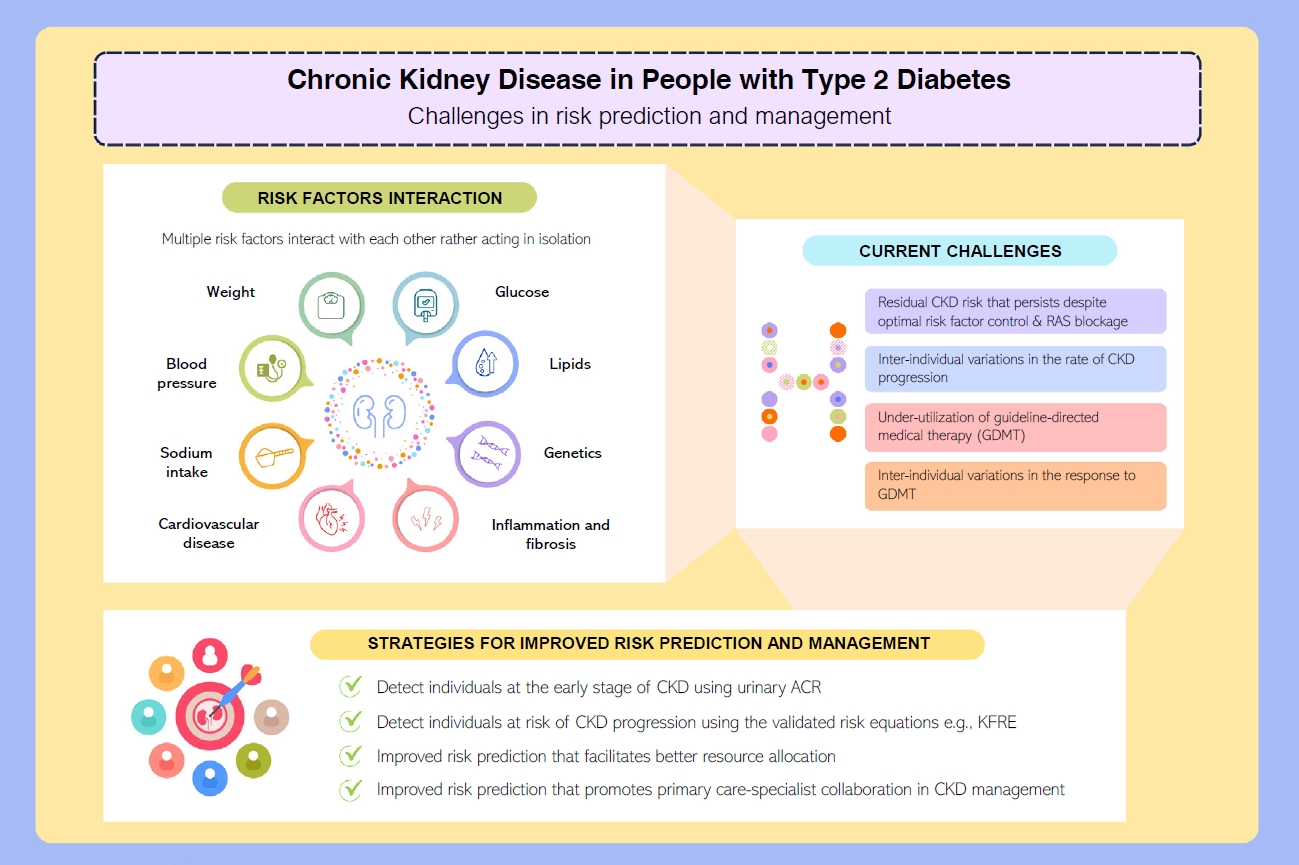
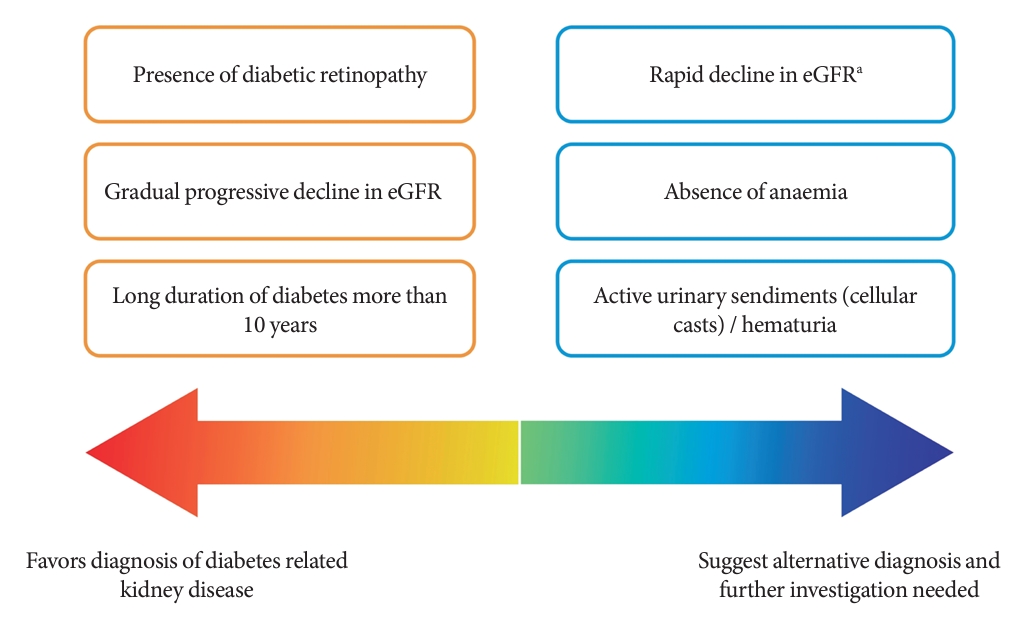
Among people with T2DM, the annual incidence rate of eGFR below 60 mL/min/1.73 m2 and albuminuria are estimated at 2% and 8%, respectively [13]. The estimated incidence of ESKD among people with CKD G3a is 0.3 per 1,000 personyears, while it increases to 4 and 43 per 1,000 person-years in CKD G3b and G4, respectively [14]. Although kidney biopsy is the gold standard to ascertain the underlying etiologies of CKD, it is not routinely performed due to procedural risks, labor-intensity, and patients’ reluctance [15]. The clinical diagnosis of CKD in T2DM and the prediction of its progression are dependent on a person’s clinical profiles and laboratory measurements in routine clinical practice (Fig. 1) [16-18].
Residual risk of CKD and cardiovascular disease
Robust evidence has also shown a bidirectional relationship between CKD and atherosclerotic cardiovascular disease (ASCVD) among people with T2DM [3,4,19]. The incidence of ASCVD in people with T2DM has reduced in most HICs due to improved care delivery, treatment target attainment and use of guideline-directed medical therapy (GDMT) including renin-angiotensin system (RAS) inhibitors and statins [20,21]. However, a similar trend is yet to be reported in LMICs. Furthermore, a large gap remains in the incidence of ASCVD, CKD and mortality between people with and without T2DM [22,23]. Despite optimal management of diabetes, blood pressure and lipids, people with diabetes still have two to three times higher risk of morbidity and mortality than those without diabetes [24].
Aging population and declining incidence of ASCVD have led to the emergence of heart failure and CKD with significant morbidity and mortality in people with T2DM [25,26]. While optimization of multiple cardiometabolic risk factors and use of RAS inhibitors and statins have reduced the incidence of CKD, residual risk persists which require additional treatment approaches [3,4,27].
Gaps in risk prediction and management of CKD in T2DM
CKD is a complex condition with heterogenous disease progression and health outcomes [28,29]. A prospective cohort study involving 6,330 people with diabetic kidney disease identified four distinct patterns in eGFR trajectories: the slow decline (84.3%), the curvilinear decline (6.5%), the progressive decline (6.1%), and the accelerated decline (3.1%) [30]. Compared to the slow decline group, those with accelerated eGFR decline reported an odds ratio of 6.9 (95% confidence interval, 5.6 to 8.4) for all-cause mortality [30].
