
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
Original Articles
- Metabolic Risk/Epidemiology
- A Prospective 1-Year Follow-Up of Glycemic Status and C-Peptide Levels of COVID-19 Survivors with Dysglycemia in Acute COVID-19 Infection
- David Tak Wai Lui, Chi Ho Lee, Ying Wong, Carol Ho Yi Fong, Kimberly Hang Tsoi, Yu Cho Woo, Kathryn Choon Beng Tan
- Received June 5, 2023 Accepted October 13, 2023 Published online March 11, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0175 [Epub ahead of print]
- 747 View
- 35 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
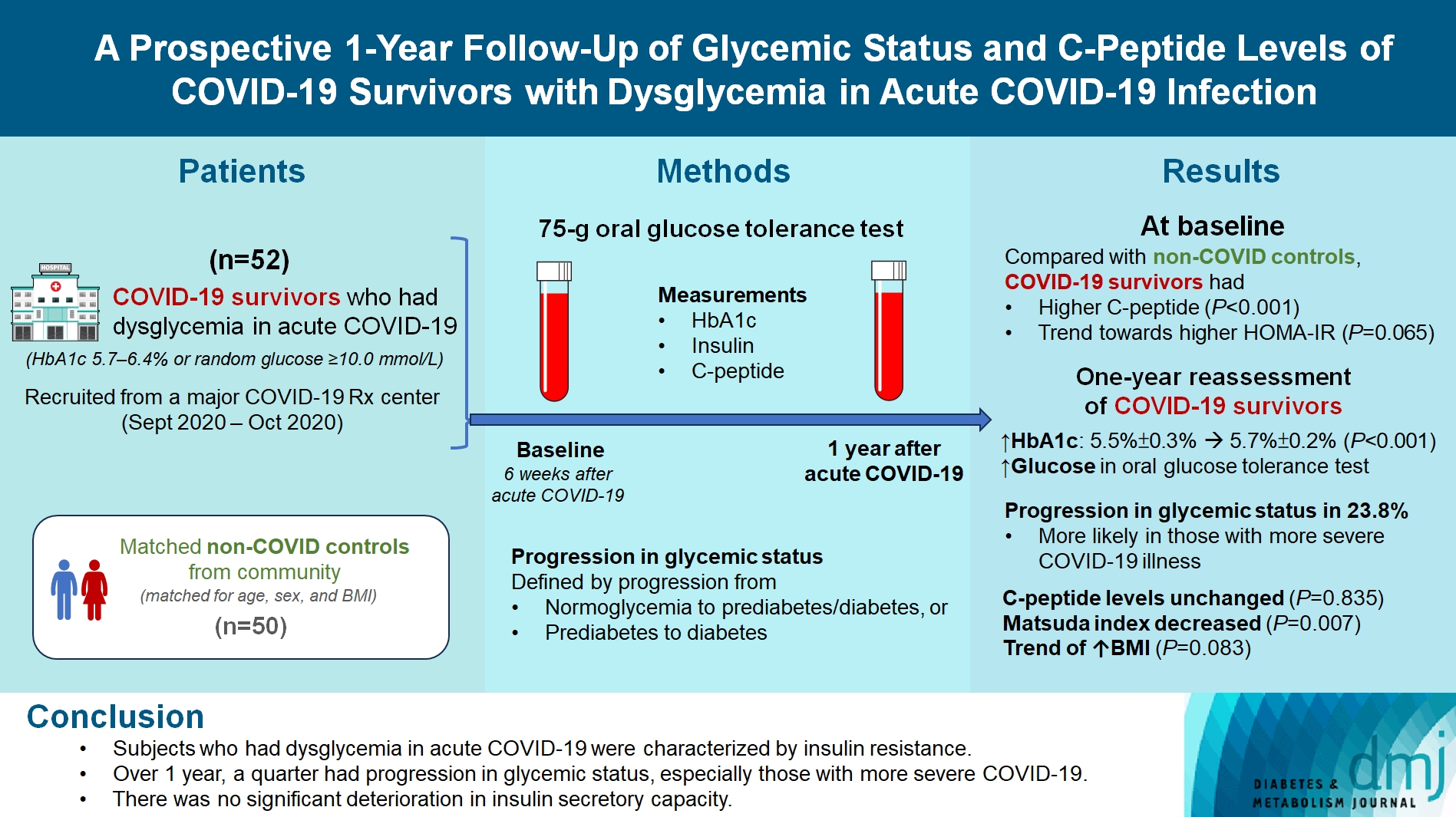
We evaluated changes in glycemic status, over 1 year, of coronavirus disease 2019 (COVID-19) survivors with dysglycemia in acute COVID-19.
Methods
COVID-19 survivors who had dysglycemia (defined by glycosylated hemoglobin [HbA1c] 5.7% to 6.4% or random glucose ≥10.0 mmol/L) in acute COVID-19 were recruited from a major COVID-19 treatment center from September to October 2020. Matched non-COVID controls were recruited from community. The 75-g oral glucose tolerance test (OGTT) were performed at baseline (6 weeks after acute COVID-19) and 1 year after acute COVID-19, with HbA1c, insulin and C-peptide measurements. Progression in glycemic status was defined by progression from normoglycemia to prediabetes/diabetes, or prediabetes to diabetes.
Results
Fifty-two COVID-19 survivors were recruited. Compared with non-COVID controls, they had higher C-peptide (P< 0.001) and trend towards higher homeostasis model assessment of insulin resistance (P=0.065). Forty-three COVID-19 survivors attended 1-year reassessment. HbA1c increased from 5.5%±0.3% to 5.7%±0.2% (P<0.001), with increases in glucose on OGTT at fasting (P=0.089), 30-minute (P=0.126), 1-hour (P=0.014), and 2-hour (P=0.165). At baseline, 19 subjects had normoglycemia, 23 had prediabetes, and one had diabetes. Over 1 year, 10 subjects (23.8%; of 42 non-diabetes subjects at baseline) had progression in glycemic status. C-peptide levels remained unchanged (P=0.835). Matsuda index decreased (P=0.007) and there was a trend of body mass index increase from 24.4±2.7 kg/m2 to 25.6±5.2 (P=0.083). Subjects with progression in glycemic status had more severe COVID-19 illness than non-progressors (P=0.030). Reassessment was not performed in the control group.
Conclusion
Subjects who had dysglycemia in acute COVID-19 were characterized by insulin resistance. Over 1 year, a quarter had progression in glycemic status, especially those with more severe COVID-19. Importantly, there was no significant deterioration in insulin secretory capacity.
- Drug/Regimen
- Efficacy and Safety of IDegAsp in a Real-World Korean Population with Type 2 Diabetes Mellitus
- Shinae Kang, Yu-Bae Ahn, Tae Keun Oh, Won-Young Lee, Sung Wan Chun, Boram Bae, Amine Dahaoui, Jin Sook Jeong, Sungeun Jung, Hak Chul Jang
- Received August 24, 2023 Accepted November 22, 2023 Published online February 27, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0297 [Epub ahead of print]
- 649 View
- 42 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
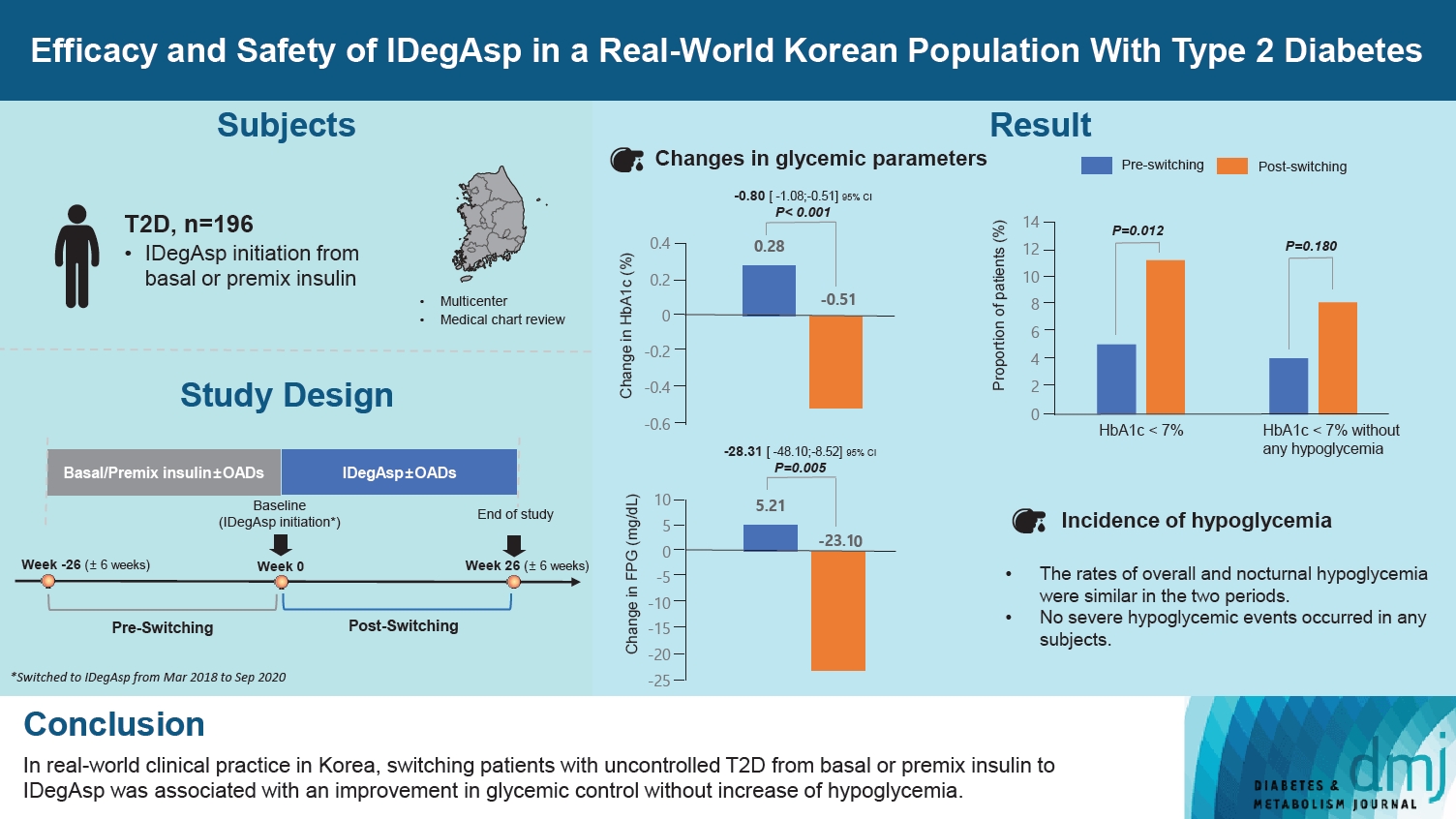
This study investigated the real-world efficacy and safety of insulin degludec/insulin aspart (IDegAsp) in Korean adults with type 2 diabetes mellitus (T2DM), whose insulin treatment was switched to IDegAsp.
