- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 47(4); 2023 > Article
-
Original ArticleMetabolic Risk/Epidemiology The Risk of Type 2 Diabetes Mellitus according to Changes in Obesity Status in Late Middle-Aged Adults: A Nationwide Cohort Study of Korea
-
Joon Ho Moon1,2*
 , Yeonhoon Jang3,4*
, Yeonhoon Jang3,4* , Tae Jung Oh1,2
, Tae Jung Oh1,2 , Se Young Jung3,4
, Se Young Jung3,4
-
Diabetes & Metabolism Journal 2023;47(4):514-522.
DOI: https://doi.org/10.4093/dmj.2022.0159
Published online: April 25, 2023
- 2,044 Views
- 133 Download
1Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
3Department of Family Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
4Department of Digital Healthcare, Seoul National University Bundang Hospital, Seongnam, Korea
-
Corresponding authors: Tae Jung Oh
 Department of Internal Medicine, Seoul National University Bundang Hospital, 82 Gumi-ro 173beon-gil, Bundang-gu, Seongnam 13620, Korea E-mail: ohtjmd@gmail.com
Department of Internal Medicine, Seoul National University Bundang Hospital, 82 Gumi-ro 173beon-gil, Bundang-gu, Seongnam 13620, Korea E-mail: ohtjmd@gmail.com -
Se Young Jung
 Department of Family Medicine, Seoul National University Bundang Hospital, 82 Gumiro 173beon-gil, Bundang-gu, Seongnam 13620, Korea E-mail: imsyjung@gmail.com
Department of Family Medicine, Seoul National University Bundang Hospital, 82 Gumiro 173beon-gil, Bundang-gu, Seongnam 13620, Korea E-mail: imsyjung@gmail.com - *Joon Ho Moon and Yeonhoon Jang contributed equally to this study as first authors.
Copyright © 2023 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Although obesity is a well-known risk factor of type 2 diabetes mellitus (T2DM), there is scant data on discriminating the contribution of previous obesity and recent weight gain on developing T2DM.
-
Methods
- We analyzed the Korean National Health Insurance Service-Health Screening Cohort data from 2002 to 2015 where Korean residents underwent biennial health checkups. Participants were classified into four groups according to their obesity status (body mass index [BMI] ≥25 kg/m2) before and after turning 50 years old: maintaining normal (MN), becoming obese (BO), becoming normal (BN), and maintaining obese (MO). Cox proportional hazards regression model was used to estimate the risk of T2DM factoring in the covariates age, sex, BMI, presence of impaired fasting glucose or hypertension, family history of diabetes, and smoking status.
-
Results
- A total of 118,438 participants (mean age, 52.5±1.1 years; men, 45.2%) were prospectively evaluated for incident T2DM. A total of 7,339 (6.2%) participants were diagnosed with T2DM during a follow-up period of 4.8±2.6 years. Incidence rates of T2DM per 1,000 person-year were 9.20 in MN, 14.81 in BO, 14.42 in BN, 21.38 in MO. After factoring in covariates, participants in the groups BN (adjusted hazard ratio [aHR], 1.15; 95% confidence interval [CI], 1.04 to 1.27) and MO (aHR, 1.14; 95% CI, 1.06 to 1.24) were at increased risk of developing T2DM compared to MN, whereas BO (hazard ratio, 1.06; 95% CI, 0.96 to 1.17) was not.
-
Conclusion
- Having been obese before 50 years old increased the risk of developing T2DM in the future, but becoming obese after 50 did not. Therefore, it is important to maintain normal weight from early adulthood to prevent future metabolic perturbations.
- Obesity and having a high body mass index (BMI) are associated with various health conditions, including type 2 diabetes mellitus (T2DM), osteoarthritis, cardiovascular disease, sleep apnea, etc. [1,2]. The global increase in the prevalence of T2DM is paralleled by the rapid increase in the prevalence of obesity [2,3]. There is a large body of evidence on how weight gain and loss have a significant impact on cardiometabolic risk factors and development of T2DM [4-7]. In the Nurses’ Health Study, more than 5 kg of weight gain increased the risk of incident T2DM by two-fold during a follow-up period of 14 years [7]. In contrast, the Diabetes Prevention Program (DPP) study [8] showed that the intensive lifestyle intervention of losing 5.6 kg in 2.8 years reduced the incidence of T2DM by 58% compared to placebo. This was significantly more effective than metformin (11.0, 7.8, and 4.8 cases per 100 person-years in placebo, metformin, and lifestyle groups, respectively) [8]. In fact, body weight can fluctuate over time, and temporal changes in body weight affect an individual’s future risk of T2DM and mortality [9]. Therefore, the metabolic effects of the past history of obesity versus recent weight gain should be elucidated, as they are currently poorly understood.
- The prevalence of obesity was highest in adults aged 40 to 59 (44.8%) among different age groups in the United States [10]. In Koreans, as of recent decades, the largest increases in the prevalence of obesity were in the young and middle-aged [11]. Given the higher prevalence and increasing trend of obesity in this age-group, it is necessary to investigate the association between obesity status and risk of developing T2DM for middle-aged persons. In fact, the prevalence of T2DM rapidly increases in middle age (4.4% in 15–49 years, 15% in 50–69 years, and 22% in >70 years) [12]. The American Diabetes Association recommends that adults aged ≥45 years and adults who are overweight or obese undergo testing for T2DM and/or prediabetes [13]. Therefore, it is of clinical importance to understand how current and previous obesity contributes to the development of T2DM in middle-aged persons.
- In this nationwide cohort study, we assess how obesity status before and after 50 years old affects the risk of incident T2DM. We hypothesize that continuous exposure to obesity is the main factor contributing to an increased risk of developing diabetes. We also analyze the impact of recent changes in obesity status such as normal BMI to obesity, or obesity to normal BMI on the risk of T2DM.
INTRODUCTION
- This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. X-2201-7732-902). Written informed consent was waived because the National Health Insurance Service (NHIS) dataset was anonymized based on strict confidentiality guidelines. The present research was conducted in accordance with the Declaration of Helsinki.
- Data source
- This study used the National Health Insurance Service-Health Screening Cohort (NHIS-HEALS) database provided by the Korean government. NHIS-HEALS is a cohort of participants who took part in health screening programs run by the NHIS [14,15]. NHIS-HEALS database was built by selecting a sample cohort from the 2002 and 2003 health screening participants, who were aged 40 to 79 in 2002 and followed up with them through 2015. The Korean government provides health screening biennially to people aged 40 to 79. In 2002 and 2003, a random selection of 10% of all health screening participants was used to create this cohort, which included 514,866 participants [16]. The national health screening program’s data consist of anthropometric measurements, laboratory test results, and a self-administered questionnaire including smoking, drinking, and exercise habits.
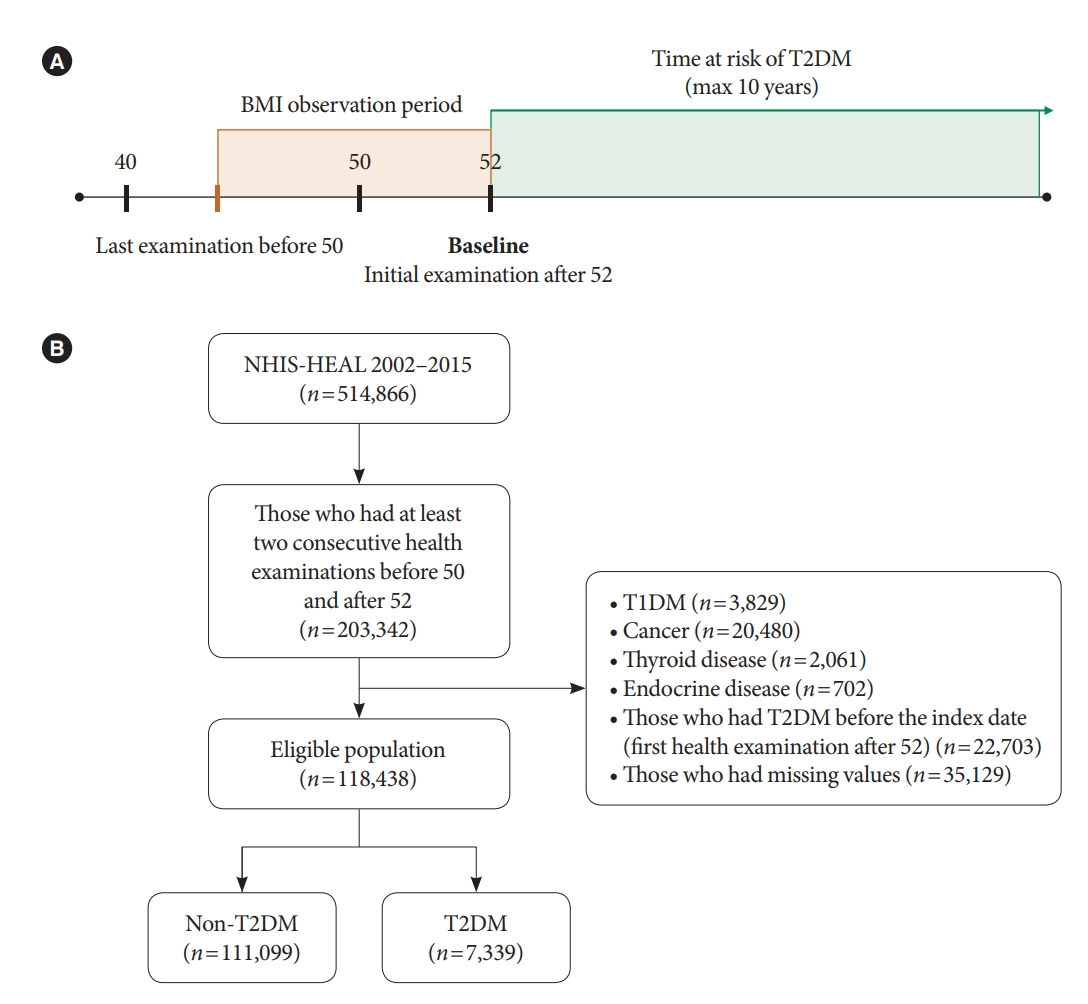
- Study participants
- We included participants who took part in the health examination at least twice, before age 50 and after age 52 (n=203,342) (Fig. 1). An interval of 2 years or more was set to observe changes in BMI for a sufficient amount of time. Participants’ first health examination after age 52 was set as their baseline. We excluded participants who met the following criteria: (1) Those who were diagnosed with type 1 diabetes mellitus (n=3,829), cancer (n=20,480), thyroid disease (n=2,061), or endocrine disease (n=702); (2) those who were diagnosed with T2DM or had fasting plasma glucose (FPG) ≥126 mg/dL on or before their first examination (n=22,703); (3) those who had at least one missing variable in the relevant covariates (FPG, total cholesterol, urinalysis, blood pressure, family history of diabetes, and smoking history) (n=35,129). The history of the abovementioned disease was defined according to the International Classification of Disease 10th Revision (ICD-10) code: type 1 diabetes mellitus (E10); cancer, all neoplasm (all C code) including pheochromocytoma (C741) but excluding thyroid cancer (C73); thyroid disease, all thyroid diseases (E00 to E09) except nontoxic goiter (E04), which includes benign thyroid nodule; and endocrine disease including pituitary disorder (E22, E23), Cushing’s syndrome (E24), hyperaldosteronism (E26), and polycystic ovarian syndrome (E282). As information on smoking was recorded through a self-administered questionnaire, most of the missing data were from participants who did not respond to the smoking questionnaire. A total of 118,438 participants were included for this study.
- Study participants were followed from their baseline to the end date of the cohort (December 31, 2015). Participants were censored at the date of T2DM diagnosis or the last follow-up (e.g., death).
- Assessment of changes in body mass index
- Obesity was defined as a BMI 25 kg/m2 and higher, as per the criteria of East Asians [17]. We classified participants into four groups according to their BMI between their closest health examinations before 50 and after 52. Participants who were not obese in both health examinations were classified as “maintaining normal (MN),” initially normal before 50 but becoming obese after 52 as “becoming obese (BO),” initially obese but becoming normal as “becoming normal (BN),” and obese in both health examinations as “maintaining obese (MO).”
- Definition of study outcome
- The primary end point was incident T2DM during the follow-up period after the age of 52. Incident T2DM was defined using the ICD-10 (E11–E14), combined with antidiabetic medication records (alpha-glucosidase inhibitor, DPP-4 inhibitor, sulfonylurea, metformin, thiazolidinedione, meglitinide, sodium glucose cotransporter-2 inhibitor, insulin, and glucagon-like peptide-1 receptor agonists) within 1 year from the diagnosis from NHIS claim database. Participants who had FPG ≥126 mg/dL without a T2DM related diagnoses or a claim for antidiabetic medications were also included as having incident T2DM. This operational definition of incident T2DM was used as suggested by the Korean Diabetes Association [14].
- Covariates
- A family history of diabetes, the presence of impaired fasting glucose (IFG), total cholesterol, the presence of hypertension, smoking history, and physical activity were included as covariates. IFG was defined as a FPG level of 100 to 125 mg/dL from a health check-up. Hypertension was defined as having high systolic blood pressure (≥140 mm Hg), high diastolic blood pressure (≥90 mm Hg), or diagnosis of hypertension (ICD-10 codes I10-I13 and I15) with antihypertensive medication. Smoking history was classified into the categories none, light, moderate, or heavy, based on cigarettes smoked per day: light (<10 cigarettes per day), moderate (10 to 19 cigarettes per day), and heavy (≥20 cigarettes per day). Physical activity was defined differently in 2002–2008 and after 2009, as the questionnaire for physical activity was updated in 2009. Physical activity was defined as exercising 3 or more days per week (from 2002 to 2008) or 4 or more days per week of >30 minutes of moderate-intensity exercise or 3 or more days per week of >30 minutes of vigorous-intensity exercise (from 2009).
- Statistical analysis
- Student’s t-test and one-way analysis of variance was used for continuous variables. For categorical variables, a chi-square test was used to compare differences between groups, and the variables were displayed as percentages. Cox proportional hazard regression model was used to estimate hazard ratios and 95% confidence intervals (CI) for incident T2DM among groups in relation to their change in BMI. The reference group was MN. Subgroup analyses were performed using the Cox model by stratifying sex, presence of IFG or hypertension, family history of diabetes, smoking status, and physical activity. We used propensity score matching to balance the clinical characteristics including age, sex, and BMI between different subgroups (MN and BN; BO and MO). Matching was done properly without significant imbalance (standardized mean difference <0.1).
- All analyses were performed with R version 3.3 software (R foundation for Statistical Computing, Vienna, Austria). Statistical significance was determined with a P value of <0.05.
- Data availability
- Data are available from the NHIS, which owns the data. Requests for data can be sent to the data owners, NHIS (http://www.nhiss.nhis.or.kr/).
METHODS
- Clinical characteristics according to the change of obesity status before and after 50 years old
- A total of 118,438 participants (mean age, 52.5±1.1 years; men, 45.2%) were prospectively evaluated with the mean follow-up duration of 4.8±2.6 years. Majority of participants were classified as MN (n=75,264, 63.5%), followed by MO (n=28,652, 24.2%), BO (n=7,970, 6.7%), and BN (n=6,552, 5.5%) (Table 1). The BN and MO groups had a higher proportion of male participants compared to MN group. The two obese groups at baseline (BO and MO) had higher FPG and a higher proportion of IFG and hypertension than the nonobese groups at baseline (MN and BN).
- Risk of incident T2DM by the change of obesity status before and after 50 years old
- A total of 7,339 (6.2%) participants were diagnosed with T2DM (6,584 [89.7%] by biennial health checkup and 755 [10.3%] by claims data). Participants who developed T2DM were more obese (BMI 25.0±3.1 kg/m2 in the T2DM group vs. 23.7±2.8 kg/m2 in the non-T2DM group) and had a higher proportion of men (62.4% vs. 44.1%), IFG (41.0% vs. 19.6%), hypertension (36.5% vs. 21.4%), and current smokers (34.9% vs. 22.5%) at baseline on their first health check-up after turning 50 years old (Supplementary Table 1).
- The incidence rates of T2DM per 1,000 person-years according to changes in obesity status were as follows: 9.20 in MN, 14.81 in BO, 14.42 in BN, 21.38 in MO (Supplementary Table 2 and Supplementary Fig. 1). In a crude analysis, the BO, BN, and MO groups had a higher risk of incident T2DM compared to the MN group. After adjusting for sex, age, and baseline BMI (model 3 in Table 2), participants who were obese before 50 years old (BN and MO group) still had a significantly increased risk of developing incident T2DM. In the fully adjusted model, the BN (adjusted hazard ratio [aHR], 1.15; 95% CI, 1.04 to 1.27) and MO groups (aHR, 1.14; 95% CI, 1.06 to 1.24) had a higher risk of developing T2DM than the MN group.
- Subgroup comparison to assess the risk of T2DM in participants who were obese before 50 years old
- Given that BN, but not BO, resulted in increased risk of incident T2DM, we hypothesized that having been obese before 50 may have a larger impact on developing T2DM compared to gaining weight near 50. To analyze this, we assessed the risk of developing T2DM according to obesity status before the age of 50 years, after controlling for recent obesity status (BO vs. MO; MN vs. BN). Furthermore, we controlled for age, sex, and BMI using propensity score matching (Supplementary Tables 3 and 4). In propensity-score matched analysis, the MO group displayed a higher risk of incident T2DM than the BO group (aHR, 1.14; 95% CI, 1.02 to 1.28; P=0.025). Similarly, the BN group had a higher risk of T2DM than the MN group (aHR, 1.16; 95% CI, 1.01 to 1.32; P=0.032).
- Subgroup analyses according to known T2DM risk factors
- To better understand which subpopulations are at a higher risk of developing incident T2DM, we compared three combinations (MN vs. BO, MN vs. BN, and MN vs. MO), which we stratified by sex, IFG, hypertension, family history of diabetes, smoking, and physical activity. There was no difference between the stratum with regards to family history of diabetes or physical activity (Supplementary Fig. 2). The association between changes in obesity status and the risk of developing T2DM was apparent in the female subgroup although statistical significance for interaction was reached in MN versus MO only. Likewise, the risk of developing T2DM for the MO group compared to the MN group was apparent in subjects without IFG or hypertension (Supplementary Fig. 2C).
- In a sex-stratified analysis, women in the BN and MO groups showed consistent results with the main finding that displayed a significantly increased risk of T2DM compared with those in the MN group (aHR, 1.23; 95% CI, 1.05 to 1.45 for BN and aHR, 1.26; 95% CI, 1.10 to 1.44 for MO) (Supplementary Table 5). The risk of T2DM in men was increased in the MO group (aHR, 1.11; 95% CI, 1.01 to 1.23), while statistical significance was not reached in the BN group (aHR, 1.11; 95% CI, 0.98 to 1.25).
RESULTS
- In this nationwide cohort study of late middle-aged Koreans, we showed that being obese before 50 years old is associated with a higher risk of developing T2DM compared to those maintaining a normal BMI. After controlling for BMI, MO, and BN had a higher risk of developing T2DM than BO and MN, respectively. Among them, women were apparently more affected by changes in obesity status in regard to a future risk of developing T2DM. We identified that previous exposure to obesity before the age of 50 years increases the risk of developing T2DM at a later age, beyond the risk of recent weight gain. This suggests that maintaining a healthy weight in early to middle age is required to minimize metabolic risk in older age.
- Beneficial effect of weight loss and clinical unmet needs
- Although obesity and weight gain are well-known risk factors for T2DM, it is controversial which has a greater impact on developing T2DM, that is, being previously obese or recently gaining weight. A large number of reports demonstrate that intentional weight loss reduces the risk of developing incident T2DM [4,6,18]. Recent studies with multiple BMI measurements have enabled our understanding of the role of weight changes in relation to cardiometabolic risks. Bailey-Davis et al. [5] assessed 63,567 participants with ≥3-weight measurements over a 2-year period and demonstrated that the risks of developing incident T2DM, hypertension, and dyslipidemia are lower in individuals maintaining weight loss than in weight loss rebounders or those maintaining their obesity, with a delayed onset of metabolic disorders in participants with a greater magnitude of weight loss. However, regardless of the fact that intentional weight loss results in beneficial metabolic effects, the harms of previous exposure to metabolic stress induced by obesity have not been elucidated.
- Previous exposure to obesity and future metabolic risk: obesity memory
- Metabolic memory and legacy effect describe the phenomenon of individuals experiencing the ongoing beneficial effects of improved and sustained glycemic control on diabetic complications [19,20]. Although current obesity is a well-known risk factor for T2DM, the metabolic consequences of being previously obese on developing incident T2DM have not been clearly addressed. For instance, many studies demonstrate positive associations between childhood obesity and cardiometabolic risk in adulthood, but majority of these studies fail to adjust for adult BMI [21]. In addition, childhood obesity per se was not sufficient and sustained obesity until early adulthood was necessary for a higher risk of developing incident T2DM later in life [22]. In this study, it was demonstrated that being obese before 50 years old increases the risk of developing incident T2DM, and more surprisingly, people who lost weight and became non-obese could not eliminate their future risk of developing diabetes. This suggests that obesity memory before the 50s may exist to affect their risk of developing T2DM in the future. This obesity memory extends to the risk of diabetic complications as pre-diagnosis BMI was positively associated with microvascular diabetic complications [23].
- Metabolic risk of previous exposure to obesity versus recent weight gain
- A large number of studies have demonstrated the decreased risk of incident T2DM in people losing weight, but this was not evident in this study. The metabolic risk of obesity is assumed to be a sum of the risk of being previously obese and the risk due to current obesity. Each risk is assumed to be a product of the duration and the extent of obesity. In most studies that assessed the effect of losing weight on diabetes, the age of the study population was not strictly controlled for further discussion of the contribution of obesity to the risk of developing diabetes at different ages (inclusion criteria: >18 years old [18], ≥25 years old [6], ≥35 years old [4], ≥40 years old [24]). In this study, we controlled the study population age of 50 years old to assess the previous contribution of obesity before 50 years age. We could conclude that recent weight loss was not sufficient to overcome the metabolic risk of being previously obese before 50 years of age for a mean follow-up duration of 4.8 years.
- Given that Asians and the study population of this study have a generally lower baseline BMI, the beneficial or harmful effect of recent weight loss or gain, respectively, may not have been significant enough to be observed. In fact, a previous study by Haase et al. [18] demonstrated that the beneficial effect of losing similar amounts of body weight was larger in participants who had a higher baseline BMI in the development of T2DM, hypertension, dyslipidemia, and chronic kidney disease. Therefore, additional studies on Westerners with higher BMIs would provide additional insights into the metabolic risk of previous exposure to obesity versus recent weight gain.
- Gender differences in the risk factor contribution to incident T2DM
- Men are diagnosed with T2DM years earlier and at lower BMI’s compared to women [25]. Men and women share conventional risk factors for incident T2DM (e.g., aging, obesity, family history of T2DM, etc.) but the extent of the contribution of each risk factor is assumed to be different. In this study, the association between obesity status and incident T2DM was more evident in women, and previous studies support this finding [25,26]. One hypothesis is that BMI overestimates body fat in men compared to that in women, because men generally have more muscle mass resulting in a higher BMI [27]. In addition, frequently engaging in unhealthy behaviors including cigarette smoking and alcohol use may attenuate the association between obesity and T2DM in men [28,29]. Notably, the majority of smokers (95.8%) in this cohort were men. Therefore, this study supports the conclusion that women are more affected by changes in obesity status in developing future T2DM. Given that menopause adversely affects metabolic risk by increasing central obesity and impairing insulin sensitivity [30], women should maintain a healthy weight in earlier periods of their life, before menopause, to limit their risk of developing future metabolic disorders.
- Strengths and limitations
- The main strength of this study is that we employed representative nationwide claims data as 97% of Koreans are enrolled in the NHIS and most receive medical service at least once a year. Based on this large sample size, we constructed a prospective cohort and strictly controlled the age of study population to demonstrate the risk of changes in obesity status on developing incident T2DM in a person’s 50s.
- There were several limitations. First, the incidence of T2DM may be underestimated because neither glycated hemoglobin nor oral glucose tolerance tests were used to diagnose T2DM. However, the validity of claim data for diagnosing T2DM has been previously reported with Kappa statistics up to 0.81 and a sensitivity and positive predictive value reaching 79% and 88%, respectively [31,32]. Second, we were unable to assess BMI at the time of T2DM diagnosis because subjects who were diagnosed with T2DM by claims data lacked health checkup data. Future studies assessing BMIs at multiple time points would give additional insights into the metabolic impact of previous versus current obesity. Third, nutritional status was not analyzed in this study, which comprise key risk factors for T2DM. Fourth, due to the observational nature of this study, we could not confirm causal relationships or exclude confounding effects.
- In conclusion, being obese before 50 years old was associated with incident T2DM in late middle-aged Koreans. Being previously obese rather than gaining weight recently had a great impact on the future risk of developing T2DM in this population. Therefore, it is important for individuals to maintain a healthy weight in the early to middle-ages before becoming 50 years old to limit the risk of developing future metabolic disorders.
DISCUSSION
SUPPLEMENTARY MATERIALS
Supplementary Table 1.
Supplementary Table 2.
Supplementary Table 3.
Supplementary Table 4.
Supplementary Table 5.
Supplementary Fig. 1.
Supplementary Fig. 2.
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: J.H.M., Y.J., T.J.O., S.Y.J.
Acquisition, analysis, or interpretation of data: J.H.M., Y.J., T.J.O., S.Y.J.
Drafting the work or revising: J.H.M., Y.J., T.J.O., S.Y.J.
Final approval of the manuscript: J.H.M., Y.J., T.J.O., S.Y.J.
-
FUNDING
This research was supported by Kyobo Insurance Company fund (Grant #06-2020-0291).
NOTES
-
Acknowledgements
- None
| Variable | Maintaining normal (n=75,264) | Becoming obese (n=7,970) | Becoming normal (n=6,552) | Maintaining obese (n=28,652) | P value | |
|---|---|---|---|---|---|---|
| Age, yr | 52.5±1.1a | 52.7±1.2b | 52.6±1.2c | 52.6±1.1d | <0.001 | |
| Male sex, % | 41.3a | 44.1b | 51.4b | 54.2c | <0.001 | |
| BMI, kg/m2 | 22.2±1.7a | 25.9±1.0b | 24.1±0.9c | 27.4±2.0d | <0.001 | |
| FHx of diabetes, % | 9.5 | 10.6 | 11.1 | 11.4 | 0.515 | |
| FPG, mg/dL | 92.5±10.8a | 95.0±11.1b | 93.7±11.3c | 95.7±11.4d | <0.001 | |
| IFG, % | 18.8a | 20.7b | 25.0c | 25.9d | <0.001 | |
| Hypertension, % | 16.7a | 26.4b | 25.8c | 35.2d | <0.001 | |
| Smoking, % | <0.001 | |||||
| None | 78.2a | 78.0a | 73.3b | 74.0c | ||
| Light | 2.8 | 2.8 | 3.1 | 3.3 | ||
| Moderate | 9.9 | 9.3 | 11.3 | 10.7 | ||
| Heavy | 9.1 | 9.9 | 12.3 | 12.1 | ||
| Physical activity, % | 24.2 | 23.0 | 28.2 | 24.2 | <0.001 | |
0.001Values are presented as mean±standard deviation unless otherwise indicated. The first health examination after age 52 is set as the baseline. P value are from one-way analysis of variance (ANOVA) or chi-square test.
BMI, body mass index; FHx, family history; FPG, fasting plasma glucose; IFG, impaired fasting glucose.
a,b,c,d The data with different superscript letters are significantly different according to the Bonferroni post hoc analysis.
Model 1: Unadjusted; Model 2: Adjusted for sex and age; Model 3: Adjusted for age, sex, body mass index; Model 4: Adjusted for age, sex, impaired fasting glucose; Model 5: Adjusted for age, sex, body mass index, impaired fasting glucose, hypertension, family history of diabetes, smoking habit.
HR, hazard ratio; CI, confidence interval; BMI, body mass index; IFG, impaired fasting glucose; FHx, family history.
- 1. Bhaskaran K, Dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol 2018;6:944-53.ArticlePubMedPMC
- 2. GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13-27.ArticlePubMedPMC
- 3. Geiss LS, Wang J, Cheng YJ, Thompson TJ, Barker L, Li Y, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. JAMA 2014;312:1218-26.ArticlePubMed
- 4. Gregg EW, Gerzoff RB, Thompson TJ, Williamson DF. Intentional weight loss and death in overweight and obese U.S. adults 35 years of age and older. Ann Intern Med 2003;138:383-9.ArticlePubMed
- 5. Bailey-Davis L, Wood GC, Benotti P, Cook A, Dove J, Mowery J, et al. Impact of sustained weight loss on cardiometabolic outcomes. Am J Cardiol 2022;162:66-72.ArticlePubMed
- 6. Delahanty LM, Pan Q, Jablonski KA, Aroda VR, Watson KE, Bray GA, et al. Effects of weight loss, weight cycling, and weight loss maintenance on diabetes incidence and change in cardiometabolic traits in the Diabetes Prevention Program. Diabetes Care 2014;37:2738-45.ArticlePubMedPMCPDF
- 7. Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 1995;122:481-6.ArticlePubMed
- 8. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-403.ArticlePubMedPMC
- 9. Oh TJ, Moon JH, Choi SH, Lim S, Park KS, Cho NH, et al. Body-weight fluctuation and incident diabetes mellitus, cardiovascular disease, and mortality: a 16-year prospective cohort study. J Clin Endocrinol Metab 2019;104:639-46.ArticlePubMedPDF
- 10. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief 2020;(360):1-8.
- 11. Nam GE, Kim YH, Han K, Jung JH, Rhee EJ, Lee WY, et al. Obesity fact sheet in Korea, 2020: prevalence of obesity by obesity class from 2009 to 2018. J Obes Metab Syndr 2021;30:141-8.ArticlePubMedPMC
- 12. Khan MA, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes: global burden of disease and forecasted trends. J Epidemiol Glob Health 2020;10:107-11.ArticlePubMedPMC
- 13. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care 2021;44(Suppl 1):S15-33.ArticlePubMedPDF
- 14. Ko SH, Han K, Lee YH, Noh J, Park CY, Kim DJ, et al. Past and current status of adult type 2 diabetes mellitus management in Korea: a National Health Insurance Service database analysis. Diabetes Metab J 2018;42:93-100.ArticlePubMedPMCPDF
- 15. Kim HK, Song SO, Noh J, Jeong IK, Lee BW. Data configuration and publication trends for the Korean National Health Insurance and Health Insurance Review & Assessment Database. Diabetes Metab J 2020;44:671-8.ArticlePubMedPMCPDF
- 16. Seong SC, Kim YY, Park SK, Khang YH, Kim HC, Park JH, et al. Cohort profile: the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 2017;7:e016640.ArticlePubMedPMC
- 17. Kim BY, Kang SM, Kang JH, Kang SY, Kim KK, Kim KB, et al. 2020 Korean Society for the Study of Obesity guidelines for the management of obesity in Korea. J Obes Metab Syndr 2021;30:81-92.ArticlePubMedPMC
- 18. Haase CL, Lopes S, Olsen AH, Satylganova A, Schnecke V, McEwan P. Weight loss and risk reduction of obesity-related outcomes in 0.5 million people: evidence from a UK primary care database. Int J Obes (Lond) 2021;45:1249-58.ArticlePubMedPMCPDF
- 19. Chalmers J, Cooper ME. UKPDS and the legacy effect. N Engl J Med 2008;359:1618-20.ArticlePubMed
- 20. Albers JW, Herman WH, Pop-Busui R, Feldman EL, Martin CL, Cleary PA, et al. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care 2010;33:1090-6.ArticlePubMedPMCPDF
- 21. Lloyd LJ, Langley-Evans SC, McMullen S. Childhood obesity and risk of the adult metabolic syndrome: a systematic review. Int J Obes (Lond) 2012;36:1-11.ArticlePubMedPMCPDF
- 22. Bjerregaard LG, Jensen BW, Angquist L, Osler M, Sorensen TI, Baker JL. Change in overweight from childhood to early adulthood and risk of type 2 diabetes. N Engl J Med 2018;378:1302-12.ArticlePubMed
- 23. Polemiti E, Baudry J, Kuxhaus O, Jager S, Bergmann MM, Weikert C, et al. BMI and BMI change following incident type 2 diabetes and risk of microvascular and macrovascular complications: the EPIC-Potsdam study. Diabetologia 2021;64:814-25.ArticlePubMedPMCPDF
- 24. Kim ES, Jeong JS, Han K, Kim MK, Lee SH, Park YM, et al. Impact of weight changes on the incidence of diabetes mellitus: a Korean nationwide cohort study. Sci Rep 2018;8:3735.ArticlePubMedPMCPDF
- 25. Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev 2016;37:278-316.ArticlePubMedPMC
- 26. Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab 2004;89:2583-9.ArticlePubMed
- 27. Power ML, Schulkin J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: possible evolutionary origins. Br J Nutr 2008;99:931-40.ArticlePubMed
- 28. Garcia M, Mulvagh SL, Merz CN, Buring JE, Manson JE. Cardiovascular disease in women: clinical perspectives. Circ Res 2016;118:1273-93.ArticlePubMedPMC
- 29. Barrett-Connor E. Sex differences in coronary heart disease: why are women so superior? The 1995 Ancel Keys Lecture. Circulation 1997;95:252-64.ArticlePubMed
- 30. Szmuilowicz ED, Stuenkel CA, Seely EW. Influence of menopause on diabetes and diabetes risk. Nat Rev Endocrinol 2009;5:553-8.ArticlePubMedPDF
- 31. Ngo DL, Marshall LM, Howard RN, Woodward JA, Southwick K, Hedberg K. Agreement between self-reported information and medical claims data on diagnosed diabetes in Oregon’s Medicaid population. J Public Health Manag Pract 2003;9:542-4.ArticlePubMed
- 32. Southern DA, Roberts B, Edwards A, Dean S, Norton P, Svenson LW, et al. Validity of administrative data claim-based methods for identifying individuals with diabetes at a population level. Can J Public Health 2010;101:61-4.ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations


 KDA
KDA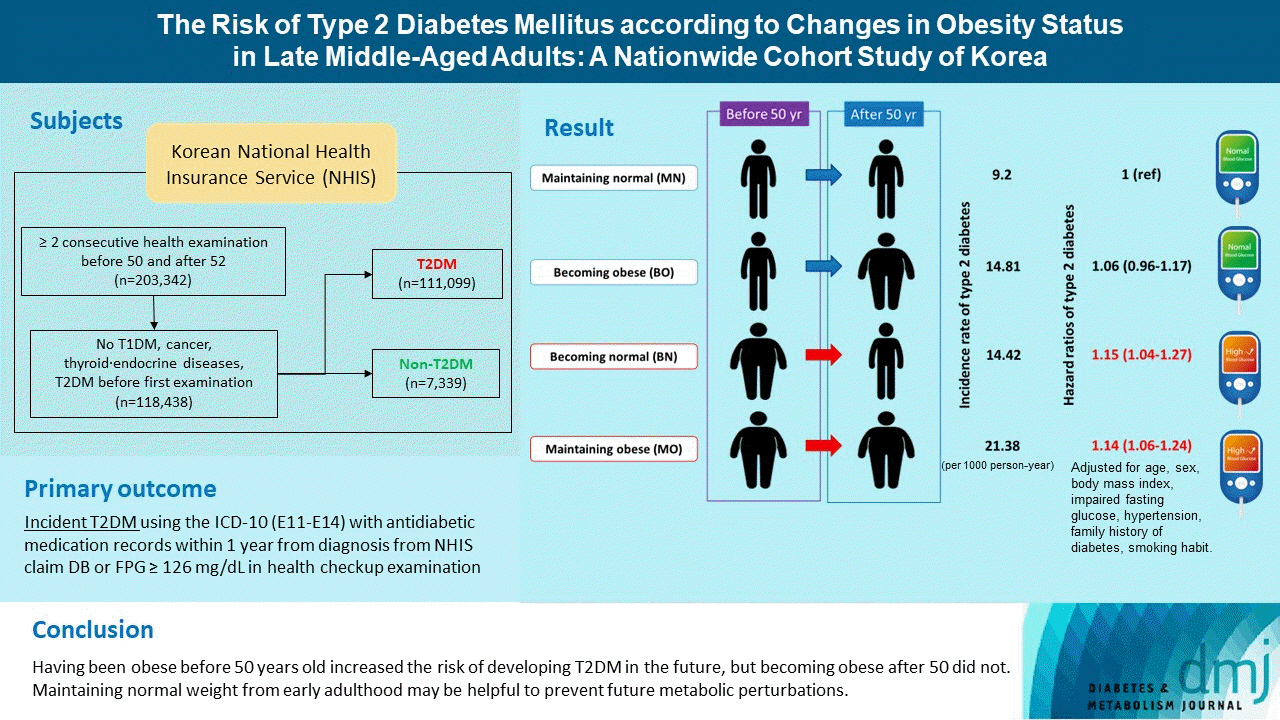
 PubReader
PubReader ePub Link
ePub Link Cite
Cite