
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Genetics
- The rs2304256 Polymorphism in TYK2 Gene Is Associated with Protection for Type 1 Diabetes Mellitus
- Felipe Mateus Pellenz, Cristine Dieter, Guilherme Coutinho Kullmann Duarte, Luís Henrique Canani, Bianca Marmontel de Souza, Daisy Crispim
- Diabetes Metab J. 2021;45(6):899-908. Published online May 24, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0194
- 4,759 View
- 157 Download
- 1 Web of Science
- 3 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub 
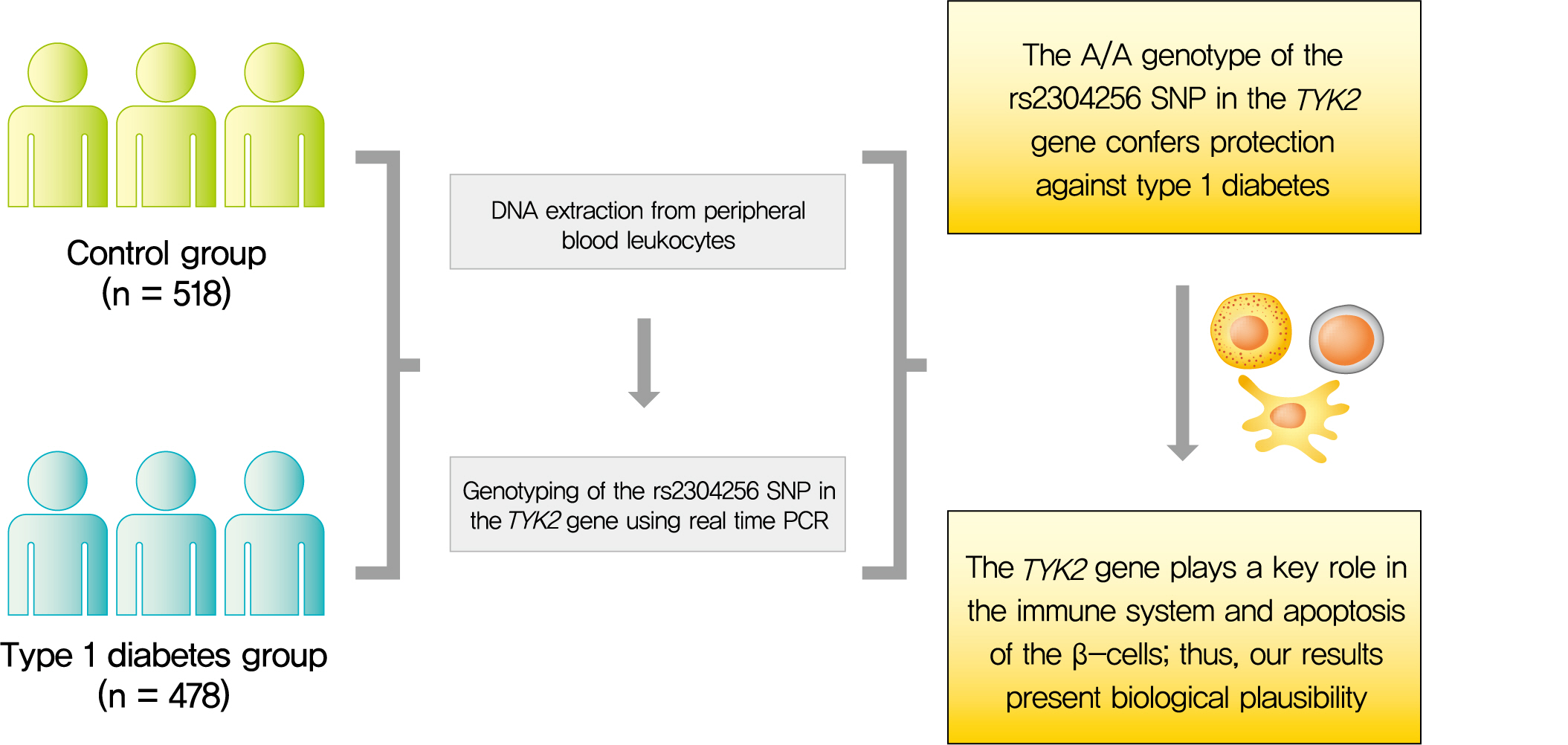
- Background
Tyrosine kinase 2 (TYK2) is a candidate gene for type 1 diabetes mellitus (T1DM) since it plays an important role in regulating apoptotic and pro-inflammatory pathways in pancreatic β-cells through modulation of the type I interferon signaling pathway. The rs2304256 single nucleotide polymorphism (SNP) in TYK2 gene has been associated with protection for different autoimmune diseases. However, to date, only two studies have evaluated the association between this SNP and T1DM, with discordant results. This study thus aimed to investigate the association between the TYK2 rs2304256 SNP and T1DM in a Southern Brazilian population.
Methods
This case-control study comprised 478 patients with T1DM and 518 non-diabetic subjects. The rs2304256 (C/A) SNP was genotyped by real-time polymerase chain reaction technique using TaqMan minor groove binder (MGB) probes.
Results
Genotype and allele frequencies of the rs2304256 SNP differed between T1DM patients and non-diabetic subjects (P<0.0001 and P=0.001, respectively). Furthermore, the A allele was associated with protection against T1DM under recessive (odds ratio [OR], 0.482; 95% confidence interval [CI], 0.288 to 0.806) and additive (OR, 0.470; 95% CI, 0.278 to 0.794) inheritance models, adjusting for human leukocyte antigen (HLA) DR/DQ genotypes, gender, and ethnicity.
Conclusion
The A/A genotype of TYK2 rs2304256 SNP is associated with protection against T1DM in a Southern Brazilian population. -
Citations
Citations to this article as recorded by- Associations of genetic variants within TYK2 with pulmonary tuberculosis among Chinese population
Mingwu Zhang, Zhengwei Liu, Yelei Zhu, Kunyang Wu, Lin Zhou, Ying Peng, Junhang Pan, Bin Chen, Xiaomeng Wang, Songhua Chen
Molecular Genetics & Genomic Medicine.2024;[Epub] CrossRef - Host genetic variants associated with COVID-19 reconsidered in a Slovak cohort
Maria Skerenova, Michal Cibulka, Zuzana Dankova, Veronika Holubekova, Zuzana Kolkova, Vincent Lucansky, Dana Dvorska, Andrea Kapinova, Michaela Krivosova, Martin Petras, Eva Baranovicova, Ivana Baranova, Elena Novakova, Peter Liptak, Peter Banovcin, Anna
Advances in Medical Sciences.2024; 69(1): 198. CrossRef - Cross-Domain Text Mining of Pathophysiological Processes Associated with Diabetic Kidney Disease
Krutika Patidar, Jennifer H. Deng, Cassie S. Mitchell, Ashlee N. Ford Versypt
International Journal of Molecular Sciences.2024; 25(8): 4503. CrossRef
- Associations of genetic variants within TYK2 with pulmonary tuberculosis among Chinese population
- Genetics
- Association of Combined TCF7L2 and KCNQ1 Gene Polymorphisms with Diabetic Micro- and Macrovascular Complications in Type 2 Diabetes Mellitus
- Rujikorn Rattanatham, Nongnuch Settasatian, Nantarat Komanasin, Upa Kukongviriyapan, Kittisak Sawanyawisuth, Phongsak Intharaphet, Vichai Senthong, Chatri Settasatian
- Diabetes Metab J. 2021;45(4):578-593. Published online March 22, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0101
- 5,610 View
- 145 Download
- 8 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Vascular complications are the major morbid consequences of type 2 diabetes mellitus (T2DM). The transcription factor 7-like 2 (TCF7L2), potassium voltage-gated channel subfamily Q member 1 (KCNQ1), and inwardly-rectifying potassium channel, subfamily J, member 11 gene (KCNJ11) are common T2DM susceptibility genes in various populations. However, the associations between polymorphisms in these genes and diabetic complications are controversial. This study aimed to investigate the effects of combined gene-polymorphisms within TCF7L2, KCNQ1, and KCNJ11 on vascular complications in Thai subjects with T2DM.
Methods
We conducted a case-control study comprising 960 T2DM patients and 740 non-diabetes controls. Single nucleotide polymorphisms in TCF7L2, KCNQ1, and KCNJ11 were genotyped and evaluated for their association with diabetic vascular complications.
Results
The gene variants TCF7L2 rs290487-T, KCNQ1 rs2237892-C, and KCNQ1 rs2237897-C were associated with increased risk of T2DM. TCF7L2 rs7903146-C, TCF7L2 rs290487-C, KCNQ1 rs2237892-T, and KCNQ1 rs2237897-T revealed an association with hypertension. The specific combination of risk-alleles that have effects on T2DM and hypertension, TCF7L2 rs7903146-C, KCNQ1 rs2237892-C, and KCNQ1 rs2237897-T, as genetic risk score (GRS), pronounced significant association with coronary artery disease (CAD), cumulative nephropathy and CAD, and cumulative microvascular and macrovascular complications (respective odds ratios [ORs] with 95% confidence interval [95% CI], comparing between GRS 2–3 and GRS 5–6, were 7.31 [2.03 to 26.35], 3.92 [1.75 to 8.76], and 2.33 [1.13 to 4.79]).
