
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Metabolic Risk/Epidemiology
- Glucagon-Like Peptide-1: New Regulator in Lipid Metabolism
- Tong Bu, Ziyan Sun, Yi Pan, Xia Deng, Guoyue Yuan
- Received August 14, 2023 Accepted January 1, 2024 Published online April 1, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0277 [Epub ahead of print]
- 466 View
- 15 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
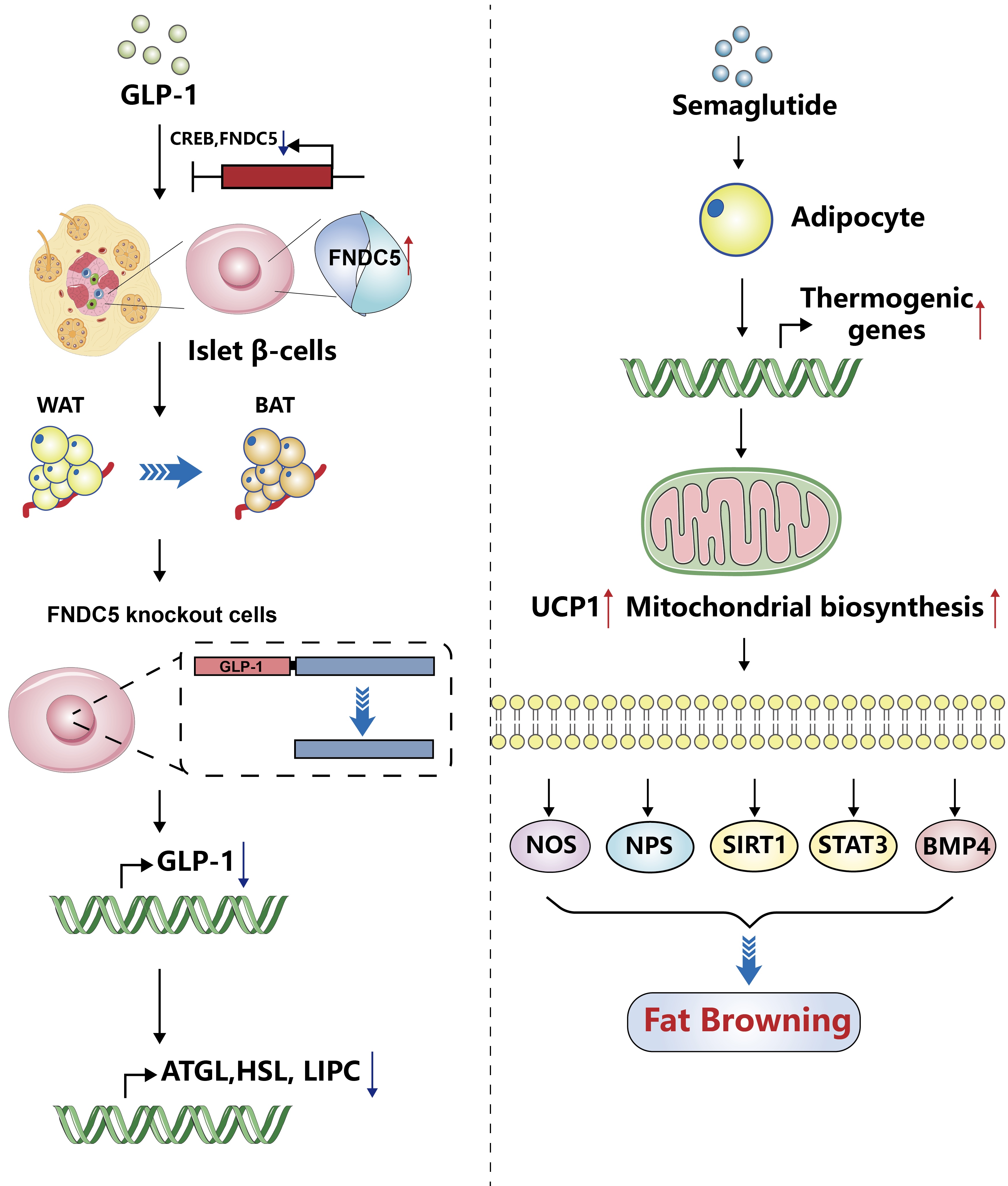
ePub - Glucagon-like peptide-1 (GLP-1) is a 30-amino acid peptide hormone that is mainly expressed in the intestine and hypothalamus. In recent years, basic and clinical studies have shown that GLP-1 is closely related to lipid metabolism, and it can participate in lipid metabolism by inhibiting fat synthesis, promoting fat differentiation, enhancing cholesterol metabolism, and promoting adipose browning. GLP-1 plays a key role in the occurrence and development of metabolic diseases such as obesity, nonalcoholic fatty liver disease, and atherosclerosis by regulating lipid metabolism. It is expected to become a new target for the treatment of metabolic disorders. The effects of GLP-1 and dual agonists on lipid metabolism also provide a more complete treatment plan for metabolic diseases. This article reviews the recent research progress of GLP-1 in lipid metabolism.
- Basic Research
- Glucagon-Like Peptide Receptor Agonist Inhibits Angiotensin II-Induced Proliferation and Migration in Vascular Smooth Muscle Cells and Ameliorates Phosphate-Induced Vascular Smooth Muscle Cells Calcification
- Jinmi Lee, Seok-Woo Hong, Min-Jeong Kim, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
- Diabetes Metab J. 2024;48(1):83-96. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0363
- 1,702 View
- 167 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
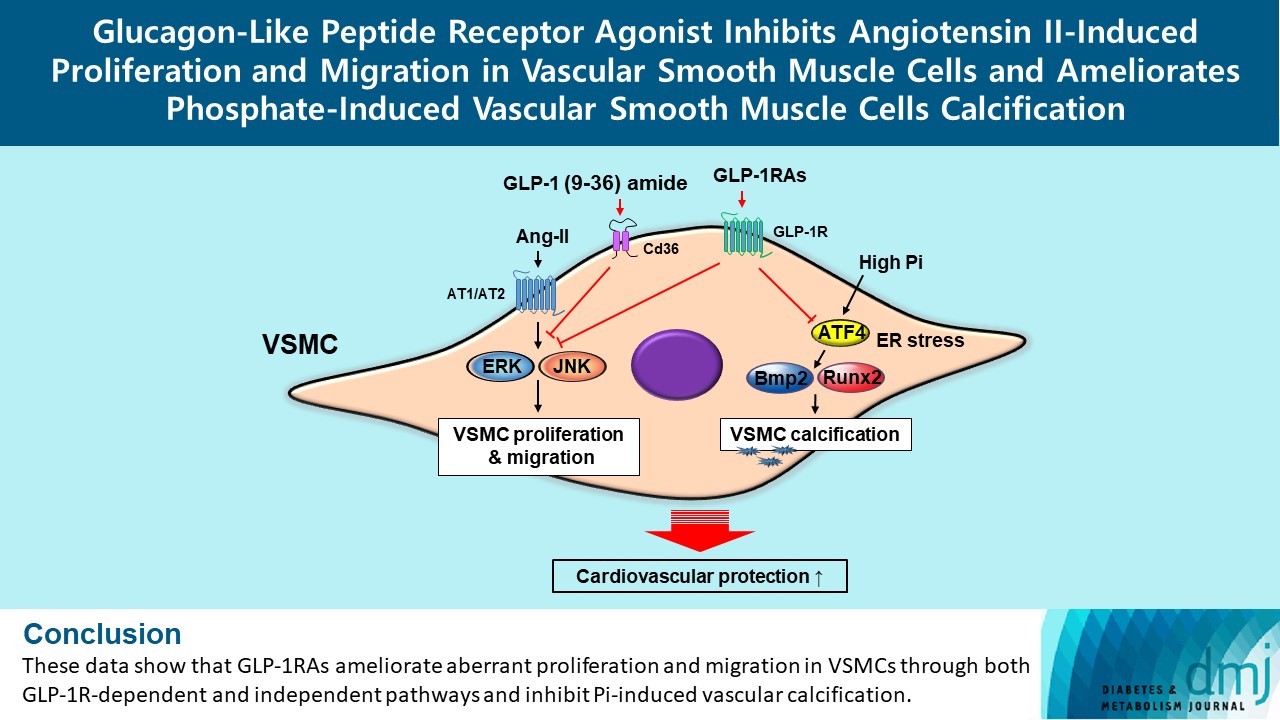
Glucagon-like peptide-1 receptor agonist (GLP-1RA), which is a therapeutic agent for the treatment of type 2 diabetes mellitus, has a beneficial effect on the cardiovascular system.
Methods
To examine the protective effects of GLP-1RAs on proliferation and migration of vascular smooth muscle cells (VSMCs), A-10 cells exposed to angiotensin II (Ang II) were treated with either exendin-4, liraglutide, or dulaglutide. To examine the effects of GLP-1RAs on vascular calcification, cells exposed to high concentration of inorganic phosphate (Pi) were treated with exendin-4, liraglutide, or dulaglutide.
Results
Ang II increased proliferation and migration of VSMCs, gene expression levels of Ang II receptors AT1 and AT2, proliferation marker of proliferation Ki-67 (Mki-67), proliferating cell nuclear antigen (Pcna), and cyclin D1 (Ccnd1), and the protein expression levels of phospho-extracellular signal-regulated kinase (p-Erk), phospho-c-JUN N-terminal kinase (p-JNK), and phospho-phosphatidylinositol 3-kinase (p-Pi3k). Exendin-4, liraglutide, and dulaglutide significantly decreased the proliferation and migration of VSMCs, the gene expression levels of Pcna, and the protein expression levels of p-Erk and p-JNK in the Ang II-treated VSMCs. Erk inhibitor PD98059 and JNK inhibitor SP600125 decreased the protein expression levels of Pcna and Ccnd1 and proliferation of VSMCs. Inhibition of GLP-1R by siRNA reversed the reduction of the protein expression levels of p-Erk and p-JNK by exendin-4, liraglutide, and dulaglutide in the Ang II-treated VSMCs. Moreover, GLP-1 (9-36) amide also decreased the proliferation and migration of the Ang II-treated VSMCs. In addition, these GLP-1RAs decreased calcium deposition by inhibiting activating transcription factor 4 (Atf4) in Pi-treated VSMCs.
Conclusion
These data show that GLP-1RAs ameliorate aberrant proliferation and migration in VSMCs through both GLP-1Rdependent and independent pathways and inhibit Pi-induced vascular calcification. -
Citations
Citations to this article as recorded by- Incretin Hormone Secretion in Women with Polycystic Ovary Syndrome: Roles of Obesity, Insulin Sensitivity and Treatment with Metformin and GLP-1s
Andrea Etrusco, Mislav Mikuš, Antonio D’Amato, Fabio Barra, Petar Planinić, Trpimir Goluža, Giovanni Buzzaccarini, Jelena Marušić, Mara Tešanović, Antonio Simone Laganà
Biomedicines.2024; 12(3): 653. CrossRef
- Incretin Hormone Secretion in Women with Polycystic Ovary Syndrome: Roles of Obesity, Insulin Sensitivity and Treatment with Metformin and GLP-1s
- Drug/Regimen
- Risk of Diabetic Retinopathy between Sodium-Glucose Cotransporter-2 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists
- Tzu-Yi Lin, Eugene Yu-Chuan Kang, Shih-Chieh Shao, Edward Chia-Cheng Lai, Sunir J. Garg, Kuan-Jen Chen, Je-Ho Kang, Wei-Chi Wu, Chi-Chun Lai, Yih-Shiou Hwang
- Diabetes Metab J. 2023;47(3):394-404. Published online March 6, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0221
- 6,549 View
- 271 Download
- 7 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
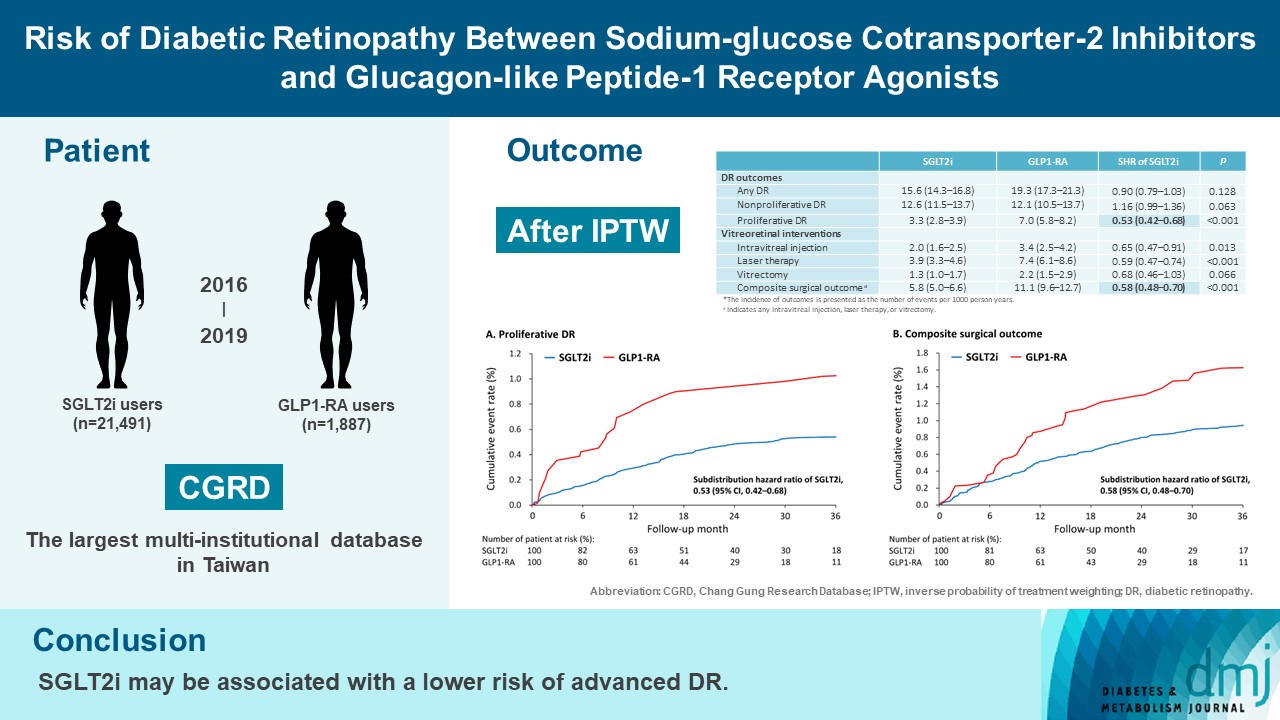
To compare risk of diabetic retinopathy (DR) between patients taking sodium-glucose cotransporter-2 inhibitors (SGLT2is) and those taking glucagon-like peptide-1 receptor agonists (GLP1-RAs) in routine care.
Methods
This retrospective cohort study emulating a target trial included patient data from the multi-institutional Chang Gung Research Database in Taiwan. Totally, 33,021 patients with type 2 diabetes mellitus using SGLT2is and GLP1-RAs between 2016 and 2019 were identified. 3,249 patients were excluded due to missing demographics, age <40 years, prior use of any study drug, a diagnosis of retinal disorders, a history of receiving vitreoretinal procedure, no baseline glycosylated hemoglobin, or no follow-up data. Baseline characteristics were balanced using inverse probability of treatment weighting with propensity scores. DR diagnoses and vitreoretinal interventions served as the primary outcomes. Occurrence of proliferative DR and DR receiving vitreoretinal interventions were regarded as vision-threatening DR.
Results
There were 21,491 SGLT2i and 1,887 GLP1-RA users included for the analysis. Patients receiving SGLT2is and GLP-1 RAs exhibited comparable rate of any DR (subdistribution hazard ratio [SHR], 0.90; 95% confidence interval [CI], 0.79 to 1.03), whereas the rate of proliferative DR (SHR, 0.53; 95% CI, 0.42 to 0.68) was significantly lower in the SGLT2i group. Also, SGLT2i users showed significantly reduced risk of composite surgical outcome (SHR, 0.58; 95% CI, 0.48 to 0.70).
