- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 47(5); 2023 > Article
-
Original ArticleOthers Glucose Regulation after Partial Pancreatectomy: A Comparison of Pancreaticoduodenectomy and Distal Pancreatectomy in the Short and Long Term
-
Jun Suh Lee1*
 , Minji Sohn2*
, Minji Sohn2* , Kyuho Kim2, Yoo-Seok Yoon1
, Kyuho Kim2, Yoo-Seok Yoon1 , Soo Lim2
, Soo Lim2
-
Diabetes & Metabolism Journal 2023;47(5):703-714.
DOI: https://doi.org/10.4093/dmj.2022.0205
Published online: June 22, 2023
- 1,690 Views
- 152 Download
1Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
2Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
-
Corresponding authors: Yoo-Seok Yoon
 Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, 82 Gumi-ro 173beon-gil, Bundang-gu, Seongnam 13620, Korea E-mail: yoonys@snubh.org
Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, 82 Gumi-ro 173beon-gil, Bundang-gu, Seongnam 13620, Korea E-mail: yoonys@snubh.org -
Soo Lim
 Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, 82 Gumi-ro 173beon-gil, Bundang-gu, Seongnam 13620, Korea E-mail: limsoo@snu.ac.kr
Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, 82 Gumi-ro 173beon-gil, Bundang-gu, Seongnam 13620, Korea E-mail: limsoo@snu.ac.kr - *Jun Suh Lee and Minji Sohn contributed equally to this study as first authors.
Copyright © 2023 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Long term quality of life is becoming increasingly crucial as survival following partial pancreatectomy rises. The purpose of this study was to investigate the difference in glucose dysregulation after pancreaticoduodenectomy (PD) or distal pancreatectomy (DP).
-
Methods
- In this prospective observational study from 2015 to 2018, 224 patients who underwent partial pancreatectomy were selected: 152 (67.9%) received PD and 72 (32.1%) received DP. Comprehensive assessment for glucose regulation, including a 75 g oral glucose tolerance test was conducted preoperatively, and 1, 12, and 52 weeks after surgery. Patients were further monitored up to 3 years to investigate development of new-onset diabetes mellitus (NODM) in patients without diabetes mellitus (DM) at baseline or worsening of glucose regulation (≥1% increase in glycosylated hemoglobin [HbA1c]) in those with preexisting DM.
-
Results
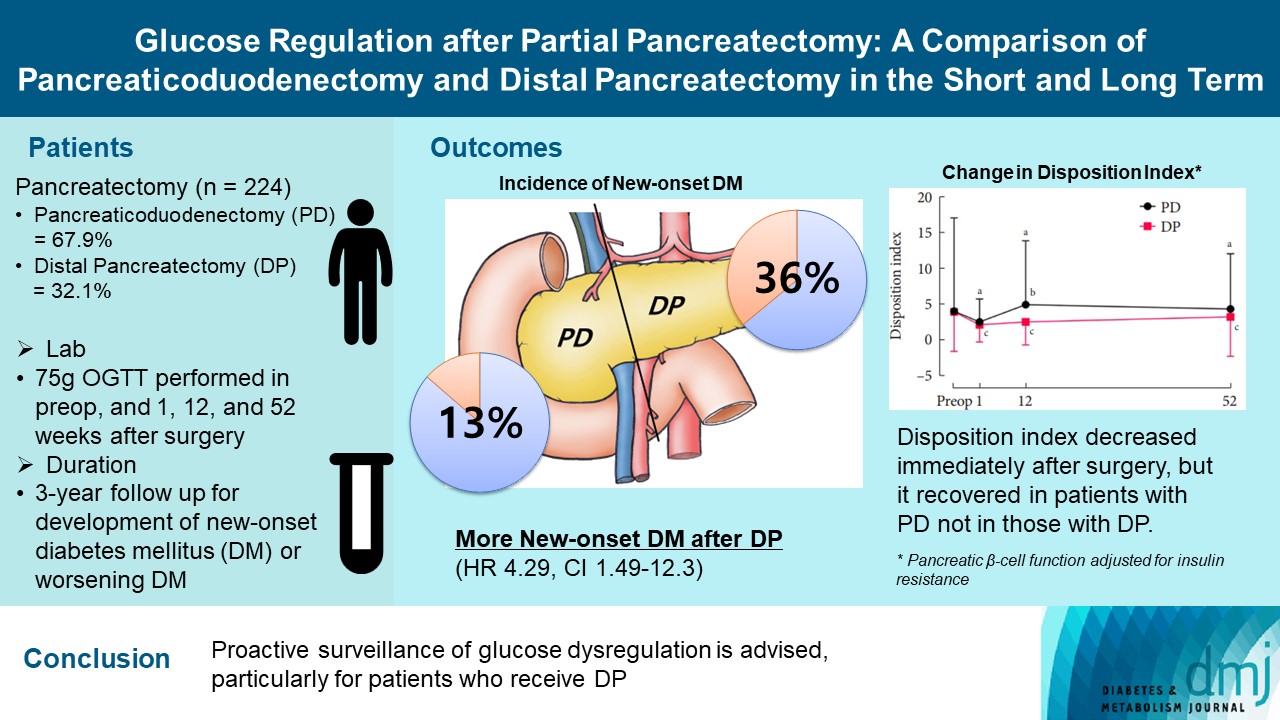
- The disposition index, an integrated measure of β-cell function, decreased 1 week after surgery in both groups, but it increased more than baseline level in the PD group while its decreased level was maintained in the DP group, resulting in a between-group difference at the 1-year examination (P<0.001). During follow-up, the DP group showed higher incidence of NODM and worsening of glucose regulation than the PD group with hazard ratio (HR) 4.29 (95% confidence interval [CI], 1.49 to 12.3) and HR 2.15 (95% CI, 1.09 to 4.24), respectively, in the multivariate analysis including dynamic glycemic excursion profile. In the DP procedure, distal DP and spleen preservation were associated with better glucose regulation. DP had a stronger association with glucose dysregulation than PD.
-
Conclusion
- Proactive surveillance of glucose dysregulation is advised, particularly for patients who receive DP.
- The number of pancreatectomies is steadily increasing in response to increased detection of premalignant or malignant diseases in this organ [1]. Increased frequency of health checkups, advances in imaging techniques, and heightened awareness of pancreatic disease contribute to this. Traditionally, pancreatic surgery was regarded as a dangerous procedure associated with high morbidity and mortality rates. However, advances in surgical techniques and perioperative care, combined with minimally invasive surgery, have resulted in improved postoperative outcomes and enhanced recovery after pancreatic resection [2,3]. With the introduction of effective chemotherapy regimens, survival outcomes after pancreatectomy have also been improving in patients with pancreatic cancer [4-6]. As the number of long term survivors after pancreatic resection increases, more emphasis is being placed on the patient’s quality of life and on late postoperative complications.
- Pancreatogenic diabetes mellitus (DM) is one of the important late complications after pancreatectomy with a debilitating influence on quality of life [7]. The American Diabetes Association classified this type of diabetes as type 3c, which has been reported to account for as much as 8% of DM [8]. The incidence of new-onset diabetes mellitus (NODM) after pancreatectomy varies with the type of surgery, at 18% to 27% after pancreatoduodenectomy (PD), and 5% to 42% after distal pancreatectomy (DP) [9,10]. Old age, high body mass index (BMI), high preoperative glycosylated hemoglobin (HbA1c) levels, type of surgery, and lower remnant pancreatic volume are known as risk factors for NODM [10-15]. However, few studies have investigated glucose regulation status comprehensively for long durations after pancreatectomy [14-16].
- In this prospective study on patients who underwent partial pancreatectomy for benign or malignant diseases, we analyzed the temporal changes in dynamic glycemic regulation parameters, and surrogate markers of pancreatic β-cell function and insulin resistance using an oral glucose tolerance test (OGTT) between types of pancreatic surgery preoperatively, and 1 and 52 weeks after surgery. For up to 3 years, we also investigated differences in NODM or worsening of glucose regulation according to each patient’s characteristics and surgical procedures, including the resection region, the volume of the pancreas resected, and spleen preservation.
INTRODUCTION
- Study design and population
- This was a prospective observational study at Seoul National University Bundang Hospital (SNUBH). Patients who underwent partial pancreatectomy for any reason and gave their consent were included in this study. A total of 398 patients were identified during 2015 to 2018. Patients who received central pancreatectomy or pancreatic enucleation were excluded from the study, as well as patients lost to follow-up, patients with incomplete laboratory measurements, or patients who died within 1 year after pancreatectomy. Finally, 224 cases were selected for this study (Supplementary Fig. 1).
- The study participants were followed up every 3 months for the 1st year after surgery. At every visit, anthropometric parameters, laboratory tests, and radiologic imaging were performed. In the biochemical parameters, fasting glucose and insulin concentrations, HbA1c levels, and liver and kidney functions were measured. For assessing dynamic glycemic excursion, a 75 g standard OGTT was conducted preoperatively, before discharge (1 week after surgery), and at 12 and 52 weeks. Then, the patients were followed up every 3–6 months for up to 3 years to assess their general health condition and glycemic regulation status. This study was approved by the SNUBH Institutional Review Board (B-1508-312-305) and has been registered at clinicaltrials.gov (NCT04409171).
- Surgical procedure
- Both PD and DP were performed using either an open or a laparoscopic approach. Our surgical procedure of PD and DP has been described in detail [17-19]. PD was performed for lesions located at the head of the pancreas, the distal common bile duct, the ampulla of Vater, or the duodenum. After removal of the head of the pancreas, common bile duct, gall bladder, and duodenum, the proximal jejunum was anastomosed to the remaining pancreas, the bile duct, and duodenum. DP was performed for lesions located in the body or tail of the pancreas. The decision on spleen preservation was made by the team according to tumor pathology and characteristics.
- In cases of PD, the pancreas was usually transected at the neck of the pancreas, which overlies the superior mesenteric vein. In most cases, approximately 40% of the pancreatic parenchyma was resected [20]. Unlike the PD procedure, with little variation in the location of the transection line, DP has variable transection lines according to the tumor pathology and location. In DP for pancreatic cancer, the transection line was mostly decided to be at the neck of the pancreas, identical to that in PD. However, when a pancreatic cancer was located at the proximal body, the pancreas was transected very proximally to obtain a safe resection margin, resulting in removal of up to 80% of the organ. In cases of premalignant tumors, which do not require a wide resection margin and lymph node dissection, the transection line was determined according to tumor location. When the lesion was located in the tail of the pancreas, the transection line could be far distal so that only 20% of the parenchyma was resected. For the DP procedure, the operation was categorized as either proximal or distal transection according to the location of the transection line. In the proximal DP approach, the transection line was proximal to the left border of the aorta, resulting in resection of the body and tail of the pancreas. For distal DP, the transection line was from the distal end of pancreas to the left border of the aorta, resulting in resection of the tail of the pancreas. These classifications are presented in Supplementary Fig. 2.
- End points
- The primary outcome of this study was deterioration in glucose regulation: the incidence of NODM, defined as having HbA1c of ≥6.5%, in patients without DM at baseline and worsening in glucose regulation, defined as an increase in HbA1c of ≥1% over the baseline value, in those without DM at baseline, for up to 3 years. Secondary outcomes were temporal changes in glucose regulation parameters related to pancreatic β-cell function or insulin resistance, which were estimated by dynamic glycemic excursion obtained from an OGTT performed during the 1st year after surgery.
- Measurements
- Height, body weight, and BMI were measured by standard methods. The subjects fasted for 10 hours overnight, and venous blood samples were taken for biochemistry assays. Plasma glucose concentration was measured using the glucose oxidase method (747 Clinical Chemistry Analyzer, Hitachi, Tokyo, Japan). Fasting plasma C-peptide and insulin levels were measured using a radioimmunoassay (Linco, St. Charles, MO, USA). HbA1c level was measured using a Variant II Turbo HPLC Analyzer (Bio-Rad, Hercules, CA, USA) in a National Glycohemoglobin Standardization Program level II certified laboratory.
- All subjects underwent a standardized 75 g OGTT following overnight fasting for 10 hours. The levels of plasma glucose and insulin were measured at baseline, 1, and 2 hours after the glucose load. To estimate the glucose regulatory function, the following surrogate markers were calculated: the homeostasis model assessment of insulin resistance (HOMA-IR) [21] and the Matsuda insulin sensitivity index (ISI) [22] for insulin resistance, and homeostasis model assessment of β-cell function (HOMA-β) and the insulinogenic index (IGI) for β-cell function. The disposition index, an integrated measure of β-cell function, was calculated as the IGI multiplied by the fasting insulin and has been validated for people with European and Asian ancestries [23]. The total area under the curve of glucose and insulin (AUCGlucose and AUCInsulin) was also derived from the results of the OGTT. The IGI60 was calculated as the ratio of the 60 minutes insulin level minus the fasting insulin level to that of the 60 minutes glucose level minus the fasting glucose level (ΔInsulin60/ΔGlucose60) [24] and it was validated against first-phase insulin secretion on intravenous glucose tolerance testing [25].
- Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (NADH-UV method) and creatinine (Jaffe’s kinetic method) levels were measured using an Architect Ci8200 Analyzer (Abbott Laboratories, Abbott Park, IL, USA). The estimated glomerular filtration rate (eGFR) was calculated using the creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation [26]. Concentrations of total cholesterol were measured using a 747 Clinical Chemistry Analyzer (Hitachi). The serum high-sensitivity C-reactive protein (hsCRP) level was measured via a high-sensitivity automated immunoturbidimetric method (CRP-Latex [II] X2, Denka Seiken Co., Tokyo, Japan).
- Statistical analysis
- Values are presented as the mean±standard deviation or as percentages. A two-sample Student’s t-test or Wilcoxon’s ranksum test was used according to the distribution to compare mean differences between treatment arms. A chi-squared test or Fisher’s exact test was used to test the significance of discrete variables. Survival is presented as Kaplan-Meier curves and compared by log-rank test. Crude or adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for NODM in nondiabetic patients and worsening in glucose regulation in patients with DM were estimated by Cox proportional hazards regression models. Analyses were adjusted for age, sex, baseline characteristics such as BMI, HbA1c, total cholesterol, ALT, eGFR, hsCRP, and the origin of pancreas disease, changes of disposition index from baseline to postoperative 1 week, surgical information including type of partial pancreatectomy and resected pancreas volume, and usage of postoperative chemotherapy. As a sensitivity analysis, the factors of transection level and whether splenectomy was performed in the DP group were further evaluated for glucose dysregulation. According to differences in baseline characteristics, propensity score matching (PSM) was developed with age, sex, BMI, fasting glucose, postprandial 2 hours glucose, and the nature of pancreas disease to compare glucose regulation between the types of surgery used. Significance was defined as a two-sided P<0.05. All statistical analyses were conducted using R software version 4.1.0 (R Development Core Team, Vienna, Austria) and RStudio version 1.4.1717 (RStudio, Boston, MA, USA).
METHODS
- Baseline characteristics
- Among 224 patients, 152 (67.9%) received PD, 72 (32.1%) received DP, and were followed up for up to 3 years (median 114 weeks). The baseline characteristics of the two groups are listed in Table 1. The PD group was older by 5 years and had a lower BMI than the DP patients. The PD group had higher AUCGlucose, and lower HOMA-β and IGI60 compared with the DP group (all P<0.05), indicating high glycemic burden and worse β-cell function. However, there were no significant differences in HbA1c, AUCInsulin, HOMA-IR, or disposition index between the two groups. The PD group had significantly higher AST and ALT levels than the DP patients. This might have been because of obstructive cholangitis caused by tumors located in the periampullary area. The duration of diabetes in patients with preexisting DM did not differ between the two groups (5.5±7.5 years in the PD group vs. 5.2±5.4 years in the DP group).
- More patients with malignant lesions were included in the PD group than in the DP group. The two groups had similar comorbidity. Approximately 30% of the patients had DM, 40% had hypertension, and 30% had dyslipidemia. There were no significant differences in the medication usage for these chronic disorders. Six patients in both groups received neoadjuvant chemotherapy (FOLFIRINOX regimen including leucovorin, fluorouracil, irinotecan, and oxaliplatin) before pancreatectomy. The PD group stayed longer after surgery in the hospital than DP group (13±10 days vs. 9±5 days, P<0.05) and their body weight change was not different between two groups. After PSM for age, sex, BMI, fasting glucose, postprandial 2 hours glucose, and the cause of disease of partial pancreatectomy, 72 patients subjected to PD were well matched to 72 patients with DP in all preoperative variables. The characteristics of matched patients are presented in Supplementary Table 1.
- Temporal changes in biochemical profiles for glucose metabolism during 1 year after surgery
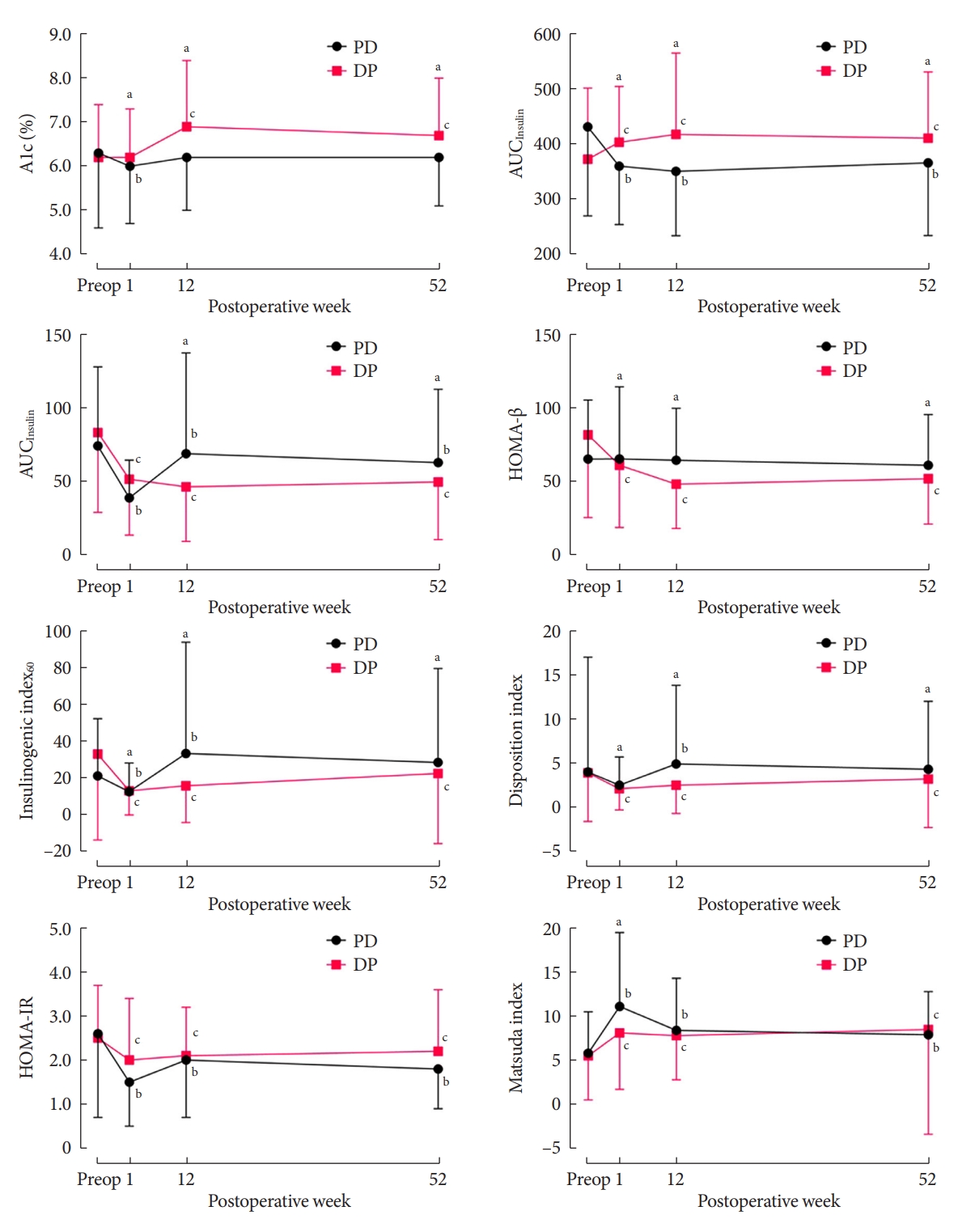
- Between the two groups, the DP group showed more deterioration in glucose regulation than did the PD group. The DP group also showed increases in HbA1c and AUCGlucose levels, and decreases in AUCInsulin, HOMA-β, IGI60 and the disposition index (all P<0.05) (Supplementary Table 2). Compared with the PD group, the HbA1c level rose by more than 0.5% in the DP group at 12 and 52 weeks postoperatively (Fig. 1). The AUCGlucose level also increased in the DP group at postoperative 1 week and maintained this increased level for 52 weeks. The HOMA-β level remained stable in the PD group after surgery, whereas it continued to decrease by about 30% in the DP group. The AUCInsulin, IGI60, and disposition index decreased at postoperative 1 week in both groups, but they rebounded above the baseline level in the PD group (Fig. 1). Eventually, the HbA1c, AUCGlucose, AUCInsulin, HOMA-β, IGI60, and disposition index showed significant differences between the two groups (all P<0.05). HOMA-IR decreased, and the Matsuda ISI increased in both groups, with no difference between the groups (Supplementary Table 2).
- The difference of changes in BMI between the two groups over time were not significant in all subjects. The total cholesterol and ALT levels significantly reduced in the PD group after partial pancreatectomy while they did not change in the DP group, resulting in a significant between-group difference (P<0.001). Similar patterns in the changes of these parameters were found in the matched populations (Supplementary Table 3).
- Development of NODM or worsened DM over time
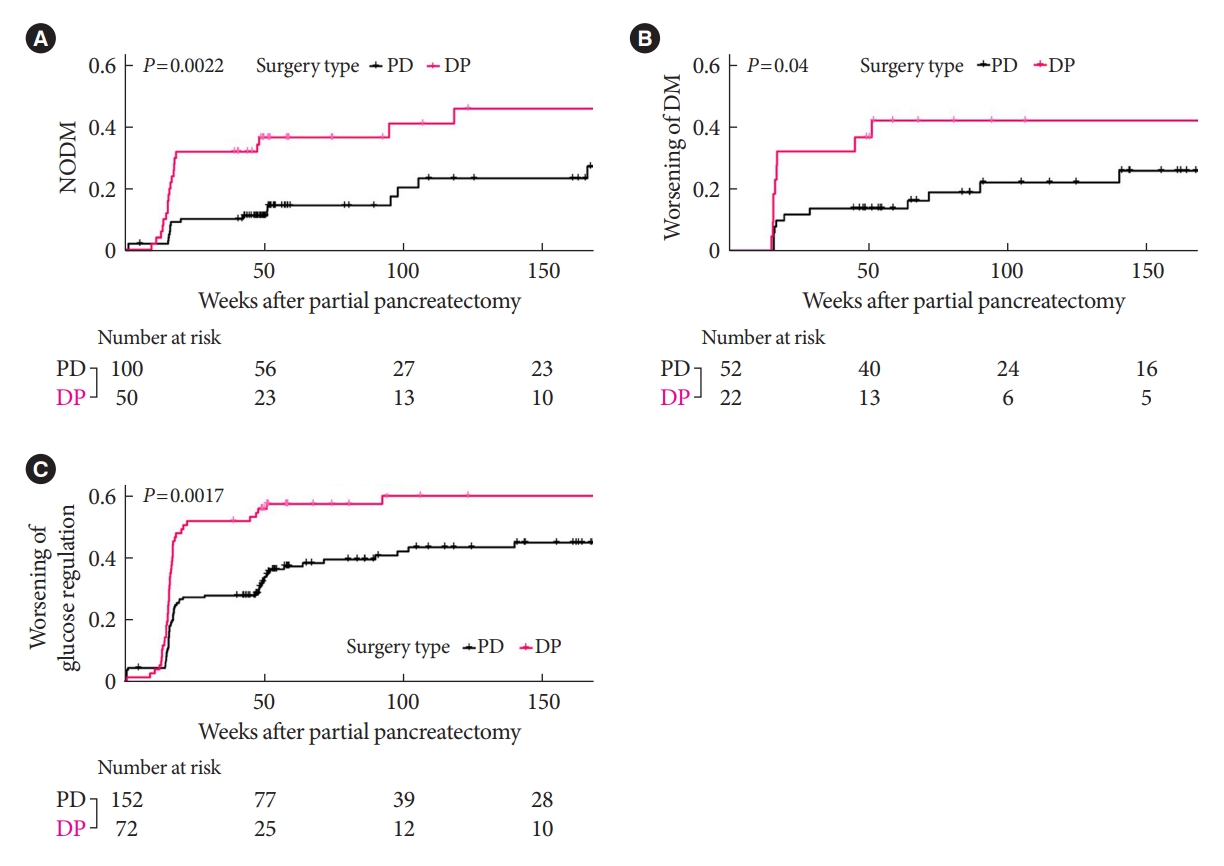
- In the patients without DM at baseline, the development of NODM was significantly higher in the DP group compared with the PD group at the 1-year follow-up (36% vs. 13%, P<0.05) (Fig. 2A). In the patients with preexisting DM, worsening of DM (defined as decreased HbA1c ≥1% within 1 year) was also significantly higher in the DP group compared with the PD group (41% vs. 14%, P<0.05) (Fig. 2B). Of note, the deterioration of glucose regulation including both NODM and worsening in glucose regulation occurred within 1 year in both groups (20/27 [74%] in the PD group and 27/29 [93%] in the DP group, P=0.053) and continued after 1 year (Fig. 2C). Similar results were found in the analysis with matched patients (the DP vs. the PD procedure for NODM: HR, 2.04; 95% CI, 1.09 to 4.15; P<0.05; for worsening in glucose regulation: HR, 5.23; 95% CI, 1.13 to 24.3; P<0.05) (Supplementary Fig. 3).
- Risk factors for NODM and worsened DM
- In the multivariable Cox regression analysis for patients without DM at baseline, DP was more than four times more significantly associated with NODM than PD at the 3-year follow-up (HR, 4.29; 95% CI, 1.49 to 12.3) (Table 2). High baseline HbA1c, greater decrease in disposition index after 1 week, pathology of pancreas cancer, and postoperative chemotherapy were also significant predictors for NODM (Table 2).
- In the same analysis with the patients with preexisting DM, DP was twice more significantly associated with worsening in glucose regulation than PD at the 3-year follow-up (HR, 2.15; 95% CI, 1.09 to 4.24) (Table 2). High baseline levels of total cholesterol and decreased disposition index at postoperative 1 week were also significant risk factors for worsened DM.
- We also investigated whether a family history of diabetes or diabetes duration was associated with NODM or worsening of DM, and these factors were not associated with impaired glucose regulation. In this study, three patients in the PD group received insulin treatment before partial pancreatectomy but no one in the DP group received insulin. This insulin therapy was not associated with worsening of DM.
- We further investigated the effect of resected pancreatic volume on impaired glucose regulation quantitatively. In the DP group, the second and third tertiles of resected volume were associated with a two times higher risk of worsening of glucose regulation, suggesting the resection of more than 69.2 cm3 in the distal part of the pancreas might be a cutoff for poorer outcome (Supplementary Table 4). However, there was no difference in the worsening of glucose regulation according to the tertiles of resected volume in the PD group. This implies that the resection volume is not relevant to glucose dysregulation in this group.
- In the DP group, the pancreas was transected proximal to the left border of the aorta in 46 (63.9%) patients, and there was a significant difference in the volume of resected pancreas between proximal and distal resections (111.4±83.9 cm3 vs. 93.4±75.4 cm3, P<0.05). In addition, 55 (76.4%) patients in the DP group had splenectomy. In this group, proximal DP and splenectomy were identified as factors affecting the deterioration in glucose regulation (proximal resection vs. PD group: HR, 2.28; 95% CI, 1.49 to 3.47; splenectomy vs. PD group: HR, 1.98; 95% CI, 1.32 to 2.99) (Supplementary Figs. 4 and 5). The risk of NODM in patients without DM was higher with proximal DP than with distal DP (HR, 5.47; 95% CI, 1.61 to 18.7).
RESULTS
- Compared with PD, DP was a potent risk factor for the development of NODM and worsening glucose regulation in the multivariate analysis during the 3-year postoperative follow-up. The transection level and splenectomy affected the risk of glucose dysregulation in the DP group. The matched analysis adjusted for baseline characteristics confirmed these results.
- In our investigation of dynamic glycemic excursions based on the OGTT, the PD group showed better changes in glucose regulation parameters compared with the DP group, which showed a progressive or sustained deterioration in glucose metabolism. The glucose burden represented by the AUCGlucose decreased in the PD group, whereas it increased in the DP group. In terms of pancreatic β-cell function, the HOMA-β measure was maintained in the PD group but it decreased in the DP group. The initially decreased level of IGI60 and disposition index, indicators for β-cell function, rebounded above the baseline level only in the PD group whereas it was maintained in the DP group.
- The higher risk of NODM and worsening glucose regulation after DP could be explained by several mechanisms. First, there are regional differences in pancreatic islet distribution. Studies of pancreas specimens from cadavers of nondiabetic subjects and a healthy subject demonstrated that the β-cell concentration was double in the body or tail regions than in the head or neck regions [27,28]. Therefore, considering that the resected volume of the pancreas was similar between the two surgical procedures, a greater decrease in the pancreatic β-cell reservoir in the DP compared with the PD procedure is likely to contribute to worsened glucose regulation. Second, the physiological and anatomical changes after PD, which bypasses the duodenum and proximal jejunum, are similar to those seen after Roux-en-Y gastric bypass (RYGB) surgery. RYGB surgery was reported to improve metabolic profiles and reduce body weight through increased incretin hormones such as glucagon-like peptide-1 (GLP-1) [29]. The enhanced incretin hormones could have been associated with improvement in glycemic control in our PD group. Although GLP-1 was not measured directly here, the β-cell reservoir or insulin secretory function was relatively well maintained in the PD group whereas it decreased or did not recover to the baseline level in the DP group. This is in accordance with a previous study showing that GLP-1 secretion increased during a mixed-meal test after the PD procedure n nondiabetic subjects [30]. However, another study demonstrated that the GLP-1 level increased after DP but not after PD, suggesting that the positive feedback regulation for impaired β-cell function after DP might result in increased GLP-1 levels [31]. Further studies are needed to clarify the role of GLP-1 in glucose regulation after such partial pancreatectomy.
- Malignant lesions of the pancreas are likely to deteriorate glucose regulation in many ways. Like other neoplasms, pancreatic cancers also secrete noxious chemokines, cytokines, and peptides, which might worsen glucose homeostasis [32]. Moreover, islet amyloid polypeptide was elevated in patients with pancreatic cancer and DM, which is known to cause insulin resistance in skeletal muscle [33,34]. Also, pancreatic cancer exacerbated insulin resistance by impairing glycogen synthesis and storage in skeletal muscle [35,36]. Notably, the HOMA-IR reflecting insulin resistance decreased and the Matsuda ISI reflecting insulin sensitivity increased in both groups in our study, indicating improved insulin resistance after partial pancreatectomy, consistent with a previous study [16]. That insulin secretion decreased only in the DP group and rebounded in the PD group suggests that the deterioration of DM with DP was caused by decreased β-cell function, as shown by our HOMA-β results.
- In this study, the baseline total cholesterol level was associated with a worsening of DM, although the HR was 1.01 (95% CI, 1.00 to 1.01). Moreover, cholesterol level was not associated with NODM. These data suggest that the contribution of total cholesterol level to impaired glucose regulation is minimal in this study.
- Of note, postoperative chemotherapy was found to be associated with NODM in the unadjusted model. Administration of the four most commonly used agents, namely, gemcitabine, 5-fluorouracil, leucovorin, and capecitabine, was comparable between the two groups. These compounds are known to have little effect on glucose regulation. However, a few cases of chemotherapy were accompanied by systemic glucocorticoid therapy, which might increase glucose levels.
- After an initial drop in the disposition index, there was an obvious difference between the PD and DP groups in the follow-up examinations. It increased above the baseline level in the PD group while it remained at a decreased level in the DP group at the 1-year follow-up. These results support that β-cell function adjusted for insulin resistance was relatively well preserved in the PD group compared with the DP group. One study reported that Korean people have smaller pancreatic volumes and higher fat content in the pancreas compared with people of European ancestry [37]. Thus, it should be recognized that the risk of aggravation of glycemic control after DP may be accentuated in Asian populations with relatively low pancreatic volumes or β-cell reservoirs.
- The transection level and removing the spleen affected the deterioration of glucose regulation. As the removed volume of pancreatic tissue was larger with proximal DP than with distal DP, the higher risk of glucose dysregulation with proximal resection supports that reduced residual pancreatic volume is one of the strongest risk factors for NODM [10]. Even though the tail area contains more β-cells, the volume of excision appears to have had a greater impact for the defined transection position. Several studies have shown a link between splenectomy and the development of DM. Thus, the spleen is a source of adult multipotent stem cells, which might serve as progenitors for pancreatic islet secretory cells [38]. A study in the mouse showed that splenocytes enhanced the neogenesis of pancreatic β-islet secretory cells [39], implying that the spleen may play a key role in the endocrine function of the pancreas.
- Our study had several strengths. First, we investigated dynamic changes of glucose metabolism profiles using the standardized 75 g OGTT. Second, deterioration in glucose regulation was assessed for up to 3 years in a prospective manner. Third, primary results were confirmed in the matched subjects with relevant variables including age, sex, BMI, fasting and postprandial 2 hours glucose levels, and the pancreas disease entity. Nevertheless, this study had some limitations. First, the hyperglycemic clamp and the hyperinsulinemic euglycemic clamp tests, the gold standard methods to assess glucose homeostasis, were not performed. However, this is not practically possible in the clinical setting. Second, because only Korean subjects were enrolled, our results cannot be generalized to other ethnicities. In addition, recurrence of cancer or administration of chemotherapy might affect glucose regulation. Because partial pancreatectomy may affect the gastric emptying [40], which is one of major determinants of postprandial glycemia [41], the OGTT test including shorter time such as 30 min point could have revealed a better mechanistic relationship to the glucose regulation and the type of partial pancreatectomy.
- In conclusion, the risk of NODM or worsening in glucose regulation was significantly higher after DP than PD in this prospective 3-year study. Moreover, insulin secretory function seemed to be attenuated significantly more in the DP group than in the PD group. Therefore, in planning the surgical strategy for DP in which the volume of resected pancreas is variable—unlike PD—it should be noted that when removing the lesions located in the tail of the pancreas, conservation of the remnant pancreatic volume might help alleviate the loss of the pancreatic β-cell reservoir. Thus, careful monitoring for deterioration in glucose regulation is needed after pancreatectomy and strategies to preserve pancreatic volume might be beneficial particularly for patients who are supposed to receive the DP.
DISCUSSION
SUPPLEMENTARY MATERIALS
Supplementary Table 1.
Supplementary Table 2.
Supplementary Table 3.
Supplementary Table 4.
Supplementary Fig. 1.
Supplementary Fig. 2.
Supplementary Fig. 3.
Supplementary Fig. 4.
Supplementary Fig. 5.
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: J.S.L., M.S., Y.S.Y., S.L.
Acquisition, analysis, or interpretation of data: J.S.L., M.S., K.K., Y.S.Y., S.L.
Drafting the work or revising: J.S.L., M.S., Y.S.Y., S.L.
Final approval of the manuscript: J.S.L., M.S., K.K., Y.S.Y., S.L.
-
FUNDING
None
NOTES
-
Acknowledgements
- None
| Variable | Pancreaticoduodenectomy (n=152) | Distal pancreatectomy (n=72) | P value | ||
|---|---|---|---|---|---|
| Age, yr | 64.8±10.4 | 59.7±13.5 | 0.002 | ||
| Male sex | 82 (53.9) | 37 (51.4) | 0.830 | ||
| Body mass index, kg/m2 | 23.3±2.8 | 24.2±3.1 | 0.022 | ||
| HbA1c, % | 6.3±1.7 | 6.2±1.2 | 0.748 | ||
| Glucose at 0 min, mg/dL | 126.0±50.3 | 114.3±33.4 | 0.073 | ||
| Glucose at 60 min, mg/dL | 246.3±88.0 | 218.5±74.6 | 0.022 | ||
| Glucose at 120 min, mg/dL | 244.5±113.8 | 194.3±89.1 | 0.001 | ||
| AUCGlucose | 431.5±161.7 | 372.8±129.2 | 0.008 | ||
| Insulin at 0 min, μIU/mL | 8.2±4.0 | 9.1±3.8 | 0.100 | ||
| Insulin at 60 min, μIU/mL | 44.0±39.6 | 51.5±39.3 | 0.185 | ||
| Insulin at 120 min, μIU/mL | 51.5±42.5 | 53.7±44.9 | 0.720 | ||
| AUCInsulin | 73.9±53.8 | 83.0±54.4 | 0.240 | ||
| C-peptide, ng/mL | 3.0±1.9 | 2.4±1.2 | 0.022 | ||
| HOMA-IR | 2.6±1.9 | 2.5±1.2 | 0.761 | ||
| HOMA-β | 65.0±40.2 | 81.5±56.3 | 0.013 | ||
| Matsuda ISI | 5.8±4.7 | 5.5±5.0 | 0.647 | ||
| IGI60 | 21.2±31.2 | 33.1±46.8 | 0.026 | ||
| Disposition index | 4.0±13.0 | 3.9±5.5 | 0.957 | ||
| Total cholesterol, mg/dL | 196.8±75.0 | 179.6±35.0 | 0.069 | ||
| Aspartate aminotransferase, IU/L | 71.9±96.0 | 23.9±10.2 | <0.001 | ||
| Alanine aminotransferase, IU/L | 96.3±123.4 | 22.7±15.5 | <0.001 | ||
| eGFR, mL/min/1.73 m2 | 95.0±15.8 | 95.6±14.2 | 0.814 | ||
| hsCRP, mg/L | 1.02±1.88 | 0.80±3.55 | 0.568 | ||
| Resected pancreas volume, cm3 | 113.5±63.4 | 104.9±80.9 | 0.390 | ||
| Family history of diabetes | 28 (18.4) | 18 (25.0) | 0.255 | ||
| Pathology (etiology of surgery) | <0.001 | ||||
| Malignant lesion | 132 (86.8) | 41 (56.9) | |||
| Pancreatic cancer | 56 (36.8) | 38 (52.8) | |||
| Nonpancreatic cancer | 76 (50.0) | 3 (4.2) | |||
| Premalignant or benign lesion | 20 (13.2) | 31 (43.1) | |||
| ASA PS classification | 0.233 | ||||
| I | 31 (20.4) | 20 (27.8) | |||
| II | 102 (67.1) | 40 (55.6) | |||
| III | 17 (11.2) | 12 (16.7) | |||
| IV | 2 (1.3) | 0 | |||
| Comorbidities | |||||
| Prediabetes | 28 (18.4) | 19 (26.4) | 0.233 | ||
| Diabetes mellitus | 52 (34.2) | 22 (30.6) | 0.696 | ||
| Hypertension | 64 (42.1) | 30 (41.7) | 1.000 | ||
| Dyslipidemia | 57 (37.5) | 26 (36.1) | 0.958 | ||
| Obesity | 34 (22.4) | 26 (36.1) | 0.045 | ||
| Nephropathy | 5 (3.3) | 1 (1.4) | 0.704 | ||
| Preoperative medications | |||||
| Antidiabetic agentsa | 42 (27.6) | 20 (27.8) | 1.000 | ||
| Antihypertensive agents | 61 (40.1) | 30 (41.7) | 0.942 | ||
| Lipid-lowering agents | 36 (23.7) | 23 (31.9) | 0.251 | ||
| Neoadjuvant chemotherapyb | 6 (3.9) | 6 (8.3) | 0.297 | ||
| Postoperative chemotherapyc | 67 (44.1) | 32 (44.4) | 0.999 | ||
Values are presented as mean±standard deviation or number (%).
HbA1c, glycosylated hemoglobin; AUC, area under curve; HOMA-IR, homeostasis model assessment of insulin resistance; HOMA-β, homeostasis model assessment of β-cell function; ISI, insulin sensitivity index; IGI, insulinogenic index; eGFR, estimated glomerular filtration rate; hsCRP, high-sensitivity C-reactive protein; ASA, American Society of Anesthesiologists; PS, performance status.
a Three patients were using insulin: one in the pancreaticoduodenectomy (PD) group and two in the distal pancreatectomy group,
b All patients were treated with FOLFIRINOX combination chemotherapy (oxaliplatin, irinotecan, leucovorin, and fluorouracil),
c Only one patient in the PD group was given glucocorticoids.
| Variable |
HR (95% CI) for NODM |
HR (95% CI) for worsening of DM |
|||
|---|---|---|---|---|---|
| Crude | Adjusted model | Crude | Adjusted model | ||
| Age, yr | 1.00 (0.98–1.03) | 0.99 (0.93–1.05) | 0.99 (0.96–1.03) | 0.99 (0.96–1.02) | |
| Female vs. male | 0.75 (0.40–1.42) | 0.59 (0.26–1.35) | 1.37 (0.57–3.32) | 0.71 (0.43–1.19) | |
| Baseline body mass index, kg/m2 | 1.08 (0.97–1.21) | 1.04 (0.89–1.22) | 0.93 (0.81–1.08) | 0.90 (0.81–1.00) | |
| Baseline HbA1c, % | 5.13 (2.49–10.6)a | 7.10 (2.49–20.2)a | 0.79 (0.58–1.09) | 1.60 (0.97–2.64) | |
| Baseline total cholesterol, mg/dL | 0.99 (0.99–1.01) | 1.01 (1.00–1.02) | 1.01 (1.00–1.01)a | 1.01 (1.00–1.01)a | |
| Baseline ALT, IU/L | 1.00 (1.00–1.00) | 1.00 (1.00–1.01) | 1.00 (0.99–1.01) | 1.00 (1.00–1.00) | |
| Baseline eGFR, mL/min/1.73 m2 | 0.99 (0.97–1.02) | 0.98 (0.93–1.02) | 0.99 (0.97–1.01) | 0.98 (0.96–1.01) | |
| Baseline hsCRP, g/dL | 0.64 (0.36–1.14) | 0.75 (0.45–1.25) | 0.89 (0.59–1.34) | 0.96 (0.82–1.12) | |
| Delta disposition index(Baseline – Week 1) | 1.03 (1.01–1.05)a | 1.03 (1.01–1.06)a | 1.04 (0.94–1.12) | 1.03 (1.02–1.04)a | |
| DP vs. PD | 2.58 (1.37–4.85)a | 4.29 (1.49–12.3)a | 2.45 (1.01–5.92)a | 2.15 (1.09–4.24)a | |
| Resected pancreas volume, cm3 | 1.00 (0.99–1.01) | 1.00 (1.00–1.01) | 0.99 (0.99–1.01) | 1.00 (1.00–1.00) | |
| Pathology (vs. premalignant or benign lesion) | |||||
| Pancreatic cancer | 2.85 (1.31–6.22)a | 3.21 (0.68–15.1) | 0.93 (0.30–2.93) | 1.12 (0.47–2.69) | |
| Nonpancreatic cancer | 0.55 (0.20–1.49) | 1.15 (0.26–5.16) | 1.18 (0.32–4.39) | 1.30 (0.56–3.03) | |
| Postoperative chemotherapyb | 2.39 (1.27–4.50)a | 2.48 (0.63–9.79) | 0.95 (0.39–2.29) | 1.64 (0.81–3.35) | |
HR, hazard ratio; NODM, new-onset diabetes mellitus; DM, diabetes mellitus; HbA1c, glycosylated hemoglobin; CI, confidence interval; ALT, alanine aminotransferase; eGFR, estimated glomerular filtration rate; hsCRP, high-sensitivity C-reactive protein; DP, distal pancreatectomy; PD, pancreaticoduodenectomy.
a P<0.05,
b Includes single or combination chemotherapy of oxaliplatin, irinotecan, leucovorin, fluorouracil, gemcitabine, capecitabine, paclitaxel, cisplatin, etoposide, carboplatin, erlotinib, or tegafur/uracil.
- 1. Zhang Q, Zeng L, Chen Y, Lian G, Qian C, Chen S, et al. Pancreatic cancer epidemiology, detection, and management. Gastroenterol Res Pract 2016;2016:8962321.ArticlePubMedPMCPDF
- 2. Feng Q, Xin Z, Zhu B, Liao M, Liao W, Zeng Y. Perioperative and short-term oncological outcomes following laparoscopic versus open pancreaticoduodenectomy after learning curve in the past 10 years: a systematic review and meta-analysis. Gland Surg 2021;10:1655-68.ArticlePubMedPMC
- 3. Lyu Y, Cheng Y, Wang B, Zhao S, Chen L. Assessment of laparoscopic versus open distal pancreatectomy: a systematic review and meta-analysis. Minim Invasive Ther Allied Technol 2022;31:350-8.ArticlePubMed
- 4. Kim K, Yu JI, Jung W, Kim TH, Seong J, Kim WC, et al. Role of adjuvant radiotherapy in extrahepatic bile duct cancer: a multicenter retrospective study (Korean Radiation Oncology Group 18-14). Eur J Cancer 2021;157:31-9.ArticlePubMed
- 5. Torphy RJ, Fujiwara Y, Schulick RD. Pancreatic cancer treatment: better, but a long way to go. Surg Today 2020;50:1117-25.ArticlePubMedPMCPDF
- 6. Lee M, Kang JS, Kim H, Kwon W, Lee SH, Ryu JK, et al. Impact of conversion surgery on survival in locally advanced pancreatic cancer patients treated with FOLFIRINOX chemotherapy. J Hepatobiliary Pancreat Sci 2023;30:111-21.Article
- 7. Shaw K, Thomas AS, Rosario V, Kwon W, Schrope BA, Sugahara K, et al. Long term quality of life amongst pancreatectomy patients with diabetes mellitus. Pancreatology 2021;21:501-8.ArticlePubMed
- 8. Cui Y, Andersen DK. Pancreatogenic diabetes: special considerations for management. Pancreatology 2011;11:279-94.ArticlePubMed
- 9. Maeda H, Hanazaki K. Pancreatogenic diabetes after pancreatic resection. Pancreatology 2011;11:268-76.ArticlePubMed
- 10. Wu L, Nahm CB, Jamieson NB, Samra J, Clifton-Bligh R, Mittal A, et al. Risk factors for development of diabetes mellitus (type 3c) after partial pancreatectomy: a systematic review. Clin Endocrinol (Oxf) 2020;92:396-406.ArticlePubMedPDF
- 11. Maxwell DW, Jajja MR, Galindo RJ, Zhang C, Nadeem SO, Sweeney JF, et al. Post-pancreatectomy diabetes index: a validated score predicting diabetes development after major pancreatectomy. J Am Coll Surg 2020;230:393-402.ArticlePubMed
- 12. Shirakawa S, Matsumoto I, Toyama H, Shinzeki M, Ajiki T, Fukumoto T, et al. Pancreatic volumetric assessment as a predictor of new-onset diabetes following distal pancreatectomy. J Gastrointest Surg 2012;16:2212-9.ArticlePubMedPMC
- 13. Kang JS, Jang JY, Kang MJ, Kim E, Jung W, Chang J, et al. Endocrine function impairment after distal pancreatectomy: incidence and related factors. World J Surg 2016;40:440-6.ArticlePubMedPDF
- 14. Maxwell DW, Jajja MR, Tariq M, Mahmooth Z, Galindo RJ, Sweeney JF, et al. Development of diabetes after pancreaticoduodenectomy: results of a 10-year series using prospective endocrine evaluation. J Am Coll Surg 2019;228:400-12.ArticlePubMed
- 15. Ishida J, Toyama H, Matsumoto I, Shirakawa S, Terai S, Yamashita H, et al. Glucose tolerance after pancreatectomy: a prospective observational follow-up study of pancreaticoduodenectomy and distal pancreatectomy. J Am Coll Surg 2021;233:753-62.ArticlePubMed
- 16. Niwano F, Babaya N, Hiromine Y, Matsumoto I, Kamei K, Noso S, et al. Glucose metabolism after pancreatectomy: opposite extremes between pancreaticoduodenectomy and distal pancreatectomy. J Clin Endocrinol Metab 2021;106:e2203-14.ArticlePubMedPMCPDF
- 17. Kim S, Yoon YS, Han HS, Cho JY, Choi Y, Lee B. Evaluation of a single surgeon’s learning curve of laparoscopic pancreaticoduodenectomy: risk-adjusted cumulative summation analysis. Surg Endosc 2021;35:2870-8.ArticlePubMedPDF
- 18. Lee B, Yoon YS, Kang CM, Choi M, Lee JS, Hwang HK, et al. Fistula risk score-adjusted comparison of postoperative pancreatic fistula following laparoscopic vs open pancreatoduodenectomy. J Hepatobiliary Pancreat Sci 2021;28:1089-97.ArticlePubMedPDF
- 19. Mendoza AS 3rd, Han HS, Yoon YS, Cho JY, Choi Y. Laparoscopy-assisted pancreaticoduodenectomy as minimally invasive surgery for periampullary tumors: a comparison of short-term clinical outcomes of laparoscopy-assisted pancreaticoduodenectomy and open pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 2015;22:819-24.ArticlePubMed
- 20. Kwon JH, Kim SC, Shim IK, Song KB, Lee JH, Hwang DW, et al. Factors affecting the development of diabetes mellitus after pancreatic resection. Pancreas 2015;44:1296-303.ArticlePubMed
- 21. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412-9.ArticlePubMedPDF
- 22. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462-70.ArticlePubMedPDF
- 23. Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009;32:335-41.ArticlePubMedPMCPDF
- 24. Wareham NJ, Phillips DI, Byrne CD, Hales CN. The 30 minute insulin incremental response in an oral glucose tolerance test as a measure of insulin secretion. Diabet Med 1995;12:931.ArticlePubMed
- 25. Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Jarvinen H, Van Haeften T, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000;23:295-301.ArticlePubMedPDF
- 26. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604-12.ArticlePubMedPMC
- 27. Wang X, Misawa R, Zielinski MC, Cowen P, Jo J, Periwal V, et al. Regional differences in islet distribution in the human pancreas: preferential beta-cell loss in the head region in patients with type 2 diabetes. PLoS One 2013;8:e67454.ArticlePubMedPMC
- 28. Ionescu-Tirgoviste C, Gagniuc PA, Gubceac E, Mardare L, Popescu I, Dima S, et al. A 3D map of the islet routes throughout the healthy human pancreas. Sci Rep 2015;5:14634.ArticlePubMedPMCPDF
- 29. Mingrone G, Cummings DE. Changes of insulin sensitivity and secretion after bariatric/metabolic surgery. Surg Obes Relat Dis 2016;12:1199-205.ArticlePubMed
- 30. Mezza T, Muscogiuri G, Sorice GP, Clemente G, Hu J, Pontecorvi A, et al. Insulin resistance alters islet morphology in nondiabetic humans. Diabetes 2014;63:994-1007.ArticlePubMedPMCPDF
- 31. Mori Y, Ohtsuka T, Tsutsumi K, Yasui T, Ueda J, Takahata S, et al. Different incretin responses after pancreatoduodenectomy and distal pancreatectomy. Pancreas 2012;41:455-60.ArticlePubMed
- 32. Padoan A, Plebani M, Basso D. Inflammation and pancreatic cancer: focus on metabolism, cytokines, and immunity. Int J Mol Sci 2019;20:676.ArticlePubMedPMC
- 33. Tabata H, Hirayama J, Sowa R, Furuta H, Negoro T, Sanke T, et al. Islet amyloid polypeptide (IAPP/amylin) causes insulin resistance in perfused rat hindlimb muscle. Diabetes Res Clin Pract 1992;15:57-61.ArticlePubMed
- 34. Permert J, Larsson J, Westermark GT, Herrington MK, Christmanson L, Pour PM, et al. Islet amyloid polypeptide in patients with pancreatic cancer and diabetes. N Engl J Med 1994;330:313-8.ArticlePubMed
- 35. Sah RP, Nagpal SJ, Mukhopadhyay D, Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol 2013;10:423-33.ArticlePubMedPMCPDF
- 36. Liu J, Knezetic JA, Strommer L, Permert J, Larsson J, Adrian TE. The intracellular mechanism of insulin resistance in pancreatic cancer patients. J Clin Endocrinol Metab 2000;85:1232-8.ArticlePubMed
- 37. Roh E, Kim KM, Park KS, Kim YJ, Chun EJ, Choi SH, et al. Comparison of pancreatic volume and fat amount linked with glucose homeostasis between healthy Caucasians and Koreans. Diabetes Obes Metab 2018;20:2642-52.ArticlePubMedPDF
- 38. Wu SC, Fu CY, Muo CH, Chang YJ. Splenectomy in trauma patients is associated with an increased risk of postoperative type II diabetes: a nationwide population-based study. Am J Surg 2014;208:811-6.ArticlePubMed
- 39. Park S, Hong SM, Ahn IS. Can splenocytes enhance pancreatic beta-cell function and mass in 90% pancreatectomized rats fed a high fat diet? Life Sci 2009;84:358-63.PubMed
- 40. Glowka TR, von Websky M, Pantelis D, Manekeller S, Standop J, Kalff JC, et al. Risk factors for delayed gastric emptying following distal pancreatectomy. Langenbecks Arch Surg 2016;401:161-7.ArticlePubMedPDF
- 41. Horowitz M, Edelbroek MA, Wishart JM, Straathof JW. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia 1993;36:857-62.ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations


 KDA
KDA
 PubReader
PubReader ePub Link
ePub Link Cite
Cite