Conclusion
This study demonstrated, for the first time, the effect conferred by specific combined genetic variants in TCF7L2 and KCNQ1 on diabetic vascular complications, predominantly with nephropathy and CAD. Such a specific pattern of gene variant combination may implicate in the progression of T2DM and life-threatening vascular complications. -
Citations
Citations to this article as recorded by- Genetic Risk Scores Identify People at High Risk of Developing Diabetic Kidney Disease: A Systematic Review
Aleena Shujaat Ali, Cecilia Pham, Grant Morahan, Elif Ilhan Ekinci
The Journal of Clinical Endocrinology & Metabolism.2024; 109(5): 1189. CrossRef - Saudi Community-Based Screening Study on Genetic Variants in β-Cell Dysfunction and Its Role in Women with Gestational Diabetes Mellitus
Amal F. Alshammary, Malak Mohammed Al-Hakeem, Imran Ali Khan
Genes.2023; 14(4): 924. CrossRef - Association between KCNJ11 E23K polymorphism and the risk of type 2 diabetes mellitus: A global meta-analysis
Yaxuan Ren, Wenfei Zhu, Jikang Shi, Aiyu Shao, Yi Cheng, Yawen Liu
Journal of Diabetes and its Complications.2022; 36(5): 108170. CrossRef - Association between carotid atherosclerosis and presence of intracranial atherosclerosis using three-dimensional high-resolution vessel wall magnetic resonance imaging in asymptomatic patients with type 2 diabetes
Ji Eun Jun, You-Cheol Hwang, Kyu Jeong Ahn, Ho Yeon Chung, Geon-Ho Jahng, Soonchan Park, In-Kyung Jeong, Chang-Woo Ryu
Diabetes Research and Clinical Practice.2022; 191: 110067. CrossRef - Multiple Single Nucleotide Polymorphism Testing Improves the Prediction of Diabetic Retinopathy Risk with Type 2 Diabetes Mellitus
Yu-Ting Hsiao, Feng-Chih Shen, Shao-Wen Weng, Pei-Wen Wang, Yung-Jen Chen, Jong-Jer Lee
Journal of Personalized Medicine.2021; 11(8): 689. CrossRef - Oxidative Stress Genes in Diabetes Mellitus Type 2: Association with Diabetic Kidney Disease
Athanasios Roumeliotis, Stefanos Roumeliotis, Fotis Tsetsos, Marianthi Georgitsi, Panagiotis I. Georgianos, Aikaterini Stamou, Anna Vasilakou, Kalliopi Kotsa, Xanthippi Tsekmekidou, Peristera Paschou, Stylianos Panagoutsos, Vassilios Liakopoulos, Elena Az
Oxidative Medicine and Cellular Longevity.2021; 2021: 1. CrossRef - Analysis of the association of polymorphisms of genes markers functions of endothelium and vascular-plate hemostasis with development of diabetic foot syndrome
N. I. Troitskaya, K. G. Shapovalov, V. A. Mudrov
Acta Biomedica Scientifica.2021; 6(4): 18. CrossRef
- Genetic Risk Scores Identify People at High Risk of Developing Diabetic Kidney Disease: A Systematic Review
- Genetics
APO A2 -265T /C Polymorphism Is Associated with Increased Inflammatory Responses in Patients with Type 2 Diabetes Mellitus- Fariba Koohdani, Haleh Sadrzadeh-Yeganeh, Mahmoud Djalali, Mohammadreza Eshraghian, Elham Zamani, Gity Sotoudeh, Mohammad-Ali Mansournia, Laleh Keramat
- Diabetes Metab J. 2016;40(3):222-229. Published online June 20, 2016
- DOI: https://doi.org/10.4093/dmj.2016.40.3.222
- 3,137 View
- 28 Download
- 12 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Apolipoprotein A2 (
APO A2 ) is the second most abundant structural apolipoprotein in high density lipoprotein. Several studies have examined the possible effect ofAPO A2 on atherosclerosis incidence. Due to the role of inflammation in atherosclerosis, we aimed to determine the relationship betweenAPO A2 -265T/C polymorphism and inflammation as a risk factor in type 2 diabetes mellitus (T2DM) patients.Methods In total, 180 T2DM patients, with known
APO A2 -265T/C polymorphism, were recruited for this comparative study and were grouped equally based on their genotypes. Dietary intakes, anthropometric parameters, lipid profile, and inflammatory markers (i.e., pentraxin 3 [PTX3], high-sensitivity C-reactive protein [hs-CRP], and interleukin 18) were measured. The data were analyzed using an independentt -test, a chi-square test, and the analysis of covariance.Results After adjusting for confounding factors, in the entire study population and in the patients with or without obesity, the patients with the CC genotype showed higher hs-CRP (
P =0.001,P =0.008, andP =0.01, respectively) and lower PTX3 (P =0.01,P =0.03, andP =0.04, respectively) in comparison with the T allele carriers. In the patients with the CC genotype, no significant differences were observed in the inflammatory markers between the obese or non-obese patients. However, regarding the T allele carriers, the plasma hs-CRP level was significantly higher in the obese patients compared to the non-obese patients (P =0.01).Conclusion In the T2DM patients, the CC genotype could be considered as a risk factor and the T allele as a protective agent against inflammation, which the latter effect might be impaired by obesity. Our results confirmed the anti-atherogenic effect of
APO A2 , though more studies are required to establish this effect.-
Citations
Citations to this article as recorded by- Proteomic Profiling of Extracellular Vesicles Isolated from Plasma and Peritoneal Exudate in Mice Induced by Crotalus scutulatus scutulatus Crude Venom and Its Purified Cysteine-Rich Secretory Protein (Css-CRiSP)
Armando Reyes, Joseph D. Hatcher, Emelyn Salazar, Jacob Galan, Anton Iliuk, Elda E. Sanchez, Montamas Suntravat
Toxins.2023; 15(7): 434. CrossRef - ApoA2–256T > C polymorphism interacts with Healthy Eating Index, Dietary Quality Index-International and Dietary Phytochemical Index to affect biochemical markers among type 2 diabetic patients
Zahra Esmaeily, Gity Sotoudeh, Masoumeh Rafiee, Fariba Koohdani
British Journal of Nutrition.2022; 127(9): 1343. CrossRef - Interaction between Apo A-II –265T > C polymorphism and dietary total antioxidant capacity on some oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus
Banafsheh Jafari Azad, Mehdi Yaseri, Elnaz Daneshzad, Fariba Koohdani
British Journal of Nutrition.2022; 128(1): 13. CrossRef - Deletion allele of Apo B gene is associated with higher inflammation, oxidative stress and dyslipidemia in obese type 2 diabetic patients: an analytical cross-sectional study
Nasim Mokhtary, Seyedeh Neda Mousavi, Gity Sotoudeh, Mostafa Qorbani, Maryam Dehghani, Fariba Koohdani
BMC Endocrine Disorders.2022;[Epub] CrossRef - Interaction between Apo A-II -265T>C polymorphism and dietary total antioxidant capacity on some anthropometric indices and serum lipid profile in patients with type 2 diabetes mellitus
Banafsheh Jafari Azad, Mehdi Yaseri, Elnaz Daneshzad, Fariba Koohdani
Journal of Nutritional Science.2021;[Epub] CrossRef - A personalised diet study: The interaction between ApoA2 −265T > C polymorphism and dietary inflammatory index on oxidative and inflammatory markers and lipid profile in patients with type 2 diabetes mellitus: A cross‐sectional study
Elmira Karimi, Pourya Tondkar, Gity Sotoudeh, Mostafa Qorbani, Masoumeh Rafiee, Fariba Koohdani
International Journal of Clinical Practice.2021;[Epub] CrossRef - Interaction between the dietary indices and PPAR‐γ Pro12Ala gene variants on cardiovascular risk factors in patients with type 2 diabetes mellitus
Faezeh Abaj, Gity Sotoudeh, Elmira Karimi, Masoumeh Rafiee, Fariba Koohdani
International Journal of Clinical Practice.2021;[Epub] CrossRef - A Genetic Score of Predisposition to Low-Grade Inflammation Associated with Obesity May Contribute to Discern Population at Risk for Metabolic Syndrome
Sebastià Galmés, Margalida Cifre, Andreu Palou, Paula Oliver, Francisca Serra
Nutrients.2019; 11(2): 298. CrossRef - Study of the relationship between APOA-II −265T>C polymorphism and HDL function in response to weight loss in overweight and obese type 2 diabetic patients
Masoumeh Moradi, Maryam Mahmoudi, Ahmad Saedisomeolia, Mohammad Ali Mansournia, Roxana Zahirihashemi, Fariba Koohdani
Clinical Nutrition.2018; 37(3): 965. CrossRef - Apolipoprotein A-II induces acute-phase response associated AA amyloidosis in mice through conformational changes of plasma lipoprotein structure
Mu Yang, Yingye Liu, Jian Dai, Lin Li, Xin Ding, Zhe Xu, Masayuki Mori, Hiroki Miyahara, Jinko Sawashita, Keiichi Higuchi
Scientific Reports.2018;[Epub] CrossRef - Prenatal Exposure to Lipopolysaccharide Induces PTX3 Expression and Results in Obesity in Mouse Offspring
Shugang Qin, Xin Chen, Meng Gao, Jianzhi Zhou, Xiaohui Li
Inflammation.2017; 40(6): 1847. CrossRef
- Proteomic Profiling of Extracellular Vesicles Isolated from Plasma and Peritoneal Exudate in Mice Induced by Crotalus scutulatus scutulatus Crude Venom and Its Purified Cysteine-Rich Secretory Protein (Css-CRiSP)
- Genetics
- Non-Association between rs7903146 and rs12255372 Polymorphisms in Transcription Factor 7-Like 2 Gene and Type 2 Diabetes Mellitus in Jahrom City, Iran
- Mohammad Pourahmadi, Saiedeh Erfanian, Malihe Moradzadeh, Abdolreza Sotoodeh Jahromi
- Diabetes Metab J. 2015;39(6):512-517. Published online November 13, 2015
- DOI: https://doi.org/10.4093/dmj.2015.39.6.512
- 3,105 View
- 32 Download
- 21 Web of Science
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Transcription factor 7-like 2 (TCF7L2) is a transcription factor in the Wnt signaling pathway. High levels of TCF7L2 have been reported in most human tissues, including the heart, lung, brain, liver, kidney, placenta, adipose tissues, and pancreatic β-cells. The purpose of this study was to assess the association between TCF7L2 polymorphisms (rs12255372 and rs7903146) and type 2 diabetes mellitus in the city of Jahrom, Iran.
Methods This case-control study was conducted with 200 patients referred to Diabetes Clinics and 200 healthy subjects in Jahrom City. Biochemical characteristics were first determined. TCF7L2 rs1255372 and rs7903146 polymorphisms were then genotyped using the polymerase chain reaction-restriction fragment length polymorphism method.
Results T-allele frequencies of both single nucleotide polymorphisms (SNPs) were significantly higher in diabetic patients than in normal glucose-tolerant subjects (rs12255372: 20.3% vs. 14.5%; rs7903146: 28.5% vs. 22.25%). The rs12255372 (G/T) polymorphism analysis showed an odds ratio of 0.473 (95% confidence interval [CI], 0.170 to 1.314;
P =0.151) for the TT genotype and 0.646 (95% CI, 0.410 to 1.019;P =0.060) for the TG genotype, compared with the GG genotype. The rs7903146 (C/T) polymorphism odds ratios for TT and TC genotypes were 0.564 (95% CI, 0.280 to 1.135;P =0.109) and 0.751 (95% CI, 0.487 to 1.157;P =0.194) compared with the CC genotype, respectively.Conclusion The rs12255372 and rs7903146 SNPs of the TCF7L2 gene were not associated with insulin resistance in the evaluated population.
-
Citations
Citations to this article as recorded by- Association between Transcription Factor 7-Like 2 Gene Polymorphisms rs7903146 and rs12255372 with the Risk of Diabetic Nephropathy among South Indian Population
Balaji Ramanathan, Kumaravel Velayutham
Chronicle of Diabetes Research and Practice.2024; 3(1): 8. CrossRef - Association between TCF7L2 polymorphism and type 2 diabetes mellitus susceptibility: a case–control study among the Bangladeshi population
Asma Salauddin, Kallyan Chakma, Md. Mahbub Hasan, Farhana Akter, Nowshad Asgar Chowdhury, Sumon Rahman Chowdhury, Adnan Mannan
Molecular Biology Reports.2023; 50(1): 609. CrossRef - Role of rs7903146 polymorphism and adropin serum level in patients with diabetes mellitus; a case–control study from Isfahan, Iran
Abbas-Ali Palizban, Abdol-Hamid Yazdani, Hamidreza Jahanbani-Ardakani
Archives of Physiology and Biochemistry.2022; 128(2): 378. CrossRef - PPARɣ2, aldose reductase, and TCF7L2 gene polymorphisms: relation to diabetes mellitus
Hadeel Ahmed Shawki, Ekbal M. Abo-hashem, Magdy M. Youssef, Maha Shahin, Rasha Elzehery
Journal of Diabetes & Metabolic Disorders.2022; 21(1): 241. CrossRef - Case-control study on the association between the GATA2 gene and premature myocardial infarction in the Iranian population
Peyman Izadpanah, Ehsan Khabbzi, Saiedeh Erfanian, Simin Jafaripour, Mohammad Shojaie
Herz.2021; 46(1): 71. CrossRef - Polymorphic genetic markers and how they are associated with clinical and metabolic indicators of type 2 diabetes mellitus in the Kazakh population
Valeriy V. Benberin, Tamara A. Vochshenkova, Gulshara Zh. Abildinova, Anna V. Borovikova, Almagul A. Nagimtayeva
Journal of Diabetes & Metabolic Disorders.2021; 20(1): 131. CrossRef - The role of transcription factor 7‐like 2 in metabolic disorders
Zhensheng Zhang, Li Xu, Xiao Xu
Obesity Reviews.2021;[Epub] CrossRef - Association of transcription factor 7-like 2 (rs7903146) gene polymorphism with diabetic retinopathy
Hadeel Ahmed Shawki, Ekbal M.Abo-Hashem, Magdy M. Youssef, Maha Shahin, Rasha Elzehery
Ophthalmic Genetics.2020; 41(5): 420. CrossRef - Association of SLC30A8, CDKAL1, TCF7L2 and HHEX Gene Polymorphisms with Type 2 Diabetes in the Population of North East India
A. Bhowmick, P. Sarkar, M. P. Baruah, D. Bodhini, V. Radha, V. Mohan, S. Banu
Cytology and Genetics.2020; 54(2): 165. CrossRef - Transcription Factor 7-Like-2 (TCF7L2) rs7903146 (C/T) Polymorphism in Patients with Type 2 Diabetes Mellitus
Ahmed Mohamed Bahaaeldin, Arig Aly Seif, Amira Ibrahim Hamed, Walaa Ahmed Yousry Kabiel
Dubai Diabetes and Endocrinology Journal.2020; 26(3): 112. CrossRef - The antileukemic effects of saffron (Crocus sativus L.) and its related molecular targets: A mini review
Maliheh Moradzadeh, Mohamad Reza Kalani, Amir Avan
Journal of Cellular Biochemistry.2019; 120(4): 4732. CrossRef - Association of transcription factor 7-like 2 (TCF7L2) gene polymorphism with type 2 diabetes mellitus in Chinese Korean ethnicity population
Kui-Chen Zhou, Hong-Wei Liu, Chen Wang, Yan-Jun Fu, Feng Jin
Medicine.2019; 98(5): e14288. CrossRef - Targeted deletion of Tcf7l2 in adipocytes promotes adipocyte hypertrophy and impaired glucose metabolism
Gisela Geoghegan, Judith Simcox, Marcus M. Seldin, Timothy J. Parnell, Chris Stubben, Steven Just, Lori Begaye, Aldons J. Lusis, Claudio J. Villanueva
Molecular Metabolism.2019; 24: 44. CrossRef - Association study of the rs46522 polymorphism in UBE2Z and rs7903146 polymorphism in TCF7L2 gene with T2DM in Arab population of Khuzestan province: case-control study
Sana Shafidelpour, Ali Mohammad Foroughmand, Merhrnoosh Zakerkish, Mahdi Pourmahdi Borujeni
Medical Journal of Tabriz University of Medical Sciences and Health Services.2019; 41(6): 59. CrossRef - Transcription factor 7-like 2 single nucleotide polymorphisms are associated with lipid profile in the Balinese
Sukma Oktavianthi, Made R. Saraswati, Ketut Suastika, Pande Dwipayana, Asri Sulfianti, Rahma F. Hayati, Hidayat Trimarsanto, Clarissa A. Febinia, Herawati Sudoyo, Safarina G. Malik
Molecular Biology Reports.2018; 45(5): 1135. CrossRef - Association of TCF7L2 mutation and atypical diabetes in a Uruguayan population
Carolina Beloso, Jorge Souto, Matias Fábregat, Gerardo Romanelli, Gerardo Javiel, Adriana Mimbacas
World Journal of Diabetes.2018; 9(9): 157. CrossRef - Associations of TCF7L2 gene polymorphisms with the risk of diabetic nephropathy
Yan Zhuang, Fukun Niu, Defeng Liu, Juanjuan Sun, Xiaowei Zhang, Jian Zhang, Shuxia Guo
Medicine.2018; 97(40): e8388. CrossRef
- Association between Transcription Factor 7-Like 2 Gene Polymorphisms rs7903146 and rs12255372 with the Risk of Diabetic Nephropathy among South Indian Population
- Resistin in Rodents and Humans
- Hyeong Kyu Park, Rexford S. Ahima
- Diabetes Metab J. 2013;37(6):404-414. Published online December 12, 2013
- DOI: https://doi.org/10.4093/dmj.2013.37.6.404
- 5,602 View
- 54 Download
- 122 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Obesity is characterized by excess accumulation of lipids in adipose tissue and other organs, and chronic inflammation associated with insulin resistance and an increased risk of type 2 diabetes. Obesity, type 2 diabetes, and cardiovascular diseases are major health concerns. Resistin was first discovered as an adipose-secreted hormone (adipokine) linked to obesity and insulin resistance in rodents. Adipocyte-derived resistin is increased in obese rodents and strongly related to insulin resistance. However, in contrast to rodents, resistin is expressed and secreted from macrophages in humans and is increased in inflammatory conditions. Some studies have also suggested an association between increased resistin levels and insulin resistance, diabetes and cardiovascular disease. Genetic studies have provided additional evidence for a role of resistin in insulin resistance and inflammation. Resistin appears to mediate the pathogenesis of atherosclerosis by promoting endothelial dysfunction, vascular smooth muscle cell proliferation, arterial inflammation, and formation of foam cells. Indeed, resistin is predictive of atherosclerosis and poor clinical outcomes in patients with coronary artery disease and ischemic stroke. There is also growing evidence that elevated resistin is associated with the development of heart failure. This review will focus on the biology of resistin in rodents and humans, and evidence linking resistin with type 2 diabetes, atherosclerosis, and cardiovascular disease.
-
Citations
Citations to this article as recorded by- Resistin – A Plausible Therapeutic Target in the Pathogenesis of Psoriasis
Manupati Srikanth, Mahaboobkhan Rasool
Immunological Investigations.2024; 53(2): 115. CrossRef - MHO or MUO? White adipose tissue remodeling
Jing Yi Zhao, Li Juan Zhou, Kai Le Ma, Rui Hao, Min Li
Obesity Reviews.2024;[Epub] CrossRef - Resistin in endocrine pancreas of sheep: Presence and expression related to different diets
Margherita Maranesi, Elisa Palmioli, Cecilia Dall'Aglio, Daniele Marini, Polina Anipchenko, Elena De Felice, Paola Scocco, Francesca Mercati
General and Comparative Endocrinology.2024; 348: 114452. CrossRef - Adipocytokines levels as potential biomarkers for discriminating patients with a diagnosis of depressive disorder from healthy controls
Elżbieta Małujło-Balcerska, Tadeusz Pietras
Journal of Psychiatric Research.2024; 171: 163. CrossRef - Adipokines in atopic dermatitis: the link between obesity and atopic dermatitis
Shiyun Zhang, Bingjie Zhang, Yuehua Liu, Li Li
Lipids in Health and Disease.2024;[Epub] CrossRef - The Role of Adipokines in the Control of Pituitary Functions
Barbara Kaminska, Beata Kurowicka, Marta Kiezun, Kamil Dobrzyn, Katarzyna Kisielewska, Marlena Gudelska, Grzegorz Kopij, Karolina Szymanska, Barbara Zarzecka, Oguzhan Koker, Ewa Zaobidna, Nina Smolinska, Tadeusz Kaminski
Animals.2024; 14(2): 353. CrossRef - Adipokine imbalance and its role in the pathogenesis of novel coronavirus infection
I. D. Bespalova, U. M. Mitrichenko, V. V. Kalyuzhin, E. S. Koroleva, Yu. I. Koshchavtseva, D. S. Romanov, D. E. Pershina
Bulletin of Siberian Medicine.2024; 22(4): 164. CrossRef - Association of adipokine levels with obesity in periodontal health and disease: A systematic review with meta‐analysis and meta‐regression
Eswar Kandaswamy, Chun‐Teh Lee, Soumya Bardvalli Gururaj, Sachin Shivanaikar, Vinayak M. Joshi
Journal of Periodontal Research.2024;[Epub] CrossRef - Role of epicardial adipose tissue in the pathogenesis of chronic inflammation in heart failure with preserved ejection fraction
O. N. Dzhioeva, Yu. S. Timofeev, V. A. Metelskaya, A. A. Bogdanova, T. Yu. Vedenikin, O. M. Drapkina
Cardiovascular Therapy and Prevention.2024; 23(3): 3928. CrossRef - Association of maternal body composition and diet on breast milk hormones and neonatal growth during the first month of lactation
David Ramiro-Cortijo, Pratibha Singh, Gloria Herranz Carrillo, Andrea Gila-Díaz, María A. Martín-Cabrejas, Camilia R. Martin, Silvia M. Arribas
Frontiers in Endocrinology.2023;[Epub] CrossRef - Upregulation of peripheral blood mononuclear cells resistin gene expression in severe obstructive sleep apnea and obstructive sleep apnea with coexisting type 2 diabetes mellitus
Branislava Rajkov, Marija Zdravković, Ana Ninić, Milica Brajković, Slobodan Klašnja, Vera Gardijan, Lidija Memon, Jelena Munjas, Marija Mihajlović, Vesna Spasojević- Kalimanovska, Vojislav Radosavljević, Miron Sopić
Sleep and Breathing.2023; 27(5): 2031. CrossRef - Fat-to-heart crosstalk in health and disease
Fleur Lodewijks, Timothy A. McKinsey, Emma L. Robinson
Frontiers in Genetics.2023;[Epub] CrossRef - Adipokines as Diagnostic and Prognostic Markers for the Severity of COVID-19
Thomas Grewal, Christa Buechler
Biomedicines.2023; 11(5): 1302. CrossRef - Role of adipokines in sarcopenia
Wenhao Lu, Wenjie Feng, Jieyu Lai, Dongliang Yuan, Wenfeng Xiao, Yusheng Li
Chinese Medical Journal.2023; 136(15): 1794. CrossRef - Resistin, TNF-α, and microRNA 124-3p expressions in peripheral blood mononuclear cells are associated with diabetic nephropathy
Amin Monjezi, Azam Khedri, Mehrnoosh Zakerkish, Ghorban Mohammadzadeh
International Journal of Diabetes in Developing Countries.2022; 42(1): 62. CrossRef - Resistin in Urine and Breast Milk: Relation to Type of Feeding and Anthropometry at 1-Month
Irena Santosa, Hiromichi Shoji, Kentaro Awata, Yoshiteru Arai, Hiroki Suganuma, Toshiaki Shimizu
Pediatric Reports.2022; 14(1): 86. CrossRef - High Serum Levels of Resistin is Associated With Acute Cerebral Infarction
Kee Ook Lee, Kyung-Yul Lee, Cheol-Young Lee, Ji Hoon Kim, Jaeku Kang, Hoi Young Lee, Sang-Jun Na, Seung-Hun Oh, Ji Hoe Heo
The Neurologist.2022; 27(2): 41. CrossRef - Resistin production does not affect outcomes in a mouse model of acute surgical sepsis
Anthony S. Bonavia, Zissis C. Chroneos, Victor Ruiz-Velasco, Charles H. Lang, Partha Mukhopadhyay
PLOS ONE.2022; 17(3): e0265241. CrossRef - Single-nucleotide polymorphisms as important risk factors of diabetes among Middle East population
Iman Akhlaghipour, Amir Reza Bina, Mohammad Reza Mogharrabi, Ali Fanoodi, Amir Reza Ebrahimian, Soroush Khojasteh Kaffash, Atefeh Babazadeh Baghan, Mohammad Erfan Khorashadizadeh, Negin Taghehchian, Meysam Moghbeli
Human Genomics.2022;[Epub] CrossRef - Synergistic Effects of Weighted Genetic Risk Scores and Resistin and sST2 Levels on the Prognostication of Long-Term Outcomes in Patients with Coronary Artery Disease
Hsin-Hua Chou, Lung-An Hsu, Jyh-Ming Jimmy Juang, Fu-Tien Chiang, Ming-Sheng Teng, Semon Wu, Yu-Lin Ko
International Journal of Molecular Sciences.2022; 23(8): 4292. CrossRef - Hypoxia Increases the Potential for Neutrophil-mediated Endothelial Damage in Chronic Obstructive Pulmonary Disease
Katharine M. Lodge, Arlette Vassallo, Bin Liu, Merete Long, Zhen Tong, Paul R. Newby, Danya Agha-Jaffar, Koralia Paschalaki, Clara E. Green, Kylie B. R. Belchamber, Victoria C. Ridger, Robert A. Stockley, Elizabeth Sapey, Charlotte Summers, Andrew S. Cowb
American Journal of Respiratory and Critical Care Medicine.2022; 205(8): 903. CrossRef - The Role of the Adipokine Resistin in the Pathogenesis and Progression of Epithelial Ovarian Cancer
Klaudia Parafiniuk, Wiktoria Skiba, Anna Pawłowska, Dorota Suszczyk, Aleksandra Maciejczyk, Iwona Wertel
Biomedicines.2022; 10(4): 920. CrossRef - Resistin Modulates Low-Density Lipoprotein Cholesterol Uptake in Human Placental Explants via PCSK9
Sonia Nava-Salazar, Arturo Flores-Pliego, Giovanni Pérez-Martínez, Sandra Parra-Hernández, America Vanoye-Carlo, Francisco Ibarguengoitia-Ochoa, Otilia Perichart-Perera, Enrique Reyes-Muñoz, Juan Mario Solis-Paredes, Salvador Espino y Sosa, Guadalupe Estr
Reproductive Sciences.2022; 29(11): 3242. CrossRef - Differential Association of Selected Adipocytokines, Adiponectin, Leptin, Resistin, Visfatin and Chemerin, with the Pathogenesis and Progression of Type 2 Diabetes Mellitus (T2DM) in the Asir Region of Saudi Arabia: A Case Control Study
Mohammad Muzaffar Mir, Rashid Mir, Mushabab Ayed Abdullah Alghamdi, Javed Iqbal Wani, Zia Ul Sabah, Mohammed Jeelani, Vijaya Marakala, Shahzada Khalid Sohail, Mohamed O’haj, Muffarah Hamid Alharthi, Mohannad Mohammad S. Alamri
Journal of Personalized Medicine.2022; 12(5): 735. CrossRef - Immune system and sarcopenia: Presented relationship and future perspective
Xuzhi Zhang, Hengzhen Li, Miao He, Jingyu Wang, Yuxiang Wu, Yusheng Li
Experimental Gerontology.2022; 164: 111823. CrossRef - Adipose Tissue Secretion Pattern Influences β-Cell Wellness in the Transition from Obesity to Type 2 Diabetes
Giuseppina Biondi, Nicola Marrano, Anna Borrelli, Martina Rella, Giuseppe Palma, Isabella Calderoni, Edoardo Siciliano, Pasquale Lops, Francesco Giorgino, Annalisa Natalicchio
International Journal of Molecular Sciences.2022; 23(10): 5522. CrossRef - Supplemental hydroxychloroquine therapy regulates adipokines in patients with systemic lupus erythematosus with stable disease
Risa Wakiya, Kiyo Ueeda, Hiromi Shimada, Shusaku Nakashima, Tomohiro Kameda, Nobuyuki Miyatake, Mikiya Kato, Taichi Miyagi, Koichi Sugihara, Mao Mizusaki, Rina Mino, Norimitsu Kadowaki, Hiroaki Dobashi
Clinical Rheumatology.2022; 41(11): 3345. CrossRef - Can soy isoflavones in combination with soy protein change serum concentration of adiponectin and resistin? A systematic review and meta‐analysis on randomized clinical trials
Mitra Hariri, Bahareh Amirkalali, Ensiyeh Mollanoroozy, Ali Gholami
Food Science & Nutrition.2022; 10(12): 4126. CrossRef - Adipokines: Deciphering the cardiovascular signature of adipose tissue
Joseph C. Galley, Shubhnita Singh, Wanessa M.C. Awata, Juliano V. Alves, Thiago Bruder-Nascimento
Biochemical Pharmacology.2022; 206: 115324. CrossRef - Evaluation of the Anti-Obesity Effect of Zeaxanthin and Exercise in HFD-Induced Obese Rats
Mona Al-thepyani, Salha Algarni, Hana Gashlan, Mohamed Elzubier, Lina Baz
Nutrients.2022; 14(23): 4944. CrossRef - Single High-Dose Vitamin D Supplementation as an Approach for Reducing Ultramarathon-Induced Inflammation: A Double-Blind Randomized Controlled Trial
Jan Mieszkowski, Andżelika Borkowska, Błażej Stankiewicz, Andrzej Kochanowicz, Bartłomiej Niespodziński, Marcin Surmiak, Tomasz Waldziński, Rafał Rola, Miroslav Petr, Jędrzej Antosiewicz
Nutrients.2021; 13(4): 1280. CrossRef - Resistin mitigates stemness and metabolic profile of human adipose-derived mesenchymal stem cells via insulin resistance
Komal Rawal, Kishan M. Purohit, Tushar P. Patel, Neeta Karont, Sarita Gupta
Cytokine.2021; 138: 155374. CrossRef - Resistin is co-secreted with adiponectin in white mouse adipocytes
Saliha Musovic, Man Mohan Shrestha, Ali M. Komai, Charlotta S. Olofsson
Biochemical and Biophysical Research Communications.2021; 534: 707. CrossRef - Resistin: Potential biomarker and therapeutic target in atherosclerosis
Li Zhou, Jun-Yi Li, Ping-Ping He, Xiao-Hua Yu, Chao-Ke Tang
Clinica Chimica Acta.2021; 512: 84. CrossRef - The circulating levels of CTRP1 and CTRP5 are associated with obesity indices and carotid intima-media thickness (cIMT) value in patients with type 2 diabetes: a preliminary study
Ziba Majidi, Solaleh Emamgholipour, Abolfazl Omidifar, Soheil Rahmani Fard, Hossein Poustchi, Mehrnoosh Shanaki
Diabetology & Metabolic Syndrome.2021;[Epub] CrossRef - Corylin reduces obesity and insulin resistance and promotes adipose tissue browning through SIRT-1 and β3-AR activation
Chin-Chuan Chen, Chen-Hsin Kuo, Yann-Lii Leu, Shu-Huei Wang
Pharmacological Research.2021; 164: 105291. CrossRef - A Focused Review of the Metabolic Side-Effects of Clozapine
Jessica W. Y. Yuen, David D. Kim, Ric M. Procyshyn, William J. Panenka, William G. Honer, Alasdair M. Barr
Frontiers in Endocrinology.2021;[Epub] CrossRef - A Negative Energy Balance Is Associated with Metabolic Dysfunctions in the Hypothalamus of a Humanized Preclinical Model of Alzheimer’s Disease, the 5XFAD Mouse
Antonio J. López-Gambero, Cristina Rosell-Valle, Dina Medina-Vera, Juan Antonio Navarro, Antonio Vargas, Patricia Rivera, Carlos Sanjuan, Fernando Rodríguez de Fonseca, Juan Suárez
International Journal of Molecular Sciences.2021; 22(10): 5365. CrossRef - Resistin in pregnancy: Analysis of determinants in pairs of umbilical cord blood and maternal serum
Anne Floeck, Nina Ferrari, Christine Joisten, Maria T. Puth, Brigitte Strizek, Ramona Dolscheid-Pommerich, Ulrich Gembruch, Waltraut M. Merz
Cytokine: X.2021; 3(2): 100052. CrossRef - Is resistin the master link between inflammation and inflammation-related chronic diseases?
Mohammed Taouis, Yacir Benomar
Molecular and Cellular Endocrinology.2021; 533: 111341. CrossRef - The dynamics of human bone marrow adipose tissue in response to feeding and fasting
Pouneh K. Fazeli, Miriam A. Bredella, Gisela Pachon-Peña, Wenxiu Zhao, Xun Zhang, Alexander T. Faje, Megi Resulaj, Sai P. Polineni, Tara M. Holmes, Hang Lee, Elizabeth K. O’Donnell, Ormond A. MacDougald, Mark C. Horowitz, Clifford J. Rosen, Anne Klibanski
JCI Insight.2021;[Epub] CrossRef - Resistin: A journey from metabolism to cancer
Ankita Deb, Bhavana Deshmukh, Pranay Ramteke, Firoz Khan Bhati, Manoj Kumar Bhat
Translational Oncology.2021; 14(10): 101178. CrossRef - Obesity is the basis of metabolic syndrome
A. F. Verbovoy, N. I. Verbovaya, Yu. A. Dolgikh
Obesity and metabolism.2021; 18(2): 142. CrossRef - Human Milk Metabolic Hormones: Analytical Methods and Current Understanding
Majed A. Suwaydi, Zoya Gridneva, Sharon L. Perrella, Mary E. Wlodek, Ching Tat Lai, Donna T. Geddes
International Journal of Molecular Sciences.2021; 22(16): 8708. CrossRef - Adipokines as Immune Cell Modulators in Multiple Sclerosis
Merel Rijnsburger, Niek Djuric, Inge A. Mulder, Helga E. de Vries
International Journal of Molecular Sciences.2021; 22(19): 10845. CrossRef - The Role of Adipokines in Cardiovascular Pathology
Valery Podzolkov , Anna Pokrovskaya, Ulyana Bazhanova , Tatyana Vargina , Svetlana Anatolievna Knyazeva , Daria Vanina
Open Access Macedonian Journal of Medical Sciences.2021; 9(F): 794. CrossRef - Measurement of Plasma Resistin Concentrations in Horses with Metabolic and Inflammatory Disorders
Beatriz Fuentes-Romero, Alberto Muñoz-Prieto, José J. Cerón, María Martín-Cuervo, Manuel Iglesias-García, Escolástico Aguilera-Tejero, Elisa Díez-Castro
Animals.2021; 12(1): 77. CrossRef - EFFECT OF DIET AND EXERCISE-INDUCE WEIGHT LOSS ON LEVEL OF RESISTIN IN PATIENT WITH OBESITY
О. I. Tokarenko, I. O. Andreieva, O. O. Tokarenko, M. M. Surmilo
Modern medical technology.2021; (4): 11. CrossRef - Alteration of gut microbiota affects expression of adiponectin and resistin through modifying DNA methylation in high-fat diet-induced obese mice
Hongyang Yao, Chaonan Fan, Yuanyuan Lu, Xiuqin Fan, Lulu Xia, Ping Li, Rui Wang, Tiantian Tang, Yuanyuan Wang, Kemin Qi
Genes & Nutrition.2020;[Epub] CrossRef - Resistin hormone in diabetic kidney disease and its relation to iron status and hepcidin
Zhian Sherzad Hayder, Zrar Saleem Kareem
International Urology and Nephrology.2020; 52(4): 749. CrossRef - Proteoglycans in Obesity-Associated Metabolic Dysfunction and Meta-Inflammation
Ariane R. Pessentheiner, G. Michelle Ducasa, Philip L. S. M. Gordts
Frontiers in Immunology.2020;[Epub] CrossRef - Resistin Is Increased in Periodontal Cells and Tissues: In Vitro and In Vivo Studies
Andressa V. B. Nogueira, Marjan Nokhbehsaim, Sema Tekin, Rafael S. de Molon, Luis C. Spolidorio, Svenja Memmert, Anna Damanaki, Andreas Jäger, Sigrun Eick, James Deschner, Joni A. Cirelli
Mediators of Inflammation.2020; 2020: 1. CrossRef - Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease
Alan Chait, Laura J. den Hartigh
Frontiers in Cardiovascular Medicine.2020;[Epub] CrossRef - The possible role of endocrine dysfunction of adipose tissue in gestational diabetes mellitus
Patrik Šimják, Kateřina Anderlová, Anna Cinkajzlová, Antonín Pařízek, Michal Kršek, Martin Haluzík
Minerva Endocrinologica.2020;[Epub] CrossRef - High Plasma Resistin Levels Portend the Insulin Resistance-Associated Susceptibility to Early Cognitive Decline in Patients with Type 2 Diabetes Mellitus
Chenchen Wang, Xi Huang, Sai Tian, Rong Huang, Dan Guo, Hongyan Lin, Jiaqi Wang, Shaohua Wang
Journal of Alzheimer's Disease.2020; 75(3): 807. CrossRef - Resistin in metabolism, inflammation, and disease
Deeksha Tripathi, Sashi Kant, Saurabh Pandey, Nasreen Z. Ehtesham
The FEBS Journal.2020; 287(15): 3141. CrossRef - Adipokines: New Potential Therapeutic Target for Obesity and Metabolic, Rheumatic, and Cardiovascular Diseases
Lucia Recinella, Giustino Orlando, Claudio Ferrante, Annalisa Chiavaroli, Luigi Brunetti, Sheila Leone
Frontiers in Physiology.2020;[Epub] CrossRef - Correlation of CCL18 with Levels of Adi-pokines in the Sera of Patients with Myocardial Infarction in a 6-Month Period: Case Series
Atefeh GamarTalepoor, Ehsan Dowlatshahi, Mehrnoush Doroudchi
Iranian South Medical Journal.2020; 23(3): 222. CrossRef - The Mesentery, Systemic Inflammation, and Crohn’s Disease
Edgardo D Rivera, John Calvin Coffey, Dara Walsh, Eli D Ehrenpreis
Inflammatory Bowel Diseases.2019; 25(2): 226. CrossRef - Resistin and adenylyl cyclase-associated protein 1 (CAP1) regulate the expression of genes related to insulin resistance in BNL CL.2 mouse liver cells
Dimiter Avtanski, Karin Chen, Leonid Poretsky
Data in Brief.2019; 25: 104112. CrossRef - Proteomic profile of patients with atrial fibrillation undergoing cardiac surgery†
Ilias P Doulamis, George Samanidis, Aspasia Tzani, Asier Antoranz, Anastasios Gkogkos, Panagiotis Konstantopoulos, Vaia Pliaka, Angeliki Minia, Leonidas G Alexopoulos, Despina N Perrea, Konstantinos Perreas
Interactive CardioVascular and Thoracic Surgery.2019; 28(1): 94. CrossRef - Angiotensin-(1-7), Adipokines and Inflammation
Deborah de Farias Lelis, Daniela Fernanda de Freitas, Amanda Souto Machado, Thaísa Soares Crespo, Sérgio Henrique Sousa Santos
Metabolism.2019; 95: 36. CrossRef - New Insights into Adipokines as Potential Biomarkers for Type-2 Diabetes Mellitus
Marta Olivera-Santa Catalina, Pedro C. Redondo, Maria P. Granados, Carlos Cantonero, Jose Sanchez-Collado, Letizia Albarran, Jose J. Lopez
Current Medicinal Chemistry.2019; 26(22): 4119. CrossRef - Myokine–adipokine cross-talk: potential mechanisms for the association between plasma irisin and adipokines and cardiometabolic risk factors in Mexican children with obesity and the metabolic syndrome
Adrian M. Gonzalez-Gil, Mariana Peschard-Franco, Elena C. Castillo, Gustavo Gutierrez-DelBosque, Victor Treviño, Christian Silva-Platas, Luisa Perez-Villarreal, Gerardo Garcia-Rivas, Leticia Elizondo-Montemayor
Diabetology & Metabolic Syndrome.2019;[Epub] CrossRef - Early Life Exposures to Perfluoroalkyl Substances in Relation to Adipokine Hormone Levels at Birth and During Childhood
Colleen Shelly, Philippe Grandjean, Youssef Oulhote, Peter Plomgaard, Ruth Frikke-Schmidt, Flemming Nielsen, Denis Zmirou-Navier, Pal Weihe, Damaskini Valvi
The Journal of Clinical Endocrinology & Metabolism.2019; 104(11): 5338. CrossRef - Overweight and obesity in childhood: Dietary, biochemical, inflammatory and lifestyle risk factors
Samah R. Albataineh, Eman F. Badran, Reema F. Tayyem
Obesity Medicine.2019; 15: 100112. CrossRef - Effects of major adipokines and the −420 C > G resistin gene polymorphism on the long-term outcome of patients with acute ischemic stroke
Stella Bouziana, Konstantinos Tziomalos, Antonis Goulas, Timoleon-Achilleas Vyzantiadis, Maria Papadopoulou, Athanasia Panderi, Apostolos Ι. Ηatzitolios
International Journal of Neuroscience.2019; 129(10): 978. CrossRef - The Complex Interactions Between Obesity, Metabolism and the Brain
Romina María Uranga, Jeffrey Neil Keller
Frontiers in Neuroscience.2019;[Epub] CrossRef - Resistin: A reappraisal
E. Acquarone, F. Monacelli, R. Borghi, A. Nencioni, P. Odetti
Mechanisms of Ageing and Development.2019; 178: 46. CrossRef - Implications of resistin in type 2 diabetes mellitus and coronary artery disease: Impairing insulin function and inducing pro‐inflammatory cytokines
Melissa Emamalipour, Khaled Seidi, Ali Jahanban‐Esfahlan, Rana Jahanban‐Esfahlan
Journal of Cellular Physiology.2019; 234(12): 21758. CrossRef - Serum-based soluble markers differentiate psoriatic arthritis from osteoarthritis
Vinod Chandran, Fatima Abji, Anthony V Perruccio, Rajiv Gandhi, Suzanne Li, Richard J Cook, Dafna D Gladman
Annals of the Rheumatic Diseases.2019; 78(6): 796. CrossRef - Telmisartan prevents diet-induced obesity and preserves leptin transport across the blood-brain barrier in high-fat diet-fed mice
Franziska Schuster, Gianna Huber, Ines Stölting, Emily E. Wing, Kathrin Saar, Norbert Hübner, William A. Banks, Walter Raasch
Pflügers Archiv - European Journal of Physiology.2018; 470(11): 1673. CrossRef - Adipokines in human breast milk
Juergen Kratzsch, Yoon Ju Bae, Wieland Kiess
Best Practice & Research Clinical Endocrinology & Metabolism.2018; 32(1): 27. CrossRef - Addressing the Perfect Storm: Biomarkers in Obesity and Pathophysiology of Cardiometabolic Risk
Krasimira Aleksandrova, Dariush Mozaffarian, Tobias Pischon
Clinical Chemistry.2018; 64(1): 142. CrossRef - Adipocytokine Involvement in Innate Immune Mechanisms
Paulina Żelechowska, Elżbieta Kozłowska, Joanna Pastwińska, Justyna Agier, Ewa Brzezińska-Błaszczyk
Journal of Interferon & Cytokine Research.2018; 38(12): 527. CrossRef - The effect of a garlic supplement on the pro-inflammatory adipocytokines, resistin and tumor necrosis factor-alpha, and on pain severity, in overweight or obese women with knee osteoarthritis
Sahar Dehghani, Elham Alipoor, Ahmad Salimzadeh, Mehdi Yaseri, Mostafa Hosseini, Christine Feinle-Bisset, Mohammad Javad Hosseinzadeh-Attar
Phytomedicine.2018; 48: 70. CrossRef - Perivascular adipose tissue (PVAT) in atherosclerosis: a double-edged sword
Xiao-Yan Qi, Shun-Lin Qu, Wen-Hao Xiong, Oren Rom, Lin Chang, Zhi-Sheng Jiang
Cardiovascular Diabetology.2018;[Epub] CrossRef - Usefulness of the Adipokines as Biomarkers of Ischemic Cardiac Dysfunction
Larisa-Diana Mocan Hognogi, Cerasela-Mihaela Goidescu, Anca-Daniela Farcaş
Disease Markers.2018; 2018: 1. CrossRef - Circulating fibroblast growth factor 21 in patients with liver cirrhosis
Sabrina Krautbauer, Lisa Rein-Fischboeck, Elisabeth M Haberl, Rebekka Pohl, Reiner Wiest, Christa Buechler
Clinical and Experimental Medicine.2018; 18(1): 63. CrossRef - Association of Cord Blood Resistin with Neonatal Birth Weight and Gestational Age
Shahnaz Pourarian, Saeed Fotouhikia, Forough Saki
Journal of Comprehensive Pediatrics.2018;[Epub] CrossRef - Major Adipokines and the −420C>G Resistin Gene Polymorphism as Predictors of Acute Ischemic Stroke Severity and In-Hospital Outcome
Styliani D. Bouziana, Konstantinos Tziomalos, Antonios Goulas, Timoleon-Achilleas Vyzantiadis, Athanasia Panderi, Apostolos Ι. Ηatzitolios
Journal of Stroke and Cerebrovascular Diseases.2018; 27(4): 963. CrossRef - Resistin and NGAL are associated with inflammatory response, endothelial activation and clinical outcomes in sepsis
Stephen P. J. Macdonald, Erika Bosio, Claire Neil, Glenn Arendts, Sally Burrows, Lisa Smart, Simon G. A. Brown, Daniel M. Fatovich
Inflammation Research.2017; 66(7): 611. CrossRef - Reference values for fasting serum resistin in healthy children and adolescents
Ulrik Lausten-Thomsen, Michael Christiansen, Paula Louise Hedley, Tenna Ruest Haarmark Nielsen, Cilius Esmann Fonvig, Oluf Pedersen, Torben Hansen, Jens-Christian Holm
Clinica Chimica Acta.2017; 469: 161. CrossRef - Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis
Alexander Kalinkovich, Gregory Livshits
Ageing Research Reviews.2017; 35: 200. CrossRef - Is There Any Relationship between Plasma 25-Hydroxyvitamin D3, Adipokine Profiles and Excessive Body Weight in Type 2 Diabetic Patients?
Joanna Kocot, Piotr Dziemidok, Małgorzata Kiełczykowska, Jacek Kurzepa, Grzegorz Szcześniak, Irena Musik
International Journal of Environmental Research and Public Health.2017; 15(1): 19. CrossRef - Exogenous Adipokine Peptide Resistin Protects Against Focal Cerebral Ischemia/Reperfusion Injury in Mice
Jiangtao Zhu, Di Wu, Chenyu Zhao, Man Luo, Ronald C. Hamdy, Balvin H. L. Chua, Xingshun Xu, Zhigang Miao
Neurochemical Research.2017; 42(10): 2949. CrossRef - Adipokines in Liver Cirrhosis
Christa Buechler, Elisabeth Haberl, Lisa Rein-Fischboeck, Charalampos Aslanidis
International Journal of Molecular Sciences.2017; 18(7): 1392. CrossRef - The role of sex steroids in white adipose tissue adipocyte function
A E Newell-Fugate
Reproduction.2017; 153(4): R133. CrossRef - Odanacatib Inhibits Resistin-induced Cardiomyocyte Hypertrophy Through the Inactivation of ERK Signaling Pathway
Xian Zheng, Guanchang Cheng, Jianwei Luo, Qunhui Ye, Yongzhi Deng, Lin Wu
International Journal of Pharmacology.2017; 13(2): 212. CrossRef - Linking resistin, inflammation, and cardiometabolic diseases
Hyeong Kyu Park, Mi Kyung Kwak, Hye Jeong Kim, Rexford S. Ahima
The Korean Journal of Internal Medicine.2017; 32(2): 239. CrossRef - Translating the biology of adipokines in atherosclerosis and cardiovascular diseases: Gaps and open questions
M. Ruscica, A. Baragetti, A.L. Catapano, G.D. Norata
Nutrition, Metabolism and Cardiovascular Diseases.2017; 27(5): 379. CrossRef - Differences in Mean Levels of Maternal Resistin Serum between Early Onset Preeclampsia (EOPE) and Late Onset Preeclampsia (LOPE)
Yusrawati ., P. Alfajra, R. Machmud
Research Journal of Obstetrics and Gynecology.2016; 10(1): 1. CrossRef - Secret talk between adipose tissue and central nervous system via secreted factors—an emerging frontier in the neurodegenerative research
Avinash Parimisetty, Anne-Claire Dorsemans, Rana Awada, Palaniyandi Ravanan, Nicolas Diotel, Christian Lefebvre d’Hellencourt
Journal of Neuroinflammation.2016;[Epub] CrossRef - The role of adipokines in ischemic stroke risk stratification
Styliani Bouziana, Konstantinos Tziomalos, Antonios Goulas, Apostolos Ι Ηatzitolios
International Journal of Stroke.2016; 11(4): 389. CrossRef - The endocrine function of human placenta: an overview
Mariana A. Costa
Reproductive BioMedicine Online.2016; 32(1): 14. CrossRef - Ursolic acid plays a protective role in obesity-induced cardiovascular diseases
Yu-Ting Lin, Ya-Mei Yu, Weng-Cheng Chang, Su-Yin Chiang, Hsu-Chin Chan, Ming-Fen Lee
Canadian Journal of Physiology and Pharmacology.2016; 94(6): 627. CrossRef - Determinants of body weight regulation in humans
Milene Moehlecke, Luis Henrique Canani, Lucas Oliveira Junqueira e Silva, Manoel Roberto Maciel Trindade, Rogerio Friedman, Cristiane Bauermann Leitão
Archives of Endocrinology and Metabolism.2016; 60(2): 152. CrossRef - Sitagliptin decreases ventricular arrhythmias by attenuated glucose-dependent insulinotropic polypeptide (GIP)-dependent resistin signalling in infarcted rats
Tsung-Ming Lee, Wei-Ting Chen, Nen-Chung Chang
Bioscience Reports.2016;[Epub] CrossRef - Les adipokines : état des lieux et nouveautés
J.-P. Bastard, C. Bastard, S. Fellahi, C. Vatier, J. Capeau, B. Fève
Obésité.2016; 11(3): 181. CrossRef - Factors that promote macrophage homing to adipose tissue in metabolic syndrome
Ishwarlal Jialal, Beverley Adams-Huet, Sridevi Devaraj
Journal of Diabetes and its Complications.2016; 30(8): 1434. CrossRef - Uncovering Factors Related to Pancreatic Beta-Cell Function
Aoife M. Curran, Miriam F. Ryan, Elaine Drummond, Eileen R. Gibney, Michael J. Gibney, Helen M. Roche, Lorraine Brennan, Nigel Irwin
PLOS ONE.2016; 11(8): e0161350. CrossRef - Resistin’s, obesity and insulin resistance: the continuing disconnect between rodents and humans
X. Huang, Z. Yang
Journal of Endocrinological Investigation.2016; 39(6): 607. CrossRef - Adipocytokines in renal transplant recipients
Kristof Nagy, Shankar Prasad Nagaraju, Connie M. Rhee, Zoltan Mathe, Miklos Z. Molnar
Clinical Kidney Journal.2016; 9(3): 359. CrossRef - Endocrine alterations from concentric vs. eccentric muscle actions: A brief review
Robert R. Kraemer, V. Daniel Castracane
Metabolism.2015; 64(2): 190. CrossRef - Non-traditional cytokines: How catecholamines and adipokines influence macrophages in immunity, metabolism and the central nervous system
Mark A. Barnes, Monica J. Carson, Meera G. Nair
Cytokine.2015; 72(2): 210. CrossRef - Local and serum levels of adipokines in patients with obesity after periodontal therapy: one‐year follow‐up
Tiago Eduardo Dias Gonçalves, Glaucia Santos Zimmermann, Luciene Cristina Figueiredo, Monique de Carvalho Souza, Daniele Ferreira da Cruz, Marta Ferreira Bastos, Hélio Doyle Pereira da Silva, Poliana Mendes Duarte
Journal of Clinical Periodontology.2015; 42(5): 431. CrossRef - Newborn Adipokines and Birth Outcomes
Edwina H. Yeung, Alexander C. McLain, Nancy Anderson, David Lawrence, Nansi S. Boghossian, Charlotte Druschel, Erin Bell
Paediatric and Perinatal Epidemiology.2015; 29(4): 317. CrossRef - The effect of a preparation of minerals, vitamins and trace elements on the cardiac gene expression pattern in male diabetic rats
Márta Sárközy, Gergő Szűcs, Márton Pipicz, Ágnes Zvara, Katalin Éder, Veronika Fekete, Csilla Szűcs, Judit Bárkányi, Csaba Csonka, László G. Puskás, Csaba Kónya, Péter Ferdinandy, Tamás Csont
Cardiovascular Diabetology.2015;[Epub] CrossRef - Diet-induced variability of the resistin gene (Retn) transcript level and methylation profile in rats
Joanna Nowacka-Woszuk, Ewa Pruszynska-Oszmalek, Maciej Szydlowski, Slawomir Sadkowski, Izabela Szczerbal
BMC Genetics.2015;[Epub] CrossRef - INFLUENCE OF RESISTIN ON THE COURSE OF ISCHEMIC HEART DISEASE IN PATIENTS WITH TYPE 2 DIABETES MELLITUS
A. T. Teplyakov, Sh. D. Akhmedov, T. Ye. Suslova, А. V. Andriyanova, A. V. Kuznetsova, N. V. Protopopova, V. V. Kalyuzhin, O. N. Nasanova
Bulletin of Siberian Medicine.2015; 14(5): 73. CrossRef - The Effects of a Single Developmentally Entrained Pulse of Testosterone in Female Neonatal Mice on Reproductive and Metabolic Functions in Adult Life
Hyeran Jang, Shalender Bhasin, Tyler Guarneri, Carlo Serra, Mary Schneider, Mi-Jeong Lee, Wen Guo, Susan K. Fried, Karol Pencina, Ravi Jasuja
Endocrinology.2015; 156(10): 3737. CrossRef - The Resin fromProtium heptaphyllumPrevents High-Fat Diet-Induced Obesity in Mice: Scientific Evidence and Potential Mechanisms
Karine Maria Martins Bezerra Carvalho, José Delano Barreto Marinho Filho, Tiago Sousa de Melo, Ana Jérsia Araújo, Josiane da Silva Quetz, Maria do Perpétuo Socorro Saldanha da Cunha, Karina Moura de Melo, Armenio Andre de Carvalho Almeida da Silva, Adrian
Evidence-Based Complementary and Alternative Medicine.2015; 2015: 1. CrossRef - Evolution of the Vertebrate Resistin Gene Family
Qingda Hu, Huanran Tan, David M. Irwin, Marc Robinson-Rechavi
PLOS ONE.2015; 10(6): e0130188. CrossRef - Obesity, adipokines and neuroinflammation
Argel Aguilar-Valles, Wataru Inoue, Christoph Rummel, Giamal N. Luheshi
Neuropharmacology.2015; 96: 124. CrossRef - Adipokines at the crossroad between obesity and cardiovascular disease
Filippo Molica, Sandrine Morel, Brenda Kwak, Françoise Rohner-Jeanrenaud, Sabine Steffens
Thrombosis and Haemostasis.2015; 113(03): 553. CrossRef - Resistin – 420 C/G polymorphism and serum resistin level in Iranian patients with gestational diabetes mellitus
Mohammad Ali Takhshid, Zinab Zare
Journal of Diabetes & Metabolic Disorders.2015;[Epub] CrossRef - Ictal adipokines are associated with pain severity and treatment response in episodic migraine
Nu Cindy Chai, Bizu Gelaye, Gretchen E. Tietjen, Paul D. Dash, Barbara A. Gower, Linda W. White, Thomas N. Ward, Ann I. Scher, B. Lee Peterlin
Neurology.2015; 84(14): 1409. CrossRef - Inflammation and insulin/IGF-1 resistance as the possible link between obesity and neurodegeneration
Lindsay J. Spielman, Jonathan P. Little, Andis Klegeris
Journal of Neuroimmunology.2014; 273(1-2): 8. CrossRef - Wild Blueberries (Vaccinium myrtillus) Alleviate Inflammation and Hypertension Associated with Developing Obesity in Mice Fed with a High-Fat Diet
Otto T. Mykkänen, Anne Huotari, Karl-Heinz Herzig, Thomas W. Dunlop, Hannu Mykkänen, Pirkka V. Kirjavainen, Michael Müller
PLoS ONE.2014; 9(12): e114790. CrossRef - Role of fat and adipokines in intestinal inflammation
LeaI Kredel, Arvind Batra, Britta Siegmund
Current Opinion in Gastroenterology.2014; 30(6): 559. CrossRef -
13C metabolic flux analysis shows that resistin impairs the metabolic response to insulin in L6E9 myotubes
Shirley Guzmán, Silvia Marin, Anibal Miranda, Vitaly A Selivanov, Josep J Centelles, Romain Harmancey, Fatima Smih, Annie Turkieh, Yves Durocher, Antonio Zorzano, Philippe Rouet, Marta Cascante
BMC Systems Biology.2014;[Epub] CrossRef - Bee Pollen Improves Muscle Protein and Energy Metabolism in Malnourished Old Rats through Interfering with the Mtor Signaling Pathway and Mitochondrial Activity
Jérôme Salles, Nicolas Cardinault, Véronique Patrac, Alexandre Berry, Christophe Giraudet, Marie-Laure Collin, Audrey Chanet, Camille Tagliaferri, Philippe Denis, Corinne Pouyet, Yves Boirie, Stéphane Walrand
Nutrients.2014; 6(12): 5500. CrossRef
- Resistin – A Plausible Therapeutic Target in the Pathogenesis of Psoriasis
- Post-Renal Transplant Diabetes Mellitus in Korean Subjects: Superimposition of Transplant-Related Immunosuppressant Factors on Genetic and Type 2 Diabetic Risk Factors
- Hyun Chul Lee
- Diabetes Metab J. 2012;36(3):199-206. Published online June 14, 2012
- DOI: https://doi.org/10.4093/dmj.2012.36.3.199
- 3,605 View
- 31 Download
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Postrenal transplantation diabetes mellitus (PTDM), or new-onset diabetes after organ transplantation, is an important chronic transplant-associated complication. Similar to type 2 diabetes, decreased insulin secretion and increased insulin resistance are important to the pathophysiologic mechanism behind the development of PTDM. However, β-cell dysfunction rather than insulin resistance seems to be a greater contributing factor in the development of PTDM. Increased age, family history of diabetes, ethnicity, genetic variation, obesity, and hepatitis C are partially accountable for an increased underlying risk of PTDM in renal allograft recipients. In addition, the use of and kinds of immunosuppressive agents are key transplant-associated risk factors. Recently, a number of genetic variants or polymorphisms susceptible to immunosuppressants have been reported to be associated with calcineurin inhibition-induced β-cell dysfunction. The identification of high risk factors of PTDM would help prevent PTDM and improve long-term patient outcomes by allowing for personalized immunosuppressant regimens and by managing cardiovascular risk factors.
-
Citations
Citations to this article as recorded by- Risk Factors Related to New-Onset Diabetes after Renal Transplantation in Patients of a High Complexity University Hospital in Colombia, 20 Years of Experience
Guillermo E. Guzmán, Angela M. Victoria, Isabella Ramos, Alejandro Maldonado, Eliana Manzi, Juan F. Contreras-Valero, Liliana Mesa, Johanna Schweineberg, Juan G. Posada, Jorge I. Villegas, Luis A. Caicedo, Carlos E. Durán
International Journal of Endocrinology.2020; 2020: 1. CrossRef - Synthesis of Fructose Biosensors and Progressing Their Efficiency Using Californium Colloidal Nanoparticles for Detecting Fructose and Triglycerides
Alireza Heidari
Advanced Science, Engineering and Medicine.2020; 12(8): 1002. CrossRef - Comparison of Glucose Tolerance between Kidney Transplant Recipients and Healthy Controls
Hisao Shimada, Junji Uchida, Shunji Nishide, Kazuya Kabei, Akihiro Kosoku, Keiko Maeda, Tomoaki Iwai, Toshihide Naganuma, Yoshiaki Takemoto, Tatsuya Nakatani
Journal of Clinical Medicine.2019; 8(7): 920. CrossRef - Diabètes post-transplantation rénale
Danièle Dubois-Laforgue
Néphrologie & Thérapeutique.2017; 13: S137. CrossRef - Risk assessment and management of post-transplant diabetes mellitus
Eugene Han, Myoung Soo Kim, Yu Seun Kim, Eun Seok Kang
Metabolism.2016; 65(10): 1559. CrossRef - Renal posttransplantation diabetes mellitus: An overview
Ana Laura Pimentel, Andrea Carla Bauer, Joíza Lins Camargo
Clinica Chimica Acta.2015; 450: 327. CrossRef - HMG CoA Reductase Inhibitor Treatment Induces Dysglycemia in Renal Allograft Recipients
Eun Yeong Choe, Hye Jin Wang, Obin Kwon, Yongin Cho, Kyu Ha Huh, Myoung Soo Kim, Yu Seun Kim, Chul Woo Ahn, Bong Soo Cha, Hyun Chul Lee, Eun Seok Kang
Transplantation.2014; 97(4): 419. CrossRef - Statin therapy is associated with the development of new-onset diabetes after transplantation in liver recipients with high fasting plasma glucose levels
Yongin Cho, Min Jung Lee, Eun Yeong Choe, Chang Hee Jung, Dong Jin Joo, Myoung Soo Kim, Bong Soo Cha, Joong-Yeol Park, Eun Seok Kang
Liver Transplantation.2014; 20(5): 557. CrossRef - Post-Transplant Diabetes Mellitus: Is It Associated With Poor Allograft Outcomes in Renal Transplants?
J.Y. Choi, O.J. Kwon
Transplantation Proceedings.2013; 45(8): 2892. CrossRef
- Risk Factors Related to New-Onset Diabetes after Renal Transplantation in Patients of a High Complexity University Hospital in Colombia, 20 Years of Experience
- The Search for Genetic Risk Factors of Type 2 Diabetes Mellitus
- Kyong Soo Park
- Diabetes Metab J. 2011;35(1):12-22. Published online February 28, 2011
- DOI: https://doi.org/10.4093/dmj.2011.35.1.12
- 4,768 View
- 58 Download
- 23 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Type 2 diabetes mellitus (T2DM) is caused by complex interplay between multiple genetic and environmental factors. The three major approaches used to identify the genetic susceptibility include candidate gene approach, familial linkage analysis and genome- wide association analysis. Recent advance in genome-wide association studies have greatly improved our understanding of the pathophysiology of T2DM. As of the end of 2010, there are more than 40 confirmed T2DM-associated genetic loci. Most of the T2DM susceptibility genes were implicated in decreased β-cell function. However, these genetic variations have a modest effect and their combination only explains less than 10% of the T2DM heritability. With the advent of the next-generation sequencing technology, we will soon identify rare variants of larger effect as well as causal variants. These advances in understanding the genetics of T2DM will lead to the development of new therapeutic and preventive strategies and individualized medicine.
-
Citations
Citations to this article as recorded by- Diabetes: Risk factor and translational therapeutic implications for Alzheimer's disease
Jeffrey Cummings, Andrew Ortiz, Janelle Castellino, Jefferson Kinney
European Journal of Neuroscience.2022; 56(9): 5727. CrossRef - Association of gene polymorphisms with body weight changes in prediabetic patients
Farida V. Valeeva, Mariya S. Medvedeva, Kamilya B. Khasanova, Elena V. Valeeva, Tatyana A. Kiseleva, Emiliya S. Egorova, Craig Pickering, Ildus I. Ahmetov
Molecular Biology Reports.2022; 49(6): 4217. CrossRef - Analysis of the association of FTO, PPARG and PPARGC1A gene polymorphisms with carbohydrate metabolism disorders
Farida V. Valeeva, Kamilya B. Khasanova, Elizaveta A. Sozinova, Tatyana A. Kiseleva, Elena V. Valeeva, Emiliya S. Egorova, Ildus I. Ahmetov
Kazan medical journal.2022; 103(4): 592. CrossRef - Associations between new and old anthropometric indices with type 2 diabetes mellitus and risk of metabolic complications: a cross-sectional analytical study
Parichehr Amiri, Ahmad Zare Javid, Leila Moradi, Neda Haghighat, Rahim Moradi, Hossein Bavi Behbahani, Milad Zarrin, Hadi Bazyar
Jornal Vascular Brasileiro.2021;[Epub] CrossRef - When will individuals meet their personalized probabilities? A philosophical note on risk prediction
Olaf M. Dekkers, Jesse M. Mulder
European Journal of Epidemiology.2020; 35(12): 1115. CrossRef - From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research
Radia Khan, Zoey Chua, Jia Tan, Yingying Yang, Zehuan Liao, Yan Zhao
Medicina.2019; 55(9): 546. CrossRef - Systematic analysis of genes and diseases using PheWAS-Associated networks
Ali Khosravi, Morteza Kouhsar, Bahram Goliaei, B. Jayaram, Ali Masoudi-Nejad
Computers in Biology and Medicine.2019; 109: 311. CrossRef - Protective effects of asiatic acid in a spontaneous type 2 diabetic mouse model
Wen Sun, Guangyuan Xu, Xuan Guo, Guangbin Luo, Lili Wu, Yi Hou, Xiangyu Guo, Jingxin Zhou, Tunhai Xu, Lingling Qin, Yixin Fan, Li Han, Motlalepula Matsabisa, Xuesheng Ma, Tonghua Liu
Molecular Medicine Reports.2017; 16(2): 1333. CrossRef - Are We in the Same Risk of Diabetes Mellitus? Gender- and Age-Specific Epidemiology of Diabetes in 2001 to 2014 in the Korean Population
Bo Kyung Koo, Min Kyong Moon
Diabetes & Metabolism Journal.2016; 40(3): 175. CrossRef - Efficient Strategy to Identify Gene-Gene Interactions and Its Application to Type 2 Diabetes
Donghe Li, Sungho Won
Genomics & Informatics.2016; 14(4): 160. CrossRef - Genetic polymorphisms associated with overweight and obesity in uncontrolled Type 2 diabetes mellitus
Nor Bahirah Kasim, Hasniza Zaman Huri, Shireene Ratna Vethakkan, Luqman Ibrahim, Bashar Mudhaffar Abdullah
Biomarkers in Medicine.2016; 10(4): 403. CrossRef - A multicenter clinical study to determine the efficacy of a novel fenugreek seed (Trigonella foenum-graecum) extract (Fenfuro™) in patients with type 2 diabetes
Narsingh Verma, Kauser Usman, Naresh Patel, Arvind Jain, Sudhir Dhakre, Anand Swaroop, Manashi Bagchi, Pawan Kumar, Harry G. Preuss, Debasis Bagchi
Food & Nutrition Research.2016; 60(1): 32382. CrossRef - Association between -308G/A TNFA Polymorphism and Susceptibility to Type 2 Diabetes Mellitus: A Systematic Review
Geisa Izetti Luna, Izabel Cristina Rodrigues da Silva, Mauro Niskier Sanchez
Journal of Diabetes Research.2016; 2016: 1. CrossRef - Metabolomics – the complementary field in systems biology: a review on obesity and type 2 diabetes
Mohamad Hafizi Abu Bakar, Mohamad Roji Sarmidi, Kian-Kai Cheng, Abid Ali Khan, Chua Lee Suan, Hasniza Zaman Huri, Harisun Yaakob
Molecular BioSystems.2015; 11(7): 1742. CrossRef - Predictive modeling for incident and prevalent diabetes risk evaluation
Katya L Masconi, Justin Basile Echouffo-Tcheugui, Tandi E Matsha, Rajiv T Erasmus, Andre Pascal Kengne
Expert Review of Endocrinology & Metabolism.2015; 10(3): 277. CrossRef - Polymorphism of gene UBE2E2 and the risk of developing diabetes type 2
Elena Vladimirovna Kazakova, Yanhui Wu, Meijun Chen, Tongtong Wang, Lulu Sun, Hong Qiao
Diabetes mellitus.2015; 18(3): 46. CrossRef - The Architecture of Risk for Type 2 Diabetes: Understanding Asia in the Context of Global Findings
Noraidatulakma Abdullah, John Attia, Christopher Oldmeadow, Rodney J. Scott, Elizabeth G. Holliday
International Journal of Endocrinology.2014; 2014: 1. CrossRef - Translational medicine as a new clinical tool and application which improves metabolic diseases: perspectives from 2012 Sino‐American symposium on clinical and translational medicine
Lin Shi, Elena López Villar, Chengshui Chen
Clinical and Translational Medicine.2014;[Epub] CrossRef - Frequency of Fat Mass and Obesity-Associated Gene rs9939609 and Peroxisome Proliferator-Activated Receptor Gamma 2 Gene rs1801282 Polymorphisms among Trinidadian Neonates of Different Ethnicities and Their Relationship to Anthropometry at Birth
Candace E. Cuthbert, D. Dan Ramdath, Jerome E. Foster
Lifestyle Genomics.2014; 7(1): 39. CrossRef - Genetics of type 2 diabetes and potential clinical implications
Soo Heon Kwak, Kyong Soo Park
Archives of Pharmacal Research.2013; 36(2): 167. CrossRef - Genetics in Diabetes Mellitus - Contribution to the Classification and Management
Jeesuk Yu
Annals of Pediatric Endocrinology & Metabolism.2012; 17(4): 211. CrossRef - Genome-wide association studies with metabolomics
Jerzy Adamski
Genome Medicine.2012; 4(4): 34. CrossRef - Typ-2-Diabetes-assoziierte Gene
J. Kriebel, H. Grallert, T. Illig
Der Diabetologe.2012; 8(1): 26. CrossRef
- Diabetes: Risk factor and translational therapeutic implications for Alzheimer's disease
- R1467H Variants of Rho Guanine Nucleotide Exchange Factor 11 (
ARHGEF11 ) are Associated with Type 2 Diabetes Mellitus in Koreans - Qing Song Jin, So Hun Kim, Shan-Ji Piao, Hyun Ae Lim, Seung Youn Lee, Seong Bin Hong, Yong Seong Kim, Hun-Jae Lee, Moonsuk Nam
- Korean Diabetes J. 2010;34(6):368-373. Published online December 31, 2010
- DOI: https://doi.org/10.4093/kdj.2010.34.6.368
- 4,139 View
- 23 Download
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The human Rho guanine nucleotide exchange factor 11 (
ARHGEF11 ) functions as an activator of Rho GTPases and is thought to influence insulin signaling. The R1467H variant ofARHGEF11 has been reported to be associated with susceptibility to type 2 diabetes mellitus (T2DM) in Western populations.Methods We investigated the effects of the R1467H variant on susceptibility to T2DM as well as related traits in a Korean population. We genotyped the R1467H (rs945508) of
ARHGEF11 in 689 unrelated T2DM patients and 249 non-diabetic individuals and compared the clinical and biochemical characteristics according to different alleles.Results The H allele was significantly more frequent in T2DM cases than in controls (
P = 0.037, 17.1% and 13.1%; respectively). H homozygocity was associated with a higher risk of T2DM compared to those with R/R or R/H genotype (odds ratio, 5.24; 95% confidence interval, 1.06 to 25.83;P = 0.042). The fasting plasma glucose, HbA1c, fasting insulin, HOMA2-IR and HOMA2-%β levels did not differ significantly between different genotypes.Conclusion Our study replicated associations of the
ARHGEF11 polymorphism with increased risk of T2DM in a Korean population and thus supports previous data implicating a potential role ofARHGEF11 in the etiology of T2DM. Further studies revealing the underlying mechanism for this association are needed.-
Citations
Citations to this article as recorded by- Epigenetic alteration of Rho guanine nucleotide exchange Factor 11 (ARHGEF11) in cord blood samples in macrosomia exposed to intrauterine hyperglycemia
Jie Yan, Rina Su, Wanyi Zhang, Yumei Wei, Chen Wang, Li Lin, Hui Feng, Huixia Yang
The Journal of Maternal-Fetal & Neonatal Medicine.2021; 34(3): 422. CrossRef -
Loss of
Arhgef11
in the Dahl Salt-Sensitive Rat Protects Against Hypertension-Induced Renal Injury
Ashley C. Johnson, Wenjie Wu, Esinam M. Attipoe, Jennifer M. Sasser, Erin B. Taylor, Kurt C. Showmaker, Patrick B. Kyle, Merry L. Lindsey, Michael R. Garrett
Hypertension.2020; 75(4): 1012. CrossRef - Transgenerational Obesity and Alteration of ARHGEF11 in the Rat Liver Induced by Intrauterine Hyperglycemia
Wanyi Zhang, Rina Su, Hui Feng, Li Lin, Chen Wang, Huixia Yang
Journal of Diabetes Research.2019; 2019: 1. CrossRef - ARHGEF11 affecting the placental insulin signaling pathway in fetal macrosomia of normal glucose tolerance pregnant women
Wanyi Zhang, Rina Su, Li Lin, Huixia Yang
Placenta.2018; 63: 7. CrossRef - Genetic variants and clinical relevance associated with gestational diabetes mellitus in Chinese women: a case-control study
Jie Yan, Rina Su, Deng Ao, Yan Wang, Haijun Wang, Huixia Yang
The Journal of Maternal-Fetal & Neonatal Medicine.2018; 31(16): 2115. CrossRef - Human Rho Guanine Nucleotide Exchange Factor 11 (ARHGEF11) Regulates Dendritic Morphogenesis
Yutaka Mizuki, Manabu Takaki, Shinji Sakamoto, Sojiro Okamoto, Makiko Kishimoto, Yuko Okahisa, Masahiko Itoh, Norihito Yamada
International Journal of Molecular Sciences.2016; 18(1): 67. CrossRef - Allelic Variants in Arhgef11 via the Rho-Rock Pathway Are Linked to Epithelial–Mesenchymal Transition and Contributes to Kidney Injury in the Dahl Salt-Sensitive Rat
Zhen Jia, Ashley C. Johnson, Xuexiang Wang, Zibiao Guo, Albert W. Dreisbach, Jack R. Lewin, Patrick B. Kyle, Michael R. Garrett, Maria Pia Rastaldi
PLOS ONE.2015; 10(7): e0132553. CrossRef - The Rho-guanine nucleotide exchange factor PDZ-RhoGEF governs susceptibility to diet-induced obesity and type 2 diabetes
Ying-Ju Chang, Scott Pownall, Thomas E Jensen, Samar Mouaaz, Warren Foltz, Lily Zhou, Nicole Liadis, Minna Woo, Zhenyue Hao, Previn Dutt, Philip J Bilan, Amira Klip, Tak Mak, Vuk Stambolic
eLife.2015;[Epub] CrossRef - Human Rho guanine nucleotide exchange factor 11 gene is associated with schizophrenia in a Japanese population
Yutaka Mizuki, Manabu Takaki, Yuko Okahisa, Shinji Sakamoto, Masafumi Kodama, Hiroshi Ujike, Yosuke Uchitomi
Human Psychopharmacology: Clinical and Experimental.2014; 29(6): 552. CrossRef - Small G proteins and their regulators in cellular signalling
Roland Csépányi-Kömi, Magdolna Lévay, Erzsébet Ligeti
Molecular and Cellular Endocrinology.2012; 353(1-2): 10. CrossRef - The Duration of Sulfonylurea Treatment Is Associated withβ-Cell Dysfunction in Patients with Type 2 Diabetes Mellitus
Mi-Seon Shin, Jee Hee Yu, Chang Hee Jung, Jenie Yoonoo Hwang, Woo Je Lee, Min-Seon Kim, Joong-Yeol Park
Diabetes Technology & Therapeutics.2012; 14(11): 1033. CrossRef
- Epigenetic alteration of Rho guanine nucleotide exchange Factor 11 (ARHGEF11) in cord blood samples in macrosomia exposed to intrauterine hyperglycemia
- Polymorphisms of the
Reg 1α Gene and Early Onset Type 2 Diabetes in the Korean Population - Bo Kyung Koo, Young Min Cho, Kuchan Kimm, Jong-Young Lee, Bermseok Oh, Byung Lae Park, Hyun Sub Cheong, Hyoung Doo Shin, Kyung Soo Ko, Sang Gyu Park, Hong Kyu Lee, Kyong Soo Park
- Korean Diabetes J. 2010;34(4):229-236. Published online August 31, 2010
- DOI: https://doi.org/10.4093/kdj.2010.34.4.229
- 3,532 View
- 23 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The
Reg gene has been reported to be expressed in regenerating islets and Reg1 protein to be up-regulated at an early stage of diabetes in mice. As humanReg 1α is homologous with murineReg 1, we investigated whether common variants inReg 1α are associated with type 2 diabetes in the Korean population.Methods We sequenced the
Reg 1α gene to identify common polymorphisms using 24 Korean DNA samples. Of 11 polymorphisms found, five common ones (g.-385T>C [rs10165462], g.-36T>G [rs25689789], g.209G>T [rs2070707], g.1385C>G [novel], and g.2199G>A [novel]) were genotyped in 752 type 2 diabetic patients and 642 non-diabetic subjects.Results No polymorphism was associated with the risk of type 2 diabetes. However, g.-385C and g.2199A lowered the risk of early-onset type 2 diabetes, defined as a diagnosis in subjects whose age at diagnosis was 25 years or more but less than 40 years (odds ratio [OR], 0.721 [0.535 to 0.971] and 0.731 [0.546 to 0.977] for g.-385C and g.2199A, respectively) and g.1385G increased the risk of early-onset diabetes (OR, 1.398 [1.055 to 1.854]). Although adjusting for errors in multiple hypotheses-testing showed no statistically significant association between the three individual polymorphisms and early-onset diabetes, the haplotype
H1 , composed of g.-385C, g.1385C, and g.2199A, was associated with a reduced risk of early-onset diabetes (OR, 0.590 [0.396 to 0.877],P = 0.009).Conclusion Polymorphisms in the
Reg 1α were not found to be associated with overall susceptibility to type 2 diabetes, though some showed modest associations with early-onset type 2 diabetes in the Korean population.-
Citations
Citations to this article as recorded by- Glycemic Effects of Once-a-Day Rapid-Acting Insulin Analogue Addition on a Basal Insulin Analogue in Korean Subjects with Poorly Controlled Type 2 Diabetes Mellitus
Eun Yeong Choe, Yong-ho Lee, Byung-Wan Lee, Eun-Seok Kang, Bong Soo Cha, Hyun Chul Lee
Diabetes & Metabolism Journal.2012; 36(3): 230. CrossRef
- Glycemic Effects of Once-a-Day Rapid-Acting Insulin Analogue Addition on a Basal Insulin Analogue in Korean Subjects with Poorly Controlled Type 2 Diabetes Mellitus
- Genetic Association of Mitochondrial DNA Polymorphisms with Type 2 Diabetes Mellitus.
- Tae Su Han, Jee Hye Choi, Jina Park, Kwang Ho Lee, Ae Ja Park
- Korean Diabetes J. 2009;33(5):382-391. Published online October 1, 2009
- DOI: https://doi.org/10.4093/kdj.2009.33.5.382
- 1,815 View
- 25 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Although many single nucleotide polymorphisms (SNPs) of mtDNA have been found to be associated with type 2 diabetes mellitus, the results of studies using different population samples and different methods are mixed. Therefore, we conducted a genetic association study of mtDNA SNPs and type 2 diabetes mellitus in a Korean sample and compared our results with those of studies conducted in other human populations. METHODS: A total of 298 blood samples from 147 type 2 diabetic patients and 151 normal controls were surveyed for SNPs via PCR directed sequencing. Sequencing analyses were performed using the SeqMan module of the DNASTAR program. The identified SNPs were compared to previously reported SNP lists on NCBI and V-mitoSNP. RESULTS: A total of 24 SNPs were identified in the MT-RNR2, MR-TL1 and MT-ND1 mtDNA genes in Korean type 2 diabetes mellitus patients and normal controls. The SNPs identified in the Korean sample were not closely associated with the type 2 diabetes mellitus phenotype, a significantly different result from those previously observed in European, Chinese and Japanese samples. Additionally, a haplotype and prevalence analysis could not detect any differences between the type 2 diabetes mellitus patients and normal controls. CONCLUSION: The 24 mtDNA SNPs were not associated with type 2 diabetes mellitus risk in our Korean sample. The results of the present study support the possibility that mtDNA SNPs have a differential effect on the risk of type 2 diabetes mellitus according to geographical origin.
- Matrix Metalloproteinase-3 Gene Polymorphism is Associated with Coronary Artery Calcification Scores in Patients with Type 2 Diabetes Mellitus.
- Sang Wook Kim, Eun Hee Cho
- Korean Diabetes J. 2009;33(2):113-123. Published online April 1, 2009
- DOI: https://doi.org/10.4093/kdj.2009.33.2.113
- 1,993 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Matrix metalloproteinase-3 (MMP-3) is expressed in human coronary atherosclerotic lesions and is known to be involved in the degradation of plaque. This study examines the association of MMP-3 gene promoter 5A/6A and -709A>G polymorphisms with coronary artery calcium scores in type 2 diabetes patients. METHODS: The study comprises 140 type 2 diabetes patients aged 34~85 years, who showed no evidence of clinical cardiovascular disease before recruitment. Recruitment was based on patient's coronary artery calcium (CAC) scores and polymorphisms were identified. RESULTS: Multiple regression analysis showed that the CAC scores were significantly associated with age (P = 0.008), waist circumference (P = 0.03), duration of diabetes (P = 0.003) and the serum creatinine level (P = 0.012). MMP-3 5A/6A and -709A>G polymorphisms were not associated with CAC across all subjects. However, in the subgroup with a duration of diabetes over 10 years, MMP-3 -709A>G were significantly associated with CAC (P = 0.037) adjusted for age, body mass index, waist circumference and duration of diabetes. CONCLUSION: Our data suggest that the CAC scores in patients with type 2 diabetes were related with age, waist circumference, duration of diabetes and higher serum creatinine levels. MMP-3 polymorphisms with -709A>G are associated with high CAC in patients with a duration of diabetes over 10 years.
- An Association between 609 C --> T Polymorphism in NAD(P)H: Quinone Oxidoreductase 1 (NQO1) Gene and Blood Glucose Levels in Korean Population.
- Dohee Kim
- Korean Diabetes J. 2009;33(1):24-30. Published online February 1, 2009
- DOI: https://doi.org/10.4093/kdj.2009.33.1.24
- 2,107 View
- 22 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
NAD(P)H: quinone oxidoreductase 1 (NQO1), which is an obligate two-electron reductase that utilizes NAD(P)H as an electron donor and is involved in the protection against oxidative stress, is likely involved in beta-cell destruction. We evaluated the frequency of the NQO1 polymorphism and its association with blood glucose levels. METHODS: Genotypes were determined using a polymerase chain reaction restriction fragment length polymorphism-based assay in 56 patients and 48 healthy subjects. Fasting blood glucose, insulin, and lipid profiles were measured and homeostasis model assessment (HOMA)-insulin resistance (IR) was calculated from fasting glucose and insulin levels in the healthy subjects. RESULTS: The genotype frequencies of NQO1 polymorphism were C/C (56.7%), C/T (42.3%), and T/T (1.0%). There were no associations between the NQO1 polymorphism and body mass index, blood pressure, lipid profile, HbA1c, postprandial glucose, and HOMA-IR. However, NQO1 mutants (C/T and T/T) showed weak but significantly higher fasting blood glucose levels compared with wild type (C/C). CONCLUSION: Our data suggest that NQO1 609 C --> T polymorphism may be associated with glucose metabolism. -
Citations
Citations to this article as recorded by- Association of Nuclear Factor-Erythroid 2-Related Factor 2, Thioredoxin Interacting Protein, and Heme Oxygenase-1 Gene Polymorphisms with Diabetes and Obesity in Mexican Patients
Angélica Saraí Jiménez-Osorio, Susana González-Reyes, Wylly Ramsés García-Niño, Hortensia Moreno-Macías, Martha Eunice Rodríguez-Arellano, Gilberto Vargas-Alarcón, Joaquín Zúñiga, Rodrigo Barquera, José Pedraza-Chaverri
Oxidative Medicine and Cellular Longevity.2016; 2016: 1. CrossRef
- Association of Nuclear Factor-Erythroid 2-Related Factor 2, Thioredoxin Interacting Protein, and Heme Oxygenase-1 Gene Polymorphisms with Diabetes and Obesity in Mexican Patients
- Association Study of the Peroxisome Proliferators-Activated Receptor gamma2 Pro12Ala Polymorphism with Diabetic Nephropathy.
- Kyu Ho Lee, Hee Seog Jeong, Khan Young Choi, Hyun Kim, Dal Sic Lee, Ji Young Kang, Hyun Jeong Jeon, Tae Keun Oh
- Korean Diabetes J. 2008;32(5):402-408. Published online October 1, 2008
- DOI: https://doi.org/10.4093/kdj.2008.32.5.402
- 1,913 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Peroxisome proliferators-activated receptor gamma (PPARgamma) is a member of the nuclear hormone receptor superfamily of ligand-activated transcription factors and known to play a role in regulating the expression of numerous genes involved in lipid metabolism, metabolic syndrome, inflammation, and atherosclerosis. The PPARgamma2 Pro12Ala polymorphism has recently been shown to be associated with diabetic nephropathy. In this study, we evaluated the relationship between PPARgamma2 Pro12Ala polymorphism and type 2 diabetic nephropathy whose duration of diabetes was over 10 years. METHODS: We conducted a case-control study, which enrolled 367 patients with type 2 diabetes. Genotyping of PPARgamma2 Pro12Ala polymorphism was performed using polymerase chain reaction followed by digestion with Hae III restriction enzyme. RESULTS: The genotype or allele frequencies of PPARgamma2 Pro12Ala polymorphism were not significantly different in diabetic patients with or without diabetic nephropathy. The genotype frequencies in terms of diabetic retinopathy and macrovascular complications such as coronary artery disease or stroke were not different either. Interestingly, nephropathy patients with Ala/Pro genotype showed lower C-peptide levels than those of Pro/Pro genotype. CONCLUSION: Our results suggest that PPARgamma2 Pro12Ala polymorphism is not associated with diabetic nephropathy in type 2 diabetic patients.
- Association of the Polymorphisms in the PSMA6 (rs1048990) and PSMB5 (rs2230087) Genes with Type 2 Diabetes in Korean Subjects.
- Hee Kyoung Kim, Su Won Kim, Yun Jeong Doh, Sae Rom Kim, Mi Kyung Kim, Keun Gyu Park, Hye Soon Kim, Kyong Soo Park, Min Yoo, Jung Guk Kim, Bo Wan Kim, In Kyu Lee
- Korean Diabetes J. 2008;32(3):204-214. Published online June 1, 2008
- DOI: https://doi.org/10.4093/kdj.2008.32.3.204
- 2,212 View
- 23 Download
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The 26S ubiquitin-proteasome system (UPS) is a principal proteolytic pathway of intracellular molecules regulating apoptosis, cell cycle, cell proliferation or differentiation, inflammation and etc. The recent study suggests that the rs1048990 (C/G) polymorphism of the proteasome subunit alpha type 6 (PSMA6) gene is associated with the increase of the risk of myocardial infarction by the dysregulation of IkappaB degradation. We hypothesized that 26S UPS is important in the development of insulin resistance and type 2 diabetes (T2DM) by controlling the degradation of IkappaB and insulin receptor substances as a substrate. We therefore investigated whether the rs1048990 (C/G) polymorphism of PSMA6 gene and the rs2230087 (G/A) polymorphism of proteasome subunit beta type 5 gene (PSMB5), that is chymotrypsin-like protease determining the rate of proteolysis, are associated with susceptibility to T2DM in Korean subjects. METHODS: We examined the polymorphisms of these genes in 309 diabetic subjects and 170 non-diabetic controls. The polymorphisms of rs1048990 (C/G) and rs2230087 (G/A) were genotyped by real-time PCR. RESULTS: The frequency of the G allele of rs1048990 (C/G) and the A allele of rs2230087 (G/A) polymorphisms was significantly higher in diabetic patients (28% and 13%) compared to that in controls (13% and 1%; P = 0.000 and P = 0.000, respectively). Logistic regression analysis of the rs1048990 (C/G) polymorphism showed that the odds ratio (OR) (adjusted for age, smoking, waist circumference, fasting plasma glucose, systolic blood pressure, HDL-C, triglyceride, and total cholesterol) was 3.93 (95% confidence interval [CI], 2.35-6.59; P = 0.000) for the G allele and 5.09 (95% CI, 2.71-9.57; P = 0.000) for CG and GG genotype when compared with the CC genotype. Logistic regression analysis of the rs2230087 (G/A) polymorphism showed that the adjusted OR was 5.70 (95% CI, 1.63-19.98; P = 0.007) for the A allele and 6.08 (95% CI, 1.66-22.29; P = 0.006) for GA and AA genotype when compared with the GG genotype. In multiple logistic regression analysis with T2DM as the independent Variable rs1048990 (C/G) and rs2230087 (G/A) polymorphisms were the predictor for T2DM. CONCLUSION: We suggest that the G allele of rs1048990 (C/G) polymorphism and the A allele of rs2230087 (G/A) polymorphism may be genetic risk factor to type 2 diabetes mellitus in Korean subjects. -
Citations
Citations to this article as recorded by- Ubiquitin-proteasome system in diabetic retinopathy
Zane Svikle, Beate Peterfelde, Nikolajs Sjakste, Kristine Baumane, Rasa Verkauskiene, Chi-Juei Jeng, Jelizaveta Sokolovska
PeerJ.2022; 10: e13715. CrossRef - 1,4‐Dihydropyridine derivatives without Ca2+‐antagonist activity up‐regulate Psma6 mRNA expression in kidneys of intact and diabetic rats
Kristīne Ošiņa, Evita Rostoka, Jelizaveta Sokolovska, Natalia Paramonova, Egils Bisenieks, Gunars Duburs, Nikolajs Sjakste, Tatjana Sjakste
Cell Biochemistry and Function.2016; 34(1): 3. CrossRef - Genetic variations in the PSMA3, PSMA6 and PSMC6 genes are associated with type 1 diabetes in Latvians and with expression level of number of UPS-related and T1DM-susceptible genes in HapMap individuals
Tatjana Sjakste, Natalia Paramonova, Kristine Osina, Kristine Dokane, Jelizaveta Sokolovska, Nikolajs Sjakste
Molecular Genetics and Genomics.2016; 291(2): 891. CrossRef
- Ubiquitin-proteasome system in diabetic retinopathy
- Association between Apolipoprotein E Polymorphism and Type 2 Diabetes in Subjects Aged 65 or Over.
- You Jin Lee, Hak Chul Jang, Eun Hye Kim, Hye Jin Kim, Seok Bum Lee, Sung Hee Choi, Soo Lim, Kyoung Un Park, Young Joo Park, Ki Woong Kim
- Korean Diabetes J. 2008;32(1):30-37. Published online February 1, 2008
- DOI: https://doi.org/10.4093/kdj.2008.32.1.30
- 2,205 View
- 27 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Increased prevalence of diabetes in recent years is linked with increased cardiovascular morbidity and mortality. Apolipoprotein E (apo E) polymorphism is well known to be related to hyperlipidemia and coronary heart disease, but only a few studies investigated the association between apo E polymorphism and diabetes or insulin resistance. In Korea, two studies with relatively small subjects reported controversial results. Therefore, we investigated the association between apo E polymorphism and diabetes in elderly community population. METHODS: 982 elderly people aged 65 or over in Seongnam city were enrolled. We measured anthropometric variables and blood pressure and performed biochemical tests including fasting glucose, fasting insulin, HbA1c, and lipid profiles. Apo E polymorphism was determined by PCR-RFLP method. RESULTS: Frequencies of apo E isoforms and alleles were similar to those of other reports. Subjects with e4 allele had significantly higher total and LDL-cholesterol levels. However, there were no differences in cholesterol levels between normal subjects and diabetes. Diabetes was not related to apo E polymorphism. CONCLUSION: In Korean aged 65 or over, subjects with diabetes didn't have increased total or LDL-cholesterol, triglyceride, and decreased HDL-cholesterol levels. Diabetes and apo E polymorphism were not related. -
Citations
Citations to this article as recorded by- Association of APOE genotype with lipid profiles and type 2 diabetes mellitus in a Korean population
Jung Yeon Seo, Byeong Ju Youn, Hyun Sub Cheong, Hyoung Doo Shin
Genes & Genomics.2021; 43(7): 725. CrossRef - Sarcopenia, Frailty, and Diabetes in Older Adults
Hak Chul Jang
Diabetes & Metabolism Journal.2016; 40(3): 182. CrossRef
- Association of APOE genotype with lipid profiles and type 2 diabetes mellitus in a Korean population

 KDA
KDA
 First
First Prev
Prev





