
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 43(3); 2019 > Article
-
Sulwon Lecture 2018Pathophysiology Mitochondrial Dysfunction in Adipocytes as a Primary Cause of Adipose Tissue Inflammation
-
Chang-Yun Woo1
 , Jung Eun Jang2, Seung Eun Lee3, Eun Hee Koh1, Ki-Up Lee1
, Jung Eun Jang2, Seung Eun Lee3, Eun Hee Koh1, Ki-Up Lee1
-
Diabetes & Metabolism Journal 2019;43(3):247-256.
DOI: https://doi.org/10.4093/dmj.2018.0221
Published online: March 27, 2019
1Department of Internal Medicine, University of Ulsan College of Medicine, Seoul, Korea.
2Department of Internal Medicine, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea.
3Department of Internal Medicine, Dongguk University Ilsan Hospital, Dongguk University College of Medicine, Goyang, Korea.
- Corresponding author: Ki-Up Lee. Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea. kulee@amc.seoul.kr
• Received: October 31, 2018 • Accepted: January 19, 2019
Copyright © 2019 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- ABSTRACT
- INTRODUCTION
- ADIPONECTIN SYNTHESIS AND MITOCHONDRIAL FUNCTION IN ADIPOCYTES
- STUDIES SUPPORTING THE ROLE OF ADIPOCYTE MITOCHONDRIAL DYSFUNCTION IN INSULIN RESISTANCE
- ADIPOCYTE MITOCHONDRIAL DYSFUNCTION IN AGING
- HYPOTHESIS: MITOCHONDRIAL DYSFUNCTION MAY BE A PRIMARY CAUSE OF ADIPOSE TISSUE INFLAMMATION
- PROINFLAMMATORY RESPONSE OF MACROPHAGES MAY EXACERBATE MITOCHONDRIAL DYSFUNCTION IN ADIPOCYTES
- MITOCHONDRIAL QUALITY CONTROL AND MITOPHAGY
- CLINICAL IMPLICATION
- COMPARISON OF OUR HYPOTHESIS WITH HYPOXIA THEORY
- CONCLUSIONS
- ACKNOWLEDGMENTS
- NOTES
- REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Prolonged Endurance Exercise Increases Macrophage Content and Mitochondrial Respiration in Adipose Tissue in Trained Men
Ronni Eg Sahl, Ioanna Patsi, Mikkel Thunestvedt Hansen, Tue Rømer, Jacob Frandsen, Hanne Kruuse Rasmusen, Arthur Ingersen, Steen Seier Poulsen, Flemming Dela, Steen Larsen, Jørn Wulff Helge
The Journal of Clinical Endocrinology & Metabolism.2024; 109(2): e799. CrossRef - Diabetes Mellitus, Energy Metabolism, and COVID-19
Caterina Conte, Elisa Cipponeri, Michael Roden
Endocrine Reviews.2024; 45(2): 281. CrossRef - The Role of Ion-Transporting Proteins in Human Disease
Yoshinori Marunaka
International Journal of Molecular Sciences.2024; 25(3): 1726. CrossRef - The Role of Obesity in Type 2 Diabetes Mellitus—An Overview
Preethi Chandrasekaran, Ralf Weiskirchen
International Journal of Molecular Sciences.2024; 25(3): 1882. CrossRef - The Metabolic Syndrome, a Human Disease
Marià Alemany
International Journal of Molecular Sciences.2024; 25(4): 2251. CrossRef - Inflammation‐mediated metabolic regulation in adipose tissue
Shujie Xu, Feng Lu, Jianhua Gao, Yi Yuan
Obesity Reviews.2024;[Epub] CrossRef - Sleeve Gastrectomy Reduces Oxidative Stress and Reverses Mitochondrial Dysfunction Associated with Metabolic Syndrome
Micaela M. Rossi, Franco J. Signorini, Tomas A. Castillo, María P. Scribano Parada, Federico Moser, Maria dC Baez
Obesity Surgery.2024;[Epub] CrossRef - The relationships between high-fat diet and metabolic syndrome: Potential mechanisms
Chao Tang, Yuxin Wang, Zeyu Xu, Dan Chen, Jingguo Xu, Duo Yang, Li Zhang, Jun Liu, Juan Kan
Food Bioscience.2024; 59: 104261. CrossRef - Could very low-calorie ketogenic diets turn off low grade inflammation in obesity? Emerging evidence
Luigi Barrea, Massimiliano Caprio, Mikiko Watanabe, Giuseppe Cammarata, Alessandra Feraco, Giovanna Muscogiuri, Ludovica Verde, Annamaria Colao, Silvia Savastano
Critical Reviews in Food Science and Nutrition.2023; 63(26): 8320. CrossRef - The emergent role of mitochondrial RNA modifications in metabolic alterations
Hatim Boughanem, Yvonne Böttcher, João Tomé‐Carneiro, María‐Carmen López de las Hazas, Alberto Dávalos, Akin Cayir, Manuel Macias‐González
WIREs RNA.2023;[Epub] CrossRef - Age‐associated adipose tissue inflammation promotes monocyte chemotaxis and enhances atherosclerosis
Jianrui Song, Diana Farris, Paola Ariza, Smriti Moorjani, Mita Varghese, Muriel Blin, Judy Chen, Daniel Tyrrell, Min Zhang, Kanakadurga Singer, Morgan Salmon, Daniel R. Goldstein
Aging Cell.2023;[Epub] CrossRef - Obesity, diabetes mellitus, and cardiometabolic risk: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2023
Harold Edward Bays, Shagun Bindlish, Tiffany Lowe Clayton
Obesity Pillars.2023; 5: 100056. CrossRef - A role of STING signaling in obesity-induced lung inflammation
Yong Qi, Zhuhua Wu, Dan Chen, Li Zhu, Yunlei Yang
International Journal of Obesity.2023; 47(4): 325. CrossRef - Estrogens in Adipose Tissue Physiology and Obesity-Related Dysfunction
Alina Kuryłowicz
Biomedicines.2023; 11(3): 690. CrossRef - White Adipose Tissue Dysfunction: Pathophysiology and Emergent Measurements
Natalia Santillana, Camila Astudillo-Guerrero, Amanda D’Espessailles, Gonzalo Cruz
Nutrients.2023; 15(7): 1722. CrossRef - Pleiotropic and multi-systemic actions of physical exercise on PGC-1α signaling during the aging process
Ivo Vieira de Sousa Neto, Ana Paula Pinto, Vitor Rosetto Muñoz, Rita de Cássia Marqueti, José Rodrigo Pauli, Eduardo Rochete Ropelle, Adelino Sanchez Ramos da Silva
Ageing Research Reviews.2023; 87: 101935. CrossRef - The impact of metabolic endotoxaemia on the browning process in human adipocytes
Farah Omran, Alice M. Murphy, Awais Z. Younis, Ioannis Kyrou, Jana Vrbikova, Vojtech Hainer, Petra Sramkova, Martin Fried, Graham Ball, Gyanendra Tripathi, Sudhesh Kumar, Philip G. McTernan, Mark Christian
BMC Medicine.2023;[Epub] CrossRef - Molecular Mechanisms of Obesity-Induced Development of Insulin Resistance and Promotion of Amyloid-β Accumulation: Dietary Therapy Using Weak Organic Acids via Improvement of Lowered Interstitial Fluid pH
Yoshinori Marunaka
Biomolecules.2023; 13(5): 779. CrossRef - From Obesity-Induced Low-Grade Inflammation to Lipotoxicity and Mitochondrial Dysfunction: Altered Multi-Crosstalk between Adipose Tissue and Metabolically Active Organs
Gina Cavaliere, Fabiano Cimmino, Giovanna Trinchese, Angela Catapano, Lidia Petrella, Margherita D’Angelo, Lucio Lucchin, Maria Pina Mollica
Antioxidants.2023; 12(6): 1172. CrossRef - Receptor for the Advanced Glycation End Products (RAGE) Pathway in Adipose Tissue Metabolism
Klaudia Gutowska, Krzysztof Czajkowski, Alina Kuryłowicz
International Journal of Molecular Sciences.2023; 24(13): 10982. CrossRef - Aerobic and Resistance Training Attenuate Differently Knee Joint Damage Caused by a High-Fat–High-Sucrose Diet in a Rat Model
Nada Abughazaleh, Kevin Boldt, Jaqueline Lourdes Rios, Stela Marcia Mattiello, Kelsey H. Collins, Ruth-Anne Seerattan, Walter Herzog
CARTILAGE.2023;[Epub] CrossRef - Exercise mitigates age-related metabolic diseases by improving mitochondrial dysfunction
Dandan Jia, Zhenjun Tian, Ru Wang
Ageing Research Reviews.2023; 91: 102087. CrossRef - Mitochondrial dynamics and metabolism across skin cells: implications for skin homeostasis and aging
Ines Martic, Federica Papaccio, Barbara Bellei, Maria Cavinato
Frontiers in Physiology.2023;[Epub] CrossRef - Influence of Breastfeeding on the State of Meta-Inflammation in Obesity—A Narrative Review
Dominika Mazur, Małgorzata Satora, Anna K. Rekowska, Zuzanna Kabała, Aleksandra Łomża, Żaneta Kimber-Trojnar, Bożena Leszczyńska-Gorzelak
Current Issues in Molecular Biology.2023; 45(11): 9003. CrossRef - AGER-1 Long Non-Coding RNA Levels Correlate with the Expression of the Advanced Glycosylation End-Product Receptor, a Regulator of the Inflammatory Response in Visceral Adipose Tissue of Women with Obesity and Type 2 Diabetes Mellitus
Klaudia Gutowska, Krzysztof Koźniewski, Michał Wąsowski, Marta Izabela Jonas, Zbigniew Bartoszewicz, Wojciech Lisik, Maurycy Jonas, Artur Binda, Paweł Jaworski, Wiesław Tarnowski, Bartłomiej Noszczyk, Monika Puzianowska-Kuźnicka, Krzysztof Czajkowski, Ali
International Journal of Molecular Sciences.2023; 24(24): 17447. CrossRef - Pharmacological treatment with FGF21 strongly improves plasma cholesterol metabolism to reduce atherosclerosis
Cong Liu, Milena Schönke, Enchen Zhou, Zhuang Li, Sander Kooijman, Mariëtte R Boon, Mikael Larsson, Kristina Wallenius, Niek Dekker, Louise Barlind, Xiao-Rong Peng, Yanan Wang, Patrick C N Rensen
Cardiovascular Research.2022; 118(2): 489. CrossRef - Obesity-Related Adipose Tissue Remodeling in the Light of Extracellular Mitochondria Transfer
Simon Lecoutre, Karine Clément, Isabelle Dugail
International Journal of Molecular Sciences.2022; 23(2): 632. CrossRef - IL-4 polarized human macrophage exosomes control cardiometabolic inflammation and diabetes in obesity
Tuan Anh Phu, Martin Ng, Ngan K. Vu, Laura Bouchareychas, Robert L. Raffai
Molecular Therapy.2022; 30(6): 2274. CrossRef - Insulin-inducible THRSP maintains mitochondrial function and regulates sphingolipid metabolism in human adipocytes
Maria A. Ahonen, Marcus Höring, Van Dien Nguyen, Sami Qadri, Juuso H. Taskinen, Meghana Nagaraj, Martin Wabitsch, Pamela Fischer-Posovszky, You Zhou, Gerhard Liebisch, P. A. Nidhina Haridas, Hannele Yki-Järvinen, Vesa M. Olkkonen
Molecular Medicine.2022;[Epub] CrossRef - Modulation of adipose inflammation by cellular retinoic acid-binding protein 1
Chin-Wen Wei, Jennifer Nhieu, Yu-Lung Lin, Li-Na Wei
International Journal of Obesity.2022; 46(10): 1759. CrossRef - The Role of Adipokines in Pancreatic Cancer
Qi Wang, Huizhi Wang, Yuntao Ding, Mengtian Wan, Min Xu
Frontiers in Oncology.2022;[Epub] CrossRef - Epigenetic Reprogramming of the Inflammatory Response in Obesity and Type 2 Diabetes
Federica Zatterale, Gregory Alexander Raciti, Immacolata Prevenzano, Alessia Leone, Michele Campitelli, Veronica De Rosa, Francesco Beguinot, Luca Parrillo
Biomolecules.2022; 12(7): 982. CrossRef - Cellular Metabolism and Bioenergetic Function in Human Fibroblasts and Preadipocytes of Type 2 Familial Partial Lipodystrophy
Cristina Algieri, Chiara Bernardini, Fabiana Trombetti, Elisa Schena, Augusta Zannoni, Monica Forni, Salvatore Nesci
International Journal of Molecular Sciences.2022; 23(15): 8659. CrossRef - Shared pathobiology identifies AMPK as a therapeutic target for obesity and autosomal dominant polycystic kidney disease
Ioan-Andrei Iliuta, Xuewen Song, Lauren Pickel, Amirreza Haghighi, Ravi Retnakaran, James Scholey, Hoon-Ki Sung, Gregory R. Steinberg, York Pei
Frontiers in Molecular Biosciences.2022;[Epub] CrossRef - Hypoxia as a Double-Edged Sword to Combat Obesity and Comorbidities
Ruwen Wang, Qin Sun, Xianmin Wu, Yiyin Zhang, Xiaorui Xing, Kaiqing Lin, Yue Feng, Mingqi Wang, Yibing Wang, Ru Wang
Cells.2022; 11(23): 3735. CrossRef - Macrophage and Adipocyte Mitochondrial Dysfunction in Obesity-Induced Metabolic Diseases
Liwen Wang, Jie Hu, Haiyan Zhou
The World Journal of Men's Health.2021; 39(4): 606. CrossRef - ESRRA (estrogen related receptor alpha) is a critical regulator of intestinal homeostasis through activation of autophagic flux via gut microbiota
Sup Kim, June-Young Lee, Seul Gi Shin, Jin Kyung Kim, Prashanta Silwal, Young Jae Kim, Na-Ri Shin, Pil Soo Kim, Minho Won, Sang-Hee Lee, Soo Yeon Kim, Miwa Sasai, Masahiro Yamamoto, Jin-Man Kim, Jin-Woo Bae, Eun-Kyeong Jo
Autophagy.2021; 17(10): 2856. CrossRef - GDF15 as a central mediator for integrated stress response and a promising therapeutic molecule for metabolic disorders and NASH
Kook Hwan Kim, Myung-Shik Lee
Biochimica et Biophysica Acta (BBA) - General Subjects.2021; 1865(3): 129834. CrossRef - The Influence of Obesity and Associated Fatty Acids on Placental Inflammation
Alison J. Eastman, Rebecca E. Moore, Steven D. Townsend, Jennifer A. Gaddy, David M. Aronoff
Clinical Therapeutics.2021; 43(2): 265. CrossRef - Targeting the G protein-coupled estrogen receptor (GPER) in obesity and diabetes
Geetanjali Sharma, Eric R. Prossnitz
Endocrine and Metabolic Science.2021; 2: 100080. CrossRef - Changes in Body Composition Are Associated with Metabolic Changes and the Risk of Metabolic Syndrome
Yun Hwan Oh, Seulggie Choi, Gyeongsil Lee, Joung Sik Son, Kyae Hyung Kim, Sang Min Park
Journal of Clinical Medicine.2021; 10(4): 745. CrossRef - N6-Adenosine Methylation (m6A) RNA Modification: an Emerging Role in Cardiovascular Diseases
Ye-shi Chen, Xin-ping Ouyang, Xiao-hua Yu, Petr Novák, Le Zhou, Ping-ping He, Kai Yin
Journal of Cardiovascular Translational Research.2021; 14(5): 857. CrossRef - From Metabolic Syndrome to Neurological Diseases: Role of Autophagy
Jessica Maiuolo, Micaela Gliozzi, Vincenzo Musolino, Cristina Carresi, Federica Scarano, Saverio Nucera, Miriam Scicchitano, Francesca Bosco, Stefano Ruga, Maria Caterina Zito, Roberta Macri, Rosamaria Bulotta, Carolina Muscoli, Vincenzo Mollace
Frontiers in Cell and Developmental Biology.2021;[Epub] CrossRef - Absent Exercise-Induced Improvements in Fat Oxidation in Women With Polycystic Ovary Syndrome After High-Intensity Interval Training
Sofie Lionett, Ida Almenning Kiel, Ragnhild Røsbjørgen, Stian Lydersen, Steen Larsen, Trine Moholdt
Frontiers in Physiology.2021;[Epub] CrossRef - Roles of interstitial fluid pH and weak organic acids in development and amelioration of insulin resistance
Yoshinori Marunaka
Biochemical Society Transactions.2021; 49(2): 715. CrossRef - The Role of Mitochondrial Adaptation and Metabolic Flexibility in the Pathophysiology of Obesity and Insulin Resistance: an Updated Overview
Dimitrios Tsilingiris, Evangelia Tzeravini, Chrysi Koliaki, Maria Dalamaga, Alexander Kokkinos
Current Obesity Reports.2021; 10(3): 191. CrossRef - Obesity-Related Inflammation and Endothelial Dysfunction in COVID-19: Impact on Disease Severity
Andrea De Lorenzo, Vanessa Estato, Hugo C Castro-Faria-Neto, Eduardo Tibirica
Journal of Inflammation Research.2021; Volume 14: 2267. CrossRef - Thermogenic Fat: Development, Physiological Function, and Therapeutic Potential
Bruna B. Brandão, Ankita Poojari, Atefeh Rabiee
International Journal of Molecular Sciences.2021; 22(11): 5906. CrossRef - Metabolic Syndrome in an Aging Society – Role of Oxidant-Antioxidant Imbalance and Inflammation Markers in Disentangling Atherosclerosis
Sylwia Dziegielewska-Gesiak
Clinical Interventions in Aging.2021; Volume 16: 1057. CrossRef - Recruitment and remodeling of peridroplet mitochondria in human adipose tissue
Rebeca Acín-Perez, Anton Petcherski, Michaela Veliova, Ilan Y. Benador, Essam A. Assali, Georgia Colleluori, Saverio Cinti, Alexandra J. Brownstein, Siyouneh Baghdasarian, Masha J. Livhits, Michael W. Yeh, Karthickeyan Chella Krishnan, Laurent Vergnes, Na
Redox Biology.2021; 46: 102087. CrossRef - New Insights Into Mitochondrial Dysfunction at Disease Susceptibility Loci in the Development of Type 2 Diabetes
Hannah Maude, Winston Lau, Nikolas Maniatis, Toby Andrew
Frontiers in Endocrinology.2021;[Epub] CrossRef - Effects of sleeve gastrectomy on bone mass, microstructure of femurs and bone metabolism associated serum factors in obese rats
Ying Xue, Ran Li, Yong Zhao, Ling Li, Yun Zhou
BMC Endocrine Disorders.2021;[Epub] CrossRef - The cyclin dependent kinase inhibitor Roscovitine prevents diet-induced metabolic disruption in obese mice
Nabil Rabhi, Kathleen Desevin, Briana Noel Cortez, Ryan Hekman, Jean Z. Lin, Andrew Emili, Stephen R. Farmer
Scientific Reports.2021;[Epub] CrossRef - Reliability and variation in mitochondrial respiration in human adipose tissue
Ronni Eg Sahl, Eva Frederikke Høy Helms, Malte Schmücker, Mathias Flensted-Jensen, Arthur Ingersen, Thomas Morville, Flemming Dela, Jørn Wulff Helge, Steen Larsen
Adipocyte.2021; 10(1): 605. CrossRef - Inhibition of protein tyrosine phosphatase improves mitochondrial bioenergetics and dynamics, reduces oxidative stress, and enhances adipogenic differentiation potential in metabolically impaired progenitor stem cells
Katarzyna Kornicka-Garbowska, Lynda Bourebaba, Michael Röcken, Krzysztof Marycz
Cell Communication and Signaling.2021;[Epub] CrossRef - microRNAs in Human Adipose Tissue Physiology and Dysfunction
Alina Kurylowicz
Cells.2021; 10(12): 3342. CrossRef - Aging, obese-insulin resistance, and bone remodeling
Napatsorn Imerb, Chanisa Thonusin, Nipon Chattipakorn, Siriporn C. Chattipakorn
Mechanisms of Ageing and Development.2020; 191: 111335. CrossRef - Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes
Federica Zatterale, Michele Longo, Jamal Naderi, Gregory Alexander Raciti, Antonella Desiderio, Claudia Miele, Francesco Beguinot
Frontiers in Physiology.2020;[Epub] CrossRef - Is Mitochondrial Dysfunction a Common Root of Noncommunicable Chronic Diseases?
Alexis Diaz-Vegas, Pablo Sanchez-Aguilera, James R Krycer, Pablo E Morales, Matías Monsalves-Alvarez, Mariana Cifuentes, Beverly A Rothermel, Sergio Lavandero
Endocrine Reviews.2020;[Epub] CrossRef - Inflammatory Signaling and Brown Fat Activity
Farah Omran, Mark Christian
Frontiers in Endocrinology.2020;[Epub] CrossRef - Omega-3 fatty acids as regulators of brown/beige adipose tissue: from mechanisms to therapeutic potential
Marta Fernández-Galilea, Elisa Félix-Soriano, Ignacio Colón-Mesa, Xavier Escoté, Maria J. Moreno-Aliaga
Journal of Physiology and Biochemistry.2020; 76(2): 251. CrossRef - Anti-Inflammatory Strategies Targeting Metaflammation in Type 2 Diabetes
Alina Kuryłowicz, Krzysztof Koźniewski
Molecules.2020; 25(9): 2224. CrossRef - Obese Adipose Tissue Secretion Induces Inflammation in Preadipocytes: Role of Toll-Like Receptor-4
Mariana Renovato-Martins, Catharina Moreira-Nunes, Georgia C. Atella, Christina Barja-Fidalgo, João Alfredo de Moraes
Nutrients.2020; 12(9): 2828. CrossRef -
Diabetes and Metabolism Journal in 2020: Good to Great
In-Kyung Jeong
Diabetes & Metabolism Journal.2020; 44(1): 1. CrossRef - The Effect of Silibinin on Protein Expression Profile in White Adipose Tissue of Obese Mice
Fei Wang, Shuchun Chen, Luping Ren, Yichao Wang, Zelin Li, Tiantian Song, He Zhang, Qiwen Yang
Frontiers in Pharmacology.2020;[Epub] CrossRef - Beneficial Effects of Bariatric Surgery-Induced by Weight Loss on the Proteome of Abdominal Subcutaneous Adipose Tissue
Bárbara María Varela-Rodríguez, Paula Juiz-Valiña, Luis Varela, Elena Outeiriño-Blanco, Susana Belén Bravo, María Jesús García-Brao, Enrique Mena, José Francisco Noguera, Javier Valero-Gasalla, Fernando Cordido, Susana Sangiao-Alvarellos
Journal of Clinical Medicine.2020; 9(1): 213. CrossRef - Impact of Skeletal Muscle Mass on Metabolic Health
Gyuri Kim, Jae Hyeon Kim
Endocrinology and Metabolism.2020; 35(1): 1. CrossRef - Sea buckthorn (Hippophae rhamnoides L.) oil enhances proliferation, adipocytes differentiation and insulin sensitivity in 3T3-L1 cells
Ting Zhang, Xuze Qin, Yuxin Cao, Jianxin Zhang, Junxing Zhao
Food Science and Biotechnology.2020; 29(11): 1511. CrossRef - Adipose tissue secretory profile and cardiometabolic risk in obesity
Pengcheng Zhang, Daniels Konja, Yu Wang
Endocrine and Metabolic Science.2020; 1(3-4): 100061. CrossRef - Mitochondrial Dynamics in the Brain Are Associated With Feeding, Glucose Homeostasis, and Whole-Body Metabolism
Jessica L. Haigh, Lauryn E. New, Beatrice M. Filippi
Frontiers in Endocrinology.2020;[Epub] CrossRef - Adipogenesis: A Necessary but Harmful Strategy
Mohammed El Hafidi, Mabel Buelna-Chontal, Fausto Sánchez-Muñoz, Roxana Carbó
International Journal of Molecular Sciences.2019; 20(15): 3657. CrossRef

 KDA
KDA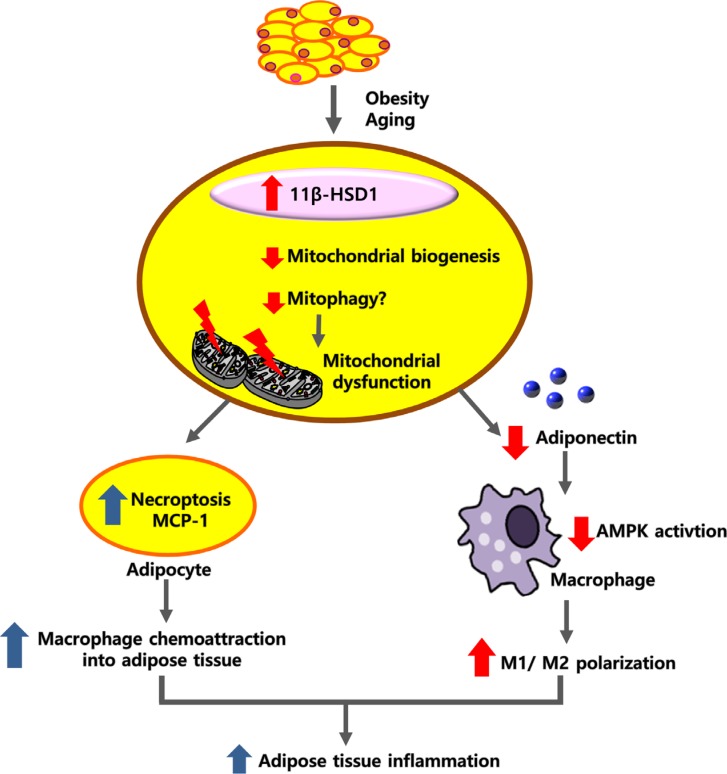


 PubReader
PubReader Cite
Cite