Methods
This was a multicenter, retrospective, observational study comprising two 26-week treatment periods, before and after switching to IDegAsp, respectively. Korean adults with uncontrolled T2DM treated with basal or premix insulin (±oral antidiabetic drugs) were enrolled. The primary objective was to compare the degree of glycosylated hemoglobin (HbA1c) change in each 26-week observation period. The analyses included changes in HbA1c, fasting plasma glucose (FPG), body weight, proportion of participants achieving HbA1c <7.0%, hypoglycemic events, and total daily insulin dose (ClinicalTrials.gov, number NCT04656106).
Results
In total, 196 adults (mean age, 65.95 years; mean T2DM duration, 18.99 years) were analyzed. The change in both HbA1c and FPG were significantly different between the pre-switching and the post-switching period (0.28% vs. –0.51%, P<0.001; 5.21 mg/dL vs. –23.10 mg/dL, P=0.005), respectively. After switching, the rate of achieving HbA1c <7.0% was significantly improved (5.10% at baseline vs. 11.22% with IDegAsp, P=0.012). No significant differences (before vs. after switching) were observed in body weight change, and total daily insulin dose. The rates of overall and severe hypoglycemia were similar in the two periods.
Conclusion
In real-world clinical practice in Korea, the change of insulin regimen to IDegAsp was associated with an improvement in glycemic control without increase of hypoglycemia, supporting the use of IDegAsp for patients with T2DM uncontrolled with basal or premix insulin.
Sulwon Lecture 2023
- Metabolic Risk/Epidemiology
- Insulin Resistance, Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Clinical and Experimental Perspective
- Inha Jung, Dae-Jeong Koo, Won-Young Lee
- Received October 4, 2023 Accepted December 26, 2024 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0350 [Epub ahead of print]
- 983 View
- 59 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
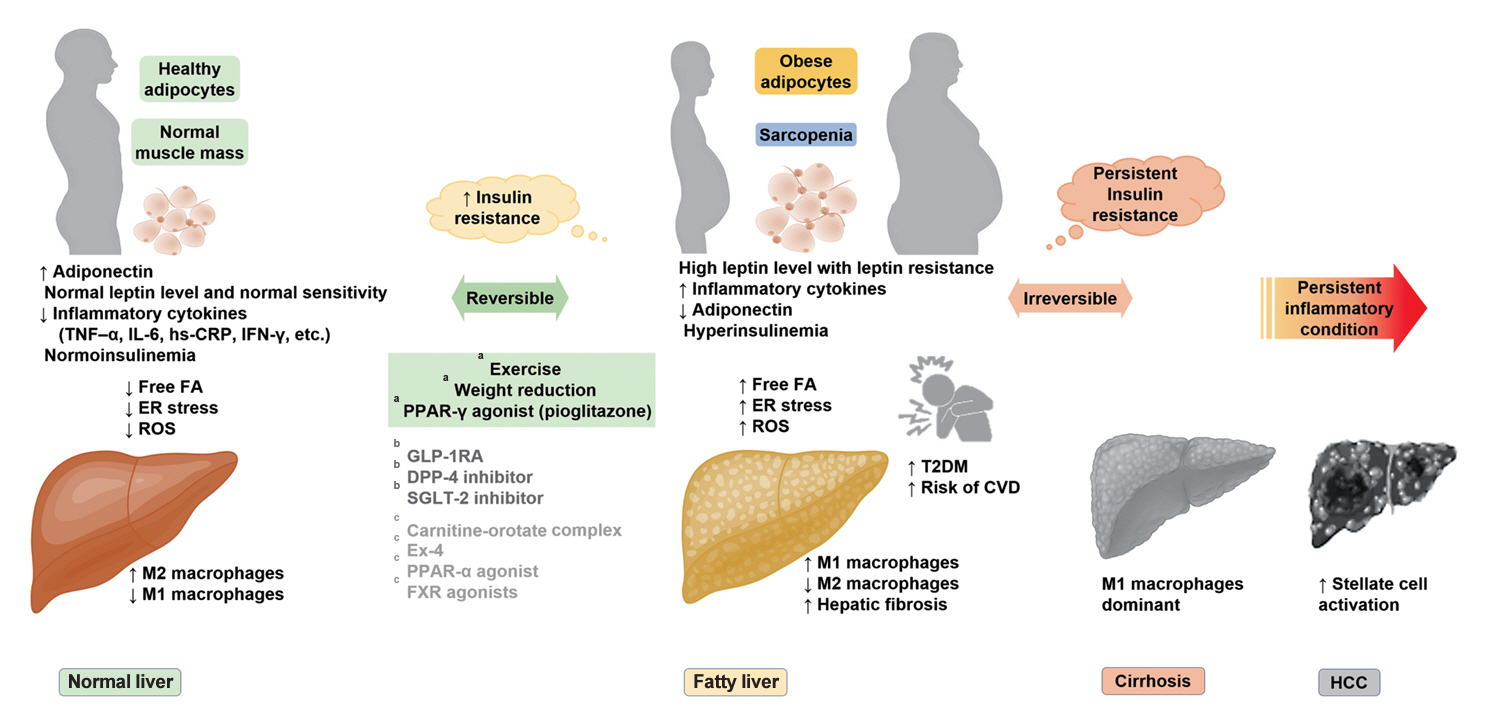
ePub - It has been generally accepted that insulin resistance (IR) and reduced insulin secretory capacity are the basic pathogenesis of type 2 diabetes mellitus (T2DM). In addition to genetic factors, the persistence of systemic inflammation caused by obesity and the associated threat of lipotoxicity increase the risk of T2DM. In particular, the main cause of IR is obesity and subjects with T2DM have a higher body mass index (BMI) than normal subjects according to recent studies. The prevalence of T2DM with IR has increased with increasing BMI during the past three decades. According to recent studies, homeostatic model assessment of IR was increased compared to that of the 1990s. Rising prevalence of obesity in Korea have contributed to the development of IR, non-alcoholic fatty liver disease and T2DM and cutting this vicious cycle is important. My colleagues and I have investigated this pathogenic mechanism on this theme through clinical and experimental studies over 20 years and herein, I would like to summarize some of our studies with deep gratitude for receiving the prestigious 2023 Sulwon Award.
Original Articles
- Drug/Regimen
- Pioglitazone as Add-on THERAPY in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Dapagliflozin and Metformin: Double-Blind, Randomized, Placebo-Controlled Trial
- Ji Hye Heo, Kyung Ah Han, Jun Hwa Hong, Hyun-Ae Seo, Eun-Gyoung Hong, Jae Myung Yu, Hye Seung Jung, Bong-Soo Cha
- Received September 1, 2023 Accepted October 25, 2023 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0314 [Epub ahead of print]
- 1,208 View
- 114 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
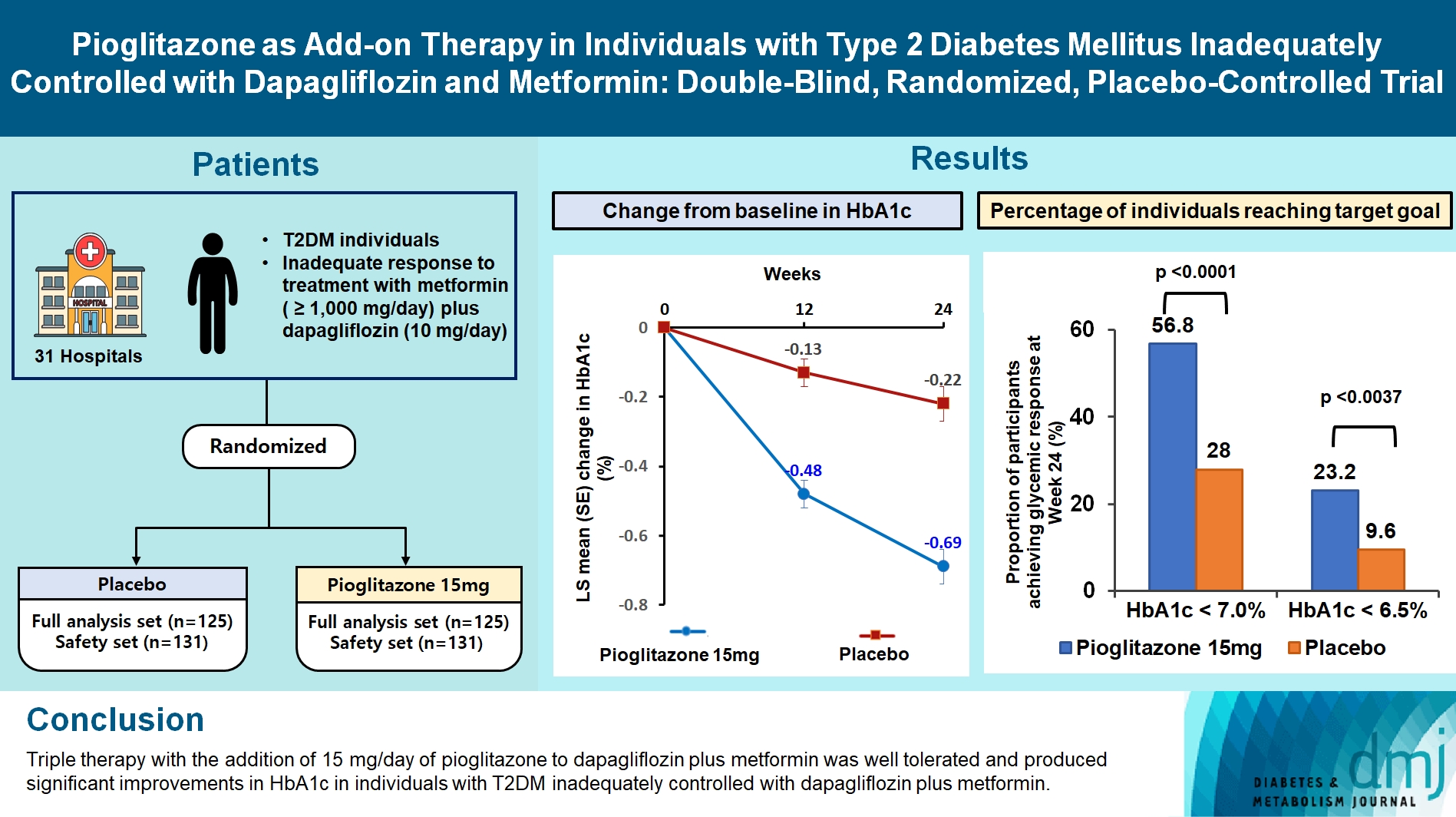
This study assessed the efficacy and safety of triple therapy with pioglitazone 15 mg add-on versus placebo in patients with type 2 diabetes mellitus (T2DM) inadequately controlled with metformin and dapagliflozin.
Methods
In this multicenter, double-blind, randomized, phase 3 study, patients with T2DM with an inadequate response to treatment with metformin (≥1,000 mg/day) plus dapagliflozin (10 mg/day) were randomized to receive additional pioglitazone 15 mg/day (n=125) or placebo (n=125) for 24 weeks. The primary endpoint was the change in glycosylated hemoglobin (HbA1c) levels from baseline to week 24 (ClinicalTrials.gov identifier: NCT05101135).
Results
At week 24, the adjusted mean change from baseline in HbA1c level compared with placebo was significantly greater with pioglitazone treatment (–0.47%; 95% confidence interval, –0.61 to –0.33; P<0.0001). A greater proportion of patients achieved HbA1c <7% or <6.5% at week 24 with pioglitazone compared to placebo as add-on to 10 mg dapagliflozin and metformin (56.8% vs. 28% for HbA1c <7%, and 23.2% vs. 9.6% for HbA1c <6.5%; P<0.0001 for all). The addition of pioglitazone also significantly improved triglyceride, highdensity lipoprotein cholesterol levels, and homeostatic model assessment of insulin resistance levels, while placebo did not. The incidence of treatment-emergent adverse events was similar between the groups, and the incidence of fluid retention-related side effects by pioglitazone was low (1.5%).
Conclusion
Triple therapy with the addition of 15 mg/day of pioglitazone to dapagliflozin plus metformin was well tolerated and produced significant improvements in HbA1c in patients with T2DM inadequately controlled with dapagliflozin plus metformin.
- Metabolic Risk/Epidemiology
- Harnessing Metabolic Indices as a Predictive Tool for Cardiovascular Disease in a Korean Population without Known Major Cardiovascular Event
- Hyun-Jin Kim, Byung Sik Kim, Yonggu Lee, Sang Bong Ahn, Dong Wook Kim, Jeong-Hun Shin
- Received June 22, 2023 Accepted August 18, 2023 Published online February 1, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0197 [Epub ahead of print]

- 934 View
- 25 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study evaluated the usefulness of indices for metabolic syndrome, non-alcoholic fatty liver disease (NAFLD), and insulin resistance (IR), as predictive tools for cardiovascular disease in middle-aged Korean adults.
Methods
The prospective data obtained from the Ansan-Ansung cohort database, excluding patients with major adverse cardiac and cerebrovascular events (MACCE). The primary outcome was the incidence of MACCE during the follow-up period.
Results
A total of 9,337 patients were included in the analysis, of whom 1,130 (12.1%) experienced MACCE during a median follow-up period of 15.5 years. The metabolic syndrome severity Z-score, metabolic syndrome severity score, hepatic steatosis index, and NAFLD liver fat score were found to significantly predict MACCE at values above the cut-off point and in the second and third tertiles. Among these indices, the hazard ratios of the metabolic syndrome severity score and metabolic syndrome severity Z-score were the highest after adjusting for confounding factors. The area under the receiver operating characteristic curve (AUC) of the 10-year atherosclerotic cardiovascular disease (ASCVD) score for predicting MACCE was 0.716, and the metabolic syndrome severity Z-score had an AUC of 0.619.
Conclusion
The metabolic syndrome severity score is a highly reliable indicator and was closely associated with the 10-year ASCVD risk score in predicting MACCE in the general population. Given the specific characteristics and limitations of metabolic syndrome severity scores as well as the indices of NAFLD and IR, a more practical scoring system that considers these factors is essential to achieve greater accuracy in forecasting cardiovascular outcomes.
