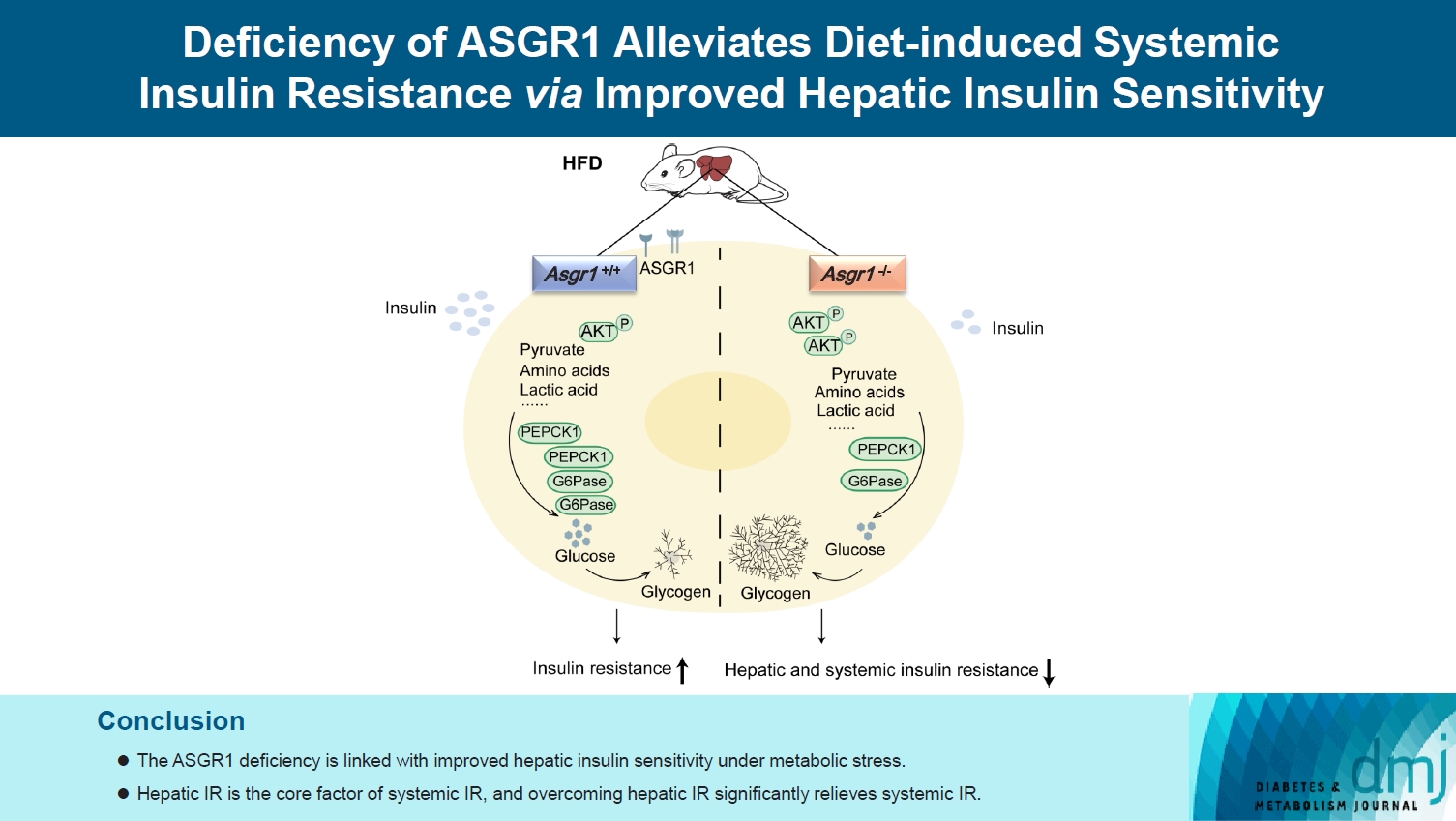
Deficiency of ASGR1 Alleviates Diet-Induced Systemic Insulin Resistance via Improved Hepatic Insulin Sensitivity
Article information
Abstract
Background
Insulin resistance (IR) is the key pathological basis of many metabolic disorders. Lack of asialoglycoprotein receptor 1 (ASGR1) decreased the serum lipid levels and reduced the risk of coronary artery disease. However, whether ASGR1 also participates in the regulatory network of insulin sensitivity and glucose metabolism remains unknown.
Methods
The constructed ASGR1 knockout mice and ASGR1-/- HepG2 cell lines were used to establish the animal model of metabolic syndrome and the IR cell model by high-fat diet (HFD) or drug induction, respectively. Then we evaluated the glucose metabolism and insulin signaling in vivo and in vitro.
Results
ASGR1 deficiency ameliorated systemic IR in mice fed with HFD, evidenced by improved insulin intolerance, serum insulin, and homeostasis model assessment of IR index, mainly contributed from increased insulin signaling in the liver, but not in muscle or adipose tissues. Meanwhile, the insulin signal transduction was significantly enhanced in ASGR1-/- HepG2 cells. By transcriptome analyses and comparison, those differentially expressed genes between ASGR1 null and wild type were enriched in the insulin signal pathway, particularly in phosphoinositide 3-kinase-AKT signaling. Notably, ASGR1 deficiency significantly reduced hepatic gluconeogenesis and glycogenolysis.
Conclusion
The ASGR1 deficiency was consequentially linked with improved hepatic insulin sensitivity under metabolic stress, hepatic IR was the core factor of systemic IR, and overcoming hepatic IR significantly relieved the systemic IR. It suggests that ASGR1 is a potential intervention target for improving systemic IR in metabolic disorders.
Highlights
• ASGR1 deficiency alleviated systemic IR under metabolic stress mainly by improving hepatic insulin sensitivity.
• Significant DEGs in the ASGR1 deficiency state were enriched in the insulin signaling pathway
• ASGR1-medicated hepatic insulin signaling primarily regulates gluconeogenesis and glycogenolysis.
INTRODUCTION
Insulin resistance (IR) is one of the earliest characteristics of a range of metabolic diseases that include obesity, type 2 diabetes mellitus (T2DM), and cardiometabolic syndrome [1,2]. Hepatic IR, manifested as the failure to respond to insulin and inhibit hepatic glucose production (HGP), is a primary driver of systemic IR [3,4]. Hepatic and systemic IR constitute the core of metabolic syndrome associated with dyslipidemia, T2DM, and cardiovascular diseases [5]. The most used clinical drugs are metformin and thiazolidinedione which can reduce circulating glucose levels and enhance tissue sensitivity to insulin, but have therapeutic limitations (existing gastrointestinal-intolerance) and side effects (weight gain) [6,7]. Thus, a quest for new therapeutic strategies and targets for improving IR is necessary.
Asialoglycoprotein receptor 1 (ASGR1) is the major subunit of ASGR complex which is specifically expressed in hepatocytes, mediating the endocytosis of desialylated glycoproteins [8]. Lack of ASGR1 decreased the serum lipid levels and reduced the risk of coronary artery disease in humans, mice, and pigs [9-11]. Our previous work suggested that ASGR1 deficiency led to a “healthy” serum lipids profile mediated by increased expression of hepatic insulin-induced gene 1 (INSIG1), causing less sterol regulatory element-binding proteins (SREBPs) to be transported into the Golgi apparatus for processing as an active transcriptional factor [12]. Glucose metabolism is closely connected with lipid metabolism and Insig1 is a downstream targeted gene of insulin signaling in the liver [13,14]. Whether ASGR1 deficiency is involved in the regulation of glucose metabolism and insulin response remains unknown.
The liver plays a fundamental role in coordinating glucose homeostasis [15]. HGP accounts for approximately 90% of endogenous glucose production [16]. The role of liver in the maintenance of blood glucose is dependent on balancing gluconeogenesis, glycogen synthesis, glycogenolysis, and glycolysis, as well as other metabolic processes [17,18]. Sustained elevation of gluconeogenesis leads to a significantly increased HGP, and it is a hallmark of IR [19]. Gluconeogenesis is regulated by two rate-limiting gluconeogenic enzymes, phosphoenolpyruvate carboxykinase 1 (PEPCK1, encoded by the Pck1 gene) and glucose-6-phosphatase (G6Pase, encoded by the glucose-6-phosphatase catalytic subunit [G6pc] gene) [20], which are upregulated by glucagon, glucocorticoids and robustly down-regulated by insulin and glucose [21]. Defective hepatic glycogenolysis is another manifestation associated with hepatic IR [22]. Therefore, effectively reducing hepatic gluconeogenesis or glycogenolysis could improve hepatic IR.
Insulin receptor substrates mediated phosphoinositide 3-kinase (PI3K)-protein kinase B (AKT) pathway initiates a signaling cascade by phosphorylation of key downstream proteins, which regulates glycogen metabolism and inhibits the expression of gluconeogenesis gene [23-26].
In the present study, the functions of ASGR1 in the regulation of insulin signal transduction were investigated in mice and human hepatic cell lines. We found that ASGR1 deficiency could alleviate systemic IR primarily by promoting hepatic insulin sensitivity under IR status, which may be mainly achieved by decreased glycogenolysis and gluconeogenesis. It suggests that targeting ASGR1 could be a potential therapeutic strategy to alleviate metabolic diseases associated with IR.
METHODS
Cell culture
HepG2 cell lines (obtained from Dr. Pan, GIBH, CAS, Guangzhou, China) were maintained in Dulbecco’s Modified Essential Medium (DMEM)-L (Gibico, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) (Nobimpex, Herbolzheim, Germany) at 37°C with humidified atmosphere of 5% CO2. ASGR1-/- HepG2 cells were constructed by clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 [12]. To induce IR, the cells were incubated with 18 mM glucosamine (GLN) (Beyotime, Nantong, China) for 18 hours in DMEM-L without FBS [27,28], or 0.25 mM sodium palmitate (PA) (Sigma, St. Louis, MO, USA) in DMEM-H for 24 hours followed by incubation in DMEM-L for 2 hours [29]. Then, the cells were stimulated with 100 nM insulin (Sigma) for 20 minutes and harvested for quantitative real-time polymerase chain reaction (qPCR) and Western blot analyses.
RNA isolation and gene expression
RNA was prepared from liver tissues and 4.0×105 cells by Trizol reagent (MCE, Monmouth Junction, NJ, USA). For more details on reverse transcription and qPCR, please refer to our previous work [12]. The relative mRNA expression was normalized to a referenced gene (β-actin). The sequence of primers was provided in Supplementary Table 1.
Western blot
Liver tissues and 8.0×105 cells were lysed on ice by radioimmunoprecipitation assay (RIPA) buffer (Beyotime) supplemented with protease inhibitor. For more details on protein sample preparation and electrophoresis, please refer to previous work [30]. The used antibodies were listed in Supplementary Table 2. Protein bands were analyzed using Immobilon Western Chemiluminescent horseradish peroxidase (HRP) Substrate (Millipore, Burlington, MA, USA).
RNA sequencing and analysis
The cells were starved for 12 hours, and about 10 million cells were harvested. A total of two tubes within each group were collected for RNA isolation. High-fat diet (HFD)-fed wild type (WT) and Asgr1-/-mice were fasted overnight and collected liver tissues. Each sample was used to extract total RNA, and the RNA quality was determined by NanoDrop, Qubit (Thermo Fisher Scientific, Waltham, MA, USA), and Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). After the libraries were constructed, the sequencing was processed by IGE Biotechnology Ltd. (Guangzhou, China). All the RNA sequencing raw and processed data were provided in the supplementary materials.
Glycogen quantification
Glycogen content was measured by Glycogen Assay Kit (Solarbio, Beijing, China). The cells and weighted liver tissues were lysed with extract buffer. Then the samples were boiled for 20 minutes, and the test tube was shaken every 5 minutes. After the sample was completely dissolved and cooled, the volume was fixed to 5 mL with distilled water, mixed well, and centrifuged at 8,000 ×g for 15 minutes. Glycogen concentrations was normalized by liver weight or protein concentration of cells.
Malondialdehyde content measurement
Malondialdehyde (MDA) levels in mice liver were measured by MDA assay kit (Beyotime). The liver tissues were homogenized with Western and IP cell lysate (Beyotime) on ice. The ratio of liver weight to lysate was 1:9, after homogenization, the mixture was centrifuged (1.2×104 ×g) at 4°C for 15 minutes. The protein content of liver tissues was determined by bicinchoninic acid (BCA) protein concentration assay kit (Thermo Fisher Scientific).
Superoxide dismutase activity measurement
Superoxide dismutase (SOD) activity in mice liver was measured by the total superoxide dismutase assay kit with WST-8 (Beyotime). The liver samples were homogenized in pre-cooled phosphate buffer saline then the homogenate was centrifuged (1.2×104×g) at 4°C for 15 minutes for subsequent assays.
Histology
Paraffin-embedded liver tissues were sectioned into 4 μm, and stained with standard hematoxylin and aqueous eosin (Beyotime). The optimal cutting temperature compound-embedded liver tissues were cut into 20 μm and stained with Oil Red O solution (Sigma-Aldrich) counterstained with hematoxylin. The morphologic characteristics of the liver tissues were acquired using a light microscope (Leica, Wetzlar, Germany).
Serum biochemistry
Serum was separated from mice and frozen at –80°C until required. Serum insulin concentration was measured by enzymelinked immunosorbent assay (ELISA) (Millipore). Aspartate aminotransferase (AST)/alanine aminotransferase (ALT) levels were measured using an AST/ALT Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Serum and hepatic lipid assays
Serum lipid levels were measured by an automatic biochemical detector (Hitachi, Tokyo, Japan). Liver lipid were measured by using the tissue triglyceride (TG)/total cholesterol (TC) content assay kit (Applygen, Beijing, China).
Glucose tolerance test and insulin tolerance test
Glucose tolerance test (GTT) or insulin tolerance test (ITT) was performed on 16-hour-fasted or 6-hour-fasted mice, and 2 g/kg body weight glucose (Sigma-Aldrich) or with 0.8 IU/kg body weight insulin (Sigma-Aldrich) was intraperitoneally injected to mice, respectively. Blood glucose was monitored from the tail veins of mice with a glucometer at different times after the glucose or insulin administration [31]. The calculation of the area under the curve (AUC) or area above the curve (AAC) were generated by the glucose value of each time point with the subtraction of those initial glucose value [32].
Mice and diets
ASGR1 knockout mice were generated in Cyagen (Guangzhou, China) [12]. All mice were of C57BL/6 genic background. Mice were maintained under a standard environment (12-hour light-dark cycle) with free access to normal chow diet and water. Considering the results of our previous studies [12], the male mice were used for experiments to reduce the potential effects of hormones on metabolism. Mice (6 weeks old) were randomly assigned and administrated with HFD (fat, 39.9%; cholesterol, 1.25%; Trophic Animal Feed High-Tech Co. Ltd., Nantong, China) for 8 weeks. For analysis of the hepatic insulin signaling status, those mice (HFD 8 weeks) were fasted for 6 hours, then injected with either vehicle or insulin (0.8 IU/kg) intraperitoneally, and the mice were euthanized 15 minutes later.
Ethics statement
All animal studies were reviewed and approved by the Animal Care and Use Committee of GIBH, CAS (IACUC approval: 2019012).
Statistical analysis
The data were analyzed with GraphPad Prism 9.0 (GraphPad Software Inc., San Diego, CA, USA). The Student’s t-test was used to assess the significance between two groups, and the one-way analysis of variance (ANOVA) was applied for three groups. All data were represented as mean±standard deviation, and P<0.05 was considered statistically significant.
RESULTS
ASGR1 deficiency alleviated systemic insulin resistance
We reported that ASGR1 deficiency significantly reduced plasma lipid content under normal physiological condition [12]. To further explore the physiological functions of ASGR1, we first tested whether the HFD influences the ASGR1 expression and found that there were no significant changes between chow and HFD-fed WT mice (Supplementary Fig. 1A and B). Then, ASGR1 deficiency and WT mice were fed with HFD for 8 weeks in total, and at the end of the sixth and seventh week, the GTT and ITT assays were performed, respectively. Except there were significant increased glucose levels in short phase of GTT (15 and 30 minutes) of those knockout mice, the overall AUC were clearly declined, particularly in Asgr1-/- mice (Fig. 1A). However, the ITT results indicated that the ASGR1-deficient mice revealed significantly improved insulin sensitivity with comparison of those WT mice, evidenced by the significantly increased AAC (Asgr1+/-, 65.5%; Asgr1-/-, 77.7%) (Fig. 1B). It is well known that the decreased of serum insulin level is an indicator of improved insulin sensitivity under metabolic stress [33,34]; therefore, the serum insulin levels were measured, and the serum insulin levels in Asgr1+/- and Asgr1-/- mice were significantly lower compared with those of WT mice (Fig. 1C). Furthermore, an important systemic IR index, the homeostasis model assessment of insulin resistance index (HOMA-IR) was also decreased in ASGR1-deficient mice (Fig. 1D). While there were no significant differences among groups in the body weight and food intake (Supplementary Fig. 1C and D), and ASGR1 deficiency had no significant changes of serum lipid under HFD condition (Supplementary Fig. 1E-H). These results suggested that deficiency of ASGR1 alleviated HFD-induced systemic IR.

Deficiency of asialoglycoprotein receptor 1 (ASGR1) protected against diet-induced insulin resistance (IR). (A, B) Glucose tolerance test (GTT) and insulin tolerance test (ITT) were performed on mice fed with high-fat diet (HFD) for 6 weeks (wild type [WT], n=8; Asgr1+/-, n=7; Asgr1-/-, n=7) and 7 weeks (WT, n=5; Asgr1+/-, n=5; Asgr1-/-, n=5). The results quantified by the area under the curve (AUC) or area above the curve (AAC) according to the line chart is on the right side. (C, D) Fasting serum insulin content and homeostasis model assessment-insulin resistance (HOMA-IR) of mice fed with HFD for 8 weeks were determined and calculated (WT, n=17; Asgr1+/-, n=14; Asgr1-/-, n=14). All data are shown as the mean±standard deviation. aP<0.05, bP<0.01, cP<0.001, dP<0.0001, as compared to the indicated WT by one-way analysis of variance (ANOVA).
ASGR1 deficiency with specifically improved insulin signaling in liver
To determine whether the improved systemic insulin sensitivity under ASGR1-deficiency has an organ-specific manner, we analyzed insulin signaling status in major insulin response tissues. It was found that insulin-induced phosphorylation of insulin receptor β (pInsRβ) and AKT was only increased significantly in livers (the pS-473 AKT, Asgr1+/-, 70%; Asgr1-/-, 50%) (Fig. 2A), but neither in adipose nor muscle tissues of ASGR1-deficient mice (Fig. 2B). This is consistent with previous report that hepatic IR is a key factor in the pathogenesis of systemic IR syndrome [4]. Furthermore, we observed that ASGR1 deficiency had no effect on the levels of AST and ALT (Supplementary Fig. 2A and B). However, although there were no significant differences among groups in liver mass and TG content, Asgr1-/- mice had higher TC content in liver (Supplementary Fig. 2C-F). Abnormal lipid metabolism induced the production of reactive oxygen species, which in turn contributed to IR [35]. Therefore, we examined oxidative stress in liver. The levels of MDA were decreased in ASGR1-deficient mice, especially in Asgr1+/- mice, along with increased SOD activity in ASGR1-deficient mice (Supplementary Fig. 2G and H). From another point of view, it confirms that ASGR1 deficiency can alleviate hepatic IR. In conclusion, the ASGR1 deficiency relieves systemic IR mainly via improved hepatic insulin sensitivity. To further explore the regulatory effects of ASGR1 deficiency on hepatocyte insulin signal transduction under IR status, the HepG2 and ASGR1-/- HepG2 cell lines were exposed to GLN for inducing IR condition. We found that the pS-473 AKT was significantly higher in ASGR1-/- HepG2 cells (increased 50%) upon insulin stimulation compared with the HepG2 cells (Fig. 2C), it also can be duplicated in PA-induced IR condition (Supplementary Fig. 3). These results suggested that ASGR1 deficiency significantly improved insulin sensitivity of hepatocytes, thus alleviated hepatic IR under metabolic stress condition.

Deficiency of asialoglycoprotein receptor 1 (ASGR1) improved hepatic and hepatocellular insulin sensitivity. (A) Western blot of key proteins in insulin signal pathway in liver. Representative images of the band and grey intensity of each band with insulin stimulation were shown (n=3). (B) Western blot of insulin-protein kinase B (AKT) signal pathway in the adipose and muscle tissues. Representative images of the band and grey intensity of phosphorylation of protein kinase B (pS-473) (pAKT) with insulin stimulation were shown (n=3). (C) Western blot analysis for insulin-induced phosphorylation of insulin receptor β (pInsRβ) and AKT in HepG2 and ASGR1-/- HepG2 cells. Representative images of the band and grey intensity of pAKT with insulin stimulation were shown (n=3). All data are shown as the mean±standard deviation. GLN, glucosamine (18 mM). aP<0.05, bP<0.01, as compared to the indicated wild type (WT) by one-way analysis of variance (ANOVA) among three groups. Statistical significance between two groups was assessed with an unpaired, 2-tailed t-test.
Differentially expressed genes under ASGR1-deficiency were enriched in the insulin signal pathway
To confirm the effect of ASGR1 on the insulin signal pathway, RNA sequencing was performed on those HFD-fed Asgr1-/- and WT livers (the detailed data of RNA-seq is listed in Supplementary Table 3). Gene set enrichment analyses showed that the genes related to PI3K-AKT signaling were significantly enriched at the top of the differentially expressed genes (DEGs), with a high normalized enrichment score (=–1.53) (Supplementary Fig. 4A), indicating that the PI3K-AKT signaling pathway was significantly improved in Asgr1-/- mice. Among those DEGs, the expressions with significant changes were 268 genes in total (fold change >2, q value <0.05) (Supplementary Fig. 4B). Importantly, these DEGs were indeed enriched in the insulin signal pathway (Supplementary Fig. 4C), that were consistent with other results from cell and mice models under metabolic stress.
To further demonstrate the effects of ASGR1 on hepatocyte metabolism, transcriptome analyses were also performed on ASGR1-/- HepG2 and HepG2 cells (detailed in Supplementary Table 4). The similarity of transcriptome between ASGR1-/- HepG2 and HepG2 cells was over 98% (Fig. 3A), except there were 249 DEGs (80 up, 169 down) with significant changes (fold change >2, P<0.05) in the ASGR1-/- HepG2 compared to the HepG2 cells. Hierarchical clustering by significant DEGs was shown by heatmap (Fig. 3B). Gene Ontology (GO) analysis and the Kyoto Encyclopedia of Genes and Genomes (KEGG) were used to characterize the functions of the DEGs and reveal pathways with significant enrichment, respectively [36]. According to the significant DEGs, the distributions between the DEGs and certain specific GO classification terms and pathways were calculated. As expected, those dysregulated genes were enriched in insulin responses (Fig. 3C), particularly in PI3K-AKT signaling (Fig. 3D and Supplementary Table 5). Once again, it indicates that ASGR1 participated in regulating hepatic insulin signaling and glucose metabolism.
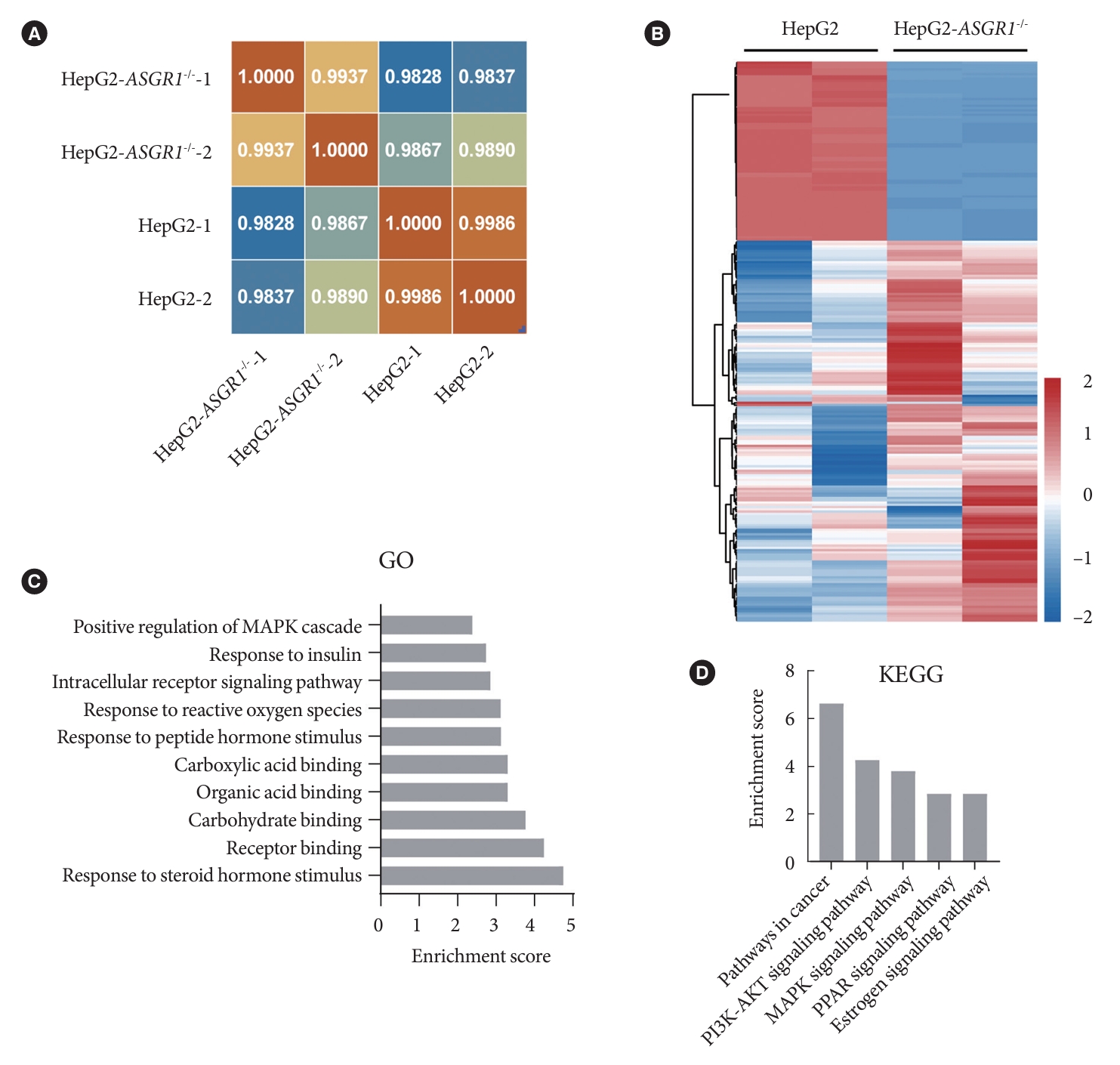
Differentially expressed genes in asialoglycoprotein receptor 1 (ASGR1) knockout cells were enriched in the insulin signal pathway. (A) Heatmap shows hierarchical clustering of Pearson’s correlation coefficient based on sequencing. The score of 1 (red) denotes perfect correlation. (B) Heatmap shows hierarchical clustering of significant differentially expressed genes (DEGs) in ASGR1-/- HepG2 cells compared to the HepG2 cells. Values are column-scaled to show expression level. (C) Gene Ontology (GO) analysis identified molecular functions and biological processes of significant DEGs. (D) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of significant DEGs. The enrichment score indicates the degree of enrichment of DEGs in the biological process or pathway. PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; MAPK, mitogen-activated protein kinase; PPAR, peroxisome proliferator-activated receptor.
Under metabolic stress, ASGR1-deficiencies led to decreased glycogenolysis and gluconeogenesis
Based on suggestions from the transcriptome analyses, we shed light on the status of hepatic glucose metabolism, in particular on the two main pathways of HGP, glycogenolysis and gluconeogenesis. After 8 weeks of HFD feeding, the glycogen analyses found that deficiency of ASGR1 significantly increased hepatic glycogen concentrations (Asgr1+/-, increased 67%; Asgr1-/-, increased 25%) (Fig. 4A), and the mRNA expression level of glycogen phosphorylase (Pygl, response for glycogenolysis) was significantly reduced in ASGR1-deficient mice (Fig. 4B). While the mRNA expression of glycogen synthase 2 (Gys2) and glucokinase (Gck) (responsible for glycogen synthase) had no significant differences among groups (Supplementary Fig. 5). However, the expressions of two key gluconeogenic genes, Pck1 and G6pc were significantly reduced in ASGR1 deficiency mice liver (Fig. 4C). It suggested that ASGR1 deficiency contributed to reductions of glycogenolysis and gluconeogenesis under IR condition. Further analysis of glucose metabolism in hepatocytes with GLN induction was consistent with the results of animal model, concretely, the glycogen content increased about 59% in ASGR1-/- HepG2 compared to the HepG2 cells (Fig. 4D), and the mRNA of PYGL showed a decreased trend (Fig. 4E); moreover, the mRNA of PCK1 and G6PC were reduced, along with their declined proteins (PEPCK1 and G6Pase, downed 90% and 43%, respectively) (Fig. 4F and G). Mechanistically, these results indicated that deficiency of ASGR1 led to improved hepatocellular insulin signal transduction linked with decreased glycogenolysis and gluconeogenesis under IR status.

Deficiency of asialoglycoprotein receptor 1 (ASGR1) represented decreased gluconeogenesis and glycogenolysis under metabolic stress conditions. (A) Glycogen content in liver tissues (wild type [WT], n=7; Asgr1+/-, n=5; Asgr1-/-, n=6). (B) The relative mRNA expression level of glycogen phosphorylase (Pygl) in liver tissues (n=4). (C) The relative mRNA expression levels of phosphoenolpyruvate carboxykinase 1 (Pck1) and glucose-6-phosphatase catalytic subunit (G6pc) in liver tissues (n=4). (D) Glycogen content (n=3). (E) The relative mRNA expression level of PYGL (n=3). (F) The relative mRNA expression level of PCK1 and G6PC (n=3). (G) Western blot of PEPCK1 and glucose-6-phosphatase (G6Pase). Representative images of the band and grey intensity of each band relative to β-actin were shown (n=3). All data are shown as the mean±standard deviation. GLN, glucosamine. aP<0.05, bP<0.01, cP<0.001, as compared to the indicated WT by one-way analysis of variance (ANOVA) among three groups. Statistical significance between two groups was assessed with an unpaired, 2-tailed t-test.
The decreased glycogenolysis and gluconeogenesis of ASGR1-deficiencies are independent of metabolic stress
To investigate whether the effects of ASGR1 on hepatic glucose metabolism and insulin signaling are metabolic stress-related, we further analyzed the insulin signal pathway status in the liver of mice fed with a standard diet, and found that there were no significant differences in the phosphorylation of the InsRβ and AKT among groups (Fig. 5A). However, the liver glycogen content was increased in ASGR1-deficient mice (Asgr1+/-, 40%; Asgr1-/-, 100%) (Fig. 5B), and the mRNA expression of Gys2 and Gck were significantly down-regulated, while the mRNA expression level of Pygl showed a slight downregulation in ASGR1-deficient mice (Fig. 5C). Moreover, the mRNA of Pck1 and G6pc, as well as their proteins were also decreased in ASGR1-deficient mice (G6Pase and PEPCK1, 45% and 34% in Asgr1+/-; 47% and 56% in Asgr1-/-, respectively) (Fig. 5D and E).

Asialoglycoprotein receptor 1 (ASGR1) deficiency affected hepatic glucose metabolism under standard diet-fed condition. (A) Western blot of insulin signal pathway in the liver from chow-fed mice. Representative images of the band and the grey intensity of each band were shown. (B) liver glycogen content. (C) The relative mRNA expression levels of glycogen synthase 2 (Gys2), glycogen phosphorylase (Pygl), and glucokinase (Gck) in liver tissues. (D) The relative mRNA expression levels of phosphoenolpyruvate carboxykinase 1 (Pck1) and glucose-6-phosphatase catalytic subunit (G6pc) in liver tissues. (E) Western blot of glucose-6-phosphatase (G6Pase) and PEPCK1 in liver tissues. Representative images of the band and the grey intensity of each band relative to β-actin were shown (n=4). All data are shown as the mean±standard deviation. AKT, protein kinase B; WT, wild type; pInsRβ, phosphorylation of insulin receptor β. aP<0.05, bP<0.01, cP<0.001, as compared to the indicated WT by one-way analysis of variance (ANOVA).
Those phenotypic changes in mice were further investigated in cell lines. Under routine culture condition, the insulin signaling had no significant changes between cells with or without ASGR1 (Fig. 6A and Supplementary Fig. 6); however, the glycogen content was 60% higher in ASGR1-/- HepG2 cells compared with HepG2 cells (Fig. 6B), and mRNA of PYGL was significantly decreased (Fig. 6C), accompanied with decreased expression of G6PC and PCK1 at mRNA and protein levels (G6Pase and PEPCK1, 28% and 90%, respectively) (Fig. 6D and E). These observations suggest that ASGR1 deficiency modulates hepatic glucose metabolism mainly by decreased glycogenolysis and gluconeogenesis in hepatocytes that were independent of metabolic stress.
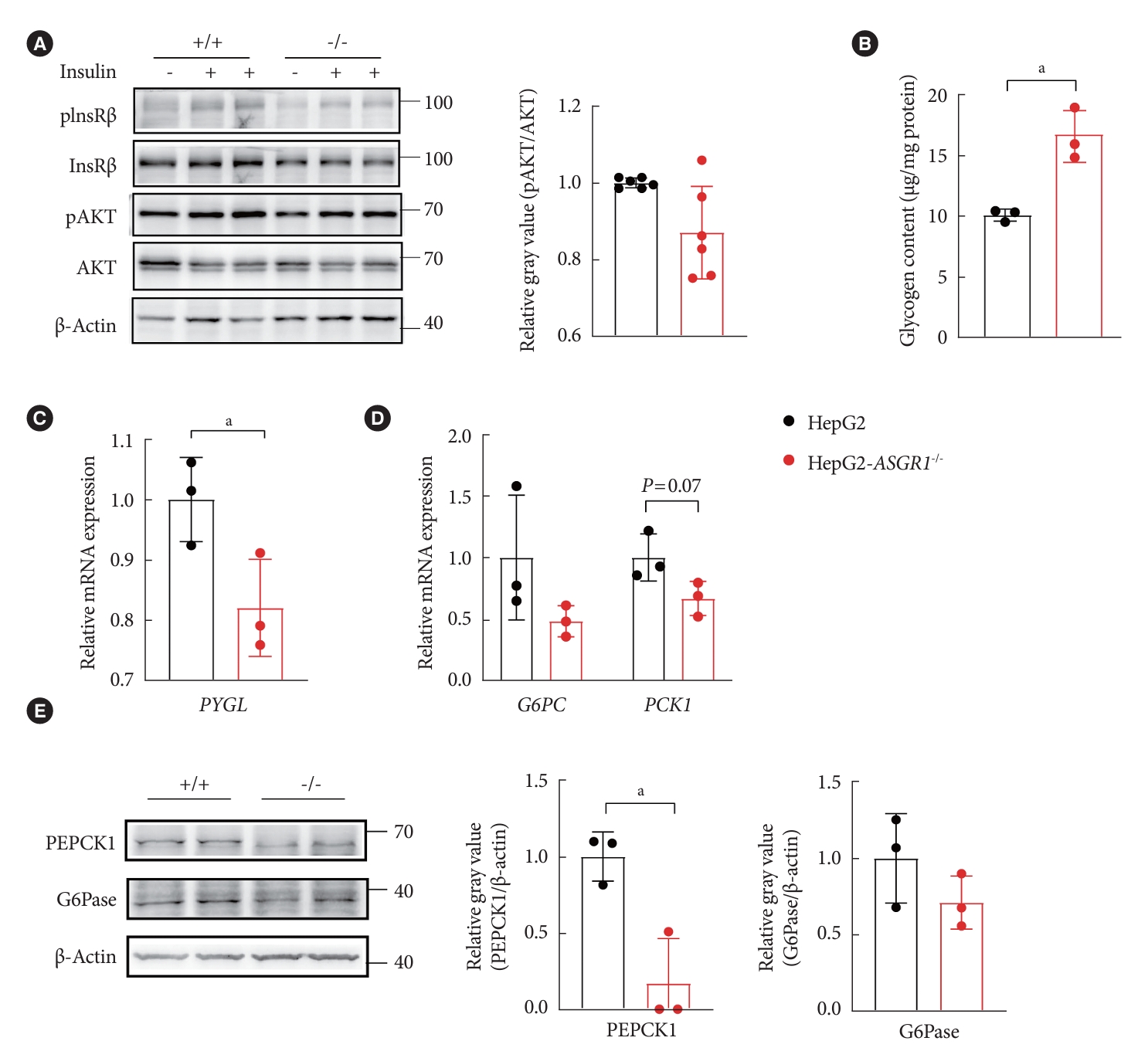
Knockout asialoglycoprotein receptor 1 (ASGR1) led to decreased gluconeogenesis and increased glycogen content in HepG2 cells under normal culture condition. (A) Western blot analysis of insulin signal pathway in HepG2 and ASGR1-/- HepG2 cells. Representative images of the band and grey intensity of phosphorylation of protein kinase B (pS-473) (pAKT) with insulin stimulation were shown, the remaining two electrophoresis are shown in Supplementary Fig. 6. (B) Glycogen content. (C) The relative mRNA expression level of glycogen phosphorylase (PYGL). (D) The relative mRNA expression levels of phosphoenolpyruvate carboxykinase 1 (PCK1) and glucose-6-phosphatase catalytic subunit (G6PC). (E) Western blot of PEPCK1 and glucose6-phosphatase (G6Pase). Representative images of the band and the grey intensity of each band relative to β-actin were shown (n=3). All data are shown as the mean±standard deviation. pInsRβ, phosphorylation of insulin receptor β. aP<0.05, unpaired 2-tailed Student’s t-test.
DISCUSSION
Our data revealed that ASGR1 deficiency significantly alleviated systemic IR under metabolic stress with reduced insulin tolerance, serum insulin level, and low HOMA-IR. Meanwhile, the hepatic insulin signaling was significantly enhanced, but not in muscle or adipose tissue. Notably, ASGR1 deficiency significantly reduced hepatic glycogenolysis and gluconeogenesis in normal condition or under IR status in vivo and in vitro. Decreased glycogen storage and increased gluconeogenesis were closely linked to metabolic abnormalities [37-40]. From a clinical perspective, targeting ASGR1 might be a promising therapeutic strategy for treating metabolic related disorders, especially in HFD-related IR.
Hepatic IR is a primary factor in systemic IR and metabolic diseases [4], and ASGR1 is predominantly expressed in the liver, therefore, the ASGR1 knockout mice provide an ideal model to clarify the relationship between hepatic IR and systemic IR. Indeed, the ITT assay results revealed that the systemic insulin sensitivity of ASGR1-deficient mice was significantly increased by 65.5% to 77.7%, accompanied by an increase in the phosphorylated AKT S-473 by 50% to 70% in a hepatic-specific manner, and no significant changes in adipose and muscle tissue, indicating the hepatic IR is the major contributor of systemic IR. It suggested that focusing on solving hepatic IR should be a drug development strategy and ASGR1 is an ideal target.
It is noticed that the glucose levels in the short phase of ASGR1 knockout mice (15 and 30 minutes) were higher than those WT mice (Fig. 1A), in contrast, the fasting (16 hours) insulin revealed significantly lower (Fig. 1C). The lower fasting insulin levels may be accounted as the major factor, lower basal insulin allow more glucose release and less glucose uptake, leading to higher glucose levels in short phase of those knockout mice. Ongoing investigation also should consider other factors, including the status of glucose effectiveness, and the function and mass of β-cell in ASGR1 deficiency mice [32].
In short-term starvation, gluconeogenesis contributes to about 35% and glycogenolysis to about 65% of HGP to maintain the serum glucose levels [41], the ASGR1 deficiency mice appeared with 34% to 56% reduction of key gluconeogenesis proteins (Fig. 5E) and relatively lower glycogenolysis activity (Fig. 5B). Therefore, the significant lower serum glucose levels were observed in short-term fasting (6 hours, ITT). And it was consistent with the results in Figs. 2A and 4A, C, the heterozygous knockout mice showed significantly improved insulin sensitivity of insulin signal transduction.