Accurate prediction of the risk of ESKD is the cornerstone of optimal CKD management. It enables better management of people at high risk of ESKD in order to slow disease progression, improve prognostication and prioritize treatment pathways such as optimization of GDMT and early preparation for KRT, as summarized in (Fig. 2) [3,31].
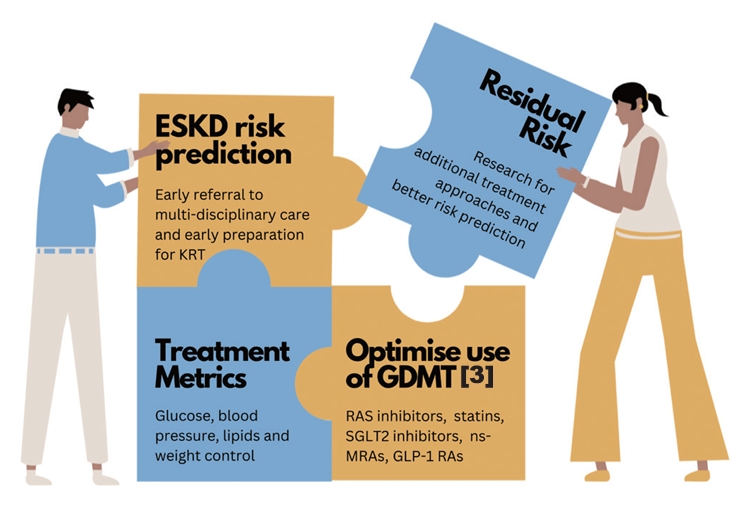
Gaps in risk prediction and management of chronic kidney disease among people with type 2 diabetes mellitus. ESKD, end-stage kidney disease; KRT, kidney replacement therapy; GDMT, guideline-directed medical therapy; RAS, renin-angiotensin system; SGLT2, sodium-glucose co-transporter 2; nsMRA, non-steroidal mineralocorticoid receptor antagonist; GLP-1 RA, glucagon-like peptide-1 receptor agonist.
In 2002, National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) published the clinical practice guideline for evaluation and classification system for CKD based on eGFR [32]. Since 2009, the Kidney Disease: Improving Global Outcomes (KDIGO) group recommends risk stratification of CKD using eGFR and urinary albumin-creatinine ratio (ACR), as the latter has an independent effect on the progression of CKD [33]. The KDIGO CKD classification informs about the risk of progression to ESKD but lacks absolute risk quantification which is essential to facilitate clinical decision-making. When resources are scarce, especially in LMICs, lack of a reliable kidney failure risk prediction model delays treatment in those who are at high risk of progression to ESKD or unnecessarily offers treatment/referral to nephrologists among those who have low risk and stable CKD [34].
These challenges call for a better CKD risk prediction model as the rates of CKD progression vary considerably between people with CKD. Cluster analysis has also suggested that the subclassification of T2DM may play a role in predicting risk of CKD. For example, the severe insulin-resistant diabetes, uric acid-related diabetes, and inheritance-related diabetes clusters were associated with an increased risk of CKD in Scandinavian populations [35,36].
Developing clinical risk scores and prediction models have become increasingly popular. As of October 27, 2023 in the preparation of the present review, a PubMed search using the medical subject headings “risk prediction” AND “chronic kidney disease” revealed 437 clinical studies on CKD risk prediction. Notwithstanding a few notable examples like the kidney failure risk equation (KFRE) developed by Tangri et al. [34], most kidney failure prediction models were neither externally validated nor considered competing risk of death prior to ESKD [37,38].