Conclusion
Compared to those taking GLP1-RAs, patients receiving SGLT2is had a lower risk of proliferative DR and vitreoretinal interventions, although the rate of any DR was comparable between the SGLT2i and GLP1-RA groups. Thus, SGLT2is may be associated with a lower risk of vision-threatening DR but not DR development. -
Citations
Citations to this article as recorded by- Incretin‐based drugs and the risk of diabetic retinopathy among individuals with type 2 diabetes: A systematic review and meta‐analysis of observational studies
Samuel Igweokpala, Naheemot Olaoluwa Sule, Antonios Douros, Oriana H. Y. Yu, Kristian B. Filion
Diabetes, Obesity and Metabolism.2024; 26(2): 721. CrossRef - Association of sodium–glucose cotransporter‐2 inhibitors and the risk of retinal vascular occlusion: A real‐world retrospective cohort study in Taiwan
Tzu‐Yi Lin, Eugene Yu‐Chuan Kang, Shih‐Chieh Shao, Edward Chia‐Cheng Lai, Nan‐Kai Wang, Sunir J. Garg, Kuan‐Jen Chen, Je‐Ho Kang, Wei‐Chi Wu, Chi‐Chun Lai, Yih‐Shiou Hwang
Diabetes/Metabolism Research and Reviews.2024;[Epub] CrossRef - Risk of rotator cuff tear and rotator cuff repair surgery comparison between sodium-glucose cotransporter 2 inhibitors and glucagon like peptide-1 receptor agonists: A real-world study
Yu-Chi Su, Pei-Chun Hsieh, Edward Chia-Cheng Lai, Yu-Ching Lin
Diabetes & Metabolism.2024; 50(2): 101522. CrossRef - Optimising renal risk parameters in type 2 diabetes mellitus: Perspectives from a retinal viewpoint
Sarita Jacob, George I. Varughese
Clinical Medicine.2024; 24(2): 100031. CrossRef - Risk of diabetic retinopathy and diabetic macular oedema with sodium–glucose cotransporter 2 inhibitors and glucagon-like peptide 1 receptor agonists in type 2 diabetes: a real-world data study from a global federated database
Aikaterini Eleftheriadou, David Riley, Sizheng S. Zhao, Philip Austin, Gema Hernández, Gregory Y. H. Lip, Timothy L. Jackson, John P. H. Wilding, Uazman Alam
Diabetologia.2024;[Epub] CrossRef - Impact of GLP-1 Agonists and SGLT-2 Inhibitors on Diabetic Retinopathy Progression: An Aggregated Electronic Health Record Data Study
Karen M. Wai, Kapil Mishra, Euna Koo, Cassie Ann Ludwig, Ravi Parikh, Prithvi Mruthyunjaya, Ehsan Rahimy
American Journal of Ophthalmology.2024;[Epub] CrossRef - Risk of Diabetic Retinopathy between Sodium-Glucose Cotransporter-2 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists (Diabetes Metab J 2023;47:394-404)
Tzu-Yi Lin, Eugene Yu-Chuan Kang, Shih-Chieh Shao, Edward Chia-Cheng Lai, Yih-Shiou Hwang
Diabetes & Metabolism Journal.2023; 47(4): 573. CrossRef - Risk of Diabetic Retinopathy between Sodium-Glucose Cotransporter-2 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists (Diabetes Metab J 2023;47:394-404)
Jihee Ko, Sun Joon Moon
Diabetes & Metabolism Journal.2023; 47(4): 571. CrossRef - Sodium-Glucose Cotransporter 2 Inhibitors and Risk of Retinopathy in Patients With Type 2 Diabetes
Fu-Shun Yen, James Cheng-Chung Wei, Teng-Shun Yu, Yu-Tung Hung, Chih-Cheng Hsu, Chii-Min Hwu
JAMA Network Open.2023; 6(12): e2348431. CrossRef
- Incretin‐based drugs and the risk of diabetic retinopathy among individuals with type 2 diabetes: A systematic review and meta‐analysis of observational studies
- Complications
- Effect of the Glucagon-Like Peptide-1 Receptor Agonists on Autonomic Function in Subjects with Diabetes: A Systematic Review and Meta-Analysis
- Carla Greco, Daniele Santi, Giulia Brigante, Chiara Pacchioni, Manuela Simoni
- Diabetes Metab J. 2022;46(6):901-911. Published online April 12, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0314
- 4,472 View
- 266 Download
- 5 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
In addition to the metabolic effects in diabetes, glucagon-like peptide 1 receptor (GLP-1R) agonists lead to a small but substantial increase in heart rate (HR). However, the GLP-1R actions on the autonomic nervous system (ANS) in diabetes remain debated. Therefore, this meta-analysis evaluates the effect of GLP-1R agonist on measures of ANS function in diabetes.
Methods
According to the Cochrane Collaboration and Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement, we conducted a meta-analysis considering clinical trials in which the autonomic function was evaluated in diabetic subjects chronically treated with GLP-1R agonists. The outcomes were the change of ANS function measured by heart rate variability (HRV) and cardiac autonomic reflex tests (CARTs).
Results
In the studies enrolled, HR significantly increased after treatment (P<0.001), whereas low frequency/high frequency ratio did not differ (P=0.410); no changes in other measures of HRV were detected. Considering CARTs, only the 30:15 value derived from lying-to-standing test was significantly lower after treatment (P=0.002), but only two studies reported this measurement. No differences in other CARTs outcome were observed.
Conclusion
The meta-analysis confirms the HR increase but seems to exclude an alteration of the sympatho-vagal balance due to chronic treatment with GLP-1R agonists in diabetes, considering the available measures of ANS function. -
Citations
Citations to this article as recorded by- Liraglutide does not increase heart rate of diabetic patients during acute myocardial infarction
Qianyi Li, Chunxuan Wu, Shiqun Sun, Lingchao Yang, Yanyan Li, Yixin Niu, Li Zhang, Wei Li, Ying Yu
Journal of Diabetes.2024;[Epub] CrossRef - Hormonal Gut–Brain Signaling for the Treatment of Obesity
Eun Roh, Kyung Mook Choi
International Journal of Molecular Sciences.2023; 24(4): 3384. CrossRef - Effects of new hypoglycemic drugs on cardiac remodeling: a systematic review and network meta-analysis
Yi-lin Huang, Xiao-zhuo Xu, Jing Liu, Pin-yao Wang, Xue-li Wang, Hong-lin Feng, Cheng-jiang Liu, Xu Han
BMC Cardiovascular Disorders.2023;[Epub] CrossRef - Obesity and hypertension: Obesity medicine association (OMA) clinical practice statement (CPS) 2023
Tiffany Lowe Clayton, Angela Fitch, Harold Edward Bays
Obesity Pillars.2023; 8: 100083. CrossRef - Incretins and microvascular complications of diabetes: neuropathy, nephropathy, retinopathy and microangiopathy
Jonathan Goldney, Jack A. Sargeant, Melanie J. Davies
Diabetologia.2023; 66(10): 1832. CrossRef - Diabetes-Induced Cardiac Autonomic Neuropathy: Impact on Heart Function and Prognosis
Susumu Z. Sudo, Tadeu L. Montagnoli, Bruna de S. Rocha, Aimeé D. Santos, Mauro P. L. de Sá, Gisele Zapata-Sudo
Biomedicines.2022; 10(12): 3258. CrossRef
- Liraglutide does not increase heart rate of diabetic patients during acute myocardial infarction
- Basic Research
- DA-1241, a Novel GPR119 Agonist, Improves Hyperglycaemia by Inhibiting Hepatic Gluconeogenesis and Enhancing Insulin Secretion in Diabetic Mice
- Youjin Kim, Si Woo Lee, Hyejin Wang, Ryeong-Hyeon Kim, Hyun Ki Park, Hangkyu Lee, Eun Seok Kang
- Diabetes Metab J. 2022;46(2):337-348. Published online January 21, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0056
- 5,522 View
- 276 Download
- 8 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
We investigated the antidiabetic effects of DA-1241, a novel G protein-coupled receptor (GPR) 119 agonist, in vitro and in vivo.
Methods
DA-1241 was administrated to high-fat diet (HFD)-fed C57BL/6J mice for 12 weeks after hyperglycaemia developed. Oral/intraperitoneal glucose tolerance test and insulin tolerance test were performed. Serum insulin and glucagon-like peptide-1 (GLP-1) levels were measured during oral glucose tolerance test. Insulinoma cell line (INS-1E) cells and mouse islets were used to find whether DA-1241 directly stimulate insulin secretion in beta cell. HepG2 cells were used to evaluate the gluconeogenesis and autophagic process. Autophagic flux was evaluated by transfecting microtubule-associated protein 1 light chain 3-fused to green fluorescent protein and monomeric red fluorescent (mRFP-GFP-LC3) expression vector to HepG2 cells.
Results
Although DA-1241 treatment did not affect body weight gain and amount of food intake, fasting blood glucose level decreased along with increase in GLP-1 level. DA-1241 improved only oral glucose tolerance test and showed no effect in intraperitoneal glucose tolerance test. No significant effect was observed in insulin tolerance test. DA-1241 did not increase insulin secretion in INS-1E cell and mouse islets. DA-1241 reduced triglyceride content in the liver thereby improved fatty liver. Additionally, DA-1241 reduced gluconeogenic enzyme expression in HepG2 cells and mouse liver. DA-1241 reduced autophagic flow in HepG2 cells.
Conclusion
These findings suggested that DA-1241 augmented glucose-dependent insulin release via stimulation of GLP-1 secretion, and reduced hepatic gluconeogenesis, which might be associated with autophagic blockage, leading to improved glycaemic control. -
Citations
Citations to this article as recorded by- G protein-coupled receptors driven intestinal glucagon-like peptide-1 reprogramming for obesity: Hope or hype?
Mohan Patil, Ilaria Casari, Leon N. Warne, Marco Falasca
Biomedicine & Pharmacotherapy.2024; 172: 116245. CrossRef - GPR119 agonists for type 2 diabetes: past failures and future hopes for preclinical and early phase candidates
Deanne H Hryciw, Rhiannon K Patten, Raymond J Rodgers, Joseph Proietto, Dana S Hutchinson, Andrew J McAinch
Expert Opinion on Investigational Drugs.2024; 33(3): 183. CrossRef - Immunomodulation through Nutrition Should Be a Key Trend in Type 2 Diabetes Treatment
Katarzyna Napiórkowska-Baran, Paweł Treichel, Marta Czarnowska, Magdalena Drozd, Kinga Koperska, Agata Węglarz, Oskar Schmidt, Samira Darwish, Bartłomiej Szymczak, Zbigniew Bartuzi
International Journal of Molecular Sciences.2024; 25(7): 3769. CrossRef - Discovery of orally active sulfonylphenyl thieno[3,2-d]pyrimidine derivatives as GPR119 agonists
Heecheol Kim, Minjung Kim, Kyujin Oh, Sohee Lee, Sunyoung Lim, Sangdon Lee, Young Hoon Kim, Kwee Hyun Suh, Kyung Hoon Min
European Journal of Medicinal Chemistry.2023; 258: 115584. CrossRef - Increased expression of sodium-glucose cotransporter 2 and O-GlcNAcylation in hepatocytes drives non-alcoholic steatohepatitis
Hye Jin Chun, Eun Ran Kim, Minyoung Lee, Da Hyun Choi, Soo Hyun Kim, Eugene Shin, Jin-Hong Kim, Jin Won Cho, Dai Hoon Han, Bong-Soo Cha, Yong-ho Lee
Metabolism.2023; 145: 155612. CrossRef - Human skin stem cell-derived hepatic cells as in vitro drug discovery model for insulin-driven de novo lipogenesis
Karolien Buyl, Martine Vrints, Ruani Fernando, Terry Desmae, Thomas Van Eeckhoutte, Mia Jans, Jan Van Der Schueren, Joost Boeckmans, Robim M. Rodrigues, Veerle De Boe, Vera Rogiers, Joery De Kock, Filip Beirinckx, Tamara Vanhaecke
European Journal of Pharmacology.2023; 957: 175989. CrossRef - GPR119 activation by DA-1241 alleviates hepatic and systemic inflammation in MASH mice through inhibition of NFκB signaling
Seung-Ho Lee, Hansu Park, Eun-Kyoung Yang, Bo Ram Lee, Il-Hoon Jung, Tae-Hyoung Kim, Moon Jung Goo, Yuna Chae, Mi-Kyung Kim
Biomedicine & Pharmacotherapy.2023; 166: 115345. CrossRef - Characteristics of the Latest Therapeutic Agent for Diabetes
Nuri Yun
The Journal of Korean Diabetes.2023; 24(3): 148. CrossRef - DA-1241, a Novel GPR119 Agonist, Improves Hyperglycaemia by Inhibiting Hepatic Gluconeogenesis and Enhancing Insulin Secretion in Diabetic Mice
Youjin Kim, Si Woo Lee, Hyejin Wang, Ryeong-Hyeon Kim, Hyun Ki Park, Hangkyu Lee, Eun Seok Kang
Diabetes & Metabolism Journal.2022; 46(2): 337. CrossRef - Autophagy Dysregulation in Metabolic Associated Fatty Liver Disease: A New Therapeutic Target
Chun-Liang Chen, Yu-Cheng Lin
International Journal of Molecular Sciences.2022; 23(17): 10055. CrossRef
- G protein-coupled receptors driven intestinal glucagon-like peptide-1 reprogramming for obesity: Hope or hype?
- Drug/Regimen
- Clinical Efficacy of Sodium-Glucose Cotransporter 2 Inhibitor and Glucagon-Like Peptide-1 Receptor Agonist Combination Therapy in Type 2 Diabetes Mellitus: Real-World Study
- Hwi Seung Kim, Taekwan Yoon, Chang Hee Jung, Joong-Yeol Park, Woo Je Lee
- Diabetes Metab J. 2022;46(4):658-662. Published online November 8, 2021
- DOI: https://doi.org/10.4093/dmj.2021.0232
- 65,535 View
- 387 Download
- 7 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Sodium-glucose cotransporter 2 inhibitor (SGLT2i) and glucagon-like peptide-1 receptor agonist (GLP-1RA) are novel anti-diabetic drugs whose glucose-lowering effect and cardiovascular and renal benefits were evidenced in clinical trials. We investigated the real-world efficacy and safety of the combination of SGLT2i and GLP-1RA in patients with type 2 diabetes mellitus in Korea. The medical records of 104 patients who maintained the combination for at least 1 year were retrospectively reviewed. The change in glycosylated hemoglobin (HbA1c) after 6 months and 1 year of treatment was evaluated. The mean age was 51 years, and 41% were female. The mean baseline HbA1c, body mass index, and duration of diabetes were 9.0%, 28.8 kg/m2, and 11.7 years, respectively. Compared with baseline, the HbA1c decreased by 1.5% (95% confidence interval [CI], 1.27 to 1.74; P<0.001) after 6 months and by 1.4% (95% CI, 1.19 to 1.70; P<0.001) after 1 year. Over 1 year, the bodyweight change was −2.8 kg (95% CI, −4.21 to −1.47; P<0.001). The combination of SGLT2i and GLP-1RA is effective and tolerable in type 2 diabetes mellitus patients in real-world practice.
-
Citations
Citations to this article as recorded by- Effectiveness and safety of the combination of sodium–glucose transport protein 2 inhibitors and glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of observational studies
Aftab Ahmad, Hani Sabbour
Cardiovascular Diabetology.2024;[Epub] CrossRef - Hormonal Gut–Brain Signaling for the Treatment of Obesity
Eun Roh, Kyung Mook Choi
International Journal of Molecular Sciences.2023; 24(4): 3384. CrossRef - All‐cause mortality and cardiovascular outcomes with sodium‐glucose Co‐transporter 2 inhibitors, glucagon‐like peptide‐1 receptor agonists and with combination therapy in people with type 2 diabetes
David R. Riley, Hani Essa, Philip Austin, Frank Preston, Isatu Kargbo, Gema Hernández Ibarburu, Ramandeep Ghuman, Daniel J. Cuthbertson, Gregory Y. H. Lip, Uazman Alam
Diabetes, Obesity and Metabolism.2023; 25(10): 2897. CrossRef - The Efficacy and Safety of the Combination Therapy With GLP-1 Receptor Agonists and SGLT-2 Inhibitors in Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis
Chen Li, Jie Luo, Mingyan Jiang, Keke Wang
Frontiers in Pharmacology.2022;[Epub] CrossRef - Clinical Efficacy of Sodium-Glucose Cotransporter 2 Inhibitor and Glucagon-Like Peptide-1 Receptor Agonist Combination Therapy in Type 2 Diabetes Mellitus: Real-World Study (Diabetes Metab J 2022;46: 658-62)
Hwi Seung Kim, Woo Je Lee
Diabetes & Metabolism Journal.2022; 46(4): 665. CrossRef - Clinical Efficacy of Sodium-Glucose Cotransporter 2 Inhibitor and Glucagon-Like Peptide-1 Receptor Agonist Combination Therapy in Type 2 Diabetes Mellitus: Real-World Study (Diabetes Metab J 2022;46: 658-62)
Tomoyuki Kawada
Diabetes & Metabolism Journal.2022; 46(4): 663. CrossRef - Durability of glucose-lowering effect of dulaglutide in patients with type 2 diabetes mellitus: A real-world data study
Hwi Seung Kim, Yun Kyung Cho, Myung Jin Kim, Chang Hee Jung, Joong-Yeol Park, Woo Je Lee
Frontiers in Endocrinology.2022;[Epub] CrossRef
- Effectiveness and safety of the combination of sodium–glucose transport protein 2 inhibitors and glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of observational studies
- Complications
- Associations of Plasma Glucagon Levels with Estimated Glomerular Filtration Rate, Albuminuria and Diabetic Kidney Disease in Patients with Type 2 Diabetes Mellitus
- Hua-Xing Huang, Liang-Lan Shen, Hai-Yan Huang, Li-Hua Zhao, Feng Xu, Dong-Mei Zhang, Xiu-Lin Zhang, Tong Chen, Xue-Qin Wang, Yan Xie, Jian-Bin Su
- Diabetes Metab J. 2021;45(6):868-879. Published online March 23, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0149
- 5,875 View
- 173 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
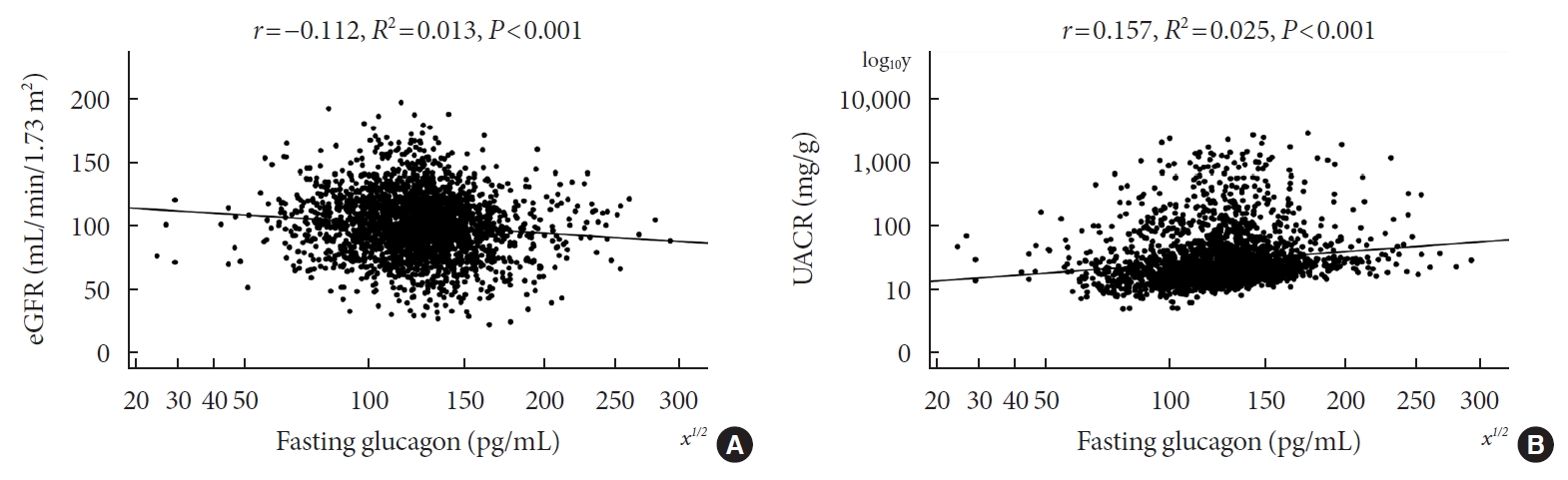
Type 2 diabetes mellitus (T2DM) is characterized by elevated fasting glucagon and impaired suppression of postprandial glucagon secretion, which may participate in diabetic complications. Therefore, we investigated the associations of plasma glucagon with estimated glomerular filtration rate (eGFR), albuminuria and diabetic kidney disease (DKD) in T2DM patients.
Methods
Fasting glucagon and postchallenge glucagon (assessed by area under the glucagon curve [AUCgla]) levels were determined during oral glucose tolerance tests. Patients with an eGFR <60 mL/min/1.73 m2 and/or a urinary albumin-to-creatinine ratio (UACR) ≥30 mg/g who presented with diabetic retinopathy were identified as having DKD.
Results
Of the 2,436 recruited patients, fasting glucagon was correlated with eGFR and UACR (r=–0.112 and r=0.157, respectively; P<0.001), and AUCgla was also correlated with eGFR and UACR (r=–0.267 and r=0.234, respectively; P<0.001). Moreover, 31.7% (n=771) presented with DKD; the prevalence of DKD was 27.3%, 27.6%, 32.5%, and 39.2% in the first (Q1), second (Q2), third (Q3), and fourth quartile (Q4) of fasting glucagon, respectively; and the corresponding prevalence for AUCgla was 25.9%, 22.7%, 33.7%, and 44.4%, respectively. Furthermore, after adjusting for other clinical covariates, the adjusted odds ratios (ORs; 95% confidence intervals) for DKD in Q2, Q3, and Q4 versus Q1 of fasting glucagon were 0.946 (0.697 to 1.284), 1.209 (0.895 to 1.634), and 1.521 (1.129 to 2.049), respectively; the corresponding ORs of AUCgla were 0.825 (0.611 to 1.114), 1.323 (0.989 to 1.769), and 2.066 (1.546 to 2.760), respectively. Additionally, when we restricted our analysis in patients with glycosylated hemoglobin <7.0% (n=471), we found fasting glucagon and AUCgla were still independently associated with DKD.
Conclusion
Both increased fasting and postchallenge glucagon levels were independently associated with DKD in T2DM patients. -
Citations
Citations to this article as recorded by- Glucagon in type 2 diabetes: Friend or foe?
Irene Caruso, Nicola Marrano, Giuseppina Biondi, Valentina Annamaria Genchi, Rossella D'Oria, Gian Pio Sorice, Sebastio Perrini, Angelo Cignarelli, Annalisa Natalicchio, Luigi Laviola, Francesco Giorgino
Diabetes/Metabolism Research and Reviews.2023;[Epub] CrossRef
- Glucagon in type 2 diabetes: Friend or foe?
- Drug/Regimen
-
- Glucagon-Like Peptide-1 Receptor Agonist Differentially Affects Brain Activation in Response to Visual Food Cues in Lean and Obese Individuals with Type 2 Diabetes Mellitus
- Jae Hyun Bae, Hyung Jin Choi, Kang Ik Kevin Cho, Lee Kyung Kim, Jun Soo Kwon, Young Min Cho
- Diabetes Metab J. 2020;44(2):248-259. Published online November 4, 2019
- DOI: https://doi.org/10.4093/dmj.2019.0018
- 7,366 View
- 222 Download
- 5 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background To investigate the effects of a glucagon-like peptide-1 receptor agonist on functional brain activation in lean and obese individuals with type 2 diabetes mellitus (T2DM) in response to visual food cues.
Methods In a randomized, single-blinded, crossover study, 15 lean and 14 obese individuals with T2DM were administered lixisenatide or normal saline subcutaneously with a 1-week washout period. We evaluated brain activation in response to pictures of high-calorie food, low-calorie food, and nonfood using functional magnetic resonance imaging and measured appetite and caloric intake in participants who were given access to an
ad libitum buffet.Results Obese individuals with T2DM showed significantly greater activation of the hypothalamus, pineal gland, parietal cortex (high-calorie food vs. low-calorie food,
P <0.05), orbitofrontal cortex (high-calorie food vs. nonfood,P <0.05), and visual cortex (food vs. nonfood,P <0.05) than lean individuals with T2DM. Lixisenatide injection significantly reduced the functional activation of the fusiform gyrus and lateral ventricle in obese individuals with T2DM compared with that in lean individuals with T2DM (nonfood vs. high-calorie food,P <0.05). In addition, in individuals who decreased their caloric intake after lixisenatide injection, there were significant interaction effects between group and treatment in the posterior cingulate, medial frontal cortex (high-calorie food vs. low-calorie food,P <0.05), hypothalamus, orbitofrontal cortex, and temporal lobe (food vs. nonfood,P <0.05).Conclusion Brain responses to visual food cues were different in lean and obese individuals with T2DM. In addition, acute administration of lixisenatide differentially affected functional brain activation in these individuals, especially in those who decreased their caloric intake after lixisenatide injection.
-
Citations
Citations to this article as recorded by- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
Jae Hyun Bae
Diabetes & Metabolism Journal.2024; 48(1): 157. CrossRef - Diabetes remission and relapse following an intensive metabolic intervention combining insulin glargine/lixisenatide, metformin and lifestyle approaches: Results of a randomised controlled trial
Natalia McInnes, Stephanie Hall, Heather A. Lochnan, Stewart B. Harris, Zubin Punthakee, Ronald J. Sigal, Irene Hramiak, Mohammed Azharuddin, Joanne F. Liutkus, Jean‐François Yale, Farah Sultan, Ada Smith, Rose E. Otto, Diana Sherifali, Yan Yun Liu, Hertz
Diabetes, Obesity and Metabolism.2023; 25(11): 3347. CrossRef - Glucagon-like peptide-1 analog therapy in rare genetic diseases: monogenic obesity, monogenic diabetes, and spinal muscular atrophy
Hussein Zaitoon, Ronit Lubetzky, Achiya Z. Amir, Hadar Moran-Lev, Liora Sagi, Michal Yacobi-Bach, Ophir Borger, Efrat Chorna, Yael Lebenthal, Avivit Brener
Acta Diabetologica.2023; 60(8): 1099. CrossRef - What can functional brain imaging teach us about remission of type 2 diabetes?
Dhruti Hirani, Shahd Alabdulkader, Alexander. D. Miras, Victoria Salem
Diabetic Medicine.2023;[Epub] CrossRef - Fasting oxyntomodulin, glicentin, and gastric inhibitory polypeptide levels are associated with activation of reward‐ and attention‐related brain centres in response to visual food cues in adults with obesity: A cross‐sectional functional MRI study
Nikolaos Perakakis, Olivia M. Farr, Christos S. Mantzoros
Diabetes, Obesity and Metabolism.2021; 23(5): 1202. CrossRef - Aberrant Brain Functional Connectivity Strength and Effective Connectivity in Patients with Type 2 Diabetes Mellitus
Xi Guo, Su Wang, Yu-Chen Chen, Heng-Le Wei, Gang-Ping Zhou, Yu-Sheng Yu, Xindao Yin, Kun Wang, Hong Zhang, Eusebio Chiefari
Journal of Diabetes Research.2021; 2021: 1. CrossRef
- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
- Obesity and Metabolic Syndrome
- Premeal Consumption of a Protein-Enriched, Dietary Fiber-Fortified Bar Decreases Total Energy Intake in Healthy Individuals
- Chang Ho Ahn, Jae Hyun Bae, Young Min Cho
- Diabetes Metab J. 2019;43(6):879-892. Published online June 25, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0202
- 4,979 View
- 84 Download
- 3 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background A premeal load of protein can increase satiety and reduce energy intake. Dietary fiber also conveys metabolic benefits by modulating energy intake. We made a protein-enriched, dietary fiber-fortified bar (PFB) and aimed to investigate its effects on food intake and gut hormone secretion in healthy individuals.
Methods Twenty subjects with normal glucose tolerance were enrolled. On three separate visits, the subjects received, in a randomized order, one of the following: a PFB containing 73 kcal with 10.7 g of protein and 12.7 g of dietary fiber; a usual bar (UB) containing the same calories as the PFB but only 0.9 g of protein and no dietary fiber; or water (control). After 15 minutes, the subjects had
ad libitum intake of a test meal. Food consumption, appetite, and plasma gut hormone levels were measured.Results Total energy intake, including the bar and the test meal, was significantly reduced with the PFB preload compared to the water (904.4±534.9 kcal vs. 1,075.0±508.0 kcal,
P =0.016). With the UB preload, only the intake of the test meal was reduced (P =0.044) but not the total energy intake (P =0.471) than the water. Fullness was also significantly increased after the PFB. In addition, postprandial glucose levels decreased and glucagon-like peptide-1 levels increased with the PFB compared with both the UB and water.Conclusion In healthy individuals, a premeal supplementation of PFB reduced total energy intake and decreased postprandial glucose excursion. This finding necessitates long-term studies regarding clinical use in obesity.
-
Citations
Citations to this article as recorded by- Citrus pectin protects mice from burn injury by modulating intestinal microbiota, GLP-1 secretion and immune response
Ji-Wei Hao, Hong-Sheng Liu, Ling-Ying Liu, Qing-Hong Zhang
International Immunopharmacology.2024; 131: 111912. CrossRef - Effect of Two Different Meal Compositions on 1-hour Plasma Ghrelin Levels in Young Men
Brinnell Annette Caszo, Sangeetha Shyam, Purushotham Krishnappa, Justin Vijay Gnanou
Malaysian Journal of Medicine and Health Sciences.2023; 19(5): 185. CrossRef - Intake of Fibre-Associated Foods and Texture Preferences in Relation to Weight Status Among 9–12 Years Old Children in 6 European Countries
Marlies Hörmann-Wallner, Raphaela Krause, Begoña Alfaro, Hannah Jilani, Monica Laureati, Valérie L. Almli, Mari Sandell, Pernilla Sandvik, Gertrude G. Zeinstra, Lisa Methven
Frontiers in Nutrition.2021;[Epub] CrossRef - Response: Premeal Consumption of a Protein-Enriched, Dietary Fiber-Fortified Bar Decreases Total Energy Intake in Healthy Individuals (Diabetes Metab J 2019;43:879–92)
Chang Ho Ahn, Jae Hyun Bae, Young Min Cho
Diabetes & Metabolism Journal.2020; 44(1): 207. CrossRef - Letter: Premeal Consumption of a Protein-Enriched, Dietary Fiber-Fortified Bar Decreases Total Energy Intake in Healthy Individuals (Diabetes Metab J 2019;43:879–92)
Mi-kyung Kim
Diabetes & Metabolism Journal.2020; 44(1): 203. CrossRef - Spent coffee (Coffea arabicaL.) grounds promote satiety and attenuate energy intake: A pilot study
Rocio Campos‐Vega, Andrea Arreguín‐Campos, Miguel A. Cruz‐Medrano, María Dolores Castillo Bilbao
Journal of Food Biochemistry.2020;[Epub] CrossRef
- Citrus pectin protects mice from burn injury by modulating intestinal microbiota, GLP-1 secretion and immune response
- Pathophysiology
- Factors Related to Blood Intact Incretin Levels in Patients with Type 2 Diabetes Mellitus
- Soyeon Yoo, Eun-Jin Yang, Gwanpyo Koh
- Diabetes Metab J. 2019;43(4):495-503. Published online February 20, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0105
- 3,918 View
- 36 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background We performed this study to identify factors related to intact incretin levels in patients with type 2 diabetes mellitus (T2DM).
Methods We cross-sectionally analyzed 336 patients with T2DM. Intact glucagon-like peptide 1 (iGLP-1) and intact glucose-dependent insulinotropic polypeptide (iGIP) levels were measured in a fasted state and 30 minutes after ingestion of a standard mixed meal. The differences between 30 and 0 minute iGLP-1 and iGIP levels were indicated as ΔiGLP-1 and ΔiGIP.
Results In simple correlation analyses, fasting iGLP-1 was positively correlated with glucose, C-peptide, creatinine, and triglyceride levels, and negatively correlated with estimated glomerular filtration rate. ΔiGLP-1 was positively correlated only with ΔC-peptide levels. Fasting iGIP showed positive correlations with glycosylated hemoglobin (HbA1c) and fasting glucose levels, and negative correlations with ΔC-peptide levels. ΔiGIP was negatively correlated with diabetes duration and HbA1c levels, and positively correlated with Δglucose and ΔC-peptide levels. In multivariate analyses adjusting for age, sex, and covariates, fasting iGLP-1 levels were significantly related to fasting glucose levels, ΔiGLP-1 levels were positively related to ΔC-peptide levels, fasting iGIP levels were related to fasting C-peptide levels, and ΔiGIP levels were positively related to ΔC-peptide and Δglucose levels.
Conclusion Taken together, intact incretin levels are primarily related to C-peptide and glucose levels. This result suggests that glycemia and insulin secretion are the main factors associated with intact incretin levels in T2DM patients.
- Clinical Diabetes & Therapeutics
-
- Asian Subpopulations May Exhibit Greater Cardiovascular Benefit from Long-Acting Glucagon-Like Peptide 1 Receptor Agonists: A Meta-Analysis of Cardiovascular Outcome Trials
- Yu Mi Kang, Yun Kyung Cho, Jiwoo Lee, Seung Eun Lee, Woo Je Lee, Joong-Yeol Park, Ye-Jee Kim, Chang Hee Jung, Michael A. Nauck
- Diabetes Metab J. 2019;43(4):410-421. Published online December 27, 2018
- DOI: https://doi.org/10.4093/dmj.2018.0070
- 6,319 View
- 137 Download
- 18 Web of Science
- 18 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background Based on reported results of three large cardiovascular outcome trials (CVOTs) of glucagon-like peptide 1 receptor agonists (GLP-1 RAs), we aimed to investigate the overall effect of GLP-1 RAs on major adverse cardiovascular events (MACEs) and to identify subpopulations exhibiting the greatest cardiovascular (CV) benefit.
Methods Three CVOTs reporting effects of long-acting GLP-1 RAs were included: LEADER (liraglutide), SUSTAIN-6 (semaglutide), and EXSCEL (exenatide once weekly). In all studies, the primary endpoint was three-point MACE, comprising CV death, non-fatal myocardial infarction, and non-fatal stroke. Overall effect estimates were calculated as hazard ratios and 95% confidence intervals (CIs) using the random-effects model; subgroup analyses reported in the original studies were similarly analyzed.
Results Overall, statistically significant risk reductions in MACE and CV death were observed. Subgroup analysis indicated a significant racial difference with respect to CV benefit (
P for interaction <0.001), and more substantial risk reductions were observed in subjects of African origin (relative risk [RR], 0.78; 95% CI, 0.60 to 0.99) and in Asians (RR, 0.35; 95% CI, 0.09 to 1.32). However,post hoc analysis (Bonferroni method) revealed that only Asians exhibited a significantly greater CV benefit from treatment, compared with white subjects (P <0.0001).Conclusion Long-acting GLP-1 RAs reduced risks of MACE and CV deaths in high-risk patients with type 2 diabetes mellitus. Our findings of a particularly effective reduction in CV events with GLP-1 RA in Asian populations merits further exploration and dedicated trials in specific populations.
-
Citations
Citations to this article as recorded by- Sex, racial, ethnic, and geographical disparities in major adverse cardiovascular outcome of glucagon-like peptide-1 receptor agonists among patients with and without diabetes mellitus: A meta-analysis of placebo-controlled randomized controlled trials,
Frederick Berro Rivera, Nathan Ross B. Bantayan, John Paul Aparece, Linnaeus Louisse A. Cruz, John Vincent Magallong, Polyn Luz Pine, Anne Mira Nicca Idian-Javier, Grace Nooriza O. Lumbang, Edgar V. Lerma, Kyla M. Lara-Breitinger, Martha Gulati, Krishnasw
Journal of Clinical Lipidology.2024;[Epub] CrossRef - Coronary Artery Disease in South Asian Patients: Cardiovascular Risk Factors, Pathogenesis and Treatments
Vincenzo Sucato, Giuseppe Coppola, Girolamo Manno, Giuseppe Vadalà, Giuseppina Novo, Egle Corrado, Alfredo Ruggero Galassi
Current Problems in Cardiology.2023; 48(8): 101228. CrossRef - Retrospective Analysis of the Effectiveness of Oral Semaglutide in Type 2 Diabetes Mellitus and Its Effect on Cardiometabolic Parameters in Japanese Clinical Settings
Hodaka Yamada, Masashi Yoshida, Shunsuke Funazaki, Jun Morimoto, Shiori Tonezawa, Asuka Takahashi, Shuichi Nagashima, Kimura Masahiko, Otsuka Kiyoshi, Kazuo Hara
Journal of Cardiovascular Development and Disease.2023; 10(4): 176. CrossRef - Efficacy of treatment with glucagon-like peptide receptor agonists-1 in Asian patients with type 2 diabetes mellitus
L. Yu. Khamnueva, L. S. Andreeva
Problems of Endocrinology.2023; 69(2): 38. CrossRef - Role of diabetes in stroke: Recent advances in pathophysiology and clinical management
Sian A. Bradley, Kevin J. Spring, Roy G. Beran, Dimitrios Chatzis, Murray C. Killingsworth, Sonu M. M. Bhaskar
Diabetes/Metabolism Research and Reviews.2022;[Epub] CrossRef - Obesity Pillars Roundtable: Obesity and East Asians
Harold Edward Bays, Jennifer Ng, Jeffrey Sicat, Michelle Look
Obesity Pillars.2022; 2: 100011. CrossRef - Pathophysiology, phenotypes and management of type 2 diabetes mellitus in Indian and Chinese populations
Calvin Ke, K. M. Venkat Narayan, Juliana C. N. Chan, Prabhat Jha, Baiju R. Shah
Nature Reviews Endocrinology.2022; 18(7): 413. CrossRef - Effect of race on cardiometabolic responses to once-weekly exenatide: insights from the Exenatide Study of Cardiovascular Event Lowering (EXSCEL)
Timothy M. E. Davis, Anna Giczewska, Yuliya Lokhnygina, Robert J. Mentz, Naveed Sattar, Rury R. Holman
Cardiovascular Diabetology.2022;[Epub] CrossRef - Generalizability of the Results of Cardiovascular Outcome Trials of Glucagon-Like Peptide 1 Receptor Agonists in Chinese Patients with Type 2 Diabetes Mellitus
Xiaoling Cai, Linong Ji
Diabetes Therapy.2021; 12(7): 1861. CrossRef - Current trends in epidemiology of cardiovascular disease and cardiovascular risk management in type 2 diabetes
Jae-Seung Yun, Seung-Hyun Ko
Metabolism.2021; 123: 154838. CrossRef - Sex and ethnic differences in the cardiovascular complications of type 2 diabetes
Jian L. Yeo, Emer M. Brady, Gerry P. McCann, Gaurav S. Gulsin
Therapeutic Advances in Endocrinology and Metabolism.2021; 12: 204201882110342. CrossRef - Efficacy and Safety of GLP-1 Receptor Agonists in Patients With Type 2 Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis
Yuan Zhu, Jiao Xu, Dong Zhang, Xingyu Mu, Yi Shi, Shangtao Chen, Zengxiang Wu, Shuangqing Li
Frontiers in Endocrinology.2021;[Epub] CrossRef - Efficacy and safety of dulaglutide in type 2 diabetes patients in endocrinology clinics of Islamabad, Pakistan
Matiullah Kamin, SajjadAli Khan, UmarYousaf Raja, Osama Ishtiaq, Asmara Malik, Tejhmal Rehman, MuhammadUmar Wahab
Indian Journal of Endocrinology and Metabolism.2021; 25(5): 456. CrossRef - Type 2 Diabetes and Atherosclerotic Cardiovascular Disease in South Asians: a Unique Population with a Growing Challenge
Afreen I. Shariff, Nitya Kumar, William S. Yancy, Leonor Corsino
Current Diabetes Reports.2020;[Epub] CrossRef - Antihypertensive and Renal Mechanisms of SGLT2 (Sodium-Glucose Linked Transporter 2) Inhibitors
Christopher S. Wilcox
Hypertension.2020; 75(4): 894. CrossRef - Subpopulation Differences in the Cardiovascular Efficacy of Long-Acting Glucagon-Like Peptide 1 Receptor Agonists in Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis
Liyun He, Na Yang, Lingling Xu, Fan Ping, Wei Li, Yuxiu Li, Huabing Zhang
Diabetes Therapy.2020; 11(9): 2121. CrossRef - 2020 Consensus of Taiwan Society of Cardiology on the pharmacological management of patients with type 2 diabetes and cardiovascular diseases
Chern-En Chiang, Kwo-Chang Ueng, Ting-Hsing Chao, Tsung-Hsien Lin, Yih-Jer Wu, Kang-Ling Wang, Shih-Hsien Sung, Hung-I Yeh, Yi-Heng Li, Ping-Yen Liu, Kuan-Cheng Chang, Kou-Gi Shyu, Jin-Long Huang, Cheng-Dao Tsai, Huei-Fong Hung, Ming-En Liu, Tze-Fan Chao,
Journal of the Chinese Medical Association.2020; 83(7): 587. CrossRef - Beneficial effect of anti-diabetic drugs for nonalcoholic fatty liver disease
Kyung-Soo Kim, Byung-Wan Lee
Clinical and Molecular Hepatology.2020; 26(4): 430. CrossRef
- Sex, racial, ethnic, and geographical disparities in major adverse cardiovascular outcome of glucagon-like peptide-1 receptor agonists among patients with and without diabetes mellitus: A meta-analysis of placebo-controlled randomized controlled trials,
- Complications
- Update on the Impact, Diagnosis and Management of Cardiovascular Autonomic Neuropathy in Diabetes: What Is Defined, What Is New, and What Is Unmet
- Vincenza Spallone
- Diabetes Metab J. 2019;43(1):3-30. Published online November 2, 2018
- DOI: https://doi.org/10.4093/dmj.2018.0259
- 16,032 View
- 402 Download
- 153 Web of Science
- 156 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The burden of diabetic cardiovascular autonomic neuropathy (CAN) is expected to increase due to the diabetes epidemic and its early and widespread appearance. CAN has a definite prognostic role for mortality and cardiovascular morbidity. Putative mechanisms for this are tachycardia, QT interval prolongation, orthostatic hypotension, reverse dipping, and impaired heart rate variability, while emerging mechanisms like inflammation support the pervasiveness of autonomic dysfunction. Efforts to overcome CAN under-diagnosis are on the table: by promoting screening for symptoms and signs; by simplifying cardiovascular reflex tests; and by selecting the candidates for screening. CAN assessment allows for treatment of its manifestations, cardiovascular risk stratification, and tailoring therapeutic targets. Risk factors for CAN are mainly glycaemic control in type 1 diabetes mellitus (T1DM) and, in addition, hypertension, dyslipidaemia, and obesity in type 2 diabetes mellitus (T2DM), while preliminary data regard glycaemic variability, vitamin B12 and D changes, oxidative stress, inflammation, and genetic biomarkers. Glycaemic control prevents CAN in T1DM, whereas multifactorial intervention might be effective in T2DM. Lifestyle intervention improves autonomic function mostly in pre-diabetes. While there is no conclusive evidence for a disease-modifying therapy, treatment of CAN manifestations is available. The modulation of autonomic function by SGLT2i represents a promising research field with possible clinical relevance.
-
Citations
Citations to this article as recorded by- Rectal sensitivity correlated with gastrointestinal‐mediated glucose disposal, but not the incretin effect
Sondre Meling, Erling Tjora, Heike Eichele, Rasmus B. Nedergaard, Filip K. Knop, Niels Ejskjaer, Siri Carlsen, Pål R. Njølstad, Christina Brock, Eirik Søfteland
Endocrinology, Diabetes & Metabolism.2024;[Epub] CrossRef - Glucose metabolism and autonomic function in healthy individuals and patients with type 2 diabetes mellitus at rest and during exercise
Takuto Hamaoka, Urs A. Leuenberger, Rachel C. Drew, Matthew Murray, Cheryl Blaha, Jonathan Carter Luck, Lawrence I. Sinoway, Jian Cui
Experimental Physiology.2024; 109(2): 214. CrossRef - Quantification of lipoproteins by proton nuclear magnetic resonance spectroscopy (1H-NMRS) improves the prediction of cardiac autonomic dysfunction in patients with type 1 diabetes
L. Nattero-Chávez, M. Insenser, N. Amigó, S. Samino, N. Martínez-Micaelo, B. Dorado Avendaño, A. Quintero Tobar, H. F. Escobar-Morreale, M. Luque-Ramírez
Journal of Endocrinological Investigation.2024;[Epub] CrossRef - Predictors of pacemaker requirement in patients with implantable loop recorder and unexplained syncope: A systematic review and meta‐analysis
Moein Zangiabadian, Kiarash Soltani, Yasaman Gholinejad, Reyhane Yahya, Shayan Bastami, Mohammad Ali Akbarzadeh, Mohammad Sharifian Ardestani, Azadeh Aletaha
Clinical Cardiology.2024;[Epub] CrossRef - No clear evidence of neuropathy among patients with high risk for the development of prediabetes/diabetes—a pilot study
Anna E. Körei, Magdolna Békeffy, Adrienn Menyhárt, Karola Osgyán, Ildikó Istenes, Viktor J. Horváth, Péter Kempler
Frontiers in Endocrinology.2024;[Epub] CrossRef - Effects of Physical Cues on Stem Cell-Derived Extracellular Vesicles toward Neuropathy Applications
Danyale Berry, Justice Ene, Aakash Nathani, Mandip Singh, Yan Li, Changchun Zeng
Biomedicines.2024; 12(3): 489. CrossRef - Oxidative stress, endothelial dysfunction, and N-acetylcysteine in type-2 diabetes mellitus
Xin Li, Junyong Zou, Aiping Lin, Jingshu Chi, Hong Hao, Hong Chen, Zhenguo Liu
Antioxidants & Redox Signaling.2024;[Epub] CrossRef - High dose cholecalciferol supplementation causing morning blood pressure reduction in patients with type 1 diabetes mellitus and cardiovascular autonomic neuropathy
João Felício, Lorena Moraes, Gabriela Lemos, Ícaro Souza, Giovana Vieira, Lilian Silva, Natércia Queiroz, Ana Carolina Souza, Franciane Melo, João Felício Abrahão Neto, Hana Britto, Manuela Lemos, Márcia Santos, Priscila Figueiredo, Ana Regina Motta, Meli
Scientific Reports.2024;[Epub] CrossRef - Transcutaneous vagal nerve stimulation for treating gastrointestinal symptoms in individuals with diabetes: a randomised, double-blind, sham-controlled, multicentre trial
Ditte S. Kornum, Davide Bertoli, Huda Kufaishi, Anne-Marie Wegeberg, Tina Okdahl, Esben B. Mark, Katrine L. Høyer, Jens B. Frøkjær, Birgitte Brock, Klaus Krogh, Christian S. Hansen, Filip K. Knop, Christina Brock, Asbjørn M. Drewes
Diabetologia.2024;[Epub] CrossRef - Independent and interactive associations of heart rate and obesity with type 2 diabetes mellites: A population‐based study
Tianxin Zhu, Qingyu Chen, Hongxing Chen, Lili You, Dan Liu, Xiaoyun Zhang, Feng Li, Hongshi Wu, Juying Tang, Diaozhu Lin, Kan Sun, Li Yan, Meng Ren
Journal of Diabetes.2024;[Epub] CrossRef - Mortality risk factors in newly diagnosed diabetic cardiac autonomic neuropathy
Bruce A. Chase, Sylwia Pocica, Roberta Frigerio, Katerina Markopoulou, Demetrius M. Maraganore, Navamon Aunaetitrakul, Alexander Epshteyn, Alexandru C. Barboi
Clinical Autonomic Research.2023; 33(6): 903. CrossRef - Autonomic symptoms and associated factors in patients with chronic heart failure
Hellen Da Silva, Sofie Pardaens, Marc Vanderheyden, Johan De Sutter, Heleen Demeyer, Michel De Pauw, Laurent Demulier, Jan Stautemas, Patrick Calders
Acta Cardiologica.2023; 78(2): 203. CrossRef - Incretins and microvascular complications of diabetes: neuropathy, nephropathy, retinopathy and microangiopathy
Jonathan Goldney, Jack A. Sargeant, Melanie J. Davies
Diabetologia.2023; 66(10): 1832. CrossRef - Functional and morphometric assessment of small-fibre damage in late-onset hereditary transthyretin amyloidosis with polyneuropathy: the controversial relation between small-fibre-related symptoms and diagnostic test findings
Eleonora Galosi, Luca Leonardi, Pietro Falco, Giuseppe Di Pietro, Alessandra Fasolino, Nicoletta Esposito, Caterina Leone, Giulia Di Stefano, Maurizio Inghilleri, Marco Luigetti, Antonini Giovanni, Andrea Truini
Amyloid.2023; 30(1): 59. CrossRef - In vivo molecular imaging of cardiac angiogenesis in persons with and without type 2 diabetes: A cross‐sectional 68 Ga‐RGD‐PET study
Jens Christian Laursen, Ida Kirstine Bull Rasmussen, Emilie Hein Zobel, Philip Hasbak, Lene Holmvang, Christian Stevns Hansen, Bernt Johan von Scholten, Marie Frimodt‐Møller, Peter Rossing, Tine Willum Hansen, Andreas Kjaer, Rasmus Sejersten Ripa
Diabetic Medicine.2023;[Epub] CrossRef - Cardiac innervations in diabetes mellitus—Anatomical evidence of neuropathy
Natalija Filipović, Maja Marinović Guić, Vana Košta, Katarina Vukojević
The Anatomical Record.2023; 306(9): 2345. CrossRef - Clinical Predictors of Pacing Device Implantation in Implantable Cardiac Monitor Recipients for Unexplained Syncope
Reina Tonegawa-Kuji, Yuko Y. Inoue, Michikazu Nakai, Koshiro Kanaoka, Yoko Sumita, Yuichiro Miyazaki, Akinori Wakamiya, Keiko Shimamoto, Nobuhiko Ueda, Kenzaburo Nakajima, Naoya Kataoka, Mitsuru Wada, Kenichiro Yamagata, Kohei Ishibashi, Koji Miyamoto, Sa
CJC Open.2023; 5(4): 259. CrossRef - Cardiovascular autonomic reflex tests using a handheld device in the diagnosis of cardiovascular autonomic neuropathy in patients with schizophrenia
Laura Blok-Husum, Milka Ane Rank Brcelic, Hanin Kawa Farman Kawal Bassi, Svend Eggert Jensen, Rene Ernst Nielsen, Kristian Kragholm, Jesper Fleischer, Esben Laugesen, Christoffer Polcwiartek
American Heart Journal Plus: Cardiology Research and Practice.2023; 26: 100252. CrossRef - Causal association between vitamin D and diabetic neuropathy: a Mendelian randomization analysis
Wei Huang, Lei Gu, Jingwen Wang, Yiqi Wang, Fangzheng Cao, Tianyu Jin, Yifan Cheng
Endocrine.2023; 80(2): 328. CrossRef - Diabetes Mellitus and Heart Failure: Epidemiology, Pathophysiologic Mechanisms, and the Role of SGLT2 Inhibitors
Panagiotis Theofilis, Evangelos Oikonomou, Konstantinos Tsioufis, Dimitris Tousoulis
Life.2023; 13(2): 497. CrossRef - Sex differences and sex steroids influence on the presentation and severity of cardiovascular autonomic neuropathy of patients with type 1 diabetes
Lía Nattero-Chávez, María Insenser, Alejandra Quintero Tobar, Elena Fernández-Durán, Beatriz Dorado Avendaño, Tom Fiers, Jean-Marc Kaufman, Manuel Luque-Ramírez, Héctor F. Escobar-Morreale
Cardiovascular Diabetology.2023;[Epub] CrossRef - Early Gastrointestinal Neuropathy Assessed by Wireless Motility Capsules in Adolescents with Type 1 Diabetes
Vinni Faber Rasmussen, Mathilde Thrysøe, Páll Karlsson, Esben Thyssen Vestergaard, Kurt Kristensen, Ann-Margrethe Rønholt Christensen, Jens Randel Nyengaard, Astrid Juhl Terkelsen, Christina Brock, Klaus Krogh
Journal of Clinical Medicine.2023; 12(5): 1925. CrossRef - Heart rate variability in people with metabolic syndrome
Kostiantyn Apykhtin, Svitlana Drozdovska, Olha Hurenko, Anastasiia Nahorna, Anatoly Pisaruk, Yuliia Panchenko, Olena Andrieieva
Ageing & Longevity.2023; (1 2023): 1. CrossRef - Heart rate variability in people with metabolic syndrome
Kostiantyn Apykhtin, Svitlana Drozdovska, Olha Hurenko, Anastasiia Nahorna, Anatoly Pisaruk, Yuliia Panchenko, Olena Andrieieva
JOURNAL OF THE NATIONAL ACADEMY OF MEDICAL SCIENCES OF UKRAINE.2023; (1 2023): 1. CrossRef - Potential of electronic devices for detection of health problems in older adults at home: A systematic review and meta-analysis
Yu-ting Cao, Xin-xin Zhao, Yi-ting Yang, Shi-jie Zhu, Liang-dong Zheng, Ting Ying, Zhou Sha, Rui Zhu, Tao Wu
Geriatric Nursing.2023; 51: 54. CrossRef - Diabetes mellitus in der Akut- und Notfallmedizin
Leo Benning, Julian Krehl, Felix Patricius Hans
Notfallmedizin up2date.2023; 18(01): 45. CrossRef - Correlation between Heart rate recovery and Left Atrial phasic functions evaluated by 2D speckle-tracking Echocardiography after Acute Myocardial infarction
Behruz Mashayekhi, Reza Mohseni-Badalabadi, Ali Hosseinsabet, Tahereh Ahmadian
BMC Cardiovascular Disorders.2023;[Epub] CrossRef - Pancreatic sympathetic innervation disturbance in type 1 diabetes
Senlin Li, Huimin Yuan, Keshan Yang, Qing Li, Ming Xiang
Clinical Immunology.2023; 250: 109319. CrossRef - A Nonrandomized Trial of the Effects of Passive Simulated Jogging on Short-Term Heart Rate Variability in Type 2 Diabetic Subjects
Jose A. Adams, Jose R. Lopez, Veronica Banderas, Marvin A. Sackner, Mark Yorek
Journal of Diabetes Research.2023; 2023: 1. CrossRef - Evaluating treatment options for cardiovascular autonomic neuropathy in patients with diabetes mellitus: a systematic review
Jasmine KaiLi Goh, Leroy Koh
Diabetology International.2023; 14(3): 224. CrossRef - Autonomic neuropathic symptoms in patients with diabetes: practical tools for screening in daily routine
Ana Raquel Souza de Azevedo Vieira, Lara Benigno Porto-Dantas, Flaviene Alves do Prado Romani, Patrícia Souza Carvalho, Rodica Pop-Busui, Hermelinda Cordeiro Pedrosa
Diabetology & Metabolic Syndrome.2023;[Epub] CrossRef - “Arterial stiffness is not associated with changes in the circadian pattern of blood pressure in patients with type 1 diabetes mellitus and cardiovascular autonomic dysfunction”
Lía Nattero-Chávez, Ane Bayona Cebada, Elena Fernández-Durán, Alejandra Quintero Tobar, Beatriz Dorado Avendaño, Héctor Escobar-Morreale, Manuel Luque-Ramírez
Diabetes and Vascular Disease Research.2023; 20(3): 147916412311736. CrossRef - Frontiers in diagnostic and therapeutic approaches in diabetic sensorimotor neuropathy (DSPN)
Sanjeev Sharma, Gerry Rayman
Frontiers in Endocrinology.2023;[Epub] CrossRef - In Type 2 Diabetes Mellitus, normalization of hemoglobin A1c accompanies reduced sensitivity to pressure at the sternum
Jens Faber, Søren Ballegaard, Nanna Ørsted, Ebbe Eldrup, Benny Karpatschof, Finn Gyntelberg, Sofie Korsgaard Hecquet, Albert Gjedde
Frontiers in Neuroscience.2023;[Epub] CrossRef - Functional status associated with postural dizziness, but not postural hypotension, in older adults: a community-based study
Hsiang-Ju Cheng, Zih-Jie Sun, Feng-Hwa Lu, Yi-Ching Yang, Chih-Jen Chang, Jin-Shang Wu
BMC Geriatrics.2023;[Epub] CrossRef - Effects of baricitinib, empagliflozin, linagliptin and telmisartan on cardiovascular autonomic neuropathy in type 1 diabetes: An exploratory, randomized, open‐label, crossover trial
Jens Christian Laursen, Viktor Rotbain Curovic, Marjolein Y. A. M. Kroonen, Niels Jongs, Emilie H. Zobel, Tine W. Hansen, Marie Frimodt‐Møller, Gozewijn D. Laverman, Adriaan Kooy, Frederik Persson, Hiddo J. L. Heerspink, Christian Stevns Hansen, Peter Ros
Diabetes, Obesity and Metabolism.2023; 25(10): 3064. CrossRef - The Retinal Nerve Fiber Layer Thickness Is Associated with Systemic Neurodegeneration in Long-Term Type 1 Diabetes
Christina Brock, Anne-Marie Wegeberg, Thomas Arendt Nielsen, Bassam Karout, Per M. Hellström, Asbjørn Mohr Drewes, Henrik Vorum
Translational Vision Science & Technology.2023; 12(6): 23. CrossRef - The Use of Empirical Mode Decomposition on Heart Rate Variability Signals to Assess Autonomic Neuropathy Progression in Type 2 Diabetes
Sandra Cossul, Felipe Rettore Andreis, Mateus Andre Favretto, Jefferson Luiz Brum Marques
Applied Sciences.2023; 13(13): 7824. CrossRef - Topical capsaicin for the management of painful diabetic neuropathy: a narrative systematic review
Brandon Goodwin, Maanas Chiplunkar, Ryan Salerno, Kylon Coombs, Umar Sannoh, Vrushank Shah, Nicholas Averell, Usmaan Al-Shebab, Deanna Janora
Pain Management.2023; 13(5): 309. CrossRef - Adynamic response to cold pain reflects dysautonomia in type 1 diabetes and polyneuropathy
Thomas Arendt Nielsen, Søren Lundbye-Christensen, Yoanna Krasimirova Dimitrova, Sam Riahi, Birgitte Brock, Asbjørn Mohr Drewes, Christina Brock
Scientific Reports.2023;[Epub] CrossRef - Autonomic Nerve Function Tests in Patients with Diabetes
Heung Yong Jin, Tae Sun Park
The Journal of Korean Diabetes.2023; 24(2): 71. CrossRef - Understanding the role of hyperglycemia and the molecular mechanism associated with diabetic neuropathy and possible therapeutic strategies
Mandeep Kaur, Sakshi Misra, Priyanka Swarnkar, Preeti Patel, Balak Das Kurmi, Ghanshyam Das Gupta, Amrita Singh
Biochemical Pharmacology.2023; 215: 115723. CrossRef - A three-month physical training program improves cardiovascular autonomic function in patients with metabolic syndrome with and without diabetes – a pilot study
Anna Vágvölgyi, Judit Erzsébet Ábrahám, Éva Máthéné Köteles, Andrea Korom, Mária Barnai, Mónika Szűcs, Andrea Orosz, Péter Kempler, Adrienn Menyhárt, Attila Nemes, Tamás Várkonyi, István Baczkó, István Kósa, Csaba Lengyel
Frontiers in Endocrinology.2023;[Epub] CrossRef - Cardiovascular autonomic neuropathy in patients with type 2 diabetes with and without sensorimotor polyneuropathy
Emil Peters, Mustapha Itani, Alexander G. Kristensen, Astrid Juhl Terkelsen, Thomas Krøigård, Hatice Tankisi, Troels S. Jensen, Nanna B. Finnerup, Sandra Sif Gylfadottir
Journal of the Peripheral Nervous System.2023; 28(3): 450. CrossRef - Cardiac Autonomic Neuropathy in Prediabetes: A Case-Control Study
Pavan Gujjar, Y. S. Ravikumar, Lakshmi Nagendra, Hiya Boro, Saptarshi Bhattacharya
Indian Journal of Endocrinology and Metabolism.2023; 27(4): 325. CrossRef - Diabetic Neuropathies
Melissa A. Elafros, Brian C. Callaghan
CONTINUUM: Lifelong Learning in Neurology.2023; 29(5): 1401. CrossRef - Determinants of the heart rate variability in type 1 diabetes mellitus
Máté Hajdu, Konstandia Garmpis, Vivien Vértes, Noémi Vorobcsuk-Varga, Gergő Attila Molnár, László Hejjel, István Wittmann, Réka Faludi
Frontiers in Endocrinology.2023;[Epub] CrossRef - The Effect of Exercise on Cardiovascular Autonomic Nervous Function in Patients with Diabetes: A Systematic Review
Hidetaka Hamasaki
Healthcare.2023; 11(19): 2668. CrossRef - Influence of Fibrinogen/Albumin Ratio and Fibrinogen/Pre-Albumin Ratio on Cardiac Autonomic Neuropathy in Type 2 Diabetes
Subei Zhao, Zheng Yang, Meng Yu, Linyu Xiang, Yuhuan Lv, Chunyan Tian, Rong Li
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 3249. CrossRef - In Ischemic Heart Disease, Reduced Sensitivity to Pressure at the Sternum Accompanies Lower Mortality after Five Years: Evidence from a Randomized Controlled Trial
Søren Ballegaard, Jens Faber, Christian Selmer, Finn Gyntelberg, Svend Kreiner, Benny Karpatschof, Tobias Wirenfeldt Klausen, Åke Hjalmarson, Albert Gjedde
Journal of Clinical Medicine.2023; 12(24): 7585. CrossRef - Assessment of the relationship of systemic vascular dysfunction and cardiac autonomic neuropathy (CAN) with diabetic retinopathy
KJ Hari Prakash, Sucheta Parija, Manisha Kar
Journal of Family Medicine and Primary Care.2023; 12(12): 3236. CrossRef - Autonomic Neuropathy in Ambulatory Type 2 Diabetes Mellitus Patients: A Single-arm Prospective, Observational Study
Kaustav Saha, Shatavisa Mukherjee, Animesh Maiti, Santanu Kumar Tripathi
Journal of the Practice of Cardiovascular Sciences.2023; 9(3): 178. CrossRef - Insomnia and type 2 diabetes: how to help the patient. Modern view of a neurologist
E. S. Akarachkova, O. V. Kotova, V. L. Klimov, D. I. Lebedeva
FOCUS. Endocrinology.2023; 4(4): 12. CrossRef - Impaired Cardiovagal Activity as a Link Between Hyperglycemia and Arterial Stiffness in Adults With Type 2 Diabetes Mellitus Patients Among an Eastern Indian Population: A Cross-sectional Study
Nibedita Priyadarsini, Devineni Likhitha, Madumathy Ramachandran, Kishore Kumar Behera
Canadian Journal of Diabetes.2023;[Epub] CrossRef - Carvedilol improves heart rate variability indices, biomarkers but not cardiac nerve density in streptozotocin-induced T2DM model of diabetic cardiac autonomic neuropathy
Olawale Mathias Akinlade, Bamidele Owoyele, Olufemi Ayodele Soladoye
Journal of Basic and Clinical Physiology and Pharmacology.2022; 33(2): 213. CrossRef - Cardiovascular autonomic responses during head-up tilt test in newly diagnosed type 2 diabetes
Esteban Jorge-Galarza, Margarita Torres-Tamayo, María del Rocío Martínez-Alvarado, Berenice Peña-Aparicio, Carmen González-Salazar, Juan Reyes-Barrera, Manuel Sierra-Beltrán, Erika Fajardo-Flores, Andrey Kostin, J. Antonio González-Hermosillo
Irish Journal of Medical Science (1971 -).2022; 191(5): 2077. CrossRef - Cardiovascular autonomic neuropathy and incident diabetic kidney disease in patients with type 2 diabetes
Ji Eun Jun, Min Sun Choi, Jae Hyeon Kim
Diabetes Research and Clinical Practice.2022; 184: 109181. CrossRef - Kardiovaskuläre Risiken in der 4.–6. Lebensdekade mit Diabetes mellitus Typ 1
Young Hee Lee-Barkey, Bernd Stratmann, Diethelm Tschöpe
Der Diabetologe.2022; 18(2): 131. CrossRef - Mechanisms of cardiac dysfunction in diabetic cardiomyopathy: molecular abnormalities and phenotypical variants
Francesca Romana Prandi, Isabella Evangelista, Domenico Sergi, Alberto Palazzuoli, Francesco Romeo
Heart Failure Reviews.2022; 28(3): 597. CrossRef - Comparison of Risk Assessment Strategies for Patients with Diabetes Mellitus and Stable Chest Pain: A Coronary Computed Tomography Angiography Study
Jia Zhao, Shuo Wang, Pengyu Zhao, Yong Huo, Chunjie Li, Jia Zhou, Pawel Kleczynski
Journal of Diabetes Research.2022; 2022: 1. CrossRef - BOND study: a randomised double-blind, placebo-controlled trial over 12 months to assess the effects of benfotiamine on morphometric, neurophysiological and clinical measures in patients with type 2 diabetes with symptomatic polyneuropathy
Gidon J Bönhof, Gundega Sipola, Alexander Strom, Christian Herder, Klaus Strassburger, Birgit Knebel, Claudia Reule, Jan-Christoph Wollmann, Andrea Icks, Hadi Al-Hasani, Michael Roden, Oliver Kuss, Dan Ziegler
BMJ Open.2022; 12(2): e057142. CrossRef - Pragmatic Clinic-Based Investigation of Glycemic Variability in Patients With Type 1 Diabetes in Routine Clinical Practice and Its Association With Cardiovascular Autonomic Neuropathy: A Pilot Study
Lucianne R.M. Tannus, Marilia B. Gomes
Endocrine Practice.2022; 28(5): 465. CrossRef - Longitudinal effects of one‐leg standing time on neuropathy outcomes in association with glycemic control in non‐elderly patients with type 2 diabetes
Kazuhiro Sugimoto, Takashi Sozu, Takehiko Hoshino, Yuko Watanabe, Akira Tamura, Toshiro Yamazaki, Setsu Ohta, Susumu Suzuki, Takuro Shimbo
Journal of Diabetes Investigation.2022; 13(6): 1039. CrossRef - Thermal quantitative sensory testing as a screening tool for cardiac autonomic neuropathy in patients with diabetes mellitus
Veronika Potockova, Sarka Mala, Lucie Hoskovcova, Vaclav Capek, Tomas Nedelka, Lucie Riedlbauchova, Daniel Baumgartner, Livie Mensova, Radim Mazanec
Brain and Behavior.2022;[Epub] CrossRef - Cardiovascular Autonomic Reflex Tests and 7 Heart Rate Variability Indices for Early Diagnosis of Cardiovascular Autonomic Neuropathy in Type 2 Diabetes Individuals
Yeelen Ballesteros Atala, Mozânia Reis De Matos, Denise Engelbrecht Zantut-Wittmann, Alejandro Rosell Castillo, Daniele P Santos-Bezerra, Maria Lucia Correa-Giannella, Maria Cândida Ribeiro Parisi
Current Diabetes Reviews.2022;[Epub] CrossRef - Design, synthesis, in vitro and in silico studies of naproxen derivatives as dual lipoxygenase and α-glucosidase inhibitors
Asma Sardar, Obaid-ur-Rahman Abid, Saima Daud, M. Fakhar-e-Alam, Muhammad Hussnain Siddique, Muhammad Ashraf, Wardah Shahid, Syeda Abida Ejaz, M. Atif, Shafiq Ahmad, Sulman Shafeeq, Muhammad Afzal
Journal of Saudi Chemical Society.2022; 26(3): 101468. CrossRef - Clinical manifestations and evaluation of cardiac autonomic neuropathy in diabetes mellitus and metabolic syndrome
L. S. Moshkhoeva, A. N. Barinov
Neurology, Neuropsychiatry, Psychosomatics.2022; 14(2): 71. CrossRef - The relationship between vitamin B12 levels and electrocardiographic ventricular repolarization markers
Emre Yılmaz, Devrim Kurt, Aslı Vural, Ertan Aydın, Sencer Çamcı, Ercan Aydın
Nutrición Hospitalaria.2022;[Epub] CrossRef - The effect of liraglutide on cardiac autonomic function in type 2 diabetes: A prespecified secondary analysis from the LIRAFLAME randomized, double‐blinded, placebo‐controlled trial
Suvanjaa Sivalingam, Emilie Hein Zobel, Christian S. Hansen, Rasmus S. Ripa, Bernt J. von Scholten, Viktor Rotbain Curovic, Andreas Kjaer, Jacob K. Jensen, Tine W. Hansen, Peter Rossing
Diabetes, Obesity and Metabolism.2022; 24(8): 1638. CrossRef - Heart Rate Variability and Chronic Kidney Disease in Patients with Type 2 Diabetes
Wei Shi, Jing Zhang, Dan Chen, Xiaolei Chen, Wei Duan, Hongmei Zhang, Fahd Abd Algalil
Applied Bionics and Biomechanics.2022; 2022: 1. CrossRef - Spectrum of cardiac autonomic neuropathy in patients with type 2 diabetes mellitus: A North India perspective
PrativaPriyadarshani Sethi, Basavraj Jatteppanavar, Ravi Kant, Monika Pathania, MukeshChand Bairwa
Journal of Cardio-diabetes and metabolic disorders.2022; 2(1): 23. CrossRef - Cardiac Autonomic Dysfunction Is Associated With Risk of Diabetic Kidney Disease Progression in Type 2 Diabetes Mellitus
Haixia Zeng, Jianmo Liu, Zheng Chen, Peng Yu, Jianping Liu
Frontiers in Endocrinology.2022;[Epub] CrossRef - Diagnostic Tools, Biomarkers, and Treatments in Diabetic polyneuropathy
and Cardiovascular Autonomic Neuropathy
Gidon J. Bönhof, Christian Herder, Dan Ziegler
Current Diabetes Reviews.2022;[Epub] CrossRef - Triglyceride glucose index is related with cardiac autonomic dysfunction in patients with metabolic syndrome
Akif Serhat Balcıoğlu, Ekrem Aksu, Ahmet Çağrı Aykan
Kardiologiia.2022; 62(6): 45. CrossRef - Pathogenesis of Distal Symmetrical Polyneuropathy in Diabetes
Sasha Smith, Pasha Normahani, Tristan Lane, David Hohenschurz-Schmidt, Nick Oliver, Alun Huw Davies
Life.2022; 12(7): 1074. CrossRef - Correlation between impaired hemodynamic response and cardiopulmonary fitness in middle-aged type 2 diabetes mellitus patients: a case–control study
Jinjin Xie, Lianhua Yin, Jia Huang, Ying Xu, Yannan Chen, Jiawei Qin, Zhizhen Liu, Jing Tao
European Journal of Applied Physiology.2022; 122(10): 2295. CrossRef - Higher frequency of cardiovascular autonomic neuropathy in youth with type 2 compared to type 1 diabetes: Role of cardiometabolic risk factors
Benjamin J. Varley, Megan L. Gow, Yoon Hi Cho, Paul Benitez‐Aguirre, Janine Cusumano, Alison Pryke, Albert Chan, Vallimayil Velayutham, Kim C. Donaghue, Maria E. Craig
Pediatric Diabetes.2022; 23(7): 1073. CrossRef - The role of protein kinase C in diabetic microvascular complications
Deng Pan, Lin Xu, Ming Guo
Frontiers in Endocrinology.2022;[Epub] CrossRef - Effect of SGLT-2 inhibitors on cardiac autonomic function in type 2 diabetes mellitus: a meta-analysis of randomized controlled trials
Dimitrios Patoulias, Alexandra Katsimardou, Nikolaos Fragakis, Christodoulos Papadopoulos, Michael Doumas
Acta Diabetologica.2022; 60(1): 1. CrossRef - Association between blood glucose levels and autonomic symptoms in Peru
Gabriel Angeles-Zurita, Margorie Narro-Fuentes, Antonio Bernabe-Ortiz
Primary Care Diabetes.2022; 16(5): 709. CrossRef - Clinical scoring systems for the risk of cardiovascular autonomic neuropathy in type 1 and type 2 diabetes: A simple tool
Marika Menduni, Cinzia D'Amato, Martina Leoni, Valentina Izzo, Mariateresa Staltari, Carla Greco, Andrea Abbatepassero, Giuseppe Seminara, Ilenia D'Ippolito, Davide Lauro, Vincenza Spallone
Journal of the Peripheral Nervous System.2022; 27(4): 259. CrossRef - Vagus nerve stimulation as a novel treatment for systemic lupus erythematous: study protocol for a randomised, parallel-group, sham-controlled investigator-initiated clinical trial, the SLE-VNS study
Amanda Hempel Zinglersen, Ida Lynghøj Drange, Katrine Aagaard Myhr, Andreas Fuchs, Mogens Pfeiffer-Jensen, Christina Brock, Søren Jacobsen
BMJ Open.2022; 12(9): e064552. CrossRef - To the interpretation of frequency components of the heart rate variability
N. V. Kuzmenko, V. A. Tsyrlin, M. G. Pliss
Translational Medicine.2022; 9(3): 35. CrossRef - Protein pyrrole adducts are associated with elevated glucose indices and clinical features of diabetic diffuse neuropathies
Xiao Chen, Zhuyi Jiang, Lianjing Zhang, Wei Liu, Xiaohu Ren, Luling Nie, Desheng Wu, Zhiwei Guo, Weimin Liu, Xifei Yang, Yan Wu, Zhen Liang, Peter Spencer, Jianjun Liu
Journal of Diabetes.2022; 14(10): 646. CrossRef - Cardiac Autonomic Neuropathy in Type 1 and 2 Diabetes: Epidemiology, Pathophysiology, and Management
Scott Williams, Siddig Abdel Raheim, Muhammad Ilyas Khan, Umme Rubab, Prathap Kanagala, Sizheng Steven Zhao, Anne Marshall, Emily Brown, Uazman Alam
Clinical Therapeutics.2022; 44(10): 1394. CrossRef - Pathophysiological and clinical aspects of the circadian rhythm of arterial stiffness in diabetes mellitus: A minireview
Victoria A. Serhiyenko, Ludmila M. Serhiyenko, Volodymyr B. Sehin, Alexandr A. Serhiyenko
Endocrine Regulations.2022; 56(4): 284. CrossRef - Heart rate-corrected QT interval prolongation is associated with decreased heart rate variability in patients with type 2 diabetes
Seon-Ah Cha
Medicine.2022; 101(45): e31511. CrossRef - Effect of the Glucagon-Like Peptide-1 Receptor Agonists on Autonomic Function in Subjects with Diabetes: A Systematic Review and Meta-Analysis
Carla Greco, Daniele Santi, Giulia Brigante, Chiara Pacchioni, Manuela Simoni
Diabetes & Metabolism Journal.2022; 46(6): 901. CrossRef - Diabetes-Induced Cardiac Autonomic Neuropathy: Impact on Heart Function and Prognosis
Susumu Z. Sudo, Tadeu L. Montagnoli, Bruna de S. Rocha, Aimeé D. Santos, Mauro P. L. de Sá, Gisele Zapata-Sudo
Biomedicines.2022; 10(12): 3258. CrossRef - The Relationship Between Orthostatic Hypotension and Vitamin D Deficiency in Patients with Uncontrolled Type 2 Diabetes Mellitus
Ece YİĞİT, Ridvan SİVRİTEPE, Dilay KARABULUT, Umut KARABULUT
Online Türk Sağlık Bilimleri Dergisi.2022; 7(2): 313. CrossRef - A study of heart rate variability in diabetic mellitus patients
Srinivasa Jayachandra, Satyanath Reddy Kodidala
INTERNATIONAL JOURNAL OF ENDOCRINOLOGY (Ukraine).2022; 18(3): 153. CrossRef - The prevalence of cardiac autonomic neuropathy in prediabetes: a systematic review
Aikaterini Eleftheriadou, Scott Williams, Sarah Nevitt, Emily Brown, Rebecca Roylance, John P. H. Wilding, Daniel J. Cuthbertson, Uazman Alam
Diabetologia.2021; 64(2): 288. CrossRef - Risk of cardiac autonomic neuropathy in latent autoimmune diabetes in adults is similar to type 1 diabetes and lower compared to type 2 diabetes: A cross‐sectional study
Ernesto Maddaloni, Chiara Moretti, Rossella Del Toro, Sara Sterpetti, Maria Vittoria Ievolella, Gabriele Arnesano, Rocky Strollo, Silvia Irina Briganti, Luca D'Onofrio, Paolo Pozzilli, Raffaella Buzzetti
Diabetic Medicine.2021;[Epub] CrossRef - Effects of lunar cycle on fasting plasma glucose, heart rate and blood pressure in type 2 diabetic patients
Sutanu Dutta Chowdhury, Subhasish Pramanik, Koena Bhattacharjee, Lakshmi Kanta Mondal
Chronobiology International.2021; 38(2): 270. CrossRef - Intensive Risk Factor Management and Cardiovascular Autonomic Neuropathy in Type 2 Diabetes: The ACCORD Trial
Yaling Tang, Hetal Shah, Carlos Roberto Bueno Junior, Xiuqin Sun, Joanna Mitri, Maria Sambataro, Luisa Sambado, Hertzel C. Gerstein, Vivian Fonseca, Alessandro Doria, Rodica Pop-Busui
Diabetes Care.2021; 44(1): 164. CrossRef - Decreased glomerular filtration rate and increased albuminuria for identification of cardiovascular autonomic neuropathy in subjects with and without diabetes
Ying-Chuen Lai, Hung-Yuan Li, Yi-Dier Jiang, Tien-Jyun Chang, Lee-Ming Chuang
Autonomic Neuroscience.2021; 230: 102757. CrossRef - Exposures influencing the developing central autonomic nervous system
Sarah D. Schlatterer, Adre J. du Plessis
Birth Defects Research.2021; 113(11): 845. CrossRef - Vitamin B12 Supplementation in Diabetic Neuropathy: A 1-Year, Randomized, Double-Blind, Placebo-Controlled Trial
Triantafyllos Didangelos, Eleni Karlafti, Evangelia Kotzakioulafi, Eleni Margariti, Parthena Giannoulaki, Georgios Batanis, Solomon Tesfaye, Kοnstantinos Kantartzis
Nutrients.2021; 13(2): 395. CrossRef - The Association Between Continuous Glucose Monitoring-Derived Metrics and Cardiovascular Autonomic Neuropathy in Outpatients with Type 2 Diabetes
Min Young Kim, Gyuri Kim, Ji Yun Park, Min Sun Choi, Ji Eun Jun, You-Bin Lee, Sang-Man Jin, Kyu Yeon Hur, Jae Hyeon Kim
Diabetes Technology & Therapeutics.2021; 23(6): 434. CrossRef - Insulin resistance is independently associated with cardiovascular autonomic neuropathy in type 2 diabetes
Yingshan Liu, Yu Peng, Jing Jin, Yanshan Chen, Chuna Chen, Zhenguo Chen, Haishan Huang, Lingling Xu
Journal of Diabetes Investigation.2021; 12(9): 1651. CrossRef - SGLT2 inhibitors and the autonomic nervous system in diabetes: A promising challenge to better understand multiple target improvement
Vincenza Spallone, Paul Valensi
Diabetes & Metabolism.2021; 47(4): 101224. CrossRef - Reduction of Pressure Pain Sensitivity as Novel Non-pharmacological Therapeutic Approach to Type 2 Diabetes: A Randomized Trial
Jens Faber, Ebbe Eldrup, Christian Selmer, Caroline Pichat, Sofie Korsgaard Hecquet, Torquil Watt, Svend Kreiner, Benny Karpatschof, Finn Gyntelberg, Søren Ballegaard, Albert Gjedde
Frontiers in Neuroscience.2021;[Epub] CrossRef - Relationship between cardiac autonomic neuropathy and cardio-metabolic risk profile in adults with type 1 diabetes
M. Serdarova, R. Dimova, N. Chakarova, G. Grozeva, A. Todorova, T. Tankova
Diabetes Research and Clinical Practice.2021; 174: 108721. CrossRef - Differences and Similarities in Neuropathy in Type 1 and 2 Diabetes: A Systematic Review
Mar Sempere-Bigorra, Iván Julián-Rochina, Omar Cauli
Journal of Personalized Medicine.2021; 11(3): 230. CrossRef - Assessment of Gastrointestinal Autonomic Dysfunction: Present and Future Perspectives
Ditte S. Kornum, Astrid J. Terkelsen, Davide Bertoli, Mette W. Klinge, Katrine L. Høyer, Huda H. A. Kufaishi, Per Borghammer, Asbjørn M. Drewes, Christina Brock, Klaus Krogh
Journal of Clinical Medicine.2021; 10(7): 1392. CrossRef - Diabetic heart disease: A clinical update
Jake Rajbhandari, Cornelius James Fernandez, Mayuri Agarwal, Beverly Xin Yi Yeap, Joseph M Pappachan
World Journal of Diabetes.2021; 12(4): 383. CrossRef - Novel and Emerging Electrophysiological Biomarkers of Diabetic Neuropathy and Painful Diabetic Neuropathy
Anne Marshall, Uazman Alam, Andreas Themistocleous, Nigel Calcutt, Andrew Marshall
Clinical Therapeutics.2021; 43(9): 1441. CrossRef - Cardiac Autonomic Neuropathy Is Not Reversed by Euglycemia Following Islet Transplantation
Tejas Deshmukh, Peter Emerson, Patricia Anderson, Eddy Kizana, Philip J. O’Connell, D. Jane Holmes-Walker, James J.H. Chong
Transplantation.2021; 105(5): 1125. CrossRef - Attenuation of Muscle Mass and Density Is Associated With Poor Outcomes Among Patients Undergoing Major Gynecologic Surgery: A Retrospective Cohort Study
Lu Che, Yan Zhang, Jiawen Yu, Li Xu, Yuguang Huang
Anesthesia & Analgesia.2021; 132(6): 1692. CrossRef - Association of Urinary N-Acetyl-β-D-Glucosaminidase with Cardiovascular Autonomic Neuropathy in Type 1 Diabetes Mellitus without Nephropathy
Min Sun Choi, Ji Eun Jun, Sung Woon Park, Jee Hee Yoo, Jiyeon Ahn, Gyuri Kim, Sang-Man Jin, Kyu Yeon Hur, Moon-Kyu Lee, Jae Hyeon Kim
Diabetes & Metabolism Journal.2021; 45(3): 349. CrossRef - Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy
Gordon Sloan, Dinesh Selvarajah, Solomon Tesfaye
Nature Reviews Endocrinology.2021; 17(7): 400. CrossRef - Heart Rate Variability as a Potential Non-invasive Marker of Blood Glucose Level
L. R. Jarman, J. L. Elliott, T. Lees, R. Clifton-Bligh, A. M. Simpson, N. Nassif, S. Lal
Human Physiology.2021; 47(2): 209. CrossRef - Cardiovascular autonomic neuropathy in diabetes: Pathophysiology, clinical assessment and implications
Alice Duque, Mauro Felippe Felix Mediano, Andrea De Lorenzo, Luiz Fernando Rodrigues Jr
World Journal of Diabetes.2021; 12(6): 855. CrossRef - Gaussian process-based kernel as a diagnostic model for prediction of type 2 diabetes mellitus risk using non-linear heart rate variability features
R. Shashikant, Uttam Chaskar, Leena Phadke, Chetankumar Patil
Biomedical Engineering Letters.2021; 11(3): 273. CrossRef - Possible Preventative/Rehabilitative Role of Gliflozins in OSA and T2DM. A Systematic Literature Review-Based Hypothesis
Vincenzo Maria Monda, Francesca Porcellati, Felice Strollo, Alessandro Fucili, Marcello Monesi, Ersilia Satta, Sandro Gentile
Advances in Therapy.2021; 38(8): 4195. CrossRef - Characteristics of cardiovascular autonomic dysfunction and association with quality of life in patients with systemic lupus erythematosus
Amanda Hempel Zinglersen, Katrine Kjær Iversen, Henrik Christian Bidstrup Leffers, Esben Laugesen, Jesper Fleischer, Søren Jacobsen
Lupus Science & Medicine.2021; 8(1): e000507. CrossRef - Impaired vagal adaptation to an exercise task in women with gestational diabetes mellitus versus women with uncomplicated pregnancies
Marieta P. Theodorakopoulou, Areti Triantafyllou, Andreas Zafeiridis, Afroditi Κ. Boutou, Iris Grigoriadou, Evangelia Kintiraki, Stella Douma, Dimitrios G. Goulis, Konstantina Dipla
Hormones.2021; 20(4): 753. CrossRef - Cardioprotective Effects of Sodium-glucose Cotransporter 2 Inhibitors Regardless of Type 2 Diabetes Mellitus: A Meta-analysis
Lucas Silva Sousa, Felipe de Araújo Nascimento, Juliano Rocha, Michelle Rocha-Parise
International Journal of Cardiovascular Sciences.2021;[Epub] CrossRef - Management of diabetic neuropathy
Simona Cernea, Itamar Raz
Metabolism.2021; 123: 154867. CrossRef - Large fibre, small fibre and autonomic neuropathy in adolescents with type 1 diabetes: A systematic review
Vinni Faber Rasmussen, Troels Staehelin Jensen, Hatice Tankisi, Páll Karlsson, Esben Thyssen Vestergaard, Kurt Kristensen, Jens Randel Nyengaard, Astrid Juhl Terkelsen
Journal of Diabetes and its Complications.2021; 35(11): 108027. CrossRef - Cardiovascular Risk Management in Type 1 Diabetes
I. H. Teoh, P. Elisaus, J. D. Schofield
Current Diabetes Reports.2021;[Epub] CrossRef - Disturbances in the intraventricular conduction system in teenagers with type 1 diabetes. A pilot study
Agnieszka Zubkiewicz-Kucharska, Anna Noczyńska, Małgorzata Sobieszczańska, Małgorzata Poręba, Joanna Chrzanowska, Rafał Poręba, Monika Seifert, Anna Janocha, Krystyna Laszki-Szcząchor
Journal of Diabetes and its Complications.2021; 35(11): 108043. CrossRef - Peripheral and Autonomic Neuropathy Status of Young Patients With Type 1 Diabetes Mellitus at the Time of Transition From Pediatric Care to Adult-Oriented Diabetes Care
Anna Vágvölgyi, Ágnes Maróti, Mónika Szűcs, Csongor Póczik, Dóra Urbán-Pap, István Baczkó, Attila Nemes, Éva Csajbók, Krisztián Sepp, Péter Kempler, Andrea Orosz, Tamás Várkonyi, Csaba Lengyel
Frontiers in Endocrinology.2021;[Epub] CrossRef - Pancreatic exocrine insufficiency in diabetes is associated with autonomic dysfunction
Dag André Sangnes, Elisabeth Sandvik Bergmann, Rose Marie Moss, Trond Engjom, Eirik Søfteland
Scandinavian Journal of Gastroenterology.2021; 56(10): 1222. CrossRef - Dependence of Heart Rate Variability Indices on the Mean Heart Rate in Women with Well-Controlled Type 2 Diabetes
Adriana Robles-Cabrera, José M. Torres-Arellano, Ruben Fossion, Claudia Lerma
Journal of Clinical Medicine.2021; 10(19): 4386. CrossRef - Normative data on cardiovascular autonomic function in Greenlandic Inuit
Marie Mathilde Bjerg Christensen, Christian Stevns Hansen, Jesper Fleischer, Dorte Vistisen, Stine Byberg, Trine Larsen, Jens Christian Laursen, Marit Eika Jørgensen
BMJ Open Diabetes Research & Care.2021; 9(1): e002121. CrossRef - What Is in the Field for Genetics and Epigenetics of Diabetic Neuropathy: The Role of MicroRNAs
V. Spallone, C. Ciccacci, A. Latini, P. Borgiani, Karim Gariani
Journal of Diabetes Research.2021; 2021: 1. CrossRef - Evaluation and treatment of peripheral nervous system dysfunction in patients with prediabetes
O. E. Zinovyeva, T. M. Ostroumova, M. V. Koniashova, N. A. Gorbachev
Neurology, Neuropsychiatry, Psychosomatics.2021; 13(5): 116. CrossRef - Manifestazioni cliniche della neuropatia autonomica diabetica: valutazione dei sintomi
Carla Greco, Chiara Pacchioni, Manuela Simoni
L'Endocrinologo.2021; 22(6): 514. CrossRef - Perspectives of glycemic variability in diabetic neuropathy: a comprehensive review
Xiaochun Zhang, Xue Yang, Bao Sun, Chunsheng Zhu
Communications Biology.2021;[Epub] CrossRef - The Association Between Cardiovascular Autonomic Function and Changes in Kidney and Myocardial Function in Type 2 Diabetes and Healthy Controls
Jens Christian Laursen, Ida Kirstine B. Rasmussen, Emilie H. Zobel, Philip Hasbak, Bernt Johan von Scholten, Lene Holmvang, Rasmus S. Ripa, Christian S. Hansen, Marie Frimodt-Moeller, Andreas Kjaer, Peter Rossing, Tine W. Hansen
Frontiers in Endocrinology.2021;[Epub] CrossRef - Dependence of heart rate variability on indicators of type 1 diabetes mellitus control.
N. O. Pertseva, O. V. Gurzhiy, K. I. Moshenets
Medicni perspektivi (Medical perspectives).2020; 25(1): 88. CrossRef - Efficacy and safety of evogliptin treatment in patients with type 2 diabetes: A multicentre, active‐controlled, randomized, double‐blind study with open‐label extension (the EVERGREEN study)
Gyuri Kim, Soo Lim, Hyuk‐Sang Kwon, Ie B. Park, Kyu J. Ahn, Cheol‐Young Park, Su K. Kwon, Hye S. Kim, Seok W. Park, Sin G. Kim, Min K. Moon, Eun S. Kim, Choon H. Chung, Kang S. Park, Mikyung Kim, Dong J. Chung, Chang B. Lee, Tae H. Kim, Moon‐Kyu Lee
Diabetes, Obesity and Metabolism.2020; 22(9): 1527. CrossRef - Diabetes mellitus and comorbidities: A bad romance
Niki Katsiki, Dimitrios Tousoulis
Hellenic Journal of Cardiology.2020; 61(1): 23. CrossRef - Features of the glucose influence on the heart activity and the changes in the potentials of the stomach and the intestines at the heart insufficiency conditions
V. V. Soltanov, L. M. Komarovskaya
Doklady of the National Academy of Sciences of Belarus.2020; 63(6): 736. CrossRef - Living Day to Day
Gerald Kayingo, Virginia McCoy Hass
Physician Assistant Clinics.2020; 5(2): 213. CrossRef - Source specific PM2.5 associated with heart rate variability in the elderly with coronary heart disease: A community-based panel study
Xi Chen, Bing Qiu, Qinpei Zou, Tian Qiu, Runkui Li, Ashley Truong, Yanmin Qi, Tao Liu, Limin Han, Tiebing Liu, Junrui Chang, Qi Sun, Ying Zhu, Dongqun Xu
Chemosphere.2020; 260: 127399. CrossRef - The Association of Autonomic Nervous System Function With Ischemic Stroke, and Treatment Strategies
Mengxi Zhao, Ling Guan, Yilong Wang
Frontiers in Neurology.2020;[Epub] CrossRef - Cardiac diabetic autonomic neuropathy
L. T. Akhmedzhanova, T. A. Belyakova, Yu. A. Podkovko, Yu. M. Shor
Medical Council.2020; (21): 94. CrossRef - Diabetes, and its treatment, as an effector of autonomic nervous system circuits and its functions
Liliana Espinoza, Carie R Boychuk
Current Opinion in Pharmacology.2020; 54: 18. CrossRef - Morning blood pressure surge is associated with autonomic neuropathy and peripheral vascular disease in patients with diabetes
Federica Di Gennaro, Cinzia D’Amato, Roberto Morganti, Carla Greco, Susanna Longo, Diana Corradini, Davide Lauro, Vincenza Spallone
Journal of Human Hypertension.2020; 34(7): 495. CrossRef - The Compound Expression of HSP90 and INOS in the Testis of Diabetic Rats as Cellular and Pathologic Adverse Effects of Diabetes
Ali Alsarhan, Kawther Faisal Amawi, Inas Saleh Al-Mazari, Hashem Abu Hurirah, Ahed J. Alkhatib
Analytical Cellular Pathology.2020; 2020: 1. CrossRef - Heart rate variability features from nonlinear cardiac dynamics in identification of diabetes using artificial neural network and support vector machine
Yogender Aggarwal, Joyani Das, Papiya Mitra Mazumder, Rohit Kumar, Rakesh Kumar Sinha
Biocybernetics and Biomedical Engineering.2020; 40(3): 1002. CrossRef - Distal Symmetric and Cardiovascular Autonomic Neuropathies in Brazilian Individuals with Type 2 Diabetes Followed in a Primary Health Care Unit: A Cross-Sectional Study
Mozania Reis de Matos, Daniele Pereira Santos-Bezerra, Cristiane das Graças Dias Cavalcante, Jacira Xavier de Carvalho, Juliana Leite, Jose Antonio Januario Neves, Sharon Nina Admoni, Marisa Passarelli, Maria Candida Parisi, Maria Lucia Correa-Giannella
International Journal of Environmental Research and Public Health.2020; 17(9): 3232. CrossRef - Awareness of hypoglycemia and spectral analysis of heart rate variability in type 1 diabetes
Ticiana Paes, L. Clemente Rolim, Celso Sallum Filho, João Roberto de Sa, Sérgio A. Dib
Journal of Diabetes and its Complications.2020; 34(8): 107617. CrossRef - Is there cardiac autonomic neuropathy in prediabetes?
Lindsay A. Zilliox, James W. Russell
Autonomic Neuroscience.2020; 229: 102722. CrossRef - Chronic Microvascular Complications in Prediabetic States—An Overview
Angelika Baranowska-Jurkun, Wojciech Matuszewski, Elżbieta Bandurska-Stankiewicz
Journal of Clinical Medicine.2020; 9(10): 3289. CrossRef - Cardiac vagal tone as a novel screening tool to recognize asymptomatic cardiovascular autonomic neuropathy: Aspects of utility in type 1 diabetes
Anne-Marie Wegeberg, Elin D Lunde, Sam Riahi, Niels Ejskjaer, Asbjørn M Drewes, Birgitte Brock, Rodica Pop-Busui, Christina Brock
Diabetes Research and Clinical Practice.2020; 170: 108517. CrossRef - Assessment of baroreceptor reflex sensitivity in young obese Saudi males at rest and in response to physiological challenges
Abdullah N. AlShahrani, Lubna I. Al‐Asoom, Ahmed A. Alsunni, Nabil S. Elbahai, Talay Yar
Physiological Reports.2020;[Epub] CrossRef - The relationship between QT interval indices with cardiac autonomic neuropathy in diabetic patients: a case control study
Maryam Vasheghani, Farzaneh Sarvghadi, Mohammad Reza Beyranvand, Habib Emami
Diabetology & Metabolic Syndrome.2020;[Epub] CrossRef - Myocardial ischaemia reperfusion injury and cardioprotection in the presence of sensory neuropathy: Therapeutic options
Péter Bencsik, Kamilla Gömöri, Tamara Szabados, Péter Sántha, Zsuzsanna Helyes, Gábor Jancsó, Péter Ferdinandy, Anikó Görbe
British Journal of Pharmacology.2020; 177(23): 5336. CrossRef - Biological Activity of c-Peptide in Microvascular Complications of Type 1 Diabetes—Time for Translational Studies or Back to the Basics?
Aleksandra Ryk, Aleksandra Łosiewicz, Arkadiusz Michalak, Wojciech Fendler
International Journal of Molecular Sciences.2020; 21(24): 9723. CrossRef - Diabetes and cardiovascular disease: inter-relation of risk factors and treatment
Aman Sharma, Shweta Mittal, Rohan Aggarwal, Meenakshi K. Chauhan
Future Journal of Pharmaceutical Sciences.2020;[Epub] CrossRef - Diabetic Autonomic Neuropathy: A Clinical Update
Jugal Kishor Sharma, Anshu Rohatgi, Dinesh Sharma
Journal of the Royal College of Physicians of Edinburgh.2020; 50(3): 269. CrossRef - Obesity, Metabolic Syndrome and the Risk of Microvascular Complications in Patients with Diabetes mellitus
Niki Katsiki, Panagiotis Anagnostis, Kalliopi Kotsa, Dimitrios G. Goulis, Dimitri P. Mikhailidis
Current Pharmaceutical Design.2019; 25(18): 2051. CrossRef - Cardiac Autonomic Neuropathy in Obesity, the Metabolic Syndrome and Prediabetes: A Narrative Review
Scott M. Williams, Aikaterini Eleftheriadou, Uazman Alam, Daniel J. Cuthbertson, John P. H. Wilding
Diabetes Therapy.2019; 10(6): 1995. CrossRef
- Rectal sensitivity correlated with gastrointestinal‐mediated glucose disposal, but not the incretin effect
- Clinical Diabetes & Therapeutics
- Glucagon-Like Peptide-1 Receptor Agonists for the Treatment of Type 2 Diabetes Mellitus: A Position Statement of the Korean Diabetes Association
- Hyun Jin Kim, Seok O Park, Seung-Hyun Ko, Sang Youl Rhee, Kyu-Yeon Hur, Nan-Hee Kim, Min Kyong Moon, Byung-Wan Lee, Jin Hwa Kim, Kyung Mook Choi
- Diabetes Metab J. 2017;41(6):423-429. Published online December 19, 2017
- DOI: https://doi.org/10.4093/dmj.2017.41.6.423
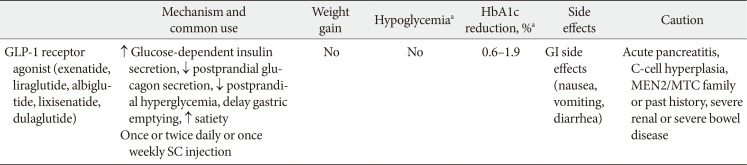
- 5,798 View
- 71 Download
- 5 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The glucagon-like peptide-1 receptor agonists (GLP-1RAs) were recommended as a monotherapy or combination therapy with oral hypoglycemic agents or basal insulin in the position statement of the Korean Diabetes Association 2017 for pharmacological therapy. Many randomized clinical trials and systematic reviews report that GLP-1RAs have considerable glucose-lowering effect and lead to weight reduction and low risk of hypoglycemia when used as a monotherapy or combination therapy. The cardiovascular safety of GLP-1RAs has been assessed in several randomized clinical trials and systematic reviews. The results of cardiovascular outcome trials of long-acting GLP-1RAs (liraglutide, semaglutide) demonstrated cardiovascular benefits in subjects with type 2 diabetes mellitus and a high risk of cardiovascular disease. The GLP-1RA may be a choice of therapy when weight control and avoidance of hypoglycemia are important, and patients with high risk of cardiovascular disease might also favor choosing GLP-1RA.
-
Citations
Citations to this article as recorded by- Anti-inflammatory effect of glucagon-like Peptide-1 receptor agonist on the neurosensory retina in an acute optic nerve injury rat model
Yeon Woong Chung, Ji Young Lee, Hyun Hee Ju, Jin A. Choi
European Journal of Pharmacology.2022; 933: 175269. CrossRef - Diabetes Risk Data Mining Method Based on Electronic Medical Record Analysis
Yang Liu, Zhaoxiang Yu, Yunlong Yang, Zhihan Lv
Journal of Healthcare Engineering.2021; 2021: 1. CrossRef - Paradigm Shift for the Treatment of Type 2 Diabetes Mellitus in Patients with Cardiovascular Disease: Cardiologist's Perspective
Doo Soo Jeon
Cardiovascular Prevention and Pharmacotherapy.2020; 2(1): 11. CrossRef - The Role of Glucagon-Like Peptide-1 Receptor Agonists in Type 2 Diabetes in Asia
Ju-Ming Lu
Advances in Therapy.2019; 36(4): 798. CrossRef - A Review of Practical Issues on the Use of Glucagon-Like Peptide-1 Receptor Agonists for the Management of Type 2 Diabetes
Irene Romera, Ana Cebrián-Cuenca, Fernando Álvarez-Guisasola, Fernando Gomez-Peralta, Jesús Reviriego
Diabetes Therapy.2019; 10(1): 5. CrossRef - Glucagon-Like Peptide-1 Receptor Agonists for the Treatment of Type 2 Diabetes Mellitus: A Position Statement of the Korean Diabetes Association
Hyun Jin Kim
The Journal of Korean Diabetes.2018; 19(1): 35. CrossRef
- Anti-inflammatory effect of glucagon-like Peptide-1 receptor agonist on the neurosensory retina in an acute optic nerve injury rat model
- Islet Studies and Transplantation
- An Update on the Effect of Incretin-Based Therapies on β-Cell Function and Mass
- Suk Chon, Jean-François Gautier
- Diabetes Metab J. 2016;40(2):99-114. Published online April 25, 2016
- DOI: https://doi.org/10.4093/dmj.2016.40.2.99
- 4,939 View
- 110 Download
- 43 Web of Science
- 41 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Type 2 diabetes mellitus (T2DM) is a multifactorial disease with a complex and progressive pathogenesis. The two primary mechanisms of T2DM pathogenesis are pancreatic β-cell dysfunction and insulin resistance. Pancreatic β-cell dysfunction is recognized to be a prerequisite for the development of T2DM. Therapeutic modalities that improve β-cell function are considered critical to T2DM management; however, blood glucose control remains a challenge for many patients due to suboptimal treatment efficacy and the progressive nature of T2DM. Incretin-based therapies are now the most frequently prescribed antidiabetic drugs in Korea. Incretin-based therapies are a favorable class of drugs due to their ability to reduce blood glucose by targeting the incretin hormone system and, most notably, their potential to improve pancreatic β-cell function. This review outlines the current understanding of the incretin hormone system in T2DM and summarizes recent updates on the effect of incretin-based therapies on β-cell function and β-cell mass in animals and humans.
-
Citations
Citations to this article as recorded by- Harnessing gut cells for functional insulin production: Strategies and challenges
Kelvin Baafi, John C. March
Biotechnology Notes.2023; 4: 7. CrossRef - Incretin and Pancreatic β-Cell Function in Patients with Type 2 Diabetes
Chang Ho Ahn, Tae Jung Oh, Se Hee Min, Young Min Cho
Endocrinology and Metabolism.2023; 38(1): 1. CrossRef - Weight loss maintenance with exercise and liraglutide improves glucose tolerance, glucagon response, and beta cell function
Simon B. K. Jensen, Christian R. Juhl, Charlotte Janus, Julie R. Lundgren, Christoffer Martinussen, Christoffer Wiingaard, Cecilie Knudsen, Ruth Frikke‐Schmidt, Bente M. Stallknecht, Jens J. Holst, Sten Madsbad, Signe S. Torekov
Obesity.2023; 31(4): 977. CrossRef - How do parasitic worms prevent diabetes? An exploration of their influence on macrophage and β-cell crosstalk
Inah Camaya, Bronwyn O’Brien, Sheila Donnelly
Frontiers in Endocrinology.2023;[Epub] CrossRef - An Imbalance of Pathophysiologic Factors in Late Postprandial Hypoglycemia Post Bariatric Surgery: A Narrative Review
Marah Alsayed Hasan, Stanley Schwartz, Victoria McKenna, Richard Ing
Obesity Surgery.2023; 33(9): 2927. CrossRef - Therapeutic Dilemma in Personalized Medicine
Ehab S. EL Desoky
Current Reviews in Clinical and Experimental Pharmacology.2022; 17(2): 94. CrossRef - Lessons from neonatal β-cell epigenomic for diabetes prevention and treatment
Amar Abderrahmani, Cécile Jacovetti, Romano Regazzi
Trends in Endocrinology & Metabolism.2022; 33(6): 378. CrossRef - Beneficial metabolic effects of recurrent periods of beta‐cell rest and stimulation using stable neuropeptide Y1 and glucagon‐like peptide‐1 receptor agonists
Neil Tanday, Ryan A. Lafferty, Peter R. Flatt, Nigel Irwin
Diabetes, Obesity and Metabolism.2022; 24(12): 2353. CrossRef - A Randomized Controlled Trial of R-Form Verapamil Added to Ongoing Metformin Therapy in Patients with Type 2 Diabetes
Chih-Yuan Wang, Kuo-Chin Huang, Chia-Wen Lu, Chih-Hsun Chu, Chien-Ning Huang, Harn-Shen Chen, I-Te Lee, Jung-Fu Chen, Ching-Chu Chen, Chung-Sen Chen, Chang-Hsun Hsieh, Kai-Jen Tien, Hung-Yu Chien, Yu-Yao Huang, Jui-Pao Hsu, Guang-Tzuu Shane, Ai-Ching Chan
The Journal of Clinical Endocrinology & Metabolism.2022; 107(10): e4063. CrossRef - Exenatide, Metformin, or Both for Prediabetes in PCOS: A Randomized, Open-label, Parallel-group Controlled Study
Tao Tao, Yi Zhang, Yu-Chen Zhu, Jia-Rong Fu, Yu-Ying Wang, Jie Cai, Jing-Yu Ma, Yu Xu, Yi-Ning Gao, Yun Sun, WuQiang Fan, Wei Liu
The Journal of Clinical Endocrinology & Metabolism.2021; 106(3): e1420. CrossRef - The utility of assessing C-peptide in patients with insulin-treated type 2 diabetes: a cross-sectional study
Tuccinardi Dario, Giorgino Riccardo, Pieralice Silvia, Watanabe Mikiko, Maggi Daria, Palermo Andrea, Defeudis Giuseppe, Fioriti Elvira, Pozzilli Paolo, Manfrini Silvia
Acta Diabetologica.2021; 58(4): 411. CrossRef - Type 2 diabetes: evidence-based medicine approach to glucose-lowering therapy
E. V. Biryukova, I. A. Morozova, S. V. Rodionova
Meditsinskiy sovet = Medical Council.2021; (21): 160. CrossRef - Emerging Role of Caveolin-1 in GLP-1 Action
Alessandra Puddu, Davide Maggi
Frontiers in Endocrinology.2021;[Epub] CrossRef - Improvements in HOMA indices and pancreatic endocrinal tissues in type 2-diabetic rats by DPP-4 inhibition and antioxidant potential of an ethanol fruit extract of Withania coagulans
Heera Ram, Pramod Kumar, Ashok Purohit, Priya Kashyap, Suresh Kumar, Shivani Kumar, Garima Singh, Abdulaziz A. Alqarawi, Abeer Hashem, Elsayed Fathi Abd-Allah, Al-Bandari Fahad Al-Arjani, Bhim Pratap Singh
Nutrition & Metabolism.2021;[Epub] CrossRef -
Chop
/
Ddit3
depletion in β cells alleviates ER stress and corrects hepatic steatosis in mice
Jing Yong, Vishal S. Parekh, Shannon M. Reilly, Jonamani Nayak, Zhouji Chen, Cynthia Lebeaupin, Insook Jang, Jiangwei Zhang, Thazha P. Prakash, Hong Sun, Sue Murray, Shuling Guo, Julio E. Ayala, Leslie S. Satin, Alan R. Saltiel, Randal J. Kaufman
Science Translational Medicine.2021;[Epub] CrossRef - Assessment of Insulin Secretion and Insulin Resistance in Human
So Young Park, Jean-François Gautier, Suk Chon
Diabetes & Metabolism Journal.2021; 45(5): 641. CrossRef - Targeted pharmacological therapy restores β-cell function for diabetes remission
Stephan Sachs, Aimée Bastidas-Ponce, Sophie Tritschler, Mostafa Bakhti, Anika Böttcher, Miguel A. Sánchez-Garrido, Marta Tarquis-Medina, Maximilian Kleinert, Katrin Fischer, Sigrid Jall, Alexandra Harger, Erik Bader, Sara Roscioni, Siegfried Ussar, Annett
Nature Metabolism.2020; 2(2): 192. CrossRef - The Relationship Between Timing of Initiation on a Glucagon-like Peptide-1 Receptor Agonist and Glycosylated Hemoglobin Values Among Patients With Type 2 Diabetes
Kristina S. Boye, Reema Mody, Maureen J. Lage, Raleigh E. Malik
Clinical Therapeutics.2020; 42(9): 1812. CrossRef - Short-term renal and metabolic effects of low dose vildagliptin treatment added-on insulin therapy in non-proteinuric patients with type 2 diabetes: open-label randomized prospective study
Valentina K. Bayrasheva, Ivan Y. Pchelin, Vladimir A. Dobronravov, Alina Yu. Babenko, Svetlana G. Chefu, Ivan S. Shatalov, Volha N. Vasilkova, Natalia V. Hudiakova, Alexandra N. Ivanova, Pavel A. Andoskin, Elena N. Grineva
Archives of Endocrinology and Metabolism.2020;[Epub] CrossRef - A Phenotypic Screen Identifies Calcium Overload as a Key Mechanism of β-Cell Glucolipotoxicity
Jennifer Vogel, Jianning Yin, Liansheng Su, Sharon X. Wang, Richard Zessis, Sena Fowler, Chun-Hao Chiu, Aaron C. Wilson, Amy Chen, Frederic Zecri, Gordon Turner, Thomas M. Smith, Brian DeChristopher, Heming Xing, Deborah M. Rothman, Xinming Cai, Alina Ber
Diabetes.2020; 69(5): 1032. CrossRef - Neuropeptide 26RFa (QRFP) is a key regulator of glucose homeostasis and its activity is markedly altered in obese/hyperglycemic mice
Gaëtan Prévost, Arnaud Arabo, Marie-Anne Le Solliec, Justine Bons, Marie Picot, Julie Maucotel, Hind Berrahmoune, Mouna El Mehdi, Saloua Cherifi, Alexandre Benani, Emmanuelle Nédélec, Moïse Coëffier, Jérôme Leprince, Anneli Nordqvist, Valéry Brunel, Pierr
American Journal of Physiology-Endocrinology and Metabolism.2019; 317(1): E147. CrossRef - Gastrin analogue administration adds no significant glycaemic benefit to a glucagon‐like peptide‐1 receptor agonist acutely or after washout of both analogues
Krister Bokvist, Ying Ding, William H. Landschulz, Vikram Sinha, Aleksandra Pastrak, Ruth M. Belin
Diabetes, Obesity and Metabolism.2019; 21(7): 1606. CrossRef - Effects of boschnaloside from Boschniakia rossica on dysglycemia and islet dysfunction in severely diabetic mice through modulating the action of glucagon-like peptide-1
Lie-Chwen Lin, Lin-Chien Lee, Cheng Huang, Chiung-Tong Chen, Jen-Shin Song, Young-Ji Shiao, Hui-Kang Liu
Phytomedicine.2019; 62: 152946. CrossRef - The future of new drugs for diabetes management
Clifford J. Bailey, Caroline Day
Diabetes Research and Clinical Practice.2019; 155: 107785. CrossRef - Compact fluidic system for functional assessment of pancreatic islets
Takeshi Hori, Kei Yamane, Takayuki Anazawa, Osamu Kurosawa, Hiroo Iwata
Biomedical Microdevices.2019;[Epub] CrossRef - Pharmacokinetics of Exenatide in nonhuman primates following its administration in the form of sustained-release PT320 and Bydureon
Yazhou Li, Kelli L. Vaughan, David Tweedie, Jin Jung, Hee Kyung Kim, Ho-Il Choi, Dong Seok Kim, Julie A. Mattison, Nigel H. Greig
Scientific Reports.2019;[Epub] CrossRef - Pharmacokinetics and efficacy of PT302, a sustained-release Exenatide formulation, in a murine model of mild traumatic brain injury
Miaad Bader, Yazhou Li, Daniela Lecca, Vardit Rubovitch, David Tweedie, Elliot Glotfelty, Lital Rachmany, Hee Kyung Kim, Ho-Il Choi, Barry J. Hoffer, Chaim G. Pick, Nigel H. Greig, Dong Seok Kim
Neurobiology of Disease.2019; 124: 439. CrossRef - Novel dual incretin agonist peptide with antidiabetic and neuroprotective potential
N.M. Pathak, V. Pathak, V.A. Gault, S. McClean, N. Irwin, P.R. Flatt
Biochemical Pharmacology.2018; 155: 264. CrossRef - Human EndoC-βH1 β-cells form pseudoislets with improved glucose sensitivity and enhanced GLP-1 signaling in the presence of islet-derived endothelial cells
Michael G. Spelios, Lauren A. Afinowicz, Regine C. Tipon, Eitan M. Akirav
American Journal of Physiology-Endocrinology and Metabolism.2018; 314(5): E512. CrossRef - Vildagliptin: ten years in the service for type 2 diabetes mellitus patients. The journey of discovery, innovation and success in clinical practice
Tatiana Yu. Demidova
Problems of Endocrinology.2018; 64(5): 336. CrossRef - Thromboxane-Dependent Platelet Activation in Obese Subjects with Prediabetes or Early Type 2 Diabetes: Effects of Liraglutide- or Lifestyle Changes-Induced Weight Loss
Paola Simeone, Rossella Liani, Romina Tripaldi, Augusto Di Castelnuovo, Maria Guagnano, Armando Tartaro, Riccardo Bonadonna, Virginia Federico, Francesco Cipollone, Agostino Consoli, Francesca Santilli
Nutrients.2018; 10(12): 1872. CrossRef - Nutrient regulation of β-cell function: what do islet cell/animal studies tell us?
R Carlessi, K N Keane, C Mamotte, P Newsholme
European Journal of Clinical Nutrition.2017; 71(7): 890. CrossRef - Effectiveness and safety of exenatide in Korean patients with type 2 diabetes inadequately controlled with oral hypoglycemic agents: an observational study in a real clinical practice
You-Cheol Hwang, Ari Kim, Euna Jo, Yeoree Yang, Jae-Hyoung Cho, Byung-Wan Lee
BMC Endocrine Disorders.2017;[Epub] CrossRef - Efficacy and safety of adding evogliptin versus sitagliptin for metformin‐treated patients with type 2 diabetes: A 24‐week randomized, controlled trial with open label extension
Sang‐Mo Hong, Cheol‐Young Park, Dong‐Min Hwang, Kyung Ah Han, Chang Beom Lee, Choon Hee Chung, Kun‐Ho Yoon, Ji‐Oh Mok, Kyong Soo Park, Sung‐Woo Park
Diabetes, Obesity and Metabolism.2017; 19(5): 654. CrossRef - The effects of vildagliptin compared with metformin on vascular endothelial function and metabolic parameters: a randomized, controlled trial (Sapporo Athero-Incretin Study 3)
Naoyuki Kitao, Hideaki Miyoshi, Tomoo Furumoto, Kota Ono, Hiroshi Nomoto, Aika Miya, Chiho Yamamoto, Atsushi Inoue, Kenichi Tsuchida, Naoki Manda, Yoshio Kurihara, Shin Aoki, Akinobu Nakamura, Tatsuya Atsumi
Cardiovascular Diabetology.2017;[Epub] CrossRef - Recent Advances in Effect‐directed Enzyme Assays based on Thin‐layer Chromatography
Sarah Bräm, Evelyn Wolfram
Phytochemical Analysis.2017; 28(2): 74. CrossRef - Efficacy and safety of gemigliptin, a dipeptidyl peptidase‐4 inhibitor, in patients with type 2 diabetes mellitus inadequately controlled with combination treatment of metformin and sulphonylurea: a 24‐week, multicentre, randomized, double‐blind, placebo‐
Chang Ho Ahn, Kyung Ah Han, Jae Myung Yu, Joo Young Nam, Kyu Jeung Ahn, Tae Keun Oh, Hyoung Woo Lee, Dae Ho Lee, Jaetaek Kim, Choon Hee Chung, Tae Sun Park, Byung Joon Kim, Seok Won Park, Hyeong Kyu Park, Kwang Jae Lee, Sang‐Wook Kim, Jeong Hyun Park, Kwa
Diabetes, Obesity and Metabolism.2017; 19(5): 635. CrossRef - Antihyperglycemic agent therapy for adult patients with type 2 diabetes mellitus 2017: a position statement of the Korean Diabetes Association
Seung-Hyun Ko, Kyu-Yeon Hur, Sang Youl Rhee, Nan-Hee Kim, Min Kyong Moon, Seok-O Park, Byung-Wan Lee, Hyun Jin Kim, Kyung Mook Choi, Jin Hwa Kim
The Korean Journal of Internal Medicine.2017; 32(6): 947. CrossRef - Antihyperglycemic Agent Therapy for Adult Patients with Type 2 Diabetes Mellitus 2017: A Position Statement of the Korean Diabetes Association
Seung-Hyun Ko, Kyu-Yeon Hur, Sang Youl Rhee, Nan-Hee Kim, Min Kyong Moon, Seok-O Park, Byung-Wan Lee, Hyun Jin Kim, Kyung Mook Choi, Jin Hwa Kim
Diabetes & Metabolism Journal.2017; 41(5): 337. CrossRef - DPP-4 inhibitors in diabetic complications: role of DPP-4 beyond glucose control
Eun Ju Bae
Archives of Pharmacal Research.2016; 39(8): 1114. CrossRef - Liraglutide Enhances the Efficacy of Human Mesenchymal Stem Cells in Preserving Islet ß-cell Function in Severe Non-obese Diabetic Mice
Li-rong Li, Jing Lu, Xiao-lei Jia, Hui Hui, Jie Zhang, Ying Liu, Wei-juan Cui, Qian-yue Xu, Da-long Zhu
Molecular Medicine.2016; 22(1): 800. CrossRef
- Harnessing gut cells for functional insulin production: Strategies and challenges
- Effects of 6-Month Sitagliptin Treatment on Insulin and Glucagon Responses in Korean Patients with Type 2 Diabetes Mellitus
- Hae Kyung Yang, Borami Kang, Seung-Hwan Lee, Hun-Sung Kim, Kun-Ho Yoon, Bong-Yun Cha, Jae-Hyoung Cho
- Diabetes Metab J. 2015;39(4):335-341. Published online July 17, 2015
- DOI: https://doi.org/10.4093/dmj.2015.39.4.335
- 3,680 View
- 35 Download
- 4 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background This study aimed to evaluate the effect of sitagliptin, an oral dipeptidyl peptidase-4 inhibitor, on insulin secretion and glucagon suppression in Korean subjects with type 2 diabetes mellitus.
Methods Twenty-four subjects underwent a 75-g oral glucose tolerance test (OGTT) before and after 6 months of sitagliptin treatment. Sitagliptin, insulin, and sulfonylurea were withdrawn for 3 days before OGTT to eliminate any acute effects on β-cell insulin or α-cell glucagon secretion. Venous samples were drawn five times during each OGTT to measure plasma glucose, insulin, and glucagon. Indices on insulin secretion and resistance were calculated.
Results Early phase insulin secretion, measured by the insulinogenic index significantly increased after 6 months of sitagliptin treatment, especially in the higher baseline body mass index group and higher baseline glycosylated hemoglobin (HbA1c) group. There were no significant differences in the insulin resistance indices before and after sitagliptin treatment. Although no significant differences were observed in the absolute levels of glucagon and the glucagon-to-insulin ratio, there was a significant reduction in the percentile change of glucagon-to-insulin ratio at 30- and 120-minute during the OGTT.
Conclusion Although the HbA1c level did not decrease significantly after 6 months of sitagliptin treatment, an increase in insulin secretion and reduction in early phase postprandial plasma glucagon-to-insulin ratio excursion was confirmed in Korean subjects with type 2 diabetes.
-
Citations
Citations to this article as recorded by- A genetic variant in GLP1R is associated with response to DPP-4 inhibitors in patients with type 2 diabetes
Eugene Han, Hye Sun Park, Obin Kwon, Eun Yeong Choe, Hye Jin Wang, Yong-ho Lee, Sang-Hak Lee, Chul Hoon Kim, Lee-Kyung Kim, Soo Heon Kwak, Kyong Soo Park, Chul Sik Kim, Eun Seok Kang
Medicine.2016; 95(44): e5155. CrossRef - Effects of sitagliptin on circulating zinc-α2-glycoprotein levels in newly diagnosed type 2 diabetes patients: a randomized trial
Mingyuan Tian, Zerong Liang, Rui Liu, Ke Li, Xinrong Tan, Yong Luo, Mengliu Yang, Harvest F Gu, Hua Liu, Ling Li, Gangyi Yang
European Journal of Endocrinology.2016; 174(2): 147. CrossRef - Effects of Sitagliptin on Insulin and Glucagon Levels in Type 2 Diabetes Mellitus
Ji Hyun Kim
Diabetes & Metabolism Journal.2015; 39(4): 304. CrossRef
- A genetic variant in GLP1R is associated with response to DPP-4 inhibitors in patients with type 2 diabetes

 KDA
KDA
 First
First Prev
Prev