While prolonged fasting time to 16 hours (GTT), the increased hepatic gluconeogenesis provides more than 50% of HGP [42]. Moreover, extrahepatic gluconeogenesis is initiated [43], these two events work together to raise the serum glucose of ASGR1 knockout mice, similar to the ones of WT mice. This explanation is in accord with current understanding and our conclusion that ASGR1 deficiency specifically improved hepatic insulin sensitivity by inhibiting hepatic gluconeogenesis and glycogenolysis.
Compared to the ASGR1 homozygous mice, the hepatic insulin sensitivity (Fig. 2A) and glucose metabolism (Fig. 4A and C) of ASGR1 heterozygous mice were more significantly improved under metabolic stress condition. This phenomenon may be due to the complexity of ASGR complex formation [44], and its functionality is in a subunit composition-dependent manner. In addition, there are alternatively spliced transcript variants encoding multiple isoforms for ASGR1, and ASGR1 null condition also caused the degradation of ASGR2 [45].
Lipid accumulation within the liver can specifically cause hepatic IR [46]. However, under HFD condition, ASGR1-deficient mice showed significant improvement in IR and no significant decrease in serum and liver lipids compared to WT mice. Meanwhile, there was little change of lipid-related genes in the liver under metabolic stress condition (data not shown). These results indicated that the improvement of insulin sensitivity in ASGR1-deficient mice is independent of lipid reduction. In addition, the decreased level of MDA and increased SOD activity suggested that ASGR1 deficiency might be involved in ameliorating oxidative stress.
Under normal feeding condition, even though there were no significant changes in insulin signaling (Fig. 5A), the ASGR1-deficient mice revealed increased liver glycogen concentrations accompanied by down-regulated Pygl transcription; and the declined gluconeogenesis was accompanied by decreased Pck1 and G6pc mRNA and the proteins (Fig. 5B-E). This phenomenon could be explained by two possibilities, one is that there is an insulin-independent regulatory signal existing in the liver; the other is the extrahepatic effect of gluconeogenesis and glycogenolysis [47].
While under metabolic stress conditions, both animal and cell models demonstrated that the ASGR1-deficiencies significantly improved hepatic insulin sensitivity and glucose metabolic disorder, as well as systemic IR. Considering similar results generated from none metabolic stress conditions, it suggests that the decrease of gluconeogenesis/glycogenolysis may contribute more than the improvement of insulin sensitivity, or both factors are interdependent to regulate the hepatic glucose homeostasis and insulin signaling. Overall, it indicated that ASGR1 plays an essential role in regulating HGP through gluconeogenesis, glycogenolysis, and insulin signaling.
Although we have demonstrated that ASGR1 deletion resulted in decreased hepatic gluconeogenesis and glycogenolysis, the complex molecular dogma regulated by ASGR1 is worthy of deep analysis. Another puzzle is that deficiency of ASGR1 had no effects on reducing serum lipid levels when fed with 8 weeks HFD, which was inconsistent with previous reports [10]. The inconsistency may be due to the differences in diet ingredients, feeding time, and mouse strains, and it may be necessary to apply more and different animal disease models to confirm the functions of ASGR1 in glucose metabolism and lipid homeostasis.
In conclusion, we uncovered an unappreciated role of ASGR1 in regulating hepatic gluconeogenesis, glycogenolysis, and insulin signaling. Overcoming hepatic IR significantly relieved the systemic IR condition. Our observations suggest that inhibition of ASGR1 is a potential novel therapeutic strategy to combat metabolic diseases with systemic IR.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2023.0124.
Primers for quantitative real-time polymerase chain reaction
Antibodies for Western blot
The differentially expressed genes of RNA sequencing data from Asgr1-/- and WT mice
The differentially expressed genes of RNA sequencing data from Asgr1-/- and WT mice
Specific DEGs in the PI3K-AKT pathway and the biological process that responds to insulin in ASGR1-/- HepG2 cells
Metabolic characterization of asialoglycoprotein receptor 1 (ASGR1)-deficient mice fed with high-fat diet (HFD). (A) Western blot of ASGR1 in the liver tissues (n=4). (B) Western blot of ASGR1 in the liver of wild type (WT) mice with or without HFD and grey intensity of each band relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were shown (n=4). (C) Body weight of the mice during the experimental period (n=7). (D) Food intake of WT, Asgr1+/- and Asgr1-/- mice per day on average (n=7). (E-H) Biochemical index including total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) contents in serum of mice (WT, n=17; Asgr1+/-, n=11; Asgr1-/-, n=13). All data are shown as the mean±standard deviation. aP<0.05, as compared to the indicated WT by one-way analysis of variance (ANOVA).
Hepatic characterization of asialoglycoprotein receptor 1 (ASGR1)-deficient mice fed with high-fat diet. (A, B) Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels in the serum of mice (wild type [WT], n=11; Asgr1+/-, n=9; Asgr1-/-, n=10). (C) Liver mass to body weight ratio (WT, n=12; Asgr1+/-, n=12; Asgr1-/-, n=13). (D) H&E (Scale bar, 100 μm) and Oil Red O staining (Scale bar, 200 μm). (E, F) Biochemical index including triglyceride (TG) (WT, n=15; Asgr1+/-, n=12; Asgr1-/-, n=15) and cholesterol (WT, n=17; Asgr1+/-, n=12; Asgr1-/-, n=14) contents in liver tissues. (G) Malondialdehyde (MDA) content and (H) superoxide dismutase (SOD) activity in the liver of mice (WT, n=9; Asgr1+/-, n=7; Asgr1-/-, n=10). All data are shown as the mean±standard deviation. aP<0.05, bP<0.01, cP<0.001, as compared to the indicated WT by one-way analysis of variance (ANOVA).
Asialoglycoprotein receptor 1 (ASGR1) deficiency repaired insulin signal transduction in cellular model stimulated with palmitate (PA) in vitro. Western blot of key proteins involved in insulin/protein kinase B (AKT) signