Attention to Innate Circadian Rhythm and the Impact of Its Disruption on Diabetes
Article information
Abstract
Novel strategies are required to reduce the risk of developing diabetes and/or clinical outcomes and complications of diabetes. In this regard, the role of the circadian system may be a potential candidate for the prevention of diabetes. We reviewed evidence from animal, clinical, and epidemiological studies linking the circadian system to various aspects of the pathophysiology and clinical outcomes of diabetes. The circadian clock governs genetic, metabolic, hormonal, and behavioral signals in anticipation of cyclic 24-hour events through interactions between a “central clock” in the suprachiasmatic nucleus and “peripheral clocks” in the whole body. Currently, circadian rhythmicity in humans can be subjectively or objectively assessed by measuring melatonin and glucocorticoid levels, core body temperature, peripheral blood, oral mucosa, hair follicles, rest-activity cycles, sleep diaries, and circadian chronotypes. In this review, we summarized various circadian misalignments, such as altered light-dark, sleep-wake, rest-activity, fasting-feeding, shift work, evening chronotype, and social jetlag, as well as mutations in clock genes that could contribute to the development of diabetes and poor glycemic status in patients with diabetes. Targeting critical components of the circadian system could deliver potential candidates for the treatment and prevention of type 2 diabetes mellitus in the future.
INTRODUCTION
Type 2 diabetes mellitus (T2DM), characterized by insulin resistance in the liver, skeletal muscle, and adipose tissue, combined with relative pancreatic β‐cell dysfunction, is the most common metabolic disease in humans [1]. Despite the development of various treatment modalities for diabetes, the number of patients with T2DM continues to increase and is expected to reach 642 million by 2040, representing a public health challenge [2]. Therefore, fundamental strategies are needed to prevent and treat diabetes.
Among lifestyle risk factors regarded as hyperglycemic contributors, the impact of circadian disruption on the development of T2DM has attracted considerable interest [3]. In modern society, the availability of artificial light has enabled the counteraction of natural biological rhythms, whether intended or not. Various studies have indicated that disruptions in circadian rhythms contribute to impaired glycemic status in humans [4-6].
Consequently, innovative approaches involving circadian rhythms are needed to prevent and treat T2DM. Therefore, the current review aimed to delineate the concept of circadian rhythms and their impact on glycemia by summarizing evidence from animal, clinical, and epidemiological research.
THE BASIC CONCEPT OF CIRCADIAN RHYTHM
All living organisms possess an intrinsic time-keeping system with an interval of approximately 24 hours, known as the circadian rhythm [4,7,8]. It governs and synchronizes genetic, metabolic, hormonal, and behavioral signals in anticipation of further signals [4,8,9]. The circadian clock system is organized as the “master clock” or “central clock” in the suprachiasmatic nucleus (SCN) of the hypothalamus, acting as a pacemaker for “peripheral clocks” in other brain regions and various organs (e.g., liver, pancreas, heart, muscle, and adrenal gland) [8]. Endogenously generated rhythms can be entrained by a variety of external cues (zeitgebers), including light and nonphotic synchronizers (e.g., meal intake, exercise, social interactions, and temperature) [10].
During molecular regulation, the central clock permeates accurate daily oscillations in gene expression via two interlocked transcriptional-translational feedback loops (TTFL) [11]. As shown in Fig. 1, a heterodimer of transcription factors, the brain and muscle Arnt-like protein (BMAL) and circadian locomotor output cycles kaput (CLOCK), promotes the transcription of period (PER), cryptochrome (CRY), reverse erythroblastosis virus (REV-ERB), and retinoic acid-related orphan receptor (ROR) [12]. PER and CRY reverse repress BMAL-CLOCK-mediated transcription [7,9]. Through ROR response elements in BMAL1, REV-ERB counteracts BMAL transcription, whereas ROR induces its activation [13]. Downstream target genes that do not interact with the BMAL-CLOCK heterodimer are clock-controlled genes (CCGs). CCGs play important roles in oxidative processes, metabolism, and immune reactions [14].
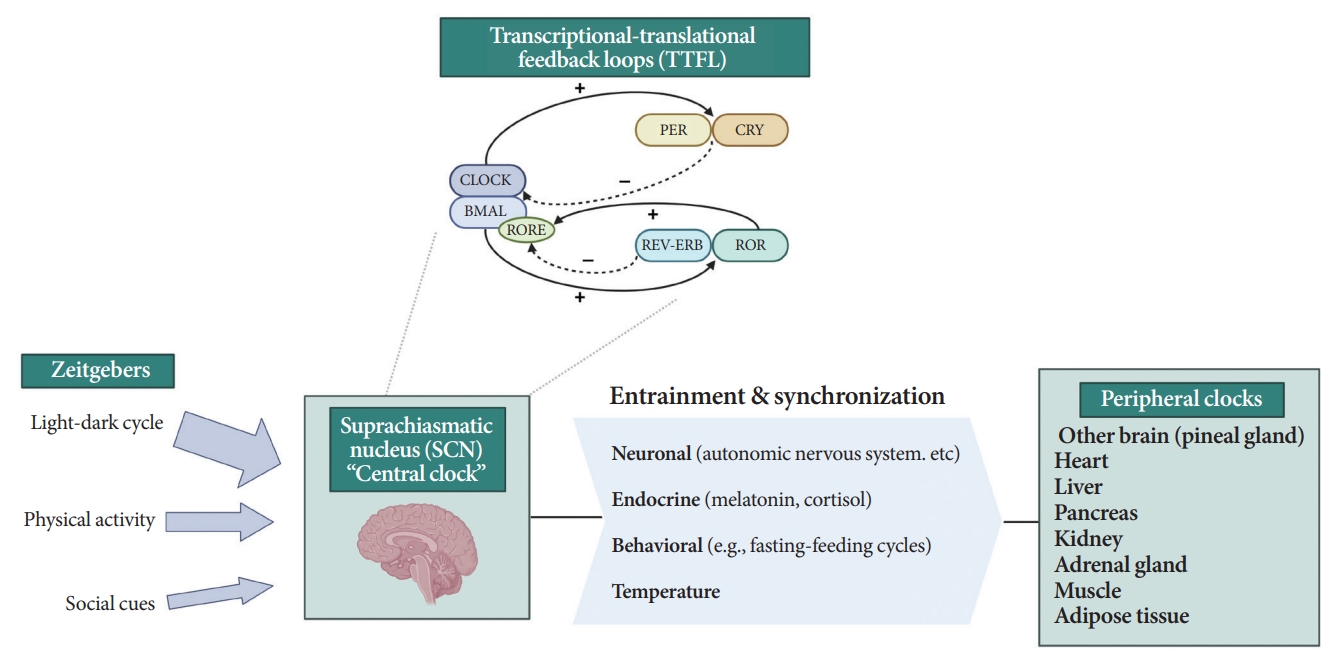
Circadian clock system. PER, period; CRY, cryptochrome; CLOCK, circadian locomotor output cycles kaput; BMAL, brain and muscle Arnt-like protein; RORE, retinoic acid-related orphan receptor response element; REV-ERB, reverse erythroblastosis virus; ROR, retinoidrelated orphan receptor.
The majority component of SCN was gamma-aminobutyric acid signaling (GABA) neuron. Its spontaneous firing activity depicts peak in the daytime and trough at nighttime [15]. About 15 brain lesions innervated by the SCN directly, such as the paraventricular nucleus (PVN), the subparaventricular zone, the arcuate nucleus, and the dorsomedial hypothalamus [16]. SCN signals propagate to the autonomous nervous system (ANS) via the PVN primarily [16,17]. Given ANS activity is related to glucose production and hepatic glycogen synthesis, disruption of SCN signals can promote hyperglycemia [18].
Peripheral clocks also possess independent, self-sustained rhythms themselves, along with those modulated by the SCN [19]. The SCN and peripheral clocks are intertwined as a hierarchical oscillator network, synchronized via molecular, neural, and endocrine routes, thereby generating 24-hour oscillations [4,8]. These rhythmicities, including the central/peripheral clocks and environmental/behavioral cues, are aligned (Fig. 2) [3].
MEASURING CIRCADIAN RHYTHM IN HUMANS
In humans, circadian rhythms can be estimated by objective or subjective methods. Common methods for objectively assessing circadian rhythms include evaluating melatonin and cortisol levels, core body temperature (CBT) modulated by the SCN, and rest-activity cycles [13]. Feasible methods targeting peripheral clocks are also available. The rhythms in the clock oscillators are represented by cosine waves [3]. In addition to objective measurements conducted in a controlled environment, sleep logs and chronotype questionnaires can also be used in real-world settings.
Nocturnal rodents are widely used as mammalian models for circadian rhythm studies [20]. However, most signal propagation from the SCN occurs in opposite directions in diurnal humans and nocturnal rodents, except for melatonin [15]. Therefore, the term “sleep-wake” rather than “light-dark” is more appropriate for designating time to describe circadian rhythms in all animals.
Melatonin
Melatonin is synthesized and secreted by the pineal gland upon the onset of darkness, peaking at 2:00 AM to 4:00 AM (Fig. 3) [21]. As the SCN clock is its primary regulator, melatonin rhythmicity is considered an indicator of the rhythm of the human master circadian clock [22]. Light inhibits melatonin synthesis [23]. Melatonin facilitates circadian phase shifting (adjusting the timing of the circadian system) [24] and can induce a sleep-permissive state by inhibiting SCN neuronal firing [25].
To determine the phasing of melatonin, melatonin levels are usually measured in the plasma or saliva every 30 minutes. To minimize the interference of light on melatonin rhythmicity, serial melatonin sampling is performed in a low-light (<50 lux) environment [22]. Dim light melatonin onset (DLMO) [13] is determined by the time point at which melatonin levels rise above specific cut-off values: 10 pg/mL for plasma, 3 pg/mL for saliva, or two standard deviations above the mean of the first three baseline values [26]. Currently, DLMO in the plasma or saliva is regarded as the gold standard for assessing circadian rhythms [13].
Glucocorticoids
Humans and rodents exhibit different rhythms of glucocorticoid (corticosterone in rodents and cortisol in humans) secretion. Corticosterone peaks at 6:00 PM to 6:30 PM in rodents [27], and cortisol peaks at 7:00 AM to 8:00 AM in humans, reaching nadir at midnight (Fig. 3) [28].
The SCN affects glucocorticoid levels in various ways [29]. It can activate the release of corticotropin-releasing hormone and adrenocorticotropic hormone via the PVN [30] and the sympathetic nervous system (SNS) [31]. As a peripheral clock, the adrenal gland itself can affect glucocorticoid rhythm [32]. Moreover, CLOCK and BMAL are involved in the rate-limiting component of steroidogenesis, resulting in a rhythmic increase in steroidogenesis [33]. When the daily rhythms of blood cortisol levels in individuals with T2DM were compared with those in controls, a flattened diurnal cortisol curve was observed throughout the day [34].
Core body temperature
CBT naturally fluctuates over a 24-hour period affected by the sleep-wake cycle in the opposite direction of the melatonin rhythm (Fig. 3) [35]. While melatonin levels increase during the night, CBT decreases, and the lowest CBT (nadir) typically occurs around 3:00 AM to 4:00 AM. Sleepiness is enhanced when CBT declines. Traditionally, the estimation of CBT rhythmicity has been used to identify circadian rhythms in humans. However, due to the substantial influence of activity and meals and the inconvenience of obtaining CBT through transrectal measurement [36], a shift towards a preference for melatonin levels as the circadian marker of choice ensued.
Peripheral clock estimation
Several minimally invasive ways to depict peripheral circadian rhythms are available [13]. Currently, clock gene expression can be studied in vivo in the peripheral blood, oral mucosa, and hair follicles [13].
Several studies have shown that the extent of clock gene expression in whole blood is related to sleep disturbances [37]. In patients with obstructive sleep apneas, the rhythmicity of BMAL1, CLOCK, and CRY2 expression in whole blood is disrupted compared to that in healthy controls [38].
Obtaining oral mucosa samples by scraping off pipette tips has recently become a valuable tool for studying clock gene expression [39]. A study performed under real-life conditions indicated that individual chronotypes affect the circadian phases of PER and REV-ERBα expression profiles in the oral mucosa [40]. However, the frequency of sampling at 1- or 2-hour intervals throughout the day limits precise measurements [13].
Hair and beard follicle samples can also serve as indicators of the human peripheral circadian clock [41]. Ferrante et al. [42] reported differences in the clock gene expression of PER in hair follicle cells of 14 individuals according to their chronotype. Despite the ability to extract high-quality RNA from approximately 10 head hairs or five beard hairs per time point [41], optimization of the amount of sampling according to sex and hair thickness is required [43].
Rest-activity cycles
The circadian rest-activity rhythm, which is a measure of circadian timing influencing behavior, is a common method of gauging the cycle of an individual [44]. Noninvasive actigraphy has traditionally been used to collect time series information [44]. Actigraphy uses a wrist-worn accelerometer that records movement, sleep-wake cycles, and light exposure in humans with 80% accuracy compared to polysomnography [44].
Resting-activity rhythms are usually analyzed using cosinor analysis [45]. Cosinor analysis enables the extraction of several circadian features from original time series data after exploring the best-fit cosine curve with the least difference [46]. To elaborate further, cosinor analysis transforms graphical oscillations into numbered features. The basic circadian indices of cosinor analysis are amplitude, acrophase, midline statistic of rhythm (MESOR), period, and phase (Fig. 4) [45-47]. The amplitude is defined as half the difference between the peak and trough values, reflecting the strength of the rhythm. In some studies, researchers defined this as the gap between the peak and trough values [48]. MESOR indicates mean activity levels. A period represents the time interval between two reference points within a rhythm (e.g., between two peaks). Phase (delayed or advanced) refers to the timing of the trough or peak. In examples of the sleep-wake cycle, a phase delay indicates a later sleep time [49]. Acrophase is the time when peak activity occurs in each cycle, and a higher value reflects a later peak [50]. Additionally, the overall rhythmicity/goodness-of-fit of the extended cosine model was estimated using pseudo-F statistics or rsquared values, with a higher value indicating a more robust overall rest-activity rhythmicity [47,50].
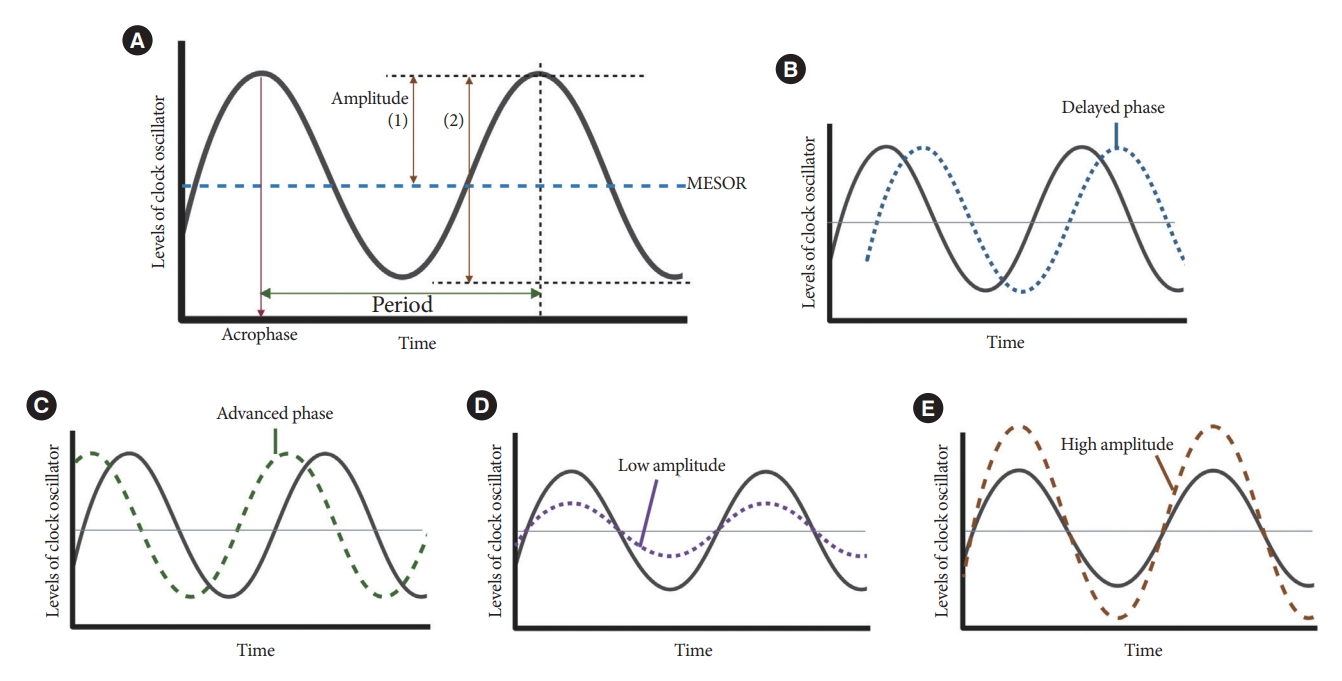
Cosine curve characteristics. (A) Parameters of circadian rhythmicity. Examples of delayed phase (B), advanced phase (C), low amplitude (D), and high amplitude (E) curves. MESOR, midline statistic of rhythm.
Currently, attempts to adapt consumer-grade wearable activity trackers and mobile technologies at a low cost instead of using conventional actigraphy are increasing, thereby enabling the construction of a population-based rest-activity cycle database; however, more reliability is required [51].
Sleep diary
Keeping a sleep log of the time they wake up, go to bed, and take a nap can offer insights into the circadian rhythm of an individual. Continuous recordings for a period of 14 days on both working and free days are required [52]. Additionally, it is useful to collect information about other behaviors that can influence the sleep-wake cycle, such as alcohol or caffeine consumption, practice of exercises, and electronic device usage.
Circadian chronotype
A chronotype is defined as the behavioral preference of an individual regarding the sleep-wake cycle, which classifies individuals as having a morning (lark) or evening (owl) chronotype [53]. Although they do not indicate circadian rhythms per se, prior research has shown that these questionnaires are equivalent to measuring melatonin levels and CBT in healthy populations [54]. Chronotypes are determined by various factors, such as genetic variations, ethnicity, sex, environmental cues (e.g., light and work schedules), country, and daylight exposure [55,56].
Chronotypes are typically assessed using self-reported questionnaires [57]. Widely-administered questionnaires are (1) the Morningness-Eveningness Questionnaire [57], (2) the Munich Chronotype Questionnaire (MCTQ), and (3) the MCTQ for Shift Workers [58]. The MCTQ calculates the midpoint of sleep duration on work-free days, adjusted by “oversleep” caused by the sleep debt on the workdays, thereby identifying the concept of “social jetlag” [55]. Social jetlag indicates a discrepancy in sleep timing between workdays and work-free days, corresponding to social and biological time [55]. Prior evidence has demonstrated that individuals with social jet lag, estimated by the MCTQ, are linked to numerous metabolic abnormalities and obesity [59].
IMPACT OF CIRCADIAN RHYTHM ON GLUCOSE LEVELS
Diurnal patterns are observed in the regulation of premeal and postprandial glucose levels (Fig. 5) [15]. In healthy humans under regular light-dark cycles, baseline or premeal glucose levels peak upon wakening and trough during sleep, independent of eating behaviors [60,61]. Diurnal oscillations of baseline premeal glucose levels are related to the rhythm of endogenous glucose production (EGP), primarily derived from hepatic gluconeogenesis [62]. The diurnal rhythm of baseline glucose and gene expression of hepatic gluconeogenic are disrupted after SCN lesioning in animal studies [63,64]. The GABA pathway in the SCN and downstream ANS is thought to be involved in the regulation of baseline glucose levels or EGP [65].
The daily oscillations of postprandial glucose levels contrast with premeal glucose level fluctuations. Prior human studies using a hyperinsulinemic-euglycemic clamp have demonstrated that an identical meal could induce greater glucose excursion at dinner than at breakfast [66,67]. That is, insulin sensitivity and glucose tolerance are greater upon waking than in the evening, and EGP suppression by insulin also results in diurnal oscillations [66,68]. This diurnal rhythm is thought to be mediated by the SCN with PVN-independent mechanisms [65,68].
Insulin secretion is also enhanced during waking hours compared to that during sleep [69]. These oscillations are derived not only from the SCN, but also from the islet-autonomous mechanism itself [68,70]. Previous animal studies have shown that BMAL1 and CLOCK regulate the oscillations of genes encoding the secretory machinery involved in insulin release [71]. Moreover, the hypothalamus, especially oxytocin neurons in the PVN, regulates insulin secretion of β-cells through pancreatic islet innervation via the SNS [70]. When these oxytocin neurons were activated, insulin secretion was suppressed, whereas ablation of these enhanced the insulin secretion in β-cells of mice [70].
In human studies on patients with diabetes, insulin rhythmicity in response to blood glucose was dampened [3]. In a postmortem analysis of patients with T2DM, the number of SCN neurons was lower than that in patients without T2DM [72].
Dawn phenomenon
The dawn phenomenon (DP) refers to spontaneous early morning hyperglycemia without nocturnal hypoglycemia [73]. In extended DP, fasting hyperglycemia is accompanied by postbreakfast hyperglycemia [74]. Although patients with DP have glycosylated hemoglobin (HbA1c) levels 0.4% higher than those without DP, no efficient strategy to manage DP exists despite treatment modalities, such as the additional administration of basal insulin before bedtime or insulin sensitizers [74,75].
DP is likely induced by increased insulin resistance and hepatic EGP rather than by decreased insulin secretion or clearance rates [76]. In a novel approach based on the circadian clock, patients with T2DM and extended DP were noted to display a different oscillatory pattern of REV-ERBα/β expression compared with those without DP [77]. Improving sleep quality, engaging in moderate-intensity aerobic exercise before breakfast, and frequently interrupting sitting could be beneficial for managing DP in patients with T2DM [78-81].
DISRUPTIVE CIRCADIAN RHYTHMS AND T2DM
As shown in Fig. 6, several studies have demonstrated a significant link between circadian disruption and T2DM [4-6].

Impact of circadian disruption on insulin resistance or diabetes. BMAL, brain and muscle Arnt-like protein; CLOCK, circadian locomotor output cycles kaput; CRY, cryptochrome.
Mutations in clock genes
Disruption of the TTFL, which consists of four core clock genes (BMAL1, CLOCK, PER, and CRY), can induce abnormalities in glucose metabolism [69,82-90]. Mice with CLOCK mutations are hyperphagic and obese, resulting in the presence of metabolic syndromes, including hyperglycemia [90]. In other animal models, disruption of the Bmal1, CLOCK, and Cry genes leads to glucose intolerance and diabetes [82-84]. In human studies, polymorphisms in the BMAL1, CLOCK, and CRY genes have been shown to increase the risk of T2DM [69], and an interaction between diet and clock gene mutations has been observed [91-93].
Altered light-dark cycles
Since the SCN receives light signals, altered light-dark cycles can substantially affect circadian rhythms [94,95]. In animal models, both a short photoperiod (5 hours a day) and constant light exposure during the day, physiological sleeping period, may lead to impaired glucose tolerance and the absence of rhythmicity in insulin sensitivity, respectively [94,95]. In studies involving a prospective cohort of an older adult population, light exposure at night, even at low levels in the bedroom, was related to an increased risk of diabetes [96]. The inhibition of melatonin coupled with elevated glucocorticoid levels at night under disrupted light-dark cycles may induce a decrease in insulin secretion and exacerbate insulin resistance [69,97].
Disruption in sleep-wake cycles
Traditionally, the association between the quantity of sleep and future risk of T2DM has been investigated in various studies. However, they were fraught with discrepancies. Several population-based studies have reported the predictive value of short sleep duration in patients with T2DM [98-100]. A recent cohort study with a 16-year follow-up period demonstrated that individuals with sleep deprivation have a higher risk of T2DM incidence than those who sleep sufficiently [99]. However, a few studies have shown that long sleep duration is significantly associated with a higher risk of T2DM [101,102]. Several meta-analyses and a study on the Chinese population found a U-shaped association between sleep duration and T2DM risk; that is, both short and long sleep durations are related with an increased risk of T2DM [103-105]. The reason for these inconsistent findings may have stemmed from the varied classifications of sleep duration.
As an explanation for these relationships, sleep deprivation can induce altered sympathovagal balance [97], resulting in decreased insulin secretion and insulin-mediated glucose uptake while stimulating hepatic glucose release [97] and elevating evening cortisol levels [19]. In the case of long sleep duration causing T2DM, sleep can still occur despite sleep fragmentation, frequent awakenings, and poor sleep quality [106], which are indicative of poor metabolic health caused by low physical activity, depression, or obesity [107]. Furthermore, poor sleep quality [108,109], daytime napping [101], and habitual late sleep initiation, from 1:00 AM to 6:00 AM [110], are significantly associated with the risk of T2DM.
Disruption in rest-activity rhythm
A small amount of evidence has established rest-activity rhythms [111-114]. In a cross-sectional analysis, a lower amplitude-to-MESOR ratio was associated with higher fasting insulin levels and homeostatic model assessment for insulin resistance, while a reverse association was found between the amplitude and presence of T2DM [114]. Using large 24-hour actigraphy data from 11,210 participants from the United States National Health and Nutrition Examination Survey (NHANES), Xiao et al. [115] suggested that individuals with a stronger cosine-like pattern of activity were less likely to be diabetic. Considering the influence of the rest-activity cycle in patients with type 1 diabetes mellitus (T1DM), Griggs et al. [111] and Farabi et al. [112], through coherence and cosinor analyses, uncovered that the relationship between glucose levels and routine activity during both wakefulness and sleep follows a circadian pattern.
Disruption in fasting-feeding rhythms
Fast-feeding rhythms, consisting of the timing, frequency, and regularity of dietary intake, are among the main components of circadian rhythms. Various studies have demonstrated the impact of diet timing, the so-called chrononutrition, on glucose metabolism [116-120].
Eating dinner late reportedly promotes insulin resistance and weight gain [116]. In an experimental study on young Japanese adults, a late eating schedule (12:00 PM, 5:00 PM, and 11:00 PM) increased the mean glucose concentration estimated using a continuous glucose monitor [117]. Kwak et al. [118] showed that individuals fasting nightly for greater than 12 hours or eating the last meal before 9:00 PM had lower odds of developing T2DM. Consuming more than 40% of their energy intake during the evening was related to a high risk of developing T2DM [118]. In contrast, an analysis using NHANES data suggested that an earlier start to eating was associated with lower fasting glucose levels and insulin resistance [119].
In patients with diabetes, misestimation of dietary intake is also a particular concern. Individuals with diabetes who eat at night, that is, consume more than 25% of their daily energy intake after regular dinnertime, have been shown to have poor compliance with glucose monitoring, higher HbA1c levels, and a greater number of complications from diabetes [120,121].
Recently, time-restricted eating (TRE), a type of intermittent fasting, was proposed as a method to reduce weight [122]. In healthy and synchronized individuals, the time from the first to last energy consumption throughout the day, called the typical eating window, spans 12 to 15 hours/day [123]. TRE shortens the eating window to around 4 to 10 hours/day [124]. Mounting evidence has similarly shown improvements in glucose metabolism, insulin sensitivity, body weight, blood pressure, lipid levels, and gut microbiome after TRE [125-127]. A recent meta-analysis of 11 studies reported significantly lower fasting glucose levels in participants on TRE than that in those eating freely [125]. Metabolic benefits have also been observed in individuals with prediabetes and T2DM [128,129]. This significance is usually explained by a spontaneous reduction in additional energy intake, a longer fasting period, and timing of food intake harmonized with circadian rhythms [123]. However, the shorter intervention duration (<16 weeks) and variation in adherence need to be resolved [130].
Shift work
Shift work is a typical example of circadian misalignment between the central and peripheral clocks and environmental/behavioral oscillations (Fig. 2). The types of shift work include rotating shifts, regular evening or night schedules, 24-hour shifts, on-call or casual shifts, split shifts, and other nonday schedules [131]. The International Labor Organization reported that almost 20% of the overall workforce, nearly 0.7 billion workers globally, is engaged in a shift work pattern [132]. Over the past decades, a growing body of evidence has indicated the adverse health outcomes of shift work, including T2DM [133-136]. In a meta-analysis of 12 cohort studies, involving 244,266 participants, the adjusted relative risks for the relationship between shift work and diabetes mellitus risk was 1.14 (95% confidence interval, 1.10 to 1.19; I2=38.9%) [134]. Importantly, individuals working rotating shifts have been reported with a higher risk of diabetes than those working fixed night shifts [133,135]. The underlying mechanism is thought to be related to shorter sleep durations and decreased insulin sensitivity due to continuous shift work [136]. In patients with T2DM, nightshift work was shown to be related to poorer glucose control with higher HbA1c levels than those who did not work night shifts, irrespective of sleep duration, chronotype, and daily carbohydrate intake [137].
Evening chronotype
Prior research shows that individuals with the evening chronotype tend to consume fewer and larger meals and eat dinner late because of later waking times [138]. Accordingly, epidemiological evidence suggests a potential association between the evening chronotype and an increased risk of T2DM, independent of sleep duration [139,140]. In patients with diabetes, valid evidence of a relationship between chronotype and glycemic control status exists. In 210 non-shift workers with T2DM in Thailand, delayed bedtime on weekends was associated with poor glycemic control [113].
Social jetlag
Social jet lag is a subtle and common example of circadian misalignment in modern societies, with a prevalence superior to 50% [55,141]. The clinical impact of social jet lag has been reported in various epidemiological studies [141,142]. Individuals with social jetlag greater than 1 hour have a 1.75 times higher prevalence of diabetes or prediabetes than those with less than 1 hour of social jetlag [141]. In a prospective cohort of patients with T2DM in the Netherlands, a cross-sectional association between moderate-to-high social jet lag and higher HbA1c was observed [142].
The extent of social jetlag partially depends on the chronotype of an individual (“morning” or “evening” preference) [57]. The detrimental effects of social jetlag have been explained by the hypothalamic-pituitary-adrenal axis disruption and the influence of sleep architecture, incretin hormones, and mood [142,143].
FUTURE DIRECTIONS
As the circadian system also affects cardiovascular physiology, kidney function, and glycemic status [144], the clinical impact of circadian disruption on the risk of complications in diabetes is an interesting subject for researchers. Recently, patients with both diabetes and disrupted rest-activity rhythms exhibited a higher risk of developing cardiovascular diseases and mortality in an analysis of the United States Biobank database [145].
Regarding the role of the circadian clock in the kidneys, various laboratory studies have shown that many cellular pathways that result in diabetic nephropathy are involved in circadian misalignment [146-148]. PER1, the main clock protein, has been reported to regulate the transcription levels of glucose transporter sodium-glucose cotransporter 1 (SGLT1) in proximal tubule cells [148], and the circadian clock located in podocytes could control the expression of the Rho GTPase activating protein 24 (ARHGAP24) gene associated with predisposition to diabetic nephropathy in both T1DM and T2DM [146]. Ansermet et al. [147] demonstrated that a Bmal1 knockout in the renal tubule aggravates hyperglycemia by accelerating renal gluconeogenesis in mouse models of T1DM. Collectively, the clinical significance of circadian perturbations on renal outcomes in patients with diabetes could be a subject of future research.
Beyond a detailed understanding of the circadian system, a novel strategy for tailoring glycemic control is needed. Small interventions, such as continuous positive airway pressure in patients with both DP and obstructive sleep apnea, frequent interruptions of sedentary time, or moderate-intensity exercise before breakfast, have been shown to improve DP [78-81].
CONCLUSIONS
The development of novel therapeutic and preventative strategies is imperative to reduce the prevalence of T2DM worldwide. Despite the considerable progress made in recent decades on the understanding of circadian physiology, its application in the treatment of diabetes remains limited. In this regard, we reviewed evidence from animal-based, clinical, and epidemiological studies linking the circadian system to various aspects of the pathophysiology and clinical outcomes of T2DM. We hope that targeting key components of the circadian system will yield a potential candidate to treat and prevent T2DM in the future.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
This work was supported by the by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2023R1A2C2003479), the Bio&Medical Technology Development Program of the NRF funded by the MSIT (NRF-2019M3E5D3073102), and the Basic Science Research Program funded by the Ministry of Education (NRF-2020R1I1A1A01071665 and RS-2023-00248873). It was also supported by the National IT Industry Promotion Agency (NIPA) grant funded by MSIT (No. S0252-21-1001, Development of AI Precision Medical Solution [Doctor Answer 2.0]), by Korea Health Industry Development Institute (KHIDI) grant funded by the Korea government (No. HI23C0679, Development and practice of main counseling doctor and patient support technology for diabetes digital healthcare), and by a Korea University grant (K2210711).
Acknowledgements
All figures were created with BioRender.com.



