
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Article category
- Page Path
- HOME > Article category > Article category
Review
- Basic Research
- Adipose Tissue and Metabolic Health
- Sung-Min An, Seung-Hee Cho, John C. Yoon
- Diabetes Metab J. 2023;47(5):595-611. Published online July 24, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0011

- 3,820 View
- 440 Download
- 3 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - In this review, we provide a brief synopsis of the connections between adipose tissue and metabolic health and highlight some recent developments in understanding and exploiting adipocyte biology. Adipose tissue plays critical roles in the regulation of systemic glucose and lipid metabolism and secretes bioactive molecules possessing endocrine, paracrine, and autocrine functions. Dysfunctional adipose tissue has a detrimental impact on metabolic health and is intimately involved in key aspects of metabolic diseases such as insulin resistance, lipid overload, inflammation, and organelle stress. Differences in the distribution of fat depots and adipose characteristics relate to divergent degrees of metabolic dysfunction found in metabolically healthy and unhealthy obese individuals. Thermogenic adipocytes increase energy expenditure via mitochondrial uncoupling or adenosine triphosphate-consuming futile substrate cycles, while functioning as a metabolic sink and participating in crosstalk with other metabolic organs. Manipulation of adipose tissue provides a wealth of opportunities to intervene and combat the progression of associated metabolic diseases. We discuss current treatment modalities for obesity including incretin hormone analogs and touch upon emerging strategies with therapeutic potential including exosome-based therapy, pharmacological activation of brown and beige adipocyte thermogenesis, and administration or inhibition of adipocyte-derived factors.
-
Citations
Citations to this article as recorded by- Pharmacological targets at the lysosomal autophagy–NLRP3 inflammasome crossroads
Srinivasa Reddy Bonam, Dylan Mastrippolito, Philippe Georgel, Sylviane Muller
Trends in Pharmacological Sciences.2024; 45(1): 81. CrossRef - Senescent adipocytes and type 2 diabetes – current knowledge and perspective concepts
Weronika Kruczkowska, Julia Gałęziewska, Mateusz Kciuk, Adrianna Gielecińska, Elżbieta Płuciennik, Zbigniew Pasieka, Lin-Yong Zhao, Yi-Jin Yu, Damian Kołat, Żaneta Kałuzińska-Kołat
Biomolecular Concepts.2024;[Epub] CrossRef - Visceral Adipose Tissue: The Hidden Culprit for Type 2 Diabetes
Sneha Dhokte, Krzysztof Czaja
Nutrients.2024; 16(7): 1015. CrossRef - Beyond the Cold: Activating Brown Adipose Tissue as an Approach to Combat Obesity
Cristina Elena Negroiu, Iulia Tudorașcu, Cristina Maria Bezna, Sanziana Godeanu, Marina Diaconu, Raluca Danoiu, Suzana Danoiu
Journal of Clinical Medicine.2024; 13(7): 1973. CrossRef
- Pharmacological targets at the lysosomal autophagy–NLRP3 inflammasome crossroads
Original Articles
- Drug/Regimen
- Glimepiride Compared to Liraglutide Increases Plasma Levels of miR-206, miR-182-5p, and miR-766-3p in Type 2 Diabetes Mellitus: A Randomized Controlled Trial
- Nikolai N. Scherbak, Robert Kruse, Thomas Nyström, Johan Jendle
- Diabetes Metab J. 2023;47(5):668-681. Published online June 22, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0342

- 2,436 View
- 135 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Diabetes is a chronic disease with several long-term complications. Several glucose-lowering drugs are used to treat type 2 diabetes mellitus (T2DM), e.g., glimepiride and liraglutide, in which both having different modes of action. Circulating microRNAs (miRNAs) are suggested as potential biomarkers that are associated with the disease development and the effects of the treatment. In the current study we evaluated the effect of glimepiride, liraglutide on the expression of the circulating miRNAs.
Methods
The present study is a post hoc trial from a previously randomized control trial comparing liraglutide versus glimepiride both in combination with metformin in subjects with T2DM, and subclinical heart failure. miRNAs were determined in the subjects’ serum samples with next generation sequencing. Expression patterns of the circulating miRNAs were analyzed using bioinformatic univariate and multivariate analyses (clinical trial registration: NCT01425580).
Results
Univariate analyses show that treatment with glimepiride altered expression of three miRNAs in patient serum, miR-206, miR-182-5p, and miR-766-3p. Both miR-182-5p and miR-766-3p were also picked up among the top contributing miRNAs with penalized regularised logistic regressions (Lasso). The highest-ranked miRNAs with respect to Lasso coefficients were miR-3960, miR-31-5p, miR-3613-3p, and miR-378a-3p. Liraglutide treatment did not significantly influence levels of circulating miRNAs.
Conclusion
Present study indicates that glucose-lowering drugs differently affect the expression of circulating miRNAs in serum in individuals with T2DM. More studies are required to investigate possible mechanisms by which glimepiride is affecting the expression of circulating miRNAs. -
Citations
Citations to this article as recorded by- Glimepiride Compared to Liraglutide Increases Plasma Levels of miR-206, miR-182-5p, and miR-766-3p in Type 2 Diabetes Mellitus: A Randomized Controlled Trial (Diabetes Metab J 2023;47:668-81)
Nikolai N. Scherbak, Robert Kruse, Thomas Nyström, Johan Jendle
Diabetes & Metabolism Journal.2023; 47(6): 882. CrossRef - Glimepiride Compared to Liraglutide Increases Plasma Levels of miR-206, miR-182-5p, and miR-766-3p in Type 2 Diabetes Mellitus: A Randomized Controlled Trial (Diabetes Metab J 2023;47:668-81)
Da Young Lee
Diabetes & Metabolism Journal.2023; 47(6): 879. CrossRef
- Glimepiride Compared to Liraglutide Increases Plasma Levels of miR-206, miR-182-5p, and miR-766-3p in Type 2 Diabetes Mellitus: A Randomized Controlled Trial (Diabetes Metab J 2023;47:668-81)
- Others
- Glucose Regulation after Partial Pancreatectomy: A Comparison of Pancreaticoduodenectomy and Distal Pancreatectomy in the Short and Long Term
- Jun Suh Lee, Minji Sohn, Kyuho Kim, Yoo-Seok Yoon, Soo Lim
- Diabetes Metab J. 2023;47(5):703-714. Published online June 22, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0205
- 1,633 View
- 150 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
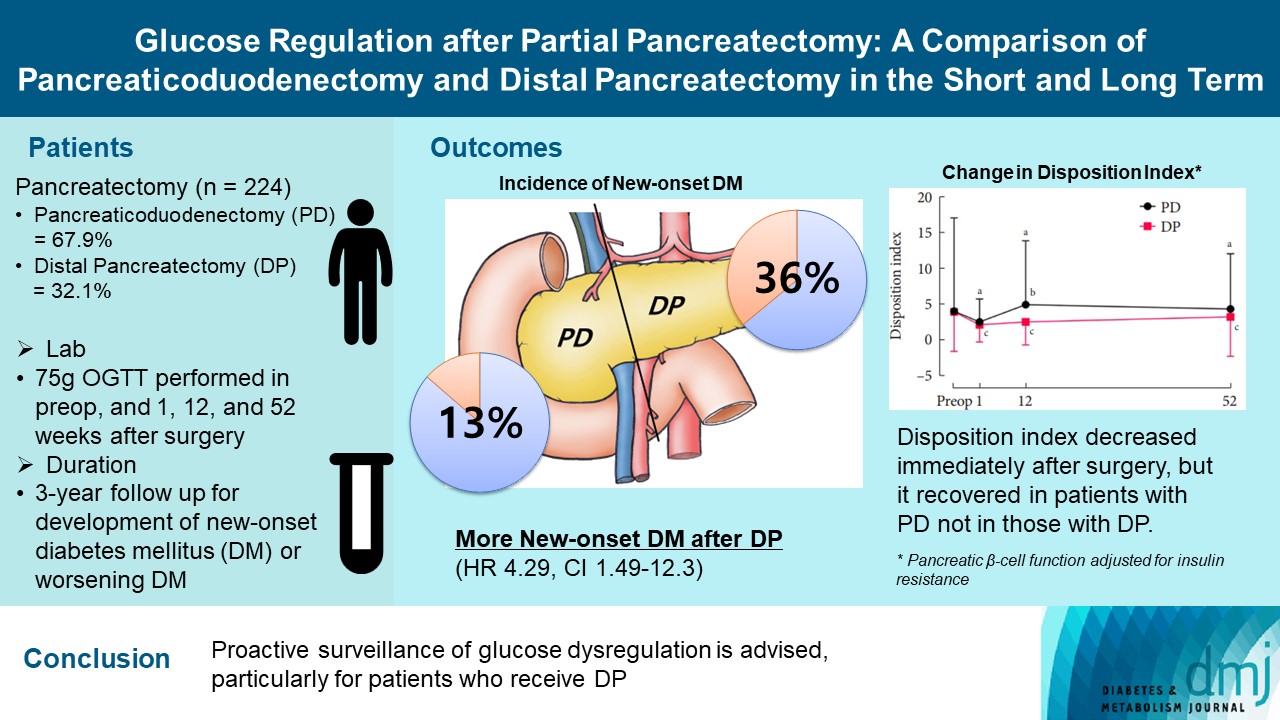
Long term quality of life is becoming increasingly crucial as survival following partial pancreatectomy rises. The purpose of this study was to investigate the difference in glucose dysregulation after pancreaticoduodenectomy (PD) or distal pancreatectomy (DP).
Methods
In this prospective observational study from 2015 to 2018, 224 patients who underwent partial pancreatectomy were selected: 152 (67.9%) received PD and 72 (32.1%) received DP. Comprehensive assessment for glucose regulation, including a 75 g oral glucose tolerance test was conducted preoperatively, and 1, 12, and 52 weeks after surgery. Patients were further monitored up to 3 years to investigate development of new-onset diabetes mellitus (NODM) in patients without diabetes mellitus (DM) at baseline or worsening of glucose regulation (≥1% increase in glycosylated hemoglobin [HbA1c]) in those with preexisting DM.
Results
The disposition index, an integrated measure of β-cell function, decreased 1 week after surgery in both groups, but it increased more than baseline level in the PD group while its decreased level was maintained in the DP group, resulting in a between-group difference at the 1-year examination (P<0.001). During follow-up, the DP group showed higher incidence of NODM and worsening of glucose regulation than the PD group with hazard ratio (HR) 4.29 (95% confidence interval [CI], 1.49 to 12.3) and HR 2.15 (95% CI, 1.09 to 4.24), respectively, in the multivariate analysis including dynamic glycemic excursion profile. In the DP procedure, distal DP and spleen preservation were associated with better glucose regulation. DP had a stronger association with glucose dysregulation than PD.
Conclusion
Proactive surveillance of glucose dysregulation is advised, particularly for patients who receive DP.
- Metabolic Risk/Epidemiology
- Low Household Income Status and Death from Pneumonia in People with Type 2 Diabetes Mellitus: A Nationwide Study
- You-Bin Lee, So Hee Park, Kyu-na Lee, Bongsung Kim, So Yoon Kwon, Jiyun Park, Gyuri Kim, Sang-Man Jin, Kyu Yeon Hur, Kyungdo Han, Jae Hyeon Kim
- Diabetes Metab J. 2023;47(5):682-692. Published online June 22, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0184
- 1,581 View
- 121 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
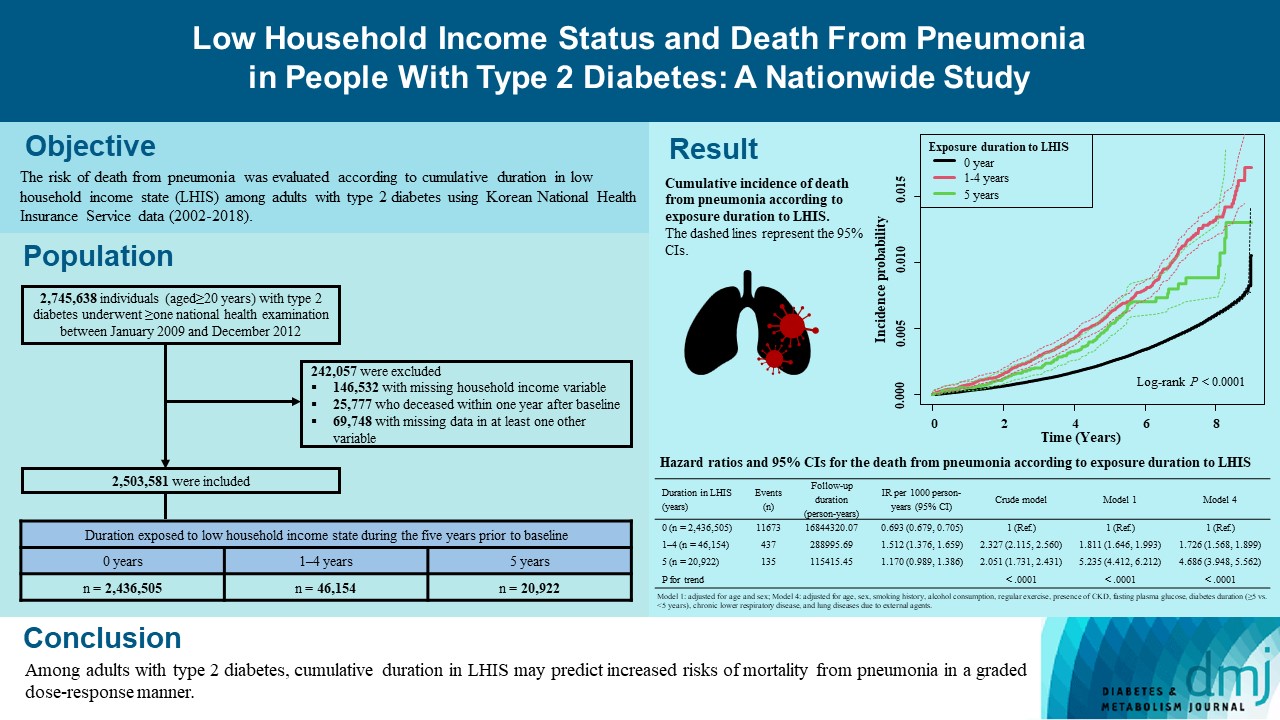
We explored the risk of death from pneumonia according to cumulative duration in low household income state (LHIS) among adults with type 2 diabetes mellitus (T2DM).
Methods
Using Korean National Health Insurance Service data (2002 to 2018), the hazards of mortality from pneumonia were analyzed according to duration in LHIS (being registered to Medical Aid) during the 5 years before baseline (0, 1–4, and 5 years) among adults with T2DM who underwent health examinations between 2009 and 2012 (n=2,503,581). Hazards of outcomes were also compared in six groups categorized by insulin use and duration in LHIS.
Results
During a median 7.18 years, 12,245 deaths from pneumonia occurred. Individuals who had been exposed to LHIS had higher hazards of death from pneumonia in a dose-response manner (hazard ratio [HR], 1.726; 95% confidence interval [CI], 1.568 to 1.899 and HR, 4.686; 95% CI, 3.948 to 5.562 in those exposed for 1–4 and 5 years, respectively) compared to the non-exposed reference. Insulin users exposed for 5 years to LHIS exhibited the highest outcome hazard among six groups categorized by insulin use and duration in LHIS.
Conclusion
Among adults with T2DM, cumulative duration in LHIS may predict increased risks of mortality from pneumonia in a graded dose-response manner. Insulin users with the longest duration in LHIS might be the group most vulnerable to death from pneumonia among adults with T2DM.
- Lifestyle
- Clinical Effects of a Home Care Pilot Program for Patients with Type 1 Diabetes Mellitus: A Retrospective Cohort Study
- Sejeong Lee, KyungYi Kim, Ji Eun Kim, Yura Hyun, Minyoung Lee, Myung-Il Hahm, Sang Gyu Lee, Eun Seok Kang
- Diabetes Metab J. 2023;47(5):693-702. Published online June 22, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0170

- 1,905 View
- 138 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Given the importance of continuous self-care for people with type 1 diabetes mellitus (T1DM), the Ministry of Health and Welfare of Korea launched a pilot program for chronic disease management. Herein, we applied a home care pilot program to people with T1DM to investigate its effects.
Methods
This retrospective cohort study was conducted at a single tertiary hospital (January 2019 to October 2021). A multidisciplinary team comprising doctors, nurses, and clinical nutritionists provided specialized education and periodically assessed patients’ health status through phone calls or text messages. A linear mixed model adjusting for age, sex, and body mass index was used to analyze the glycemic control changes before and after implementing the program between the intervention and control groups.
Results
Among 408 people with T1DM, 196 were enrolled in the intervention group and 212 in the control group. The reduction in glycosylated hemoglobin (HbA1c) after the program was significantly greater in the intervention group than in the control group (estimated marginal mean, –0.57% vs. –0.23%, P=0.008); the same trend was confirmed for glycoalbumin (GA) (–3.2% vs. –0.39%, P<0.001). More patients achieved the target values of HbA1c (<7.0%) and GA (<20%) in the intervention group than in the control group at the 9-month follow-up (34.5% vs. 19.6% and 46.7% vs. 28.0%, respectively).
Conclusion
The home care program for T1DM was clinically effective in improving glycemic control and may provide an efficient care option for people with T1DM, and positive outcomes are expected to expand the program to include more patients. -
Citations
Citations to this article as recorded by- Glycemic outcomes and patient satisfaction and self-management improves in transition from standard to virtual multidisciplinary care
Noga Minsky, Liat Arnon Klug, Tatyana Kolobov, Elizabeth Tarshish, Yuval Shalev Many, Aviva Lipsitz, Amna Jabarin, Nicole Morozov, Dania Halperin, Moshe Shalom, Rachel Nissanholtz-Gannot, Genya Aharon-Hananel, Amir Tirosh, Orly Tamir
Diabetes Research and Clinical Practice.2024; 209: 111587. CrossRef
- Glycemic outcomes and patient satisfaction and self-management improves in transition from standard to virtual multidisciplinary care
Editorial
- Navigating the Seas of Glycemic Control: The Role of Continuous Glucose Monitoring in Type 1 Diabetes Mellitus
- Jun Sung Moon
- Diabetes Metab J. 2023;47(3):345-346. Published online May 26, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0125
- 1,208 View
- 91 Download
Response
- Association of Body Mass Index and Fracture Risk Varied by Affected Bones in Patients with Diabetes: A Nationwide Cohort Study (Diabetes Metab J 2023;47:242-54)
- Se-Won Lee, Kyungdo Han, Hyuk-Sang Kwon
- Diabetes Metab J. 2023;47(3):439-440. Published online May 26, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0104
- [Original]
- 1,205 View
- 57 Download
Letter
- Association of Body Mass Index and Fracture Risk Varied by Affected Bones in Patients with Diabetes: A Nationwide Cohort Study (Diabetes Metab J 2023;47:242-54)
- So Young Park
- Diabetes Metab J. 2023;47(3):437-438. Published online May 26, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0100
- [Original]
- 1,035 View
- 60 Download
Review
- Basic Research
- Rediscovering Primary Cilia in Pancreatic Islets
- Eun Young Lee, Jing W. Hughes
- Diabetes Metab J. 2023;47(4):454-469. Published online April 28, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0442
- 2,584 View
- 240 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
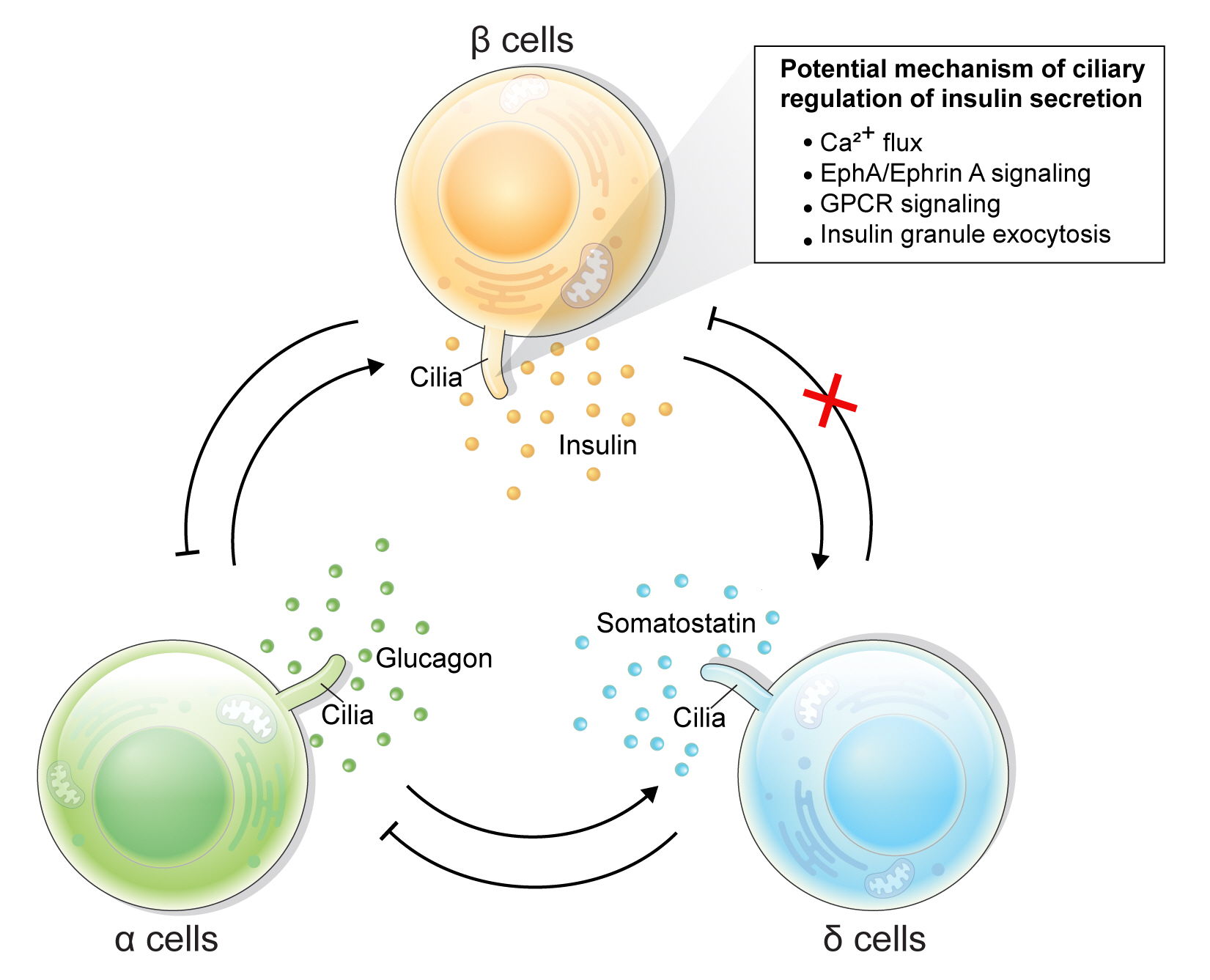
ePub - Primary cilia are microtubule-based sensory and signaling organelles on the surfaces of most eukaryotic cells. Despite their early description by microscopy studies, islet cilia had not been examined in the functional context until recent decades. In pancreatic islets as in other tissues, primary cilia facilitate crucial developmental and signaling pathways in response to extracellular stimuli. Many human developmental and genetic disorders are associated with ciliary dysfunction, some manifesting as obesity and diabetes. Understanding the basis for metabolic diseases in human ciliopathies has been aided by close examination of cilia action in pancreatic islets at cellular and molecular levels. In this article, we review the evidence for ciliary expression on islet cells, known roles of cilia in pancreas development and islet hormone secretion, and summarize metabolic manifestations of human ciliopathy syndromes. We discuss emerging data on primary cilia regulation of islet cell signaling and the structural basis of cilia-mediated cell crosstalk, and offer our interpretation on the role of cilia in glucose homeostasis and human diseases.
-
Citations
Citations to this article as recorded by- Beta cell primary cilia mediate somatostatin responsiveness via SSTR3
Samantha E. Adamson, Zipeng A. Li, Jing W. Hughes
Islets.2023;[Epub] CrossRef
- Beta cell primary cilia mediate somatostatin responsiveness via SSTR3
Original Articles
- Basic Research
- CycloZ Improves Hyperglycemia and Lipid Metabolism by Modulating Lysine Acetylation in KK-Ay Mice
- Jongsu Jeon, Dohyun Lee, Bobae Kim, Bo-Yoon Park, Chang Joo Oh, Min-Ji Kim, Jae-Han Jeon, In-Kyu Lee, Onyu Park, Seoyeong Baek, Chae Won Lim, Dongryeol Ryu, Sungsoon Fang, Johan Auwerx, Kyong-Tai Kim, Hoe-Yune Jung
- Diabetes Metab J. 2023;47(5):653-667. Published online April 26, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0244

- 2,720 View
- 193 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
CycloZ, a combination of cyclo-His-Pro and zinc, has anti-diabetic activity. However, its exact mode of action remains to be elucidated.
Methods
KK-Ay mice, a type 2 diabetes mellitus (T2DM) model, were administered CycloZ either as a preventive intervention, or as a therapy. Glycemic control was evaluated using the oral glucose tolerance test (OGTT), and glycosylated hemoglobin (HbA1c) levels. Liver and visceral adipose tissues (VATs) were used for histological evaluation, gene expression analysis, and protein expression analysis.
Results
CycloZ administration improved glycemic control in KK-Ay mice in both prophylactic and therapeutic studies. Lysine acetylation of peroxisome proliferator-activated receptor gamma coactivator 1-alpha, liver kinase B1, and nuclear factor-κB p65 was decreased in the liver and VATs in CycloZ-treated mice. In addition, CycloZ treatment improved mitochondrial function, lipid oxidation, and inflammation in the liver and VATs of mice. CycloZ treatment also increased the level of β-nicotinamide adenine dinucleotide (NAD+), which affected the activity of deacetylases, such as sirtuin 1 (Sirt1).
Conclusion
Our findings suggest that the beneficial effects of CycloZ on diabetes and obesity occur through increased NAD+ synthesis, which modulates Sirt1 deacetylase activity in the liver and VATs. Given that the mode of action of an NAD+ booster or Sirt1 deacetylase activator is different from that of traditional T2DM drugs, CycloZ would be considered a novel therapeutic option for the treatment of T2DM.
- Lifestyle
- Ultra-Processed Food Consumption and Obesity in Korean Adults
- Jee-Seon Shim, Kyoung Hwa Ha, Dae Jung Kim, Hyeon Chang Kim
- Diabetes Metab J. 2023;47(4):547-558. Published online April 26, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0026
- 2,847 View
- 139 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
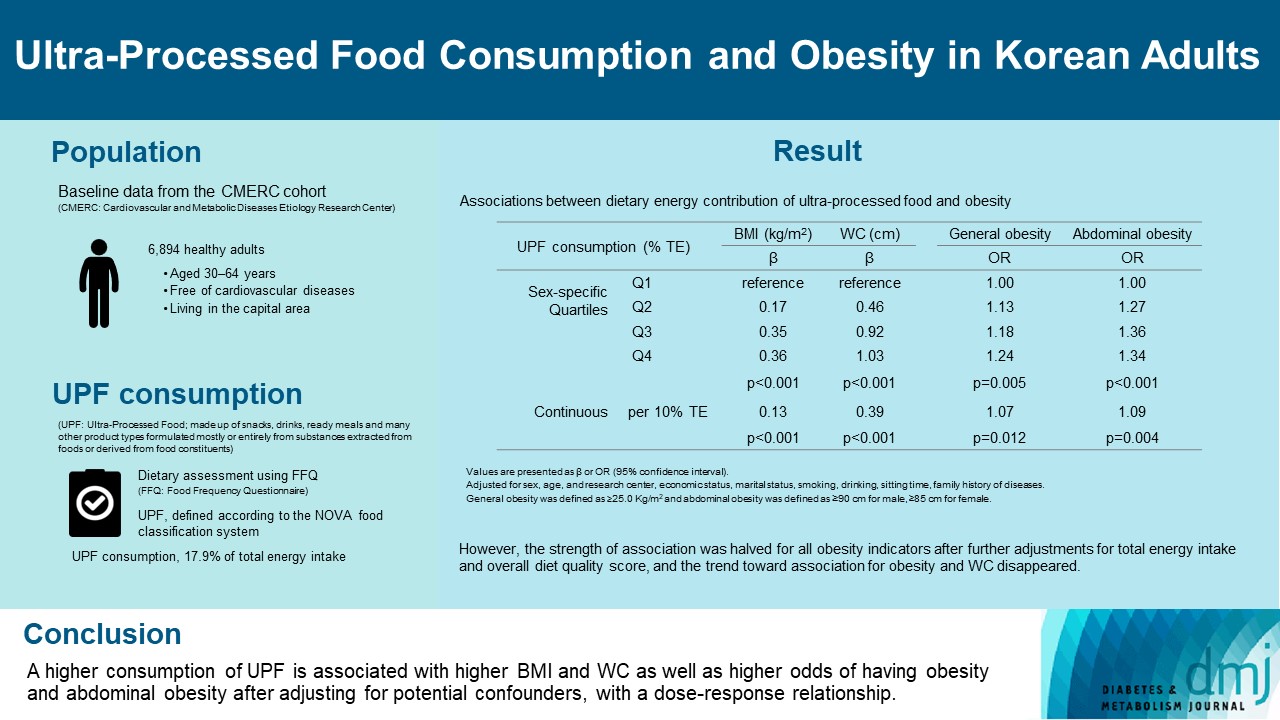
This study aimed to investigate the association between consumption of ultra-processed foods (UPF) and obesity in Korean adults.
Methods
We included the Cardiovascular and Metabolic Diseases Etiology Research Center cohort study baseline data of adults aged 30 to 64 years who completed a validated food frequency questionnaire. UPF was defined using the NOVA food classification. Multivariable linear and logistic regression analyses were performed to assess the association of dietary energy contribution of UPF with obesity indicators (body mass index [BMI], obesity, waist circumference [WC], and abdominal obesity).
Results
Consumption of UPF accounted for 17.9% of total energy intake and obesity and abdominal obesity prevalence was 35.4% and 30.2%, respectively. Compared with those in the lowest quartile of UPF consumption, adults in the highest quartile had greater BMI (β=0.36; 95% confidence interval [CI], 0.15 to 0.56), WC (β=1.03; 95% CI, 0.46 to 1.60), higher odds of having obesity (odds ratio [OR], 1.24; 95% CI, 1.07 to 1.45), and abdominal obesity (OR, 1.34; 95% CI, 1.14 to 1.57), after adjusting for sociodemographic characteristics, health-related behaviors, and family history of diseases. Dose-response associations between UPF consumption and obesity indicators were consistently found (all P trend <0.01). However, the strength of association was halved for all obesity indicators after further adjustments for total energy intake and overall diet quality score, and the trend toward association for obesity and WC disappeared.
Conclusion
Our finding supports the evidence that consumption of UPF is positively associated with obesity among Korean adults. -
Citations
Citations to this article as recorded by- Ultra-processed food consumption and increased risk of metabolic syndrome in Korean adults: A cross-sectional analysis of the KNHANES 2016–2020
Hansol Park, Youngmi Lee, Jinah Hwang, Yujin Lee
Nutrition.2024; 122: 112374. CrossRef - Diet quality partially mediates the association between ultraprocessed food consumption and adiposity indicators
Jee‐Seon Shim, Kyoung Hwa Ha, Dae Jung Kim, Hyeon Chang Kim
Obesity.2023; 31(9): 2430. CrossRef - Development of a Semi-Quantitative Food-Frequency Questionnaire for Korean Adults with Obesity
Jina Chung, Seoeun Ahn, Hyojee Joung, Sangah Shin
Nutrients.2023; 15(22): 4848. CrossRef
- Ultra-processed food consumption and increased risk of metabolic syndrome in Korean adults: A cross-sectional analysis of the KNHANES 2016–2020
- Others
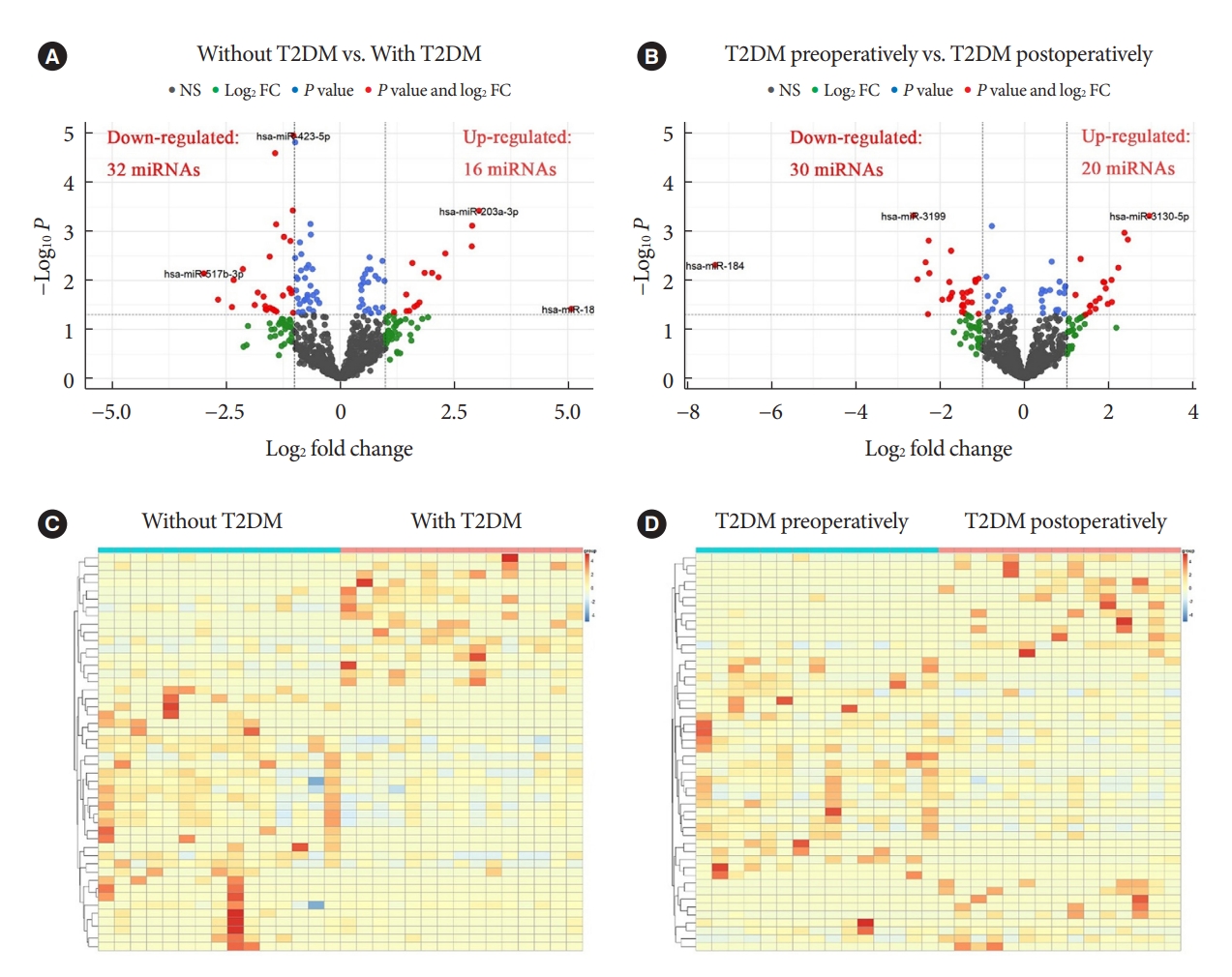
- Change Profiles and Functional Targets of MicroRNAs in Type 2 Diabetes Mellitus Patients with Obesity
- Guanhua Lu, Huanhuan Gao, Zhiyong Dong, Shuwen Jiang, Ruixiang Hu, Cunchuan Wang
- Diabetes Metab J. 2023;47(4):559-570. Published online April 25, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0226
- 1,692 View
- 76 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
MicroRNAs (miRNAs) exert an essential contribution to obesity and type 2 diabetes mellitus (T2DM). This study aimed to investigate the differences of miRNAs in the presence and absence of T2DM in patients with obesity, as well as before and after bariatric surgery in T2DM patients with obesity. Characterization of the common changes in both was further analyzed.
Methods
We enrolled 15 patients with obesity but without T2DM and 15 patients with both obesity and T2DM. Their preoperative clinical data and serum samples were collected, as well as 1 month after bariatric surgery. The serum samples were analyzed by miRNA sequencing, and the miRNAs profiles and target genes characteristics were compared.
Results
Patients with T2DM had 16 up-regulated and 32 down-regulated miRNAs compared to patients without T2DM. Improvement in metabolic metrics after bariatric surgery of T2DM patients with obesity was correlated with changes in miRNAs, as evidenced by the upregulation of 20 miRNAs and the downregulation of 30 miRNAs. Analysis of the two miRNAs profiles identified seven intersecting miRNAs that showed opposite changes. The target genes of these seven miRNAs were substantially enriched in terms or pathways associated with T2DM.
Conclusion
We determined the expression profiles of miRNAs in the obese population, with and without diabetes, before and after bariatric surgery. The miRNAs that intersected in the two comparisons were discovered. Both the miRNAs discovered and their target genes were closely associated with T2DM, demonstrating that they might be potential targets for the regulation of T2DM.
- Metabolic Risk/Epidemiology
- The Risk of Type 2 Diabetes Mellitus according to Changes in Obesity Status in Late Middle-Aged Adults: A Nationwide Cohort Study of Korea
- Joon Ho Moon, Yeonhoon Jang, Tae Jung Oh, Se Young Jung
- Diabetes Metab J. 2023;47(4):514-522. Published online April 25, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0159

- 2,056 View
- 133 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Although obesity is a well-known risk factor of type 2 diabetes mellitus (T2DM), there is scant data on discriminating the contribution of previous obesity and recent weight gain on developing T2DM.
Methods
We analyzed the Korean National Health Insurance Service-Health Screening Cohort data from 2002 to 2015 where Korean residents underwent biennial health checkups. Participants were classified into four groups according to their obesity status (body mass index [BMI] ≥25 kg/m2) before and after turning 50 years old: maintaining normal (MN), becoming obese (BO), becoming normal (BN), and maintaining obese (MO). Cox proportional hazards regression model was used to estimate the risk of T2DM factoring in the covariates age, sex, BMI, presence of impaired fasting glucose or hypertension, family history of diabetes, and smoking status.
Results
A total of 118,438 participants (mean age, 52.5±1.1 years; men, 45.2%) were prospectively evaluated for incident T2DM. A total of 7,339 (6.2%) participants were diagnosed with T2DM during a follow-up period of 4.8±2.6 years. Incidence rates of T2DM per 1,000 person-year were 9.20 in MN, 14.81 in BO, 14.42 in BN, 21.38 in MO. After factoring in covariates, participants in the groups BN (adjusted hazard ratio [aHR], 1.15; 95% confidence interval [CI], 1.04 to 1.27) and MO (aHR, 1.14; 95% CI, 1.06 to 1.24) were at increased risk of developing T2DM compared to MN, whereas BO (hazard ratio, 1.06; 95% CI, 0.96 to 1.17) was not.
Conclusion
Having been obese before 50 years old increased the risk of developing T2DM in the future, but becoming obese after 50 did not. Therefore, it is important to maintain normal weight from early adulthood to prevent future metabolic perturbations.
- Basic Research
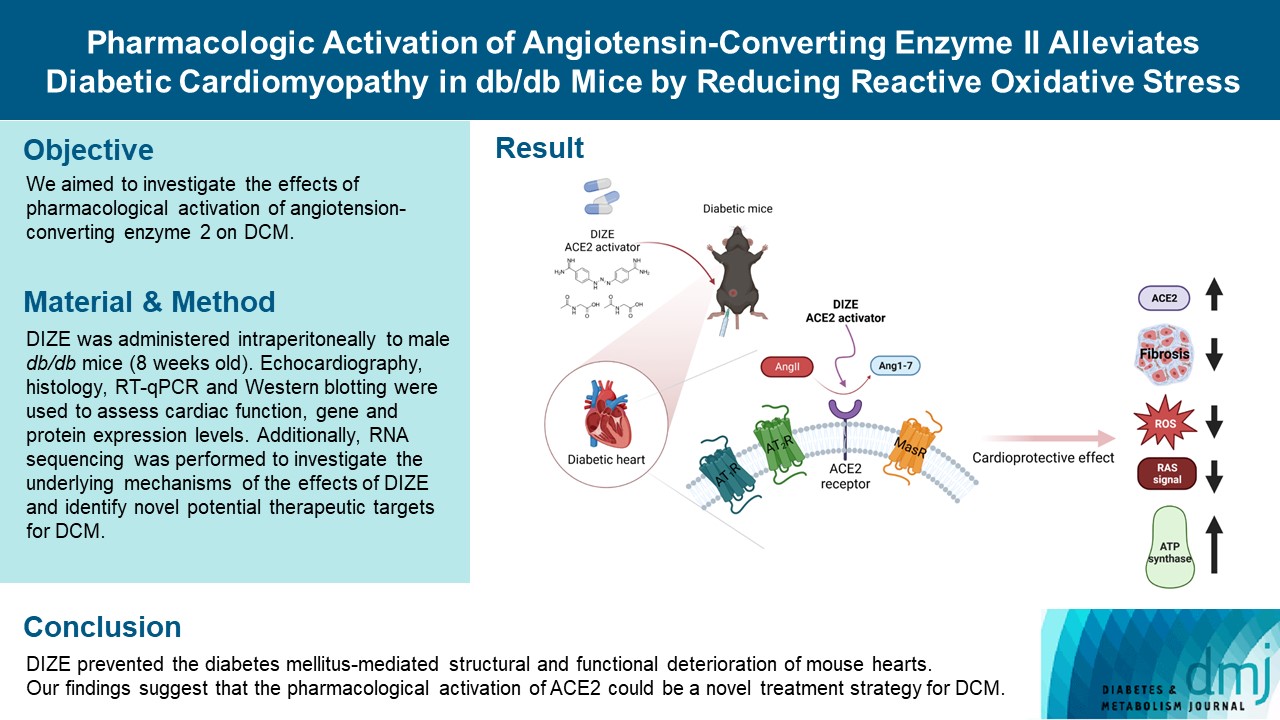
- Pharmacologic Activation of Angiotensin-Converting Enzyme II Alleviates Diabetic Cardiomyopathy in db/db Mice by Reducing Reactive Oxidative Stress
- Donghyun Kim, Wooju Jeong, Yumin Kim, Jibeom Lee, Sung Woo Cho, Chang-Myung Oh, Raekil Park
- Diabetes Metab J. 2023;47(4):487-499. Published online April 25, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0125
- 2,250 View
- 149 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Diabetes mellitus is one of the most common chronic diseases worldwide, and cardiovascular disease is the leading cause of morbidity and mortality in diabetic patients. Diabetic cardiomyopathy (DCM) is a phenomenon characterized by a deterioration in cardiac function and structure, independent of vascular complications. Among many possible causes, the renin-angiotensin-aldosterone system and angiotensin II have been proposed as major drivers of DCM development. In the current study, we aimed to investigate the effects of pharmacological activation of angiotensin-converting enzyme 2 (ACE2) on DCM.
Methods
The ACE2 activator diminazene aceturate (DIZE) was administered intraperitoneally to male db/db mice (8 weeks old) for 8 weeks. Transthoracic echocardiography was used to assess cardiac mass and function in mice. Cardiac structure and fibrotic changes were examined using histology and immunohistochemistry. Gene and protein expression levels were examined using quantitative reverse transcription polymerase chain reaction and Western blotting, respectively. Additionally, RNA sequencing was performed to investigate the underlying mechanisms of the effects of DIZE and identify novel potential therapeutic targets for DCM.
Results
Echocardiography revealed that in DCM, the administration of DIZE significantly improved cardiac function as well as reduced cardiac hypertrophy and fibrosis. Transcriptome analysis revealed that DIZE treatment suppresses oxidative stress and several pathways related to cardiac hypertrophy.
Conclusion
DIZE prevented the diabetes mellitus-mediated structural and functional deterioration of mouse hearts. Our findings suggest that the pharmacological activation of ACE2 could be a novel treatment strategy for DCM.
- Cardiovascular Risk/Epidemiology
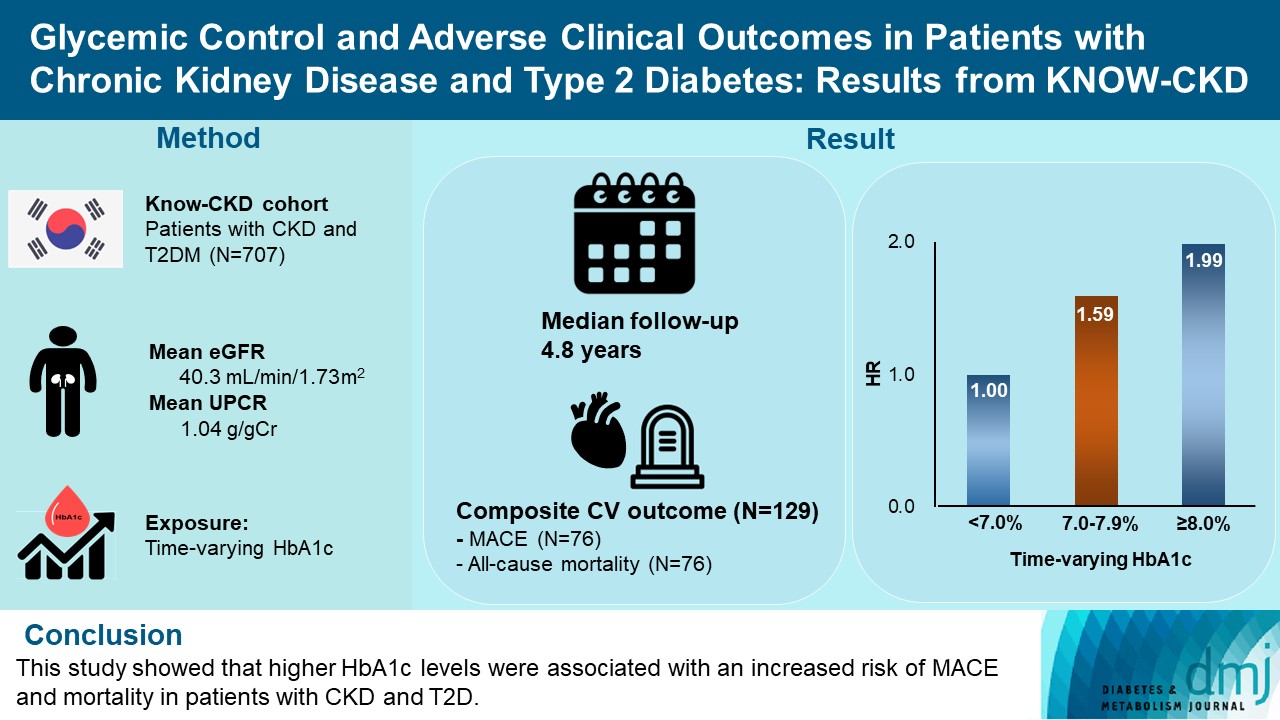
- Glycemic Control and Adverse Clinical Outcomes in Patients with Chronic Kidney Disease and Type 2 Diabetes Mellitus: Results from KNOW-CKD
- Ga Young Heo, Hee Byung Koh, Hyung Woo Kim, Jung Tak Park, Tae-Hyun Yoo, Shin-Wook Kang, Jayoun Kim, Soo Wan Kim, Yeong Hoon Kim, Su Ah Sung, Kook-Hwan Oh, Seung Hyeok Han
- Diabetes Metab J. 2023;47(4):535-546. Published online April 25, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0112
- 2,645 View
- 162 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The optimal level of glycosylated hemoglobin (HbA1c) to prevent adverse clinical outcomes is unknown in patients with chronic kidney disease (CKD) and type 2 diabetes mellitus (T2DM).
Methods
We analyzed 707 patients with CKD G1-G5 without kidney replacement therapy and T2DM from the KoreaN Cohort Study for Outcome in Patients With Chronic Kidney Disease (KNOW-CKD), a nationwide prospective cohort study. The main predictor was time-varying HbA1c level at each visit. The primary outcome was a composite of development of major adverse cardiovascular events (MACEs) or all-cause mortality. Secondary outcomes included the individual endpoint of MACEs, all-cause mortality, and CKD progression. CKD progression was defined as a ≥50% decline in the estimated glomerular filtration rate from baseline or the onset of end-stage kidney disease.
Results
During a median follow-up of 4.8 years, the primary outcome occurred in 129 (18.2%) patients. In time-varying Cox model, the adjusted hazard ratios (aHRs) for the primary outcome were 1.59 (95% confidence interval [CI], 1.01 to 2.49) and 1.99 (95% CI, 1.24 to 3.19) for HbA1c levels of 7.0%–7.9% and ≥8.0%, respectively, compared with <7.0%. Additional analysis of baseline HbA1c levels yielded a similar graded association. In secondary outcome analyses, the aHRs for the corresponding HbA1c categories were 2.17 (95% CI, 1.20 to 3.95) and 2.26 (95% CI, 1.17 to 4.37) for MACE, and 1.36 (95% CI, 0.68 to 2.72) and 2.08 (95% CI, 1.06 to 4.05) for all-cause mortality. However, the risk of CKD progression did not differ between the three groups.
Conclusion
This study showed that higher HbA1c levels were associated with an increased risk of MACE and mortality in patients with CKD and T2DM. -
Citations
Citations to this article as recorded by- The Beneficial Effect of Glycemic Control against Adverse Outcomes in Patients with Type 2 Diabetes Mellitus and Chronic Kidney Disease
Dong-Hwa Lee
Diabetes & Metabolism Journal.2023; 47(4): 484. CrossRef - Prevalence and predictors of chronic kidney disease among type 2 diabetic patients worldwide, systematic review and meta-analysis
Eneyew Talie Fenta, Habitu Birhan Eshetu, Natnael Kebede, Eyob Ketema Bogale, Amare Zewdie, Tadele Derbew Kassie, Tadele Fentabil Anagaw, Elyas Melaku Mazengia, Sintayehu Shiferaw Gelaw
Diabetology & Metabolic Syndrome.2023;[Epub] CrossRef - Efficacy and safety of teneligliptin in patients with type 2 diabetes mellitus: a Bayesian network meta-analysis
Miao Zhu, Ruifang Guan, Guo Ma
Frontiers in Endocrinology.2023;[Epub] CrossRef
- The Beneficial Effect of Glycemic Control against Adverse Outcomes in Patients with Type 2 Diabetes Mellitus and Chronic Kidney Disease

 KDA
KDA
 First
First Prev
Prev