- Pathophysiology
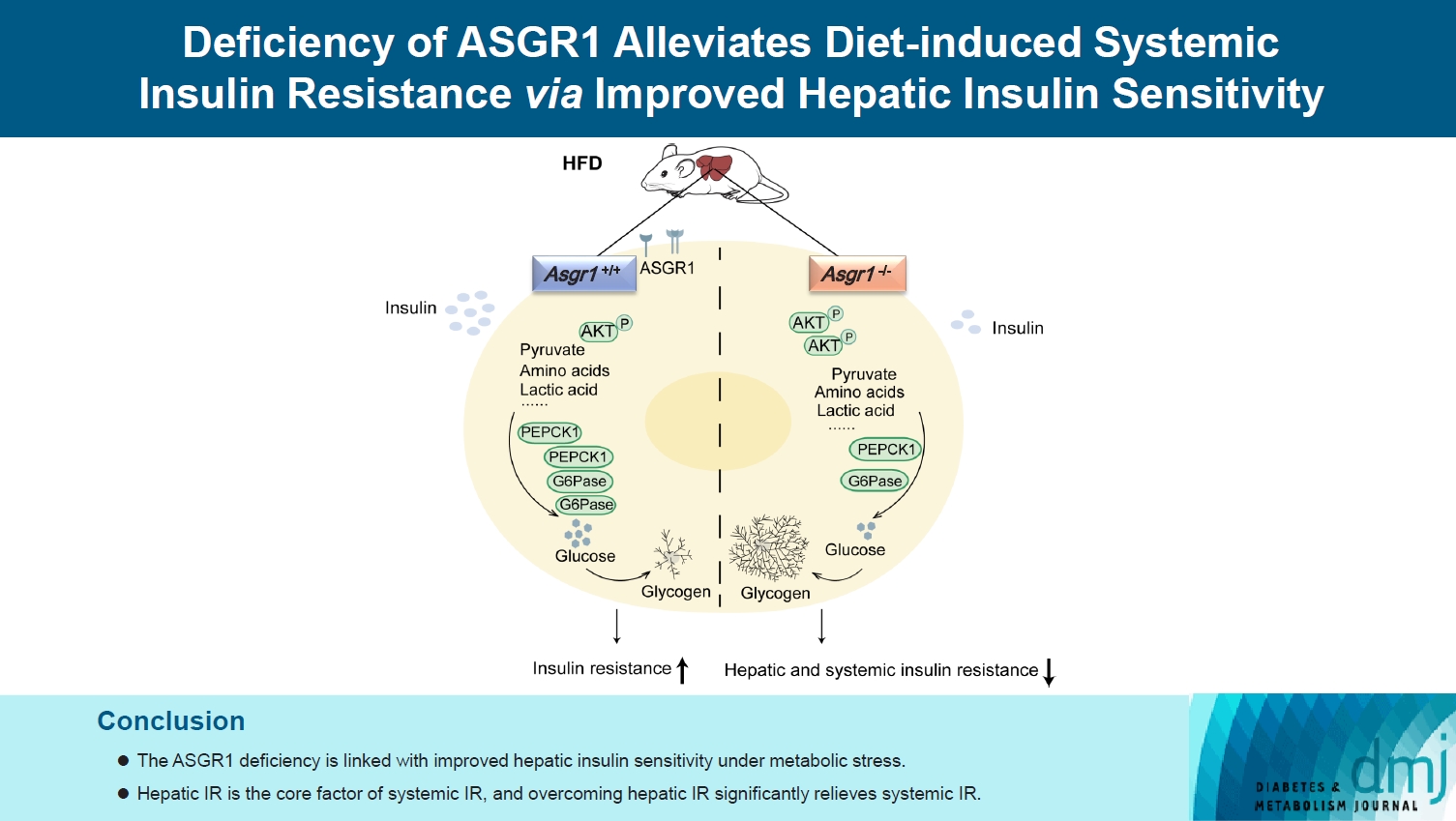
- Deficiency of ASGR1 Alleviates Diet-Induced Systemic Insulin Resistance via Improved Hepatic Insulin Sensitivity
- Xiaorui Yu, Jiawang Tao, Yuhang Wu, Yan Chen, Penghui Li, Fan Yang, Miaoxiu Tang, Abdul Sammad, Yu Tao, Yingying Xu, Yin-Xiong Li
- Published online February 1, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0124 [Epub ahead of print]
- 856 View
- 59 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Insulin resistance (IR) is the key pathological basis of many metabolic disorders. Lack of asialoglycoprotein receptor 1 (ASGR1) decreased the serum lipid levels and reduced the risk of coronary artery disease. However, whether ASGR1 also participates in the regulatory network of insulin sensitivity and glucose metabolism remains unknown.
Methods
The constructed ASGR1 knockout mice and ASGR1-/- HepG2 cell lines were used to establish the animal model of metabolic syndrome and the IR cell model by high-fat diet (HFD) or drug induction, respectively. Then we evaluated the glucose metabolism and insulin signaling in vivo and in vitro.
Results
ASGR1 deficiency ameliorated systemic IR in mice fed with HFD, evidenced by improved insulin intolerance, serum insulin, and homeostasis model assessment of IR index, mainly contributed from increased insulin signaling in the liver, but not in muscle or adipose tissues. Meanwhile, the insulin signal transduction was significantly enhanced in ASGR1-/- HepG2 cells. By transcriptome analyses and comparison, those differentially expressed genes between ASGR1 null and wild type were enriched in the insulin signal pathway, particularly in phosphoinositide 3-kinase-AKT signaling. Notably, ASGR1 deficiency significantly reduced hepatic gluconeogenesis and glycogenolysis.
Conclusion
The ASGR1 deficiency was consequentially linked with improved hepatic insulin sensitivity under metabolic stress, hepatic IR was the core factor of systemic IR, and overcoming hepatic IR significantly relieved the systemic IR. It suggests that ASGR1 is a potential intervention target for improving systemic IR in metabolic disorders.
Review
- Pathophysiology
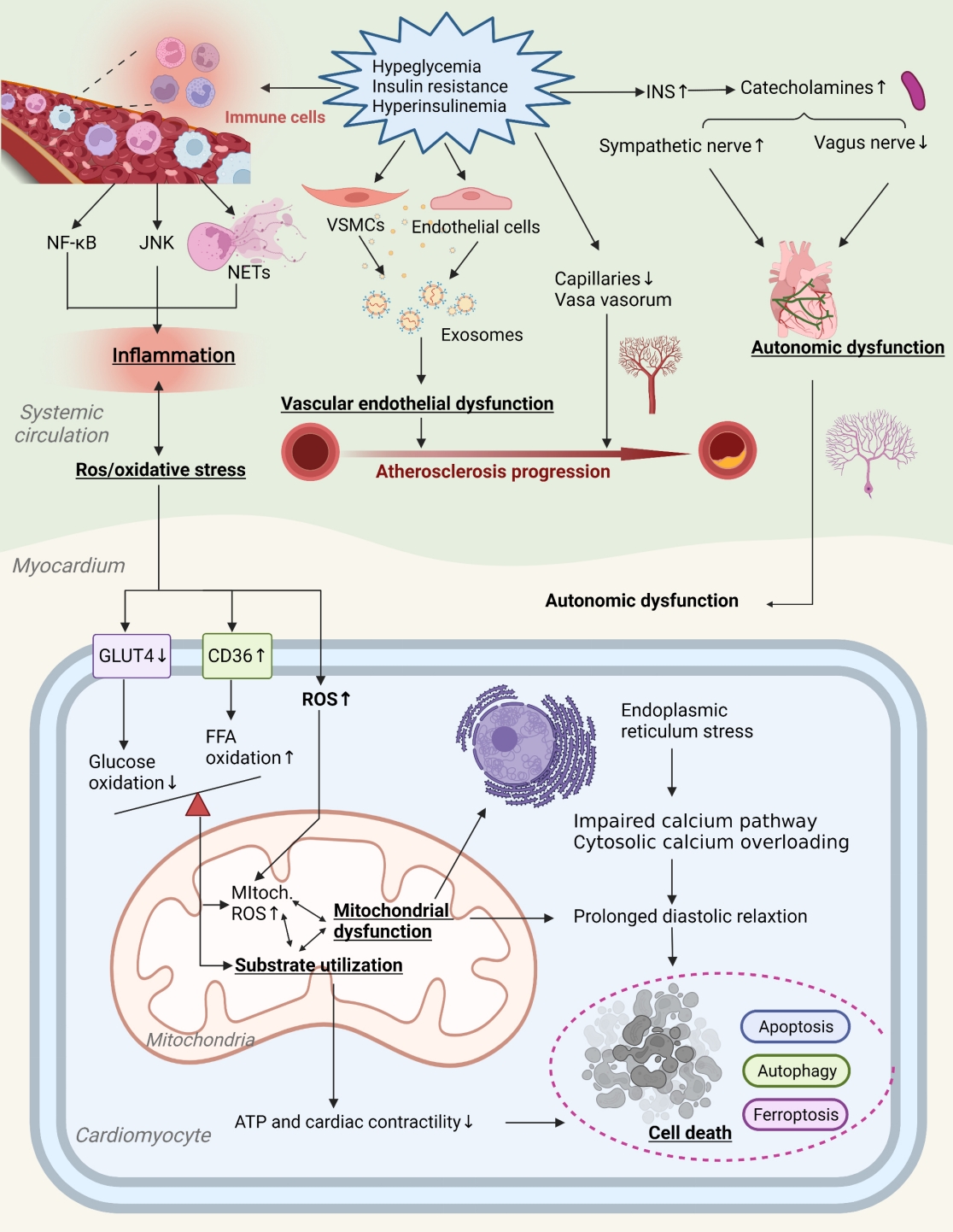
- Primordial Drivers of Diabetes Heart Disease: Comprehensive Insights into Insulin Resistance
- Yajie Fan, Zhipeng Yan, Tingting Li, Aolin Li, Xinbiao Fan, Zhongwen Qi, Junping Zhang
- Diabetes Metab J. 2024;48(1):19-36. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0110
- 2,174 View
- 182 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Insulin resistance has been regarded as a hallmark of diabetes heart disease (DHD). Numerous studies have shown that insulin resistance can affect blood circulation and myocardium, which indirectly cause cardiac hypertrophy and ventricular remodeling, participating in the pathogenesis of DHD. Meanwhile, hyperinsulinemia, hyperglycemia, and hyperlipidemia associated with insulin resistance can directly impair the metabolism and function of the heart. Targeting insulin resistance is a potential therapeutic strategy for the prevention of DHD. Currently, the role of insulin resistance in the pathogenic development of DHD is still under active research, as the pathological roles involved are complex and not yet fully understood, and the related therapeutic approaches are not well developed. In this review, we describe insulin resistance and add recent advances in the major pathological and physiological changes and underlying mechanisms by which insulin resistance leads to myocardial remodeling and dysfunction in the diabetic heart, including exosomal dysfunction, ferroptosis, and epigenetic factors. In addition, we discuss potential therapeutic approaches to improve insulin resistance and accelerate the development of cardiovascular protection drugs.
Original Articles
- Drug/Regimen
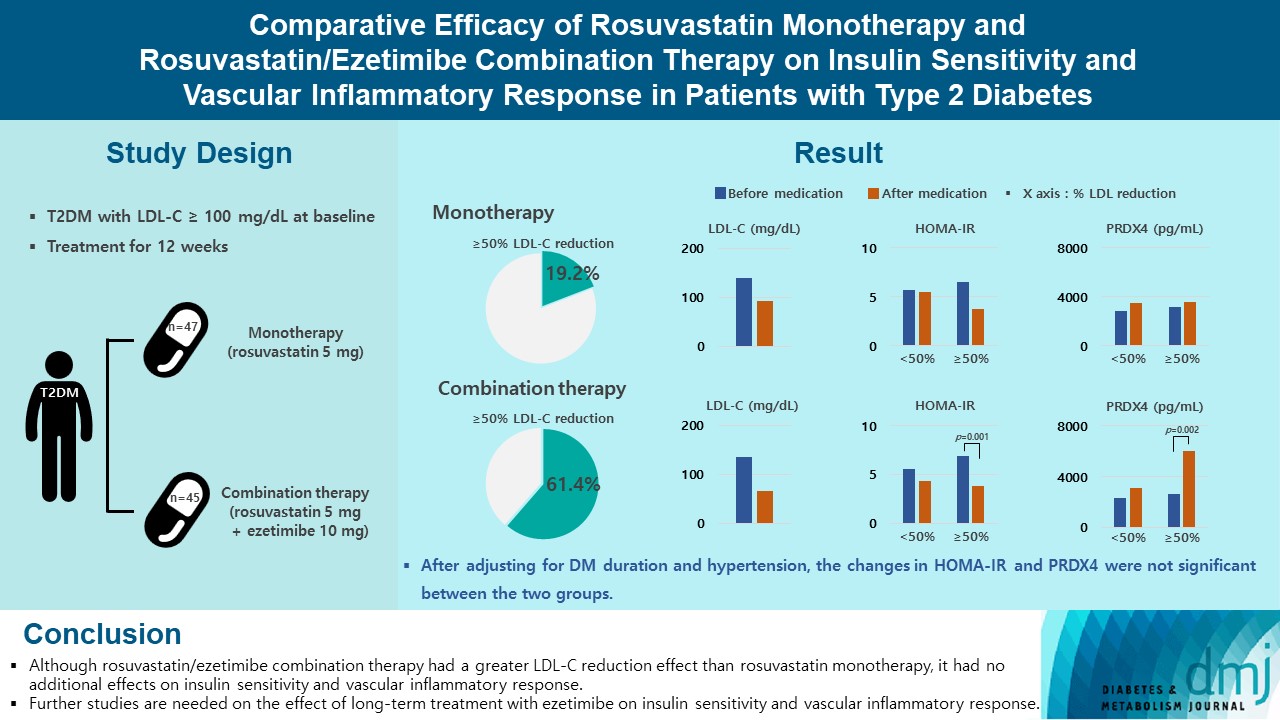
- Comparative Efficacy of Rosuvastatin Monotherapy and Rosuvastatin/Ezetimibe Combination Therapy on Insulin Sensitivity and Vascular Inflammatory Response in Patients with Type 2 Diabetes Mellitus
- Ji Hye Han, Kyong Hye Joung, Jun Choul Lee, Ok Soon Kim, Sorim Choung, Ji Min Kim, Yea Eun Kang, Hyon-Seung Yi, Ju Hee Lee, Bon Jeong Ku, Hyun Jin Kim
- Diabetes Metab J. 2024;48(1):112-121. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0402
- 2,026 View
- 222 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Type 2 diabetes mellitus (T2DM) induces endothelial dysfunction and inflammation, which are the main factors for atherosclerosis and cardiovascular disease. The present study aimed to compare the effects of rosuvastatin monotherapy and rosuvastatin/ezetimibe combination therapy on lipid profile, insulin sensitivity, and vascular inflammatory response in patients with T2DM.
Methods
A total of 101 patients with T2DM and dyslipidemia were randomized to either rosuvastatin monotherapy (5 mg/day, n=47) or rosuvastatin/ezetimibe combination therapy (5 mg/10 mg/day, n=45) and treated for 12 weeks. Serum lipids, glucose, insulin, soluble intercellular adhesion molecule-1 (sICAM-1), and peroxiredoxin 4 (PRDX4) levels were determined before and after 12 weeks of treatment.
Results
The reduction in low density lipoprotein cholesterol (LDL-C) by more than 50% from baseline after treatment was more in the combination therapy group. The serum sICAM-1 levels increased significantly in both groups, but there was no difference between the two groups. The significant changes in homeostasis model assessment of insulin resistance (HOMA-IR) and PRDX4 were confirmed only in the subgroup in which LDL-C was reduced by 50% or more in the combination therapy group. However, after adjusting for diabetes mellitus duration and hypertension, the changes in HOMA-IR and PRDX4 were not significant between the two groups.
Conclusion
Although rosuvastatin/ezetimibe combination therapy had a greater LDL-C reduction effect than rosuvastatin monotherapy, it had no additional effects on insulin sensitivity and vascular inflammatory response. Further studies are needed on the effect of long-term treatment with ezetimibe on insulin sensitivity and vascular inflammatory response. -
Citations
Citations to this article as recorded by- Combining Ezetimibe and Rosuvastatin: Impacts on Insulin Sensitivity and Vascular Inflammation in Patients with Type 2 Diabetes Mellitus
Eun Roh
Diabetes & Metabolism Journal.2024; 48(1): 55. CrossRef
- Combining Ezetimibe and Rosuvastatin: Impacts on Insulin Sensitivity and Vascular Inflammation in Patients with Type 2 Diabetes Mellitus
- Metabolic Risk/Epidemiology
- Association of Measures of Glucose Metabolism with Colorectal Cancer Risk in Older Chinese: A 13-Year Follow-up of the Guangzhou Biobank Cohort Study-Cardiovascular Disease Substudy and Meta-Analysis
- Shu Yi Wang, Wei Sen Zhang, Chao Qiang Jiang, Ya Li Jin, Tong Zhu, Feng Zhu, Lin Xu
- Diabetes Metab J. 2024;48(1):134-145. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0383

- 1,127 View
- 140 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Abnormal glucose metabolism is a risk factor for colorectal cancer (CRC). However, association of glycosylated hemoglobin (HbA1c) with CRC risk remains under-reported. We examined the association between glycemic indicators (HbA1c, fasting plasma glucose, fasting insulin, 2-hour glucose, 2-hour insulin, and homeostasis model of risk assessment-insulin resistance index) and CRC risk using prospective analysis and meta-analysis.
Methods
Participants (n=1,915) from the Guangzhou Biobank Cohort Study-Cardiovascular Disease Substudy were included. CRC events were identified through record linkage. Cox regression was used to assess the associations of glycemic indicators with CRC risk. A meta-analysis was performed to investigate the association between HbA1c and CRC risk.
Results
During an average of 12.9 years follow-up (standard deviation, 2.8), 42 incident CRC cases occurred. After adjusting for potential confounders, the hazard ratio (95% confidence interval [CI]) of CRC for per % increment in HbA1c was 1.28 (95% CI, 1.01 to 1.63) in overall population, 1.51 (95% CI, 1.13 to 2.02) in women and 1.06 (95% CI, 0.68 to 1.68) in men. No significant association of other measures of glycemic indicators and baseline diabetes with CRC risk was found. Meta-analyses of 523,857 participants including our results showed that per % increment of HbA1c was associated with 13% higher risk of CRC, with the pooled risk ratio being 1.13 (95% CI, 1.01 to 1.27). Subgroupanalyses found stronger associations in women, colon cancer, Asians, and case-control studies.
Conclusion
Higher HbA1c was a significant predictor of CRC in the general population. Our findings shed light on the pathology of glucose metabolism and CRC, which warrants more in-depth investigation.
- Basic Research
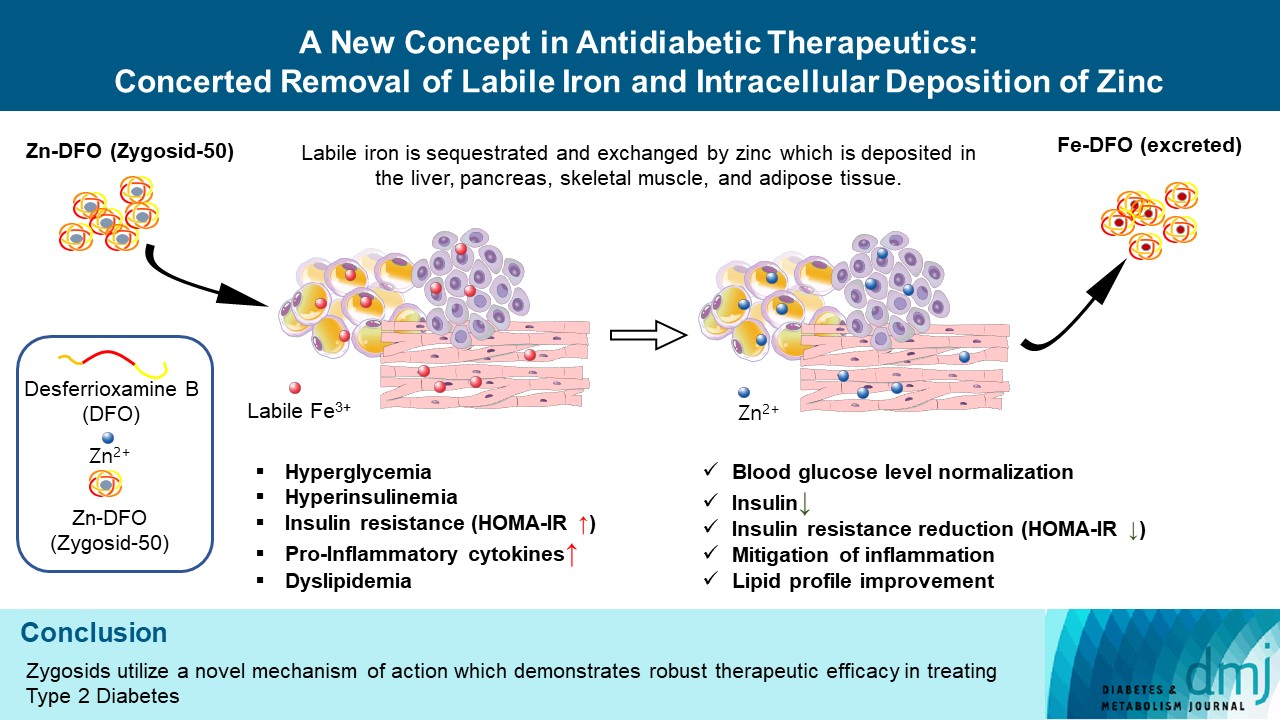
- A New Concept in Antidiabetic Therapeutics: A Concerted Removal of Labile Iron and Intracellular Deposition of Zinc
- Vladimir Vinokur, Eduard Berenshtein, Mordechai Chevion, Dror Chevion
- Diabetes Metab J. 2024;48(1):59-71. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0292
- Retraction in: Diabetes Metab J 2024;48(2):325
- 1,570 View
- 178 Download
- Basic Research
- Glucolipotoxicity Suppressed Autophagy and Insulin Contents in Human Islets, and Attenuation of PERK Activity Enhanced Them in an ATG7-Dependent Manner
- Seoil Moon, Ji Yoon Lim, Mirang Lee, Youngmin Han, Hongbeom Kim, Wooil Kwon, Jin-Young Jang, Mi Na Kim, Kyong Soo Park, Hye Seung Jung
- Diabetes Metab J. 2024;48(2):231-241. Published online September 6, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0366
- 1,695 View
- 155 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
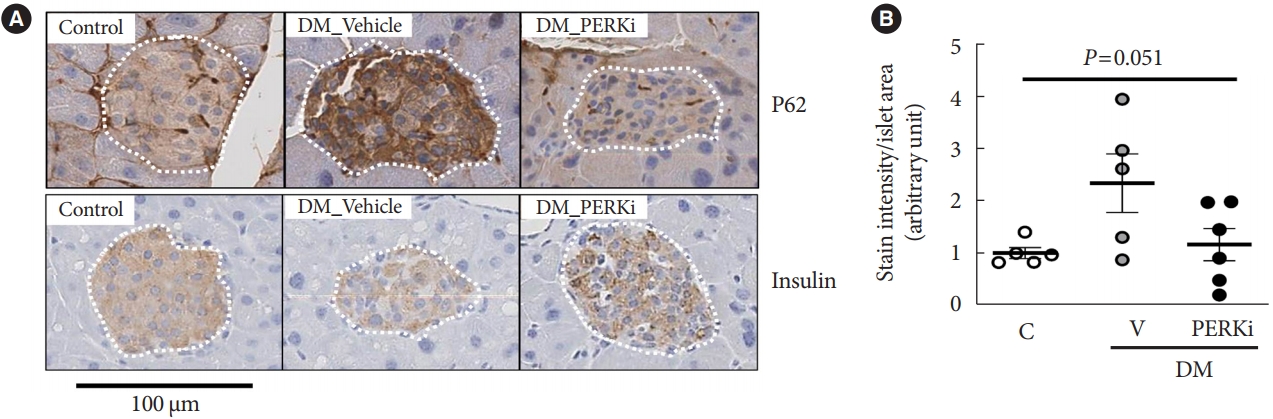
Administration of pancreatic endoplasmic reticulum kinase inhibitor (PERKi) improved insulin secretion and hyperglycemia in obese diabetic mice. In this study, autophagic balance was studied whether to mediate it.
Methods
Human islets were isolated from living patients without diabetes. PERKi GSK2606414 effects were evaluated in the islets under glucolipotoxicity by palmitate. Islet insulin contents and secretion were measured. Autophagic flux was assessed by microtubule associated protein 1 light chain 3 (LC3) conversion, a red fluorescent protein (RFP)-green fluorescent protein (GFP)- LC3 tandem assay, and P62 levels. For mechanical analyses, autophagy was suppressed using 3-methyladenine in mouse islets. Small interfering RNA for an autophagy-related gene autophagy related 7 (Atg7) was transfected to interfere autophagy.
Results
PERKi administration to mice decreased diabetes-induced P62 levels in the islets. Glucolipotoxicity significantly increased PERK phosphorylation by 70% and decreased insulin contents by 50% in human islets, and addition of PERKi (40 to 80 nM) recovered both. PERKi also enhanced glucose-stimulated insulin secretion (6-fold). PERKi up-regulated LC3 conversion suppressed by glucolipotoxicity, and down-regulated P62 contents without changes in P62 transcription, indicating enhanced autophagic flux. Increased autophagosome-lysosome fusion by PERKi was visualized in mouse islets, where PERKi enhanced ATG7 bound to LC3. Suppression of Atg7 eliminated PERKi-induced insulin contents and secretion.
Conclusion
This study provided functional changes of human islets with regard to autophagy under glucolipotoxicity, and suggested modulation of autophagy as an anti-diabetic mechanism of PERKi.
Reviews
- Basic Research
- Rediscovering Primary Cilia in Pancreatic Islets
- Eun Young Lee, Jing W. Hughes
- Diabetes Metab J. 2023;47(4):454-469. Published online April 28, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0442
- 2,598 View
- 240 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
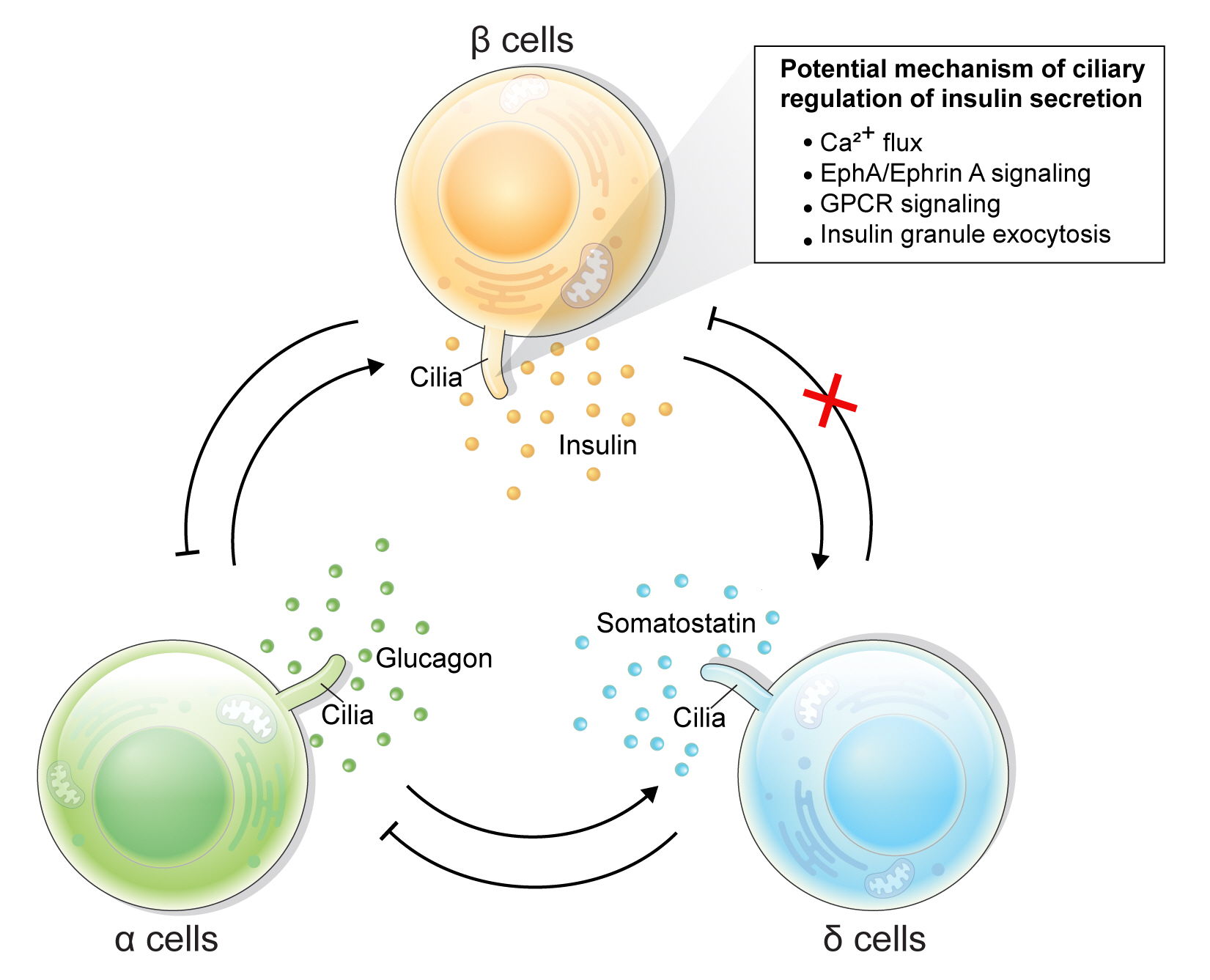
ePub - Primary cilia are microtubule-based sensory and signaling organelles on the surfaces of most eukaryotic cells. Despite their early description by microscopy studies, islet cilia had not been examined in the functional context until recent decades. In pancreatic islets as in other tissues, primary cilia facilitate crucial developmental and signaling pathways in response to extracellular stimuli. Many human developmental and genetic disorders are associated with ciliary dysfunction, some manifesting as obesity and diabetes. Understanding the basis for metabolic diseases in human ciliopathies has been aided by close examination of cilia action in pancreatic islets at cellular and molecular levels. In this article, we review the evidence for ciliary expression on islet cells, known roles of cilia in pancreas development and islet hormone secretion, and summarize metabolic manifestations of human ciliopathy syndromes. We discuss emerging data on primary cilia regulation of islet cell signaling and the structural basis of cilia-mediated cell crosstalk, and offer our interpretation on the role of cilia in glucose homeostasis and human diseases.
-
Citations
Citations to this article as recorded by- Beta cell primary cilia mediate somatostatin responsiveness via SSTR3
Samantha E. Adamson, Zipeng A. Li, Jing W. Hughes
Islets.2023;[Epub] CrossRef
- Beta cell primary cilia mediate somatostatin responsiveness via SSTR3
- Basic Research
- Regulation of Cellular Senescence in Type 2 Diabetes Mellitus: From Mechanisms to Clinical Applications
- Kanako Iwasaki, Cristian Abarca, Cristina Aguayo-Mazzucato
- Diabetes Metab J. 2023;47(4):441-453. Published online March 6, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0416

- 4,708 View
- 419 Download
- 3 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Cellular senescence is accelerated by hyperglycemia through multiple pathways. Therefore, senescence is an important cellular mechanism to consider in the pathophysiology of type 2 diabetes mellitus (T2DM) and an additional therapeutic target. The use of drugs that remove senescent cells has led to improvements in blood glucose levels and diabetic complications in animal studies. Although the removal of senescent cells is a promising approach for the treatment of T2DM, two main challenges limit its clinical application: the molecular basis of cellular senescence in each organ is yet to be understood, and the specific effect of removing senescent cells in each organ has to be determined. This review aims to discuss future applications of targeting senescence as a therapeutic option in T2DM and elucidate the characteristics of cellular senescence and senescence-associated secretory phenotype in the tissues important for regulating glucose levels: pancreas, liver, adipocytes, and skeletal muscle.
-
Citations
Citations to this article as recorded by- Amide Alkaloids as Privileged Sources of Senomodulators for Therapeutic Purposes in Age-Related Diseases
Mazzarine Dotou, Aurore L’honoré, Roba Moumné, Chahrazade El Amri
Journal of Natural Products.2024; 87(3): 617. CrossRef - Study on the Pathogenesis of Cell Senescence in Non-Alcoholic Fatty Liver
丽媛 黄
Medical Diagnosis.2024; 14(01): 76. CrossRef - Senescent adipocytes and type 2 diabetes – current knowledge and perspective concepts
Weronika Kruczkowska, Julia Gałęziewska, Mateusz Kciuk, Adrianna Gielecińska, Elżbieta Płuciennik, Zbigniew Pasieka, Lin-Yong Zhao, Yi-Jin Yu, Damian Kołat, Żaneta Kałuzińska-Kołat
Biomolecular Concepts.2024;[Epub] CrossRef - The Effect of Long-Term Passage on Porcine SMCs’ Function and the Improvement of TGF-β1 on Porcine SMCs’ Secretory Function in Late Passage
Yan-Yan Zheng, Ze-Nan Hu, Zheng Liu, Yi-Chen Jiang, Ren-Peng Guo, Shi-Jie Ding, Guang-Hong Zhou
Foods.2023; 12(14): 2682. CrossRef - Exploring the Relationship between Cellular Senescence Markers and Aging-Related Diseases
怡 罗
Advances in Clinical Medicine.2023; 13(08): 12298. CrossRef
- Amide Alkaloids as Privileged Sources of Senomodulators for Therapeutic Purposes in Age-Related Diseases
Original Articles
- Guideline/Fact Sheet
- Insulin Fact Sheet in Type 1 and 2 Diabetes Mellitus and Trends of Antidiabetic Medication Use in Insulin Users with Type 2 Diabetes Mellitus: 2002 to 2019
- Jiyun Park, Gyuri Kim, Bong-Sung Kim, Kyung-Do Han, So Yoon Kwon, So Hee Park, You-Bin Lee, Sang-Man Jin, Jae Hyeon Kim
- Diabetes Metab J. 2023;47(2):211-219. Published online February 7, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0346
- 3,543 View
- 258 Download
- 1 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study investigated the trends of insulin use among Korean patients with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM). Changes in prescription of antidiabetic medications in T2DM patients taking insulin therapy were evaluated.
Methods
We analyzed data from the National Health Insurance Service database in Korea to evaluate the prevalence of insulin users and trends of insulin use in T1DM and T2DM patients from January 2002 to December 2019. We also investigated numbers and types of antidiabetic medications in insulin users with T2DM.
Results
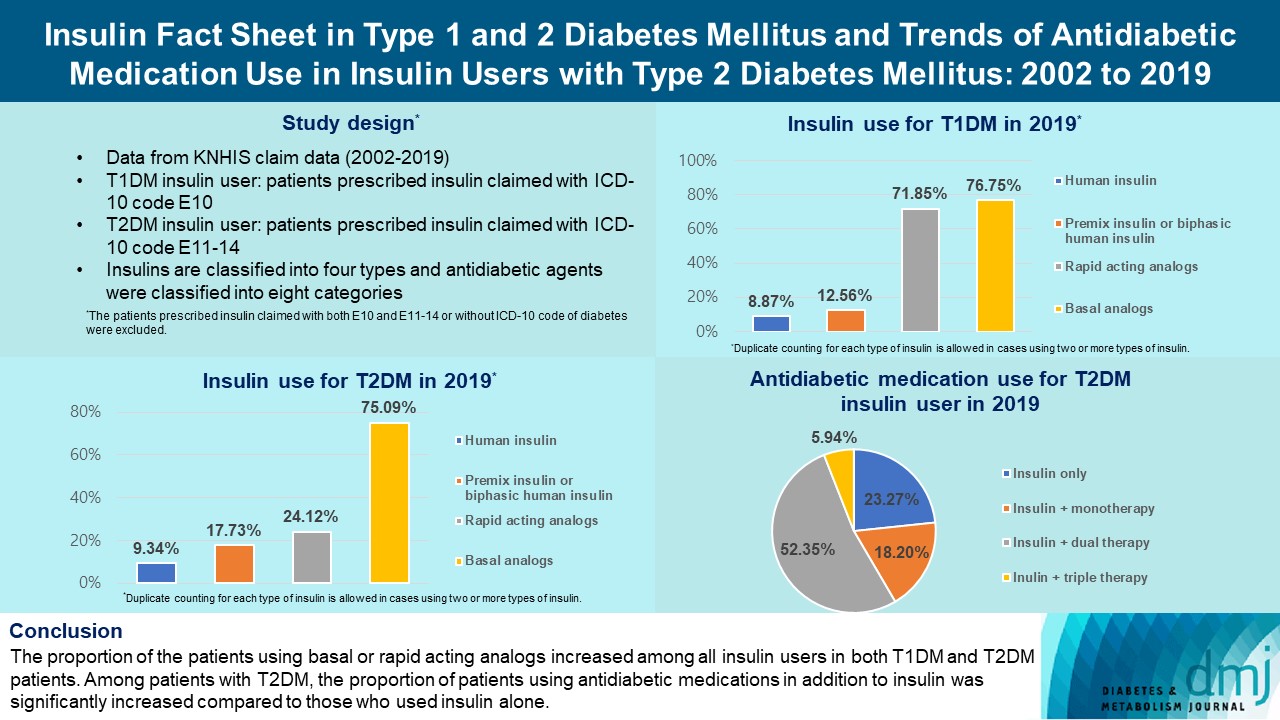
The overall total number of insulin users increased from 2002 to 2019, reaching 348,254 for T2DM and 20,287 for T1DM in 2019 compared with 109,974 for T2DM and 34,972 for T1DM in 2002. The proportion of patients using basal analogs and short acting analogs have increased and those using human insulin, premixed insulin, or biphasic human insulin have decreased (rapid acting analogs: 71.85% and 24.12% in T1DM and T2DM, respectively, in 2019; basal analogs: 76.75% and 75.09% in T1DM and T2DM, respectively, in 2019). The use of other antidiabetic medication in addition to insulin increased for T2DM, especially in dual therapy, reaching up to 52.35% in 2019 compared with 16.72% in 2002.
Conclusion
The proportion of the patients using basal or rapid acting analogs increased among all insulin users in both T1DM and T2DM patients. Among patients with T2DM, the proportion of patients using antidiabetic medications in addition to insulin was significantly increased compared to those who used insulin alone. -
Citations
Citations to this article as recorded by- Real-World Continuous Glucose Monitoring Data from a Population with Type 1 Diabetes in South Korea: Nationwide Single-System Analysis
Ji Yoon Kim, Sang-Man Jin, Sarah B. Andrade, Boyang Chen, Jae Hyeon Kim
Diabetes Technology & Therapeutics.2024;[Epub] CrossRef - Evaluation of pharmacokinetic interactions between lobeglitazone, empagliflozin, and metformin in healthy subjects
Heeyoung Kim, Choon Ok Kim, Hyeonsoo Park, Min Soo Park, Dasohm Kim, Taegon Hong, Yesong Shin, Byung Hak Jin
Translational and Clinical Pharmacology.2023; 31(1): 59. CrossRef - Smart Insulin Pen: Managing Insulin Therapy for People with Diabetes in the Digital Era
Jee Hee Yoo, Jae Hyeon Kim
The Journal of Korean Diabetes.2023; 24(4): 190. CrossRef
- Real-World Continuous Glucose Monitoring Data from a Population with Type 1 Diabetes in South Korea: Nationwide Single-System Analysis
- Basic Research
- Long Non-Coding RNA TUG1 Attenuates Insulin Resistance in Mice with Gestational Diabetes Mellitus via Regulation of the MicroRNA-328-3p/SREBP-2/ERK Axis
- Xuwen Tang, Qingxin Qin, Wenjing Xu, Xuezhen Zhang
- Diabetes Metab J. 2023;47(2):267-286. Published online January 19, 2023
- DOI: https://doi.org/10.4093/dmj.2021.0216
- 2,852 View
- 188 Download
- 6 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Long non-coding RNAs (lncRNAs) have been illustrated to contribute to the development of gestational diabetes mellitus (GDM). In the present study, we aimed to elucidate how lncRNA taurine upregulated gene 1 (TUG1) influences insulin resistance (IR) in a high-fat diet (HFD)-induced mouse model of GDM.
Methods
We initially developed a mouse model of HFD-induced GDM, from which islet tissues were collected for RNA and protein extraction. Interactions among lncRNA TUG1/microRNA (miR)-328-3p/sterol regulatory element binding protein 2 (SREBP-2) were assessed by dual-luciferase reporter assay. Fasting blood glucose (FBG), fasting insulin (FINS), homeostasis model assessment of insulin resistance (HOMA-IR), HOMA pancreatic β-cell function (HOMA-β), insulin sensitivity index for oral glucose tolerance tests (ISOGTT) and insulinogenic index (IGI) levels in mouse serum were measured through conducting gain- and loss-of-function experiments.
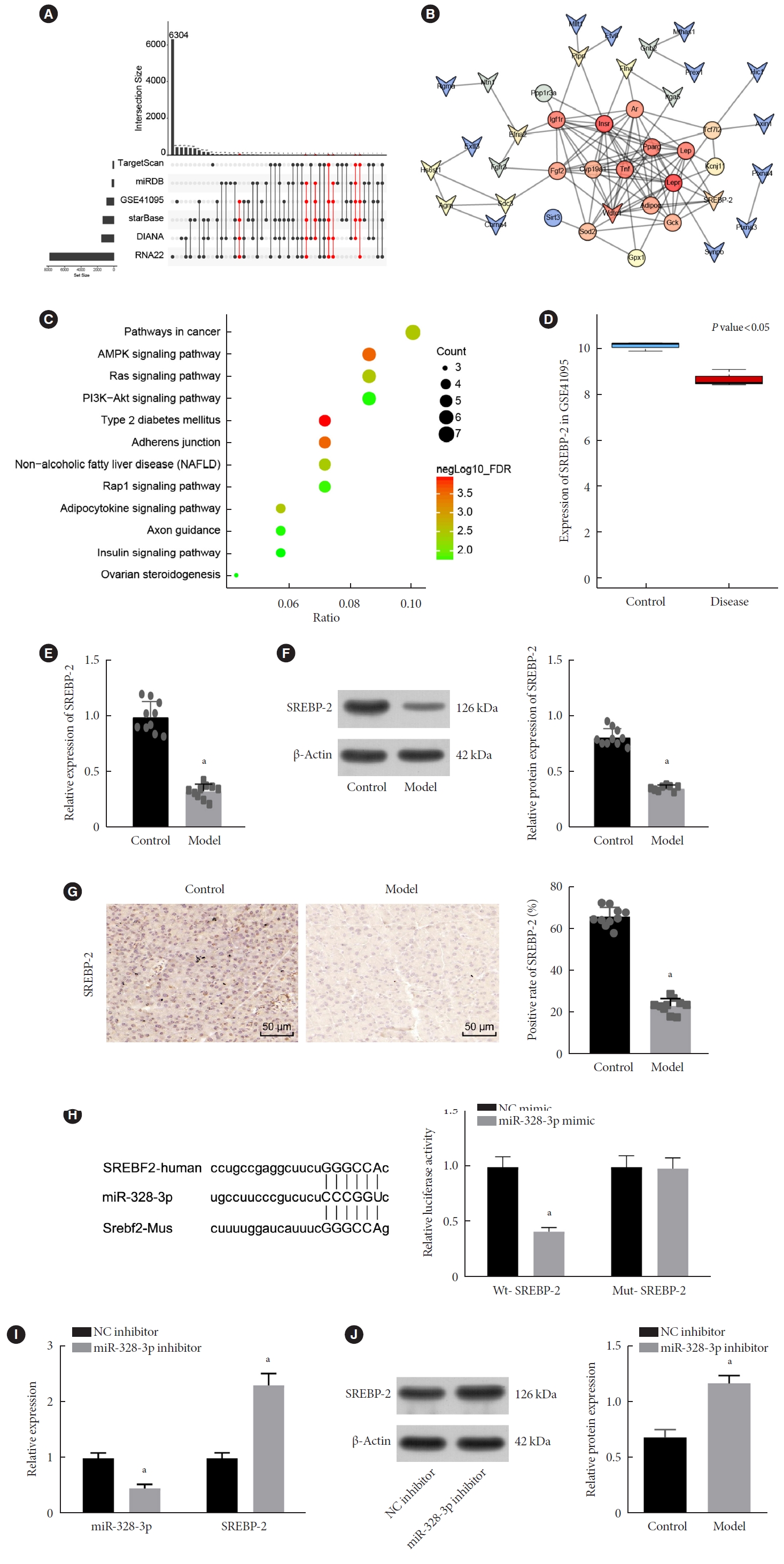
Results
Abundant expression of miR-328 and deficient expression of lncRNA TUG1 and SREBP-2 were characterized in the islet tissues of mice with HFD-induced GDM. LncRNA TUG1 competitively bound to miR-328-3p, which specifically targeted SREBP-2. Either depletion of miR-328-3p or restoration of lncRNA TUG1 and SREBP-2 reduced the FBG, FINS, HOMA-β, and HOMA-IR levels while increasing ISOGTT and IGI levels, promoting the expression of the extracellular signal-regulated kinase (ERK) signaling pathway-related genes, and inhibiting apoptosis of islet cells in GDM mice. Upregulation miR-328-3p reversed the alleviative effects of SREBP-2 and lncRNA TUG1 on IR.
Conclusion
Our study provides evidence that the lncRNA TUG1 may prevent IR following GDM through competitively binding to miR-328-3p and promoting the SREBP-2-mediated ERK signaling pathway inactivation. -
Citations
Citations to this article as recorded by- Diabetes and diabetic associative diseases: An overview of epigenetic regulations of TUG1
Mohammed Ageeli Hakami
Saudi Journal of Biological Sciences.2024; 31(5): 103976. CrossRef - Effect of Tinospora cordifolia on gestational diabetes mellitus and its complications
Ritu Rani, Havagiray Chitme, Avinash Kumar Sharma
Women & Health.2023; 63(5): 359. CrossRef - Therapeutic Effect of Tinospora cordifolia (Willd) Extracts on Letrozole-Induced Polycystic Ovarian Syndrome and its Complications in Murine Model
Ritu Rani, Avinash Kumar Sharma, Havagiray R Chitme
Clinical Medicine Insights: Endocrinology and Diabetes.2023;[Epub] CrossRef - The role of ncRNA regulatory mechanisms in diseases—case on gestational diabetes
Dong Gao, Liping Ren, Yu-Duo Hao, Nalini Schaduangrat, Xiao-Wei Liu, Shi-Shi Yuan, Yu-He Yang, Yan Wang, Watshara Shoombuatong, Hui Ding
Briefings in Bioinformatics.2023;[Epub] CrossRef - lncRNA TUG1 as potential novel biomarker for prognosis of cardiovascular diseases
Habib Haybar, Narjes Sadat Sadati, Daryush Purrahman, Mohammad Reza Mahmoudian-Sani, Najmaldin Saki
Epigenomics.2023; 15(23): 1273. CrossRef
- Diabetes and diabetic associative diseases: An overview of epigenetic regulations of TUG1

 KDA
KDA
 First
First Prev
Prev