Of note, the emergence of biomarkers such as plasma soluble tumor necrosis factor receptors 1 (TNFR1), soluble tumor necrosis factor receptors 2 (TNFR2), and kidney injury molecule-1 (KIM-1) for early detection of people at high risk for CKD progression could help to guide management for better health outcomes [39]. On top of known clinical parameters included in the KFRE (eGFR, urinary ACR, and serum calcium), a machine-learning CKD risk prediction algorithm called KidneyIntelX has been developed by incorporating the aforementioned emerging biomarkers with glycosylated hemoglobin, systolic blood pressure, platelet count, and aspartate transferase [40]. The artificial intelligence-enabled KidneyIntelX algorithm stratifies people with T2DM and CKD G1–3b or albuminuria into three risk tiers namely low, intermediate, and high risk, with the corresponding recommendations on monitoring and treatment [40]. The KidneyIntelX algorithm has been externally validated in multinational cohorts from HICs [41-43] with proven clinical utility [44,45]. However, it is not widely available due to the high cost involved in testing the emerging biomarkers (TNFR1, TNFR2, and KIM-1) and has not been validated in LMICs. Nevertheless, there is an emergence of studies investigating the use of machine-learning algorithms to predict the risk of ESKD with good prediction ability, and algorithms incorporated into electronic health systems could potentially achieve large-scale population screening for CKD in regions with limited resources [46-49].
Management of T2DM requires a holistic approach which includes attaining control of multiple cardiometabolic risk factors (glucose, blood pressure, lipids, and body weight) and cardiovascular risk reduction strategies to prevent complications and death [3,4,50]. This has been discussed extensively in a 2023 review that summarizes the latest evidence in cardiorenal risk reduction among people with and without T2DM [3]. Use of RAS inhibitors and statins have reduced the incidence of cardiovascular-renal events and related death, but the incidence remains higher than those without diabetes [3]. At a global level, there is also under-utilization of newer GDMT including glucagon-like peptide-1 receptor agonists (GLP-1 RA) and sodium-glucose co-transporter 2 (SGLT2) inhibitors at 9%–15% and 8%–15% among people with either T2DM or ASCVD, respectively [51].
Although SGLT2 inhibitors are now included in the World Health Organization Essential Medications list, high acquisition cost, lack of reimbursement, insufficient risk assessment for timely intervention, and therapeutic inertia remain the major barriers to their implementation in clinical practice [52]. Nonetheless, SGLT2 inhibitors and non-steroidal mineralocorticoid receptor antagonists have shown positive cardiorenal benefits in the dedicated kidney end-points trials (Supplementary Table 1) [53-57]. Meanwhile, GLP-1 RA has proven benefits in cardiovascular outcome trials and recently, a dedicated kidney end-points trial (FLOW A Research Study to See How Semaglutide Works Compared to Placebo in People with Type 2 Diabetes and Chronic Kidney Disease; NCT03819153) is terminated early due to positive results [58].
The 2023 Global Kidney Health Atlas reported significant gaps persist between HICs and LMICs in most key components of CKD care [6]. These disparities encompass areas such as the availability of public funding, screening practices, access to specialist care, essential medicines and technology, KRT and the establishment of CKD registries. Dialysis withdrawal due to cost contributed to mortality rates in 18% of LICs and 7% of LMICs, but none was reported in HICs.
Taken together, early identification and risk stratification of people who have increased risk of rapid CKD progression will facilitate timely intervention to prevent ESKD and premature mortality. Significant disparities related to comprehensive CKD care in LMICs should be proactively addressed by policymakers by increasing health care financing for CKD care, addressing workforce shortages, developing a surveillance system and ensuring access to KRT [6].
KIDNEY FAILURE RISK EQUATIONS
Development
To address the need for improved prediction of 2- and 5-year risk of progression to ESKD, the KFRE has been developed in 2011 using a large Canadian cohort involving people with CKD G3a–5 [34]. There were four KFREs developed in the original cohort: the 3-variable (age, sex, and eGFR), the 4-variable (3-variable plus urinary ACR), the 6-variable (4-variable plus diabetes and hypertension), and the 8-variable equations (4-variable plus corrected serum calcium, phosphate, bicarbonate, and albumin). The 4-variable and 8-variable equations demonstrated the best performance in the original cohort [34].
Validation
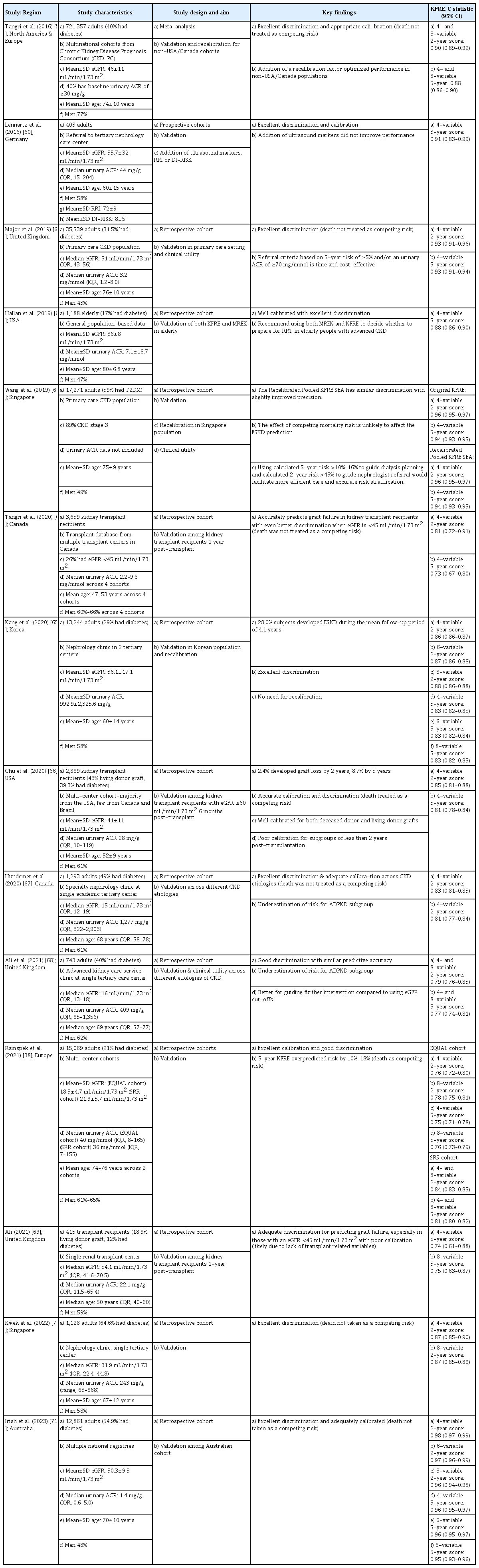
To date, KFRE is the most well-validated kidney failure risk prediction tool with good discrimination for people with CKD stages 3a–5, taking into account the status of diabetes and the competing risk of death [34,37,38,59-71]. It has been externally validated in more than 30 countries, and recalibration factor was included for non-USA/Canada populations in 2016 and South East Asia in 2019 (Table 1).
The KFRE was also validated among different populations including (1) kidney transplant recipients in Canada and USA with reduced eGFR after transplantation for 6 months to 1 year [64,66,69]; (2) elderly people [62]; and (3) across different etiologies of CKD such as diabetes, hypertension, glomerulonephritis, and autosomal dominant polycystic kidney disease (ADPKD) [67,68]. Of note, KFRE tends to underestimate the risk for CKD due to ADPKD [68]. This could be related to the unique pathophysiology of ADPKD in which its disease progression is generally associated with cyst growth and total kidney volume, rather than the KFRE variables such as degree of albuminuria [72]. It is also worth noting that the predictive performance of KFRE across different CKD etiologies were subjected to potential errors as these etiologies were not proven by kidney biopsy in most study populations, which was particularly challenging in LMICs and low-resource areas in HICs [67,68].
CLINICAL AND RESEARCH UTILITY
To date, the utilization of KFRE is limited by the access to and availability of urinary ACR measurement. In a large CKD cohort of over 60,000 people, it was found that only one-third of them had urinary ACR measurement performed [73]. To overcome this challenge, home-based albuminuria screening has shown promise with high participation rate, reliability, and accuracy [74]. Following that, an automated reporting of the validated KFRE in the presence of eGFR and urinary ACR measurements can increase physicians’ awareness, improve clinical decision-making, and reduce therapeutic inertia [73].
Compared to those at lower risk, the direct medical costs associated with people with CKD G3 and G4 were 42% and 70% higher, respectively [64,75]. These were related to a high number of hospitalizations, outpatient specialist visits, and drug dispensaries [64,75]. Therefore, the KFRE has been examined to facilitate risk-based triage for facilitating referral to nephrology care and resource allocations [61,68,76,77]. In a 5-year retrospective cohort study involving 35,000 people managed in the UK primary care setting, utilization of the 4-variable KFRE with a triage threshold of ≥5% at 5-year reduced unnecessary referrals, shortened waiting time and prompted early referrals for those who went on to develop ESKD at a younger age, which could offer long-term cost-saving [61]. Another experimental study in tertiary care centers in Canada utilized ESKD risk of ≥3% at 5-year generated from the 4-variable KFRE as the triage threshold for referral to nephrology care. Although the median number of monthly referrals increased by 45%, the median waiting time was shortened by 172 days than the pretriage period [77]. Another study in China compared the performance of 3-variable KFRE (age, gender, and eGFR) with machine-learning algorithms applied in a resource-constraint setting wherein urinary ACR was not readily available. Both 3-variables KFRE and machine-learning algorithms showed similar predictive performance, although the KFRE which based on existing risk factors had better practicality among the LMICs [46].
KFRE can provide accurate stratification of people at high risk of ESKD and guide dialysis planning [63]. Surveys are needed for evaluating healthcare providers and community satisfaction to risk-based CKD care before and after implementation of KFRE. Future research on the associations between these risk-based approaches to CKD care and a wide range of health outcomes including healthcare resource utilization, treatment metrics, cardiovascular and mortality outcomes, especially in resource-constrained settings, is very much in need [78].
CONCLUSIONS
While there is robust evidence supporting cardiorenal risk reduction strategies among people with T2DM, the next essential step is to ensure their equitable and affordable access, especially in resource-constrained settings and under-privileged populations. The delivery of care needs to be optimized for reducing residual cardiorenal risks. Simple and inexpensive risk-based approaches such as KFRE can improve detection, risk stratification, patient empowerment and timely intervention to reduce therapeutic inertia and improve health outcomes. Although the KFRE has shown promising results in facilitating CKD risk stratification, extensive validations in multiethnic populations and different healthcare settings are needed to scale up its clinical utility.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2023.0244.
Kidney outcome trials among people with or without type 2 diabetes mellitus
Notes
CONFLICTS OF INTEREST
Lee-Ling Lim has been international editorial board members of the Diabetes & Metabolism Journal since 2022. She was not involved in the review process of this article. Otherwise, there was no conflict of interest.
Ying-Guat Ooi and Shireene R. Vethakkan report no conflict of interest; Tharsini Sarvanandan reports receiving honoraria for giving lectures from Novo Nordisk; Nicholas Ken Yoong Hee reports receiving honoraria for giving lectures from AstraZeneca, Novo Nordisk and Zuellig Pharma; Quan-Hziung Lim reports receiving honoraria for giving lectures from AstraZeneca, Boehringer Ingelheim, Novo Nordisk and Zuellig Pharma; Sharmila S. Paramasivam reports receiving honoraria for consultancy or giving lectures from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk and Zuellig Pharma; Jeyakantha Ratnasingam reports receiving honoraria for consultancy or giving lectures from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Sanofi, Servier, Novartis, Ipsen and Zuellig Pharma; Soo-Kun Lim reports receiving grants and/or honoraria for consultancy or giving lectures from Abbott, AstraZeneca, Astellas, Baxter, Boehringer Ingelheim, Duopharma, Fresenius-Kabi, MSD, Novartis, Novo Nordisk, Roche, Sanofi and Taisho; Lee-Ling Lim reports receiving grants and/or honoraria for consultancy or giving lectures from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Novartis, Novo Nordisk, Roche, Sanofi, Servier and Zuellig Pharma.
FUNDING
This work was supported by the University of Malaya (UM) International Collaboration Grant (Grant number: ST048-2023). The funding source did not have any role in the design, interpretation of the study or the decision to publish the results.
Acknowledgements
None