
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Technology/Device
- Do-It-Yourself Open Artificial Pancreas System in Children and Adolescents with Type 1 Diabetes Mellitus: Real-World Data
- Min Sun Choi, Seunghyun Lee, Jiwon Kim, Gyuri Kim, Sung Min Park, Jae Hyeon Kim
- Diabetes Metab J. 2022;46(1):154-159. Published online November 23, 2021
- DOI: https://doi.org/10.4093/dmj.2021.0011
- 5,297 View
- 192 Download
- 5 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
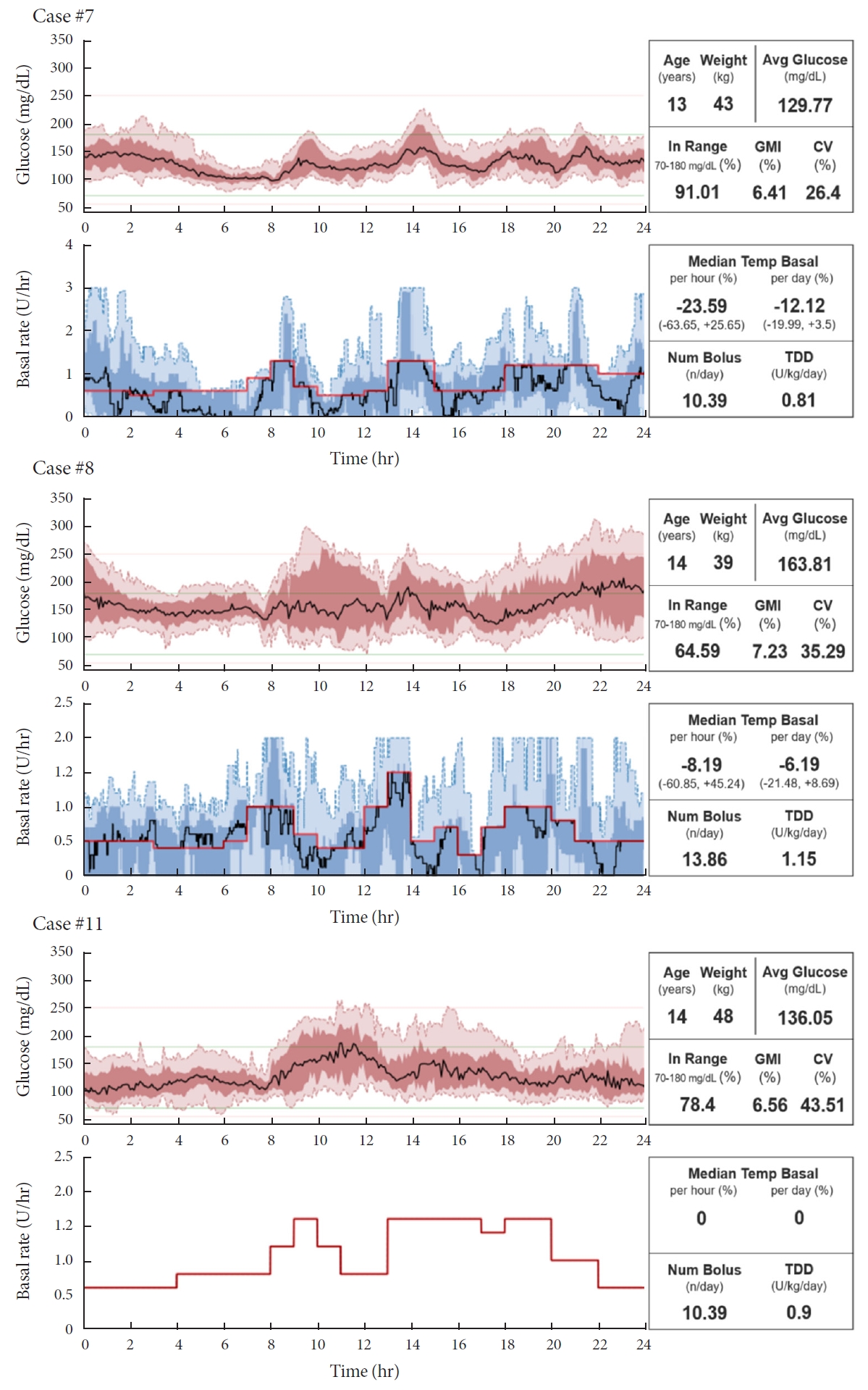
ePub - Few studies have been conducted among Asian children and adolescents with type 1 diabetes mellitus (T1DM) using do-it-yourself artificial pancreas system (DIY-APS). We evaluated real-world data of pediatric T1DM patients using DIY-APS. Data were obtained for 10 patients using a DIY-APS with algorithms. We collected sensor glucose and insulin delivery data from each participant for a period of 4 weeks. Average glycosylated hemoglobin was 6.2%±0.3%. The mean percentage of time that glucose level remained in the target range of 70 to 180 mg/dL was 82.4%±7.8%. Other parameters including time above range, time below range and mean glucose were also within the recommended level, similar to previous commercial and DIY-APS studies. However, despite meeting the target range, unadjusted gaps were still observed between the median basal setting and temporary basal insulin, which should be handled by healthcare providers.
-
Citations
Citations to this article as recorded by- Advances in Continuous Glucose Monitoring and Integrated Devices for Management of Diabetes with Insulin-Based Therapy: Improvement in Glycemic Control
Jee Hee Yoo, Jae Hyeon Kim
Diabetes & Metabolism Journal.2023; 47(1): 27. CrossRef - Open-source automated insulin delivery systems (OS-AIDs) in a pediatric population with type 1 diabetes in a real-life setting: the AWeSoMe study group experience
Judith Nir, Marianna Rachmiel, Abigail Fraser, Yael Lebenthal, Avivit Brener, Orit Pinhas-Hamiel, Alon Haim, Eve Stern, Noa Levek, Tal Ben-Ari, Zohar Landau
Endocrine.2023; 81(2): 262. CrossRef - Efficacy and safety of Android artificial pancreas system use at home among adults with type 1 diabetes mellitus in China: protocol of a 26-week, free-living, randomised, open-label, two-arm, two-phase, crossover trial
Mengyun Lei, Beisi Lin, Ping Ling, Zhigu Liu, Daizhi Yang, Hongrong Deng, Xubin Yang, Jing Lv, Wen Xu, Jinhua Yan
BMJ Open.2023; 13(8): e073263. CrossRef - Barriers to Uptake of Open-Source Automated Insulin Delivery Systems: Analysis of Socioeconomic Factors and Perceived Challenges of Caregivers of Children and Adolescents With Type 1 Diabetes From the OPEN Survey
Antonia Huhndt, Yanbing Chen, Shane O’Donnell, Drew Cooper, Hanne Ballhausen, Katarzyna A. Gajewska, Timothée Froment, Mandy Wäldchen, Dana M. Lewis, Klemens Raile, Timothy C. Skinner, Katarina Braune
Frontiers in Clinical Diabetes and Healthcare.2022;[Epub] CrossRef - Toward Personalized Hemoglobin A1c Estimation for Type 2 Diabetes
Namho Kim, Da Young Lee, Wonju Seo, Nan Hee Kim, Sung-Min Park
IEEE Sensors Journal.2022; 22(23): 23023. CrossRef
- Advances in Continuous Glucose Monitoring and Integrated Devices for Management of Diabetes with Insulin-Based Therapy: Improvement in Glycemic Control
- Technology/Device
- Current Advances of Artificial Pancreas Systems: A Comprehensive Review of the Clinical Evidence
- Sun Joon Moon, Inha Jung, Cheol-Young Park
- Diabetes Metab J. 2021;45(6):813-839. Published online November 22, 2021
- DOI: https://doi.org/10.4093/dmj.2021.0177
- 14,428 View
- 796 Download
- 28 Web of Science
- 28 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
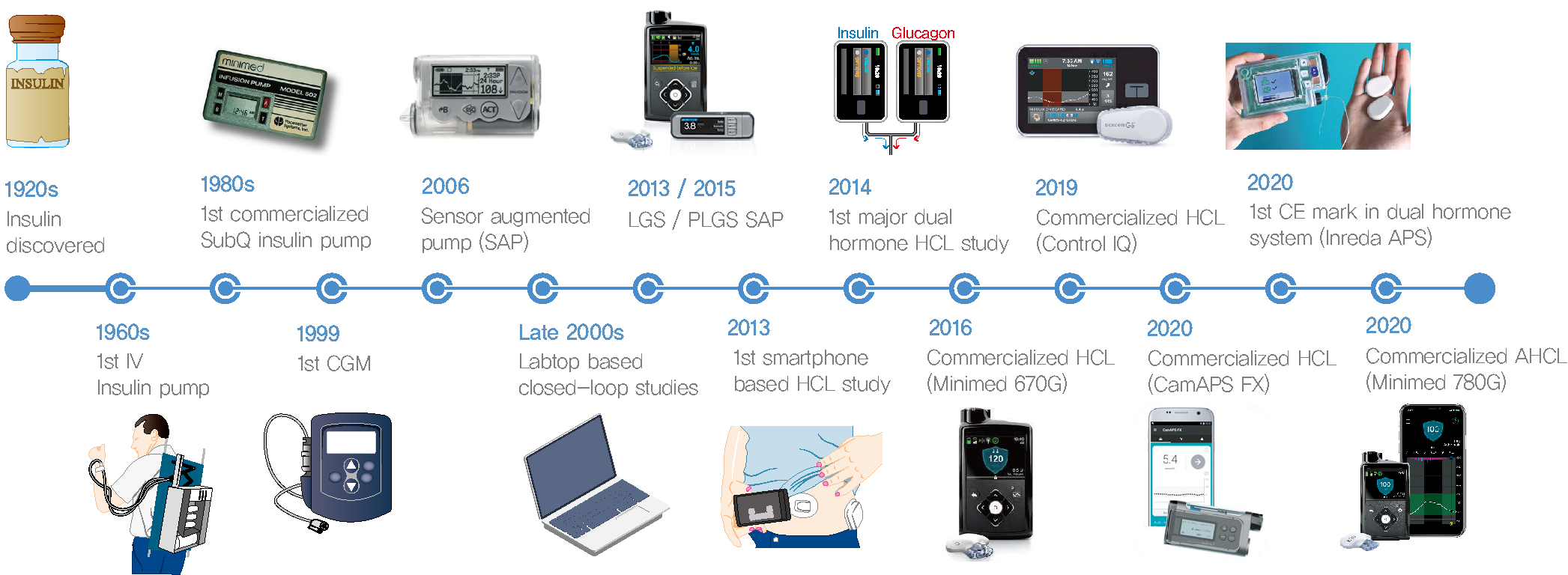
- Since Banting and Best isolated insulin in the 1920s, dramatic progress has been made in the treatment of type 1 diabetes mellitus (T1DM). However, dose titration and timely injection to maintain optimal glycemic control are often challenging for T1DM patients and their families because they require frequent blood glucose checks. In recent years, technological advances in insulin pumps and continuous glucose monitoring systems have created paradigm shifts in T1DM care that are being extended to develop artificial pancreas systems (APSs). Numerous studies that demonstrate the superiority of glycemic control offered by APSs over those offered by conventional treatment are still being published, and rapid commercialization and use in actual practice have already begun. Given this rapid development, keeping up with the latest knowledge in an organized way is confusing for both patients and medical staff. Herein, we explore the history, clinical evidence, and current state of APSs, focusing on various development groups and the commercialization status. We also discuss APS development in groups outside the usual T1DM patients and the administration of adjunct agents, such as amylin analogues, in APSs.
-
Citations
Citations to this article as recorded by- Integration of a Safety Module to Prevent Rebound Hypoglycemia in Closed-Loop Artificial Pancreas Systems
María F. Villa-Tamayo, Patricio Colmegna, Marc D. Breton
Journal of Diabetes Science and Technology.2024; 18(2): 318. CrossRef - The effects of acute hyperglycaemia on sports and exercise performance in type 1 diabetes: A systematic review and meta-analysis
Bonar McGuire, Hashim Dadah, Dominic Oliver
Journal of Science and Medicine in Sport.2024; 27(2): 78. CrossRef - A new approach to stabilize diabetes systems with time-varying delays and disturbance rejection
S. Syafiie, Fahd Alharbi, Abdullah Ali Alshehri, Bassam Hasanain
Journal of the Franklin Institute.2024; 361(1): 543. CrossRef - Effects of Low-Dose Glucagon on Subcutaneous Insulin Absorption in Pigs
Ingrid Anna Teigen, Marte Kierulf Åm, Misbah Riaz, Sverre Christian Christiansen, Sven Magnus Carlsen
Current Therapeutic Research.2024; 100: 100736. CrossRef - Robust Online Correlation Method for Identification of a Nonparametric Model of Type 1 Diabetes
Martin Dodek, Eva Miklovičová
IEEE Access.2024; 12: 35899. CrossRef - 100 Years of insulin: A chemical engineering perspective
B. Wayne Bequette
Korean Journal of Chemical Engineering.2023; 40(1): 1. CrossRef - Efficacy of intermittent short‐term use of a real‐time continuous glucose monitoring system in non‐insulin–treated patients with type 2 diabetes: A randomized controlled trial
Sun Joon Moon, Kyung‐Soo Kim, Woo Je Lee, Mi Yeon Lee, Robert Vigersky, Cheol‐Young Park
Diabetes, Obesity and Metabolism.2023; 25(1): 110. CrossRef - Identifiable prediction animal model for the bi-hormonal intraperitoneal artificial pancreas
Karim Davari Benam, Hasti Khoshamadi, Marte Kierulf Åm, Øyvind Stavdahl, Sebastien Gros, Anders Lyngvi Fougner
Journal of Process Control.2023; 121: 13. CrossRef - Advances in Continuous Glucose Monitoring and Integrated Devices for Management of Diabetes with Insulin-Based Therapy: Improvement in Glycemic Control
Jee Hee Yoo, Jae Hyeon Kim
Diabetes & Metabolism Journal.2023; 47(1): 27. CrossRef - CGM accuracy: Contrasting CE marking with the governmental controls of the USA (FDA) and Australia (TGA): A narrative review
John S Pemberton, Emma G Wilmot, Katharine Barnard‐Kelly, Lalantha Leelarathna, Nick Oliver, Tabitha Randell, Craig E Taplin, Pratik Choudhary, Peter Adolfsson
Diabetes, Obesity and Metabolism.2023; 25(4): 916. CrossRef - Evaluation of awareness and attitude of paediatric nursing students, nurses, and adolescents regarding type one diabetes advanced devices and virtual nursing
Howaida Moawad Ahmed Ali
Kontakt.2023; 25(2): 100. CrossRef - Predicting the output error of the suboptimal state estimator to improve the performance of the MPC-based artificial pancreas
Martin Dodek, Eva Miklovičová
Control Theory and Technology.2023; 21(4): 541. CrossRef - A Markov Model of Gap Occurrence in Continuous Glucose Monitoring Data for Realistic in Silico Clinical Trials
Martina Vettoretti, Martina Drecogna, Simone Del Favero, Andrea Facchinetti, Giovanni Sparacino
Computer Methods and Programs in Biomedicine.2023; 240: 107700. CrossRef - Drug delivery breakthrough technologies – A perspective on clinical and societal impact
Beate Bittner, Manuel Sánchez-Félix, Dennis Lee, Athanas Koynov, Joshua Horvath, Felix Schumacher, Simon Matoori
Journal of Controlled Release.2023; 360: 335. CrossRef - Importance of continuous glucose monitoring in the treatment of diabetes mellitus
Sun Joon Moon, Won-Young Lee
Journal of the Korean Medical Association.2023; 66(7): 432. CrossRef - Constrained Versus Unconstrained Model Predictive Control for Artificial Pancreas
Chiara Toffanin, Lalo Magni
IEEE Transactions on Control Systems Technology.2023; 31(5): 2288. CrossRef - Intelligent Insulin vs. Artificial Intelligence for Type 1 Diabetes: Will the Real Winner Please Stand Up?
Valentina Maria Cambuli, Marco Giorgio Baroni
International Journal of Molecular Sciences.2023; 24(17): 13139. CrossRef - Artificial Intelligence in Efficient Diabetes Care
Gopal Bhagwan Khodve, Sugato Banerjee
Current Diabetes Reviews.2023;[Epub] CrossRef - The artificial pancreas: two alternative approaches to achieve a fully closed-loop system with optimal glucose control
M. K. Åm, I. A. Teigen, M. Riaz, A. L. Fougner, S. C. Christiansen, S. M. Carlsen
Journal of Endocrinological Investigation.2023; 47(3): 513. CrossRef - Multivariable Automated Insulin Delivery System for Handling Planned and Spontaneous Physical Activities
Mohammad Reza Askari, Mohammad Ahmadasas, Andrew Shahidehpour, Mudassir Rashid, Laurie Quinn, Minsun Park, Ali Cinar
Journal of Diabetes Science and Technology.2023; 17(6): 1456. CrossRef - Advanced Technology (Continuous Glucose Monitoring and Advanced Hybrid Closed-Loop Systems) in Diabetes from the Perspective of Gender Differences
Maria Grazia Nuzzo, Marciano Schettino
Diabetology.2023; 4(4): 519. CrossRef - Artificial Pancreas under a Zone Model Predictive Control based on Gaussian Process models: toward the personalization of the closed loop
Marco Polver, Beatrice Sonzogni, Mirko Mazzoleni, Fabio Previdi, Antonio Ferramosca
IFAC-PapersOnLine.2023; 56(2): 9642. CrossRef - Personalized Constrained MPC for glucose regulation
Chiara Toffanin, Lalo Magni
IFAC-PapersOnLine.2023; 56(2): 9648. CrossRef - Automated Insulin Delivery Systems in Children and Adolescents With Type 1 Diabetes: A Systematic Review and Meta-analysis of Outpatient Randomized Controlled Trials
Baoqi Zeng, Le Gao, Qingqing Yang, Hao Jia, Feng Sun
Diabetes Care.2023; 46(12): 2300. CrossRef - Novel Glycemic Index Based on Continuous Glucose Monitoring to Predict Poor Clinical Outcomes in Critically Ill Patients: A Pilot Study
Eun Yeong Ha, Seung Min Chung, Il Rae Park, Yin Young Lee, Eun Young Choi, Jun Sung Moon
Frontiers in Endocrinology.2022;[Epub] CrossRef - Dual‐hormone artificial pancreas for glucose control in type 1 diabetes: A meta‐analysis
Baoqi Zeng, Hao Jia, Le Gao, Qingqing Yang, Kai Yu, Feng Sun
Diabetes, Obesity and Metabolism.2022; 24(10): 1967. CrossRef - Dual-Hormone Insulin-and-Pramlintide Artificial Pancreas for Type 1 Diabetes: A Systematic Review
Alezandra Torres-Castaño, Amado Rivero-Santana, Lilisbeth Perestelo-Pérez, Andrea Duarte-Díaz, Analia Abt-Sacks, Vanesa Ramos-García, Yolanda Álvarez-Pérez, Ana M. Wäagner, Mercedes Rigla, Pedro Serrano-Aguilar
Applied Sciences.2022; 12(20): 10262. CrossRef - History of insulin treatment of pediatric patients with diabetes in Korea
Jae Hyun Kim, Choong Ho Shin, Sei Won Yang
Annals of Pediatric Endocrinology & Metabolism.2021; 26(4): 237. CrossRef
- Integration of a Safety Module to Prevent Rebound Hypoglycemia in Closed-Loop Artificial Pancreas Systems
- Basic Research
- A Novel Pancreatic Imaging Window for Stabilized Longitudinal
In Vivo Observation of Pancreatic Islets in Murine Model - Inwon Park, Sujung Hong, Yoonha Hwang, Pilhan Kim
- Diabetes Metab J. 2020;44(1):193-198. Published online May 29, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0268
- 4,679 View
- 137 Download
- 13 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Longitudinal imaging of murine pancreas is technically challenging due to the mechanical softness of the tissue influenced by peristalsis. Here, we report a novel pancreatic imaging window for long-term stabilized cellular-level observation of the islets in the pancreas
in vivo . By spatially separating the pancreas from the bowel movement and physiologic respiration with a metal plate integrated in the imaging window, we successfully tracked the pancreatic islets up to three weeks and visualized the dumbbell-shape transformation from the single islet. This window can be a useful tool for long-term cellular-level visualization of the microstructure in the pancreas.-
Citations
Citations to this article as recorded by-
Apollo-NADP
+
reveals in vivo adaptation of NADPH/NADP
+
metabolism in electrically activated pancreatic β cells
Cindy V. Bui, Curtis W. Boswell, Brian Ciruna, Jonathan V. Rocheleau
Science Advances.2023;[Epub] CrossRef - Intravital imaging of the functions of immune cells in the tumor microenvironment during immunotherapy
Xuwen Peng, Yuke Wang, Jie Zhang, Zhihong Zhang, Shuhong Qi
Frontiers in Immunology.2023;[Epub] CrossRef - Minimizing Motion Artifacts in Intravital Microscopy Using the Sedative Effect of Dexmedetomidine
Youngkyu Kim, Minju Cho, Bjorn Paulson, Sung-Hoon Kim, Jun Ki Kim
Microscopy and Microanalysis.2022; 28(5): 1679. CrossRef - SWIP—a stabilized window for intravital imaging of the murine pancreas
Wei Du, Christian Adkisson, Xianjun Ye, Camille L. Duran, Benson Chellakkan Selvanesan, Claudia Gravekamp, Maja H. Oktay, John C. McAuliffe, John S. Condeelis, Nicole C. Panarelli, Robert J. Norgard, Yogev Sela, Ben Z. Stanger, David Entenberg
Open Biology.2022;[Epub] CrossRef - Intravital longitudinal cellular visualization of oral mucosa in a murine model based on rotatory side-view confocal endomicroscopy
Sujung Hong, Jingu Lee, Jieun Moon, Eunji Kong, Jehwi Jeon, Yeon soo Kim, Hyung-Ryong Kim, Pilhan Kim
Biomedical Optics Express.2022; 13(8): 4160. CrossRef - Improved in vivo imaging method for individual islets across the mouse pancreas reveals a heterogeneous insulin secretion response to glucose
Henriette Frikke-Schmidt, Peter Arvan, Randy J. Seeley, Corentin Cras-Méneur
Scientific Reports.2021;[Epub] CrossRef - The frontier of live tissue imaging across space and time
Qiang Huang, Aliesha Garrett, Shree Bose, Stephanie Blocker, Anne C. Rios, Hans Clevers, Xiling Shen
Cell Stem Cell.2021; 28(4): 603. CrossRef - Intravital Laser-scanning Two-photon and Confocal Microscopy for Biomedical Research
Jieun Moon, Pilhan Kim
Medical Lasers.2021; 10(1): 1. CrossRef - The Eye as a Transplantation Site to Monitor Pancreatic Islet Cell Plasticity
Erwin Ilegems, Per-Olof Berggren
Frontiers in Endocrinology.2021;[Epub] CrossRef - Longitudinal Intravital Imaging of Tumor-Infiltrating Lymphocyte Motility in Breast Cancer Models
Inwon Park, Sujung Hong, Joon Seok, Stephani Edwina Lucia, Eunjoo Song, Mingyo Kim, Eunji Kong, Howon Seo, Yoonha Hwang, Soyeon Ahn, Seonghye Kim, Dong-Hyun Jang, Jae Hyuk Lee, Su-Hyung Park, Pilhan Kim, You Hwan Jo
Journal of Breast Cancer.2021; 24(5): 463. CrossRef - Intravital longitudinal imaging of hepatic lipid droplet accumulation in a murine model for nonalcoholic fatty liver disease
Jieun Moon, Eunji Kong, Jingu Lee, Jinjoo Jung, Eunha Kim, Seung Bum Park, Pilhan Kim
Biomedical Optics Express.2020; 11(9): 5132. CrossRef
-
Apollo-NADP
+
reveals in vivo adaptation of NADPH/NADP
+
metabolism in electrically activated pancreatic β cells
- Pathophysiology
-
- Essential Role of Protein Arginine Methyltransferase 1 in Pancreas Development by Regulating Protein Stability of Neurogenin 3
- Kanghoon Lee, Hyunki Kim, Joonyub Lee, Chang-Myung Oh, Heein Song, Hyeongseok Kim, Seung-Hoi Koo, Junguee Lee, Ajin Lim, Hail Kim
- Diabetes Metab J. 2019;43(5):649-658. Published online April 8, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0232
- 5,192 View
- 70 Download
- 4 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Protein arginine methyltransferase 1 (PRMT1) is a major enzyme responsible for the formation of methylarginine in mammalian cells. Recent studies have revealed that PRMT1 plays important roles in the development of various tissues. However, its role in pancreas development has not yet been elucidated.
Methods Pancreatic progenitor cell-specific
Prmt1 knock-out (Prmt1 PKO) mice were generated and characterized for their metabolic and histological phenotypes and their levels ofNeurog3 gene expression and neurogenin 3 (NGN3) protein expression. Protein degradation assays were performed in mPAC cells.Results Prmt1 PKO mice showed growth retardation and a severely diabetic phenotype. The pancreatic size and β-cell mass were significantly reduced inPrmt1 PKO mice. Proliferation of progenitor cells during the secondary transition was decreased and endocrine cell differentiation was impaired. These defects in pancreas development could be attributed to the sustained expression of NGN3 in progenitor cells. Protein degradation assays in mPAC cells revealed that PRMT1 was required for the rapid degradation of NGN3.Conclusion PRMT1 critically contributes to pancreas development by destabilizing the NGN3 protein.
-
Citations
Citations to this article as recorded by- Arginine 65 methylation of Neurogenin 3 by PRMT1 is required for pancreatic endocrine development of hESCs
Gahyang Cho, Kwangbeom Hyun, Jieun Choi, Eunji Shin, Bumsoo Kim, Hail Kim, Jaehoon Kim, Yong-Mahn Han
Experimental & Molecular Medicine.2023; 55(7): 1506. CrossRef - Protein arginine methyltransferase 1 in the generation of immune megakaryocytes: A perspective review
Xinyang Zhao, Zechen Chong, Yabing Chen, X. Long Zheng, Qian-Fei Wang, Yueying Li
Journal of Biological Chemistry.2022; 298(11): 102517. CrossRef - Arginine 65 Methylation of Neurogenin 3 by PRMT1 Is Required for Pancreatic Endocrine Development of hESCs
Gahyang Cho, Kwangbeom Hyun, Jieun Choi, Eun Ji Shin, Bumsoo Kim, Hail Kim, Jaehoon Kim, Yong-Mahn Han
SSRN Electronic Journal .2022;[Epub] CrossRef - Protein Arginine Methyltransferase 1 Is Essential for the Meiosis of Male Germ Cells
Sahar Waseem, Sudeep Kumar, Kanghoon Lee, Byoung-Ha Yoon, Mirang Kim, Hail Kim, Keesook Lee
International Journal of Molecular Sciences.2021; 22(15): 7951. CrossRef - Proteome-Wide Alterations of Asymmetric Arginine Dimethylation Associated With Pancreatic Ductal Adenocarcinoma Pathogenesis
Meijin Wei, Chaochao Tan, Zhouqin Tang, Yingying Lian, Ying Huang, Yi Chen, Congwei Chen, Wen Zhou, Tao Cai, Jiliang Hu
Frontiers in Cell and Developmental Biology.2020;[Epub] CrossRef
- Arginine 65 methylation of Neurogenin 3 by PRMT1 is required for pancreatic endocrine development of hESCs
- Complications
- The Association between Pancreatic Steatosis and Diabetic Retinopathy in Type 2 Diabetes Mellitus Patients
- Jee Sun Jeong, Mee Kyung Kim, Kyung Do Han, Oak Kee Hong, Ki-Hyun Baek, Ki-Ho Song, Dong Jin Chung, Jung-Min Lee, Hyuk-Sang Kwon
- Diabetes Metab J. 2018;42(5):425-432. Published online August 9, 2018
- DOI: https://doi.org/10.4093/dmj.2017.0107
- 4,118 View
- 43 Download
- 5 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Whether pancreatic steatosis has a local or systemic effect, like ectopic fat of other major organs, remains unknown. Data on the influence of pancreatic steatosis on microvascular complication are rare. Therefore, we investigated the relationship between pancreatic steatosis and diabetic retinopathy (DR) in patients with type 2 diabetes mellitus (T2DM).
Methods The attenuation of three pancreatic regions (head, body, and tail) and the spleen (S) in 186 patients with T2DM was measured using non-enhanced computed tomography imaging. We used three parameters for the assessment of pancreatic steatosis (‘P’ mean: mean attenuation of three pancreatic regions; P–S: difference between ‘P’ mean and ‘S’; P/S: the ‘P’ mean to ‘S’ ratio). The presence of DR was assessed by an expert ophthalmologist using dilated fundoscopy.
Results The average P mean was 29.02 Hounsfield units (HU), P–S was −18.20 HU, and P/S was 0.61. The three pancreatic steatosis parameters were significantly associated with the prevalence of DR in non-obese T2DM patients. In the non-obese group, the odds ratios of P mean, P–S, and P/S for the prevalence of DR, after adjustment for age, sex, and glycosylated hemoglobin level, were 2.449 (
P =0.07), 2.639 (P =0.04), and 2.043 (P =0.02), respectively.Conclusion In this study, pancreatic steatosis was significantly associated with DR in non-obese patients with T2DM. Further studies are necessary to clarify the causal relationship between pancreatic steatosis and the development of DR.
-
Citations
Citations to this article as recorded by- Intra‐pancreatic fat is associated with continuous glucose monitoring metrics
Yutong Liu, Wandia Kimita, Xiatiguli Shamaitijiang, Loren Skudder‐Hill, Ivana R. Sequeira‐Bisson, Maxim S. Petrov
Diabetes, Obesity and Metabolism.2024;[Epub] CrossRef - Association between Intrapancreatic Fat Deposition and Lower High-Density Lipoprotein Cholesterol in Individuals with Newly Diagnosed T2DM
Jianliang Wang, Qingyun Cai, Xiaojuan Wu, Jiaxuan Wang, Xiaona Chang, Xiaoyu Ding, Jia Liu, Guang Wang, Muhittin Yurekli
International Journal of Endocrinology.2023; 2023: 1. CrossRef - The comparison of pancreatic and hepatic steatosis in healthy liver donor candidates
Bedriye Koyuncu Sokmen, Tolga Sahin, Alihan Oral, Erdem Kocak, Nagihan Inan
Scientific Reports.2021;[Epub] CrossRef - Computed Tomography-Estimated Pancreatic Steatosis is Associated with Carotid Plaque in Type 2 Diabetes Mellitus Patients: A Cross-Sectional Study from China
Pengtao Sun, Chunzhi Fan, Rengui Wang, Tongwei Chu, Xiaoli Sun, Dongxue Zhang, Xuechao Du
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2021; Volume 14: 1329. CrossRef
- Intra‐pancreatic fat is associated with continuous glucose monitoring metrics
- Clinical Diabetes & Therapeutics
- Recent Updates on Type 1 Diabetes Mellitus Management for Clinicians
- Ahmed Iqbal, Peter Novodvorsky, Simon R. Heller
- Diabetes Metab J. 2018;42(1):3-18. Published online February 23, 2018
- DOI: https://doi.org/10.4093/dmj.2018.42.1.3
- 6,767 View
- 89 Download
- 18 Web of Science
- 19 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Type 1 diabetes mellitus (T1DM) is a chronic autoimmune condition that requires life-long administration of insulin. Optimal management of T1DM entails a good knowledge and understanding of this condition both by the physician and the patient. Recent introduction of novel insulin preparations, technological advances in insulin delivery and glucose monitoring, such as continuous subcutaneous insulin infusion (CSII) and continuous glucose monitoring and improved understanding of the detrimental effects of hypoglycaemia and hyperglycaemia offer new opportunities and perspectives in T1DM management. Evidence from clinical trials suggests an important role of structured patient education. Our efforts should be aimed at improved metabolic control with concomitant reduction of hypoglycaemia. Despite recent advances, these goals are not easy to achieve and can put significant pressure on people with T1DM. The approach of physicians should therefore be maximally supportive. In this review, we provide an overview of the recent advances in T1DM management focusing on novel insulin preparations, ways of insulin administration and glucose monitoring and the role of metformin or sodium-glucose co-transporter 2 inhibitors in T1DM management. We then discuss our current understanding of the effects of hypoglycaemia on human body and strategies aimed at mitigating the risks associated with hypoglycaemia.
-
Citations
Citations to this article as recorded by- Health-Related Quality of Life of Adolescents and Children With Type 1 Diabetes in the Jazan Region of Saudi Arabia
Gassem A Gohal, Aqilah Majhali, Esaam Moafa, Sarah H Talebi, Bushra I Maashi, Amani Mutaen, Walaa J Alhamdan, Ibrahim M Dighriri
Cureus.2024;[Epub] CrossRef - Nose-to-brain delivery of insulin nanoparticles for diabetes management: A review
Manoj Kumbhare, Ajaykumar Surana, Pravin Morankar
Baghdad Journal of Biochemistry and Applied Biological Sciences.2023; 4(02): 39. CrossRef - Clinical Effects of a Home Care Pilot Program for Patients with Type 1 Diabetes Mellitus: A Retrospective Cohort Study
Sejeong Lee, KyungYi Kim, Ji Eun Kim, Yura Hyun, Minyoung Lee, Myung-Il Hahm, Sang Gyu Lee, Eun Seok Kang
Diabetes & Metabolism Journal.2023; 47(5): 693. CrossRef - Impact of an Acceptance and Commitment Therapy programme on HbA1c, self-management and psychosocial factors in adults with type 1 diabetes and elevated HbA1c levels: a randomised controlled trial
Ingrid Wijk, Susanne Amsberg, Unn-Britt Johansson, Fredrik Livheim, Eva Toft, Therese Anderbro
BMJ Open.2023; 13(12): e072061. CrossRef - Role of sirtuin-1 (SIRT1) in hypoxic injury in pancreatic β-cells
Ye-Jee Lee, Esder Lee, Young-Hye You, Yu-Bae Ahn, Ki-Ho Song, Ji-Won Kim, Seung-Hyun Ko
Journal of Drug Targeting.2021; 29(1): 88. CrossRef - Age at Diagnosis and the Risk of Diabetic Nephropathy in Young Patients with Type 1 Diabetes Mellitus
Jong Ha Baek, Woo Je Lee, Byung-Wan Lee, Soo Kyoung Kim, Gyuri Kim, Sang-Man Jin, Jae Hyeon Kim
Diabetes & Metabolism Journal.2021; 45(1): 46. CrossRef - The impact of chemical engineering and technological advances on managing diabetes: present and future concepts
Sabine Szunerits, Sorin Melinte, Alexandre Barras, Quentin Pagneux, Anna Voronova, Amar Abderrahmani, Rabah Boukherroub
Chemical Society Reviews.2021; 50(3): 2102. CrossRef - Surrogate markers and predictors of endogenous insulin secretion in children and adolescents with type 1 diabetes
Jin-Na Yuan, Jian-Wei Zhang, Wayne S. Cutfield, Guan-Ping Dong, You-Jun Jiang, Wei Wu, Ke Huang, Xiao-Chun Chen, Yan Zheng, Bi-Hong Liu, José G. B. Derraik, Jun-Fen Fu
World Journal of Pediatrics.2021; 17(1): 99. CrossRef - Nano-based drug delivery systems used as vehicles to enhance polyphenols therapeutic effect for diabetes mellitus treatment
Sónia Rocha, Mariana Lucas, Daniela Ribeiro, M. Luísa Corvo, Eduarda Fernandes, Marisa Freitas
Pharmacological Research.2021; 169: 105604. CrossRef - Dapagliflozin: an effective adjunctive treatment in type 1 diabetes
Ghasem Yadegarfar, Mark Livingston, Gabriela Cortes, Ramadan Alshames, Kate Leivesley, Ann Metters, Linda Horne, Tom Steele, Adrian H. Heald
Cardiovascular Endocrinology & Metabolism.2021; 10(2): 132. CrossRef - Association between reduced serum levels of magnesium and the presence of poor glycemic control and complications in type 1 diabetes mellitus: A systematic review and meta-analysis
Ana Kelen Rodrigues, Ana Elisa Melo, Caroline Pereira Domingueti
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2020; 14(2): 127. CrossRef - Insulin-Producing Cell Transplantation Platform for Veterinary Practice
Suryo Kuncorojakti, Sayamon Srisuwatanasagul, Krishaporn Kradangnga, Chenphop Sawangmake
Frontiers in Veterinary Science.2020;[Epub] CrossRef - Type 1 Diabetes Home Care Project and Educational Consultation
Eun Chong Shin
The Journal of Korean Diabetes.2020; 21(2): 88. CrossRef - Decision-Making in Artificial Intelligence: Is It Always Correct?
Hun-Sung Kim
Journal of Korean Medical Science.2020;[Epub] CrossRef - The FreeStyle Libre flash glucose monitoring system: how it has improved glycaemic control for people with type 1 diabetes in Eastern Cheshire, UK
Ghasem Yadegarfar, Simon G. Anderson, Zohaib Khawaja, Gabriela Cortes, Kathryn Leivesley, Ann Metters, Linda Horne, Tom Steele, Adrian H. Heald
Cardiovascular Endocrinology & Metabolism.2020; 9(4): 171. CrossRef - Dose-dependent effects of necrostatin-1 supplementation to tissue culture media of young porcine islets
Hien Lau, Nicole Corrales, Samuel Rodriguez, Colleen Luong, Mohammadreza Mohammadi, Veria Khosrawipour, Shiri Li, Michael Alexander, Paul de Vos, Jonathan R. T. Lakey, Zoltán Rakonczay
PLOS ONE.2020; 15(12): e0243506. CrossRef - New Insulin Pumps and Open Source Artificial Pancreas System in Korea
Jae Hyeon Kim
The Journal of Korean Diabetes.2020; 21(4): 197. CrossRef - Perspective and general approach of diabetes in palliative care
Díaz Rodríguez Juan Javier
Hospice and Palliative Medicine International Journal.2018;[Epub] CrossRef - The effects of safranal, a constitute of saffron, and metformin on spatial learning and memory impairments in type-1 diabetic rats: behavioral and hippocampal histopathological and biochemical evaluations
Fatemeh Delkhosh-Kasmaie, Amir Abbas Farshid, Esmaeal Tamaddonfard, Mehdi Imani
Biomedicine & Pharmacotherapy.2018; 107: 203. CrossRef
- Health-Related Quality of Life of Adolescents and Children With Type 1 Diabetes in the Jazan Region of Saudi Arabia
- The Effects of Dexamethasone on the Expansion and Transdifferentiation of Transplanted Porcine Neonatal Pancreas Cell Clusters into beta-cells in Normal Nude Mice.
- Ji Hun Yang, Sun Hee Suh, Sung Yoon Jeon, Oak Kee Hong, Kun Ho Yoon
- Korean Diabetes J. 2004;28(5):356-366. Published online October 1, 2004
- 883 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Several studies have suggested that glucocorticoid has an influence on the development and function of the -cells. Thus, we undertook this study to determine whether exposure to dexamethasone (Dx) has an influence on the expansion or transdifferentiation of transplanted porcine NPCCs. METHODS: After transplantation (Tx) of 4,000 islet equivalents (IEQs) of porcine NPCCs into normal nude mice, Dx (1mg/kg) or the control vehicle were injected daily for 10 weeks. To clarify the effects of timing and duration of the Dx, one group was treated by Dx at the first 2 weeks (n=10) and the other group was treated later 8 weeks (n=10) during the 10 weeks treatment period. Thr total graft and beta-cell masses were determined by morphometric analysis. We preformed semi-quantitative RT-PCR for evaluating the pancreas transcription factors. RESULTS: The relative volume and absolute mass of the beta-cells and the total graft were significantly decreased by 10 weeks Dx treatment. Moreover, Dx treatment at thr first 2 weeks (n=10) also significantly decreased the total graft mass and absolute mass of the beta-cells. The relative volume of the beta-cells was negatively correlated and the area of the duct cysts was positively correlated with the duration of the Dx treatment. Pancreas transcription factors including PDX1, Ngn 3, ISL1 and NKx6.1 were decreased in the graft by 2 days treatment of Dx. CONCLUSION: These results suggest that Dx treatment suppresses the expansion and transdifferentiation of transplanted pancreas precursor cells into beta-cell.
- High Carbohydrate Diet Effects on the Development of Diabetes Mellitus and Modification of Pancreatic Islets in OLETF Rats.
- Sung Ki Kim, Seong Bin Hong, Hwi Ra Park, Eun A Kim, Kyung Wook Lee, Moon Suk Nam, Yong Seong Kim
- Korean Diabetes J. 2004;28(3):187-198. Published online June 1, 2004
- 974 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Diet has long been believed to be an important risk factor for type 2 diabetes. The composition of carbohydrates in the diet was higher in the past, where as now it is considerably reduced in the diet of Korean peoples, which is probably associated with the risk of developing type 2 diabetes. The aim of the present study was to investigate the long-term effect of high carbohydrate/low protein diets on the glucose and lipid metabolism and the pancreatic islet in OLETF(Otsuka Long-Evans Tokushima Fatty) rats, the animal model of type 2 diabetes. METHODS: Seven week old male OLETF rat were fed a high carbohydrate/low protein diet(carbohydrate 71.0%, fat 14.5%, protein 14.5%) as the experimental group, with an ordinary chow diet(carbohydrate 63.5%, fat 14.5%, protein 22%) fed to the controls. The plasma insulin, lipid profiles, free fatty acid and oral glucose tolerance were analyzed at 16 and 32 weeks. After the glucose tolerance test, the pancreas was excised, and immunohistochemical staining was conducted for the islet morphology and insulin mRNA to quantify the insulin secretory capacity. RESULTS: The basal glucose levels tended to be higher in the control group, but with no significant statistical difference. There were no differences in the serum insulin, total cholesterol, triglyceride, HDL-cholesterol and plasma free fatty acid levels between the two groups. The pancreatic islets of the control group showed multilobulation, with fibrotic changes; where as those of the experimental group were maintained normal profiles. A higher expression of insulin mRNA was observed in the experimental than in the control group. CONCLUSION: A high carbohydrate diet induced lower body weight increases, and protected against beta cell injury and decreased the development of abnormal glucose tolerance in OLETF rats. This may explain the growing incidence of diabetes with respect to the change in carbohydrate composition in the diet of Korean peoples. However, whether the protective effect of a high carbohydrate diet, against the development of diabetes in OLETF rats, can be attributed to small weight increases or if the change in food composition itself, or both needs to be determined.
- Development of Adult Porcine Islet Isolation Method for Xenotransplantation.
- Sung Rae Kim, Kun Ho Yoon, Hyuk Sang Kwon, Sun Hee Suh, Seung Hyun Ko, Jung Min Lee, Soon Jib Yoo, Yoo Bae Ahn, Ki Ho Song, Hyun Shik Son, Moo Il Kang, Bong Yun Cha, Kwang Woo Lee, Ho Young Son, Sung Koo Kang
- Korean Diabetes J. 2004;28(2):75-87. Published online April 1, 2004
- 1,093 View
- 24 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
AND PURPOSE: Xenotransplantation using porcine islet cells might be an alternative to allotransplantation, which has been limited due to the lack of donors. Various researches using porcine islet cells have been performed in foreign countries; however, they have never been studies in Korea. Therefore, the purpose of this study was to explore the possibility of thise new treatment for cases of diabetes by establishing of improved islet isolation skill. METHODS: The pancreas and islets were extracted from pigs weighing around 100kg. To establish an islet isolation method, the islet yield, purity and the distribution size of the isolated islets were step wise compared in various ways, and then the superior method adopted. To determine the conveyance method after organ extraction, the conveyance method of pouring collagenase P was compared with the conveyance method of injecting Custidol. For digestion, the mechanical shaking and static incubation methods were also compared. To isolate islets from the digested pancreata, isolation methods were analyzed using 3 and 4 layers' Ficoll. The islet yield was appraised after their isolation using the optimized islet isolation method. To assess the results of the islet isolation, appraised the purity and the survival rates of cells, the insulin secretion resulting from the glucose stimulation test was examined. RESULTS: The method of injecting 4degrees C Custidol was effective for the conveyance and storage of the isolated pancreas in comparison with an injection of collagenase P(3465+/-1488 IEQ/g pancreas vs. 48+/-1.7 IEQ/g pancreas, p<0.01). The digestion method was superior to the mechanical shaking method at keeping a stable condition(3465+/-1488 IEQ/g pancreas vs. 1265+/-141.4 IEQ/g pancreas, p<0.01). Ficoll isolation using 3 layers gave the same results as using 4 layers. The average weights of the isolate Pancreatic islets was 23.8+/-3.3g. The numbers of islets per gram was 3465+/-1488.2(IEQ), with a the purity of 86.3+/-2.0%, and a survival rate of over 95%. The insulin secretion caused by glucose stimulation substantially increased in concentration from 24 to 72 hours(24hr: 5mM 3.12mU/mL --< 20mM 6.79mU/mL(2.17 fold), 72hr: 5mM 2.38mU/mL --< 9.93mU/mL(4.17fold))
- The Effects of High Glucose, Insulin and TGF-beta 1 on Proliferation and Differentiation of the Pancreatic Stellate Cells.
- Oak Kee Hong, Hyuk Sang Kwon, Kyu Hyun Yeom, Marie Lee, Ji Hun Yang, Seung Hyeon Ko, Soon Jib Yoo, Hyun Sik Son, Kun Ho Yoon, Bong Yeon Cha, Kwang Woo Lee, Ho Yong Son, Sung Koo Kang
- Korean Diabetes J. 2003;27(3):228-240. Published online June 1, 2003
- 885 View
- 23 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Although chronic pancreatitis gives rise to fibrosis of pancreatic exocrine tissue, and type 2 diabetes is accompanied by pancreatic fibrosis, the mechanisms of fibrogenesis in the pancreas have been insufficiently studied. The activated Pancreatic stellate cells (PSC) have recently been identified in human and experimental fibrotic areas from chronic panceatitis tissues. As PSC are similar in their morphology and biochemistry to hepatic stellate cells, they are suspected to play the same role in pancreatic fibrogenesis as the hepatic stellate cells in liver fibrosis. The PSC were isolated from the rat pancreata, and mediators stimulating the proliferation and differentiation identified. METHODS: The pancreatic stellate shaped cells were isolated by a minor modification to the method described by Apte et al (ref), using a Nycodenz gradient. The isolated PSCs were confirmed by phase-contrast and by the immunofluorescence of vimentin, desmin and smooth muscle a-actin (a-SMA). The level of alpha-SMA was quantified by Western blot in the PSCs in the culture, over time, and the cell proliferation was measured by 3[H]-Thymidine incorporation. The effect of the proliferation and differentiation of the PSC were assessed in relation to D-glucose (500 mg/dL), Insulin (10 IU/mL) and TGF-beta (10 ng/mL) treatment of the culture medium. RESULTS: The stellate shaped cells from the rat pancreata grew readily in the culture. Unactivated PSCs, cultured for 3 days, had an angular appearance, contained lipid droplets, manifesting positive vitamin A autofliuorescence, and stained positively for vimentin and desmin, but negatively for alpha-SMA. Within 4~8 days of primary culturing, the PSCs were activated, the sizes and numbers of the fat droplets decreased, the cells flattened, developed long cytoplasmic extensions and expressed alpha-SMA. After 3 passages, almost 100% of the cells were positive for alpha-SMA expression, indicating a myofibroblast type of differentiation in vitro. The addition of high-glucose concentrations and insulin to the activated PSCs significantly stimulated cell proliferation (194.4+/-8.3, 175.0+/-31.0 vs. control), and when the combination of high- glucose and insulin was applied, the cell proliferation was increased to an even greater extent (247.0+/-21.8 vs. control). CONCLUSIONS: Pancreata stellate cells can be isolated, and cultured in vitro, from normal SD rats. High concentrations of glucose and insulin in culture medium activated the PSC proliferation.
- Role of Nitric Oxide on the Insulin Secretion of Rat Pancreas.
- Moon Suk Nam, Sung Ki Kim, Seong Bin Hong, Yeo Joo Kim, Mi Rim Kim, Yong Seong Kim, Young Duk Song, Hyun Chul Lee, Kap Bum Huh
- Korean Diabetes J. 1999;23(6):748-756. Published online January 1, 2001
- 991 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Diabetes mellitus could occur when insulin secretion of pancreas is inadequate in response to blood glucose. The mechanisms on failure of pancreatic beta cell are still not known. Several recent experiments have reported that nitric oxide (NO) may be considered as a modulator of insulin secretion and impairment associated with the beta cell. The present study was purposed to investigate the role of nitric oxide on the secretion of insulin of rat pancreas in vivo and in vitro. METHODS: The plasma insulin and glucose were measured after intravenous injection of nitric oxide synthase (NOS) inhibitor (NG-nitro-L-arginine methyl. ester, L-NAME) in male rat. Insulin release was determmed during stimulation of NOS inhibitor and nitric oxide donor (hydroxylamine) in the isolated pancreatic islets. RESULT: 1. The insulin secretory response with L-arginine stimulation after injection of NOS inhibitor (L-NAME) in rat was increased resulting in mild hypoglycemia which recovered promptly. This showed that NO were related with L-arginine induced insulin secretion. 2. After isolation of pancreatic islet, 11,0 mM glucose induced insulin release was increased in culture media and L-arginine (1.0 mM) induced insulin release was also increased compared with control (6.72+/-0.66 vs. 3.48+/-0.42 prnol/islet/hour, p<0.05). 3. L-arginine induced insulin release was increased with L-NAME in the isolated rat pancreatic islets (12.5+/-1.38 vs, 7.23+/-0.93 ng/islet/ hour, p<0.05). 4. Glucose induced insulin release was progressively inhibited by NO donor hydroxylamine in the isolated rat pancreas islet (6.72+/-0.75 vs. 2.46+/-0.60 pmol/islet/hour p<0.05). CONCLUSION: These results strongly suggest that nitric oxide is a negative modulator of insulin release in normal rats induced by the nutrient secretagogues L-arginine and glucose in vivo and in vitro. Further investigation on the mechanism of nitric oxide in insulin secretory pathway will be necessary.
- Insulin Secretory Dysfunction in the Patients with Untreated Hyperthyroidism.
- Moon Suk Nam, Seung Yong Shin, Young Wan Kim, Seong Bin Hong, Yeo Joo Kim, Mi Rim Kim, Won Sick Choe, Yong Seong Kim
- Korean Diabetes J. 1998;22(3):320-327. Published online January 1, 2001
- 881 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Abnormal glucose metabolism with impaired glucose tolerance has been documented in patients with thyrotoxicosis, but the pathogenesis is not fully understood. Therefore, the aim of the present study was to study the secretory dysfunction of pancreatic 9-cell and to confirm hyperinsulinemia and hyperproinsulinemia during oral glucose tolerance test(OGTT) in patients with thyrotoxicosis. METHODS: After an overnight fast, 75 g OGTT was performed in 10 patients with hyperthyroidism and in 10 healthy control subjects matched for age, sex and hody mass index. Plasma insulin(immuno-reactive insulin, IRI), C-peptide, proinsulin levels were measured by radioimmunoassay. RESULTS: Fasting plasma glucose, insulin and C-peptide levels were similar in the two groups, but plasma proinsulin level was increased in patients with hyperthyroidism(p<0.05). A twofold rise of plasma proinsulin and the proinsulin/insulin ratio was also found in patients with hyperthyroidism during OGTT. The molar ratio of C-peptide and insulin(IRI) was similar in the two groups. CONCLUSION: Hyperinsulinemia and hyperproinsulinemia were found in patients with hyperthyroidism compared with controls. Disproportionally increased proinsulin level suggested a pancreatic secretory dysfunction in the patients with hyperthyroidism.
- Changes of CGRP- and VIP-containing Nerve Fibers in the pancreas of Streptozotocin-Induced Diabetic Rat.
- Young Joo Kim, Moo Ho Won, Gun Gyo Suh, Je Kyung Seong, Joon Sup Lee, Hwan Mook Kim, Yang Seok Oh
- Korean Diabetes J. 1998;22(3):299-311. Published online January 1, 2001
- 899 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The peptidergic nerves, namely the third nerve, are distributed in the pancreas with adreniergic and cholinergic nerves. However, it has been known that the peptidergic nerves control the pancreatic endocrine and exocrine functions, only a few morphological studies has been done. Therefore we tried to investigate the distribution patterns of the pancreatic calcitonin gene-related peptide(CGRP) and vasoactive intestinal polypeptide(VIP) containing nerves in the pancreas of the streptozotocin-induced diabetic rats. METHODS: Eight week old male WKY rats were used in this study. Insulin-dependent diabetic animals were produced by the intraperitoneal injection of streptozotocin(60 mg/Kg) dissolved in cold citrate buffer(pH 4.2) to WKY rats. The control rats were treated with the vehicle. Immnunohistochemical studies were carried out to observe the changes of CGRP containing nerve fibers and VIP containing nerve fibers in the pancreas at 0, 3, 14, and 28 days after the injection of streptozotocin. RESULTS: In insulin-dependent diabetic animal group, the density and the innervation patr.erns of CGRP containing nerve fitrs were significantly increased on day 3 and thereafter decreased to the level of control group at the end of experiment. The immunoreactivities of VIP containing nerve fibers were not altered in the pancreatic islers. The immunoreaetivities, however, were gradually increased in the periphery of the acini, the connective tissues, and the periphery of the ducts and vasculatures. CONCLUSION: The results of the present imvestigation suggest that the distribution patterns of CGRP and VIP containing nerve fibers in the insulin-dependent diabetic rats are related to the development of diabetes and may provide a morphological bases for the possible role of nueropeptides in pancreas of the diabetic rats. Further study are required about the relationships between the distribution patterns and the functions of the peptidergic nerve in pancreas.

 KDA
KDA
 First
First Prev
Prev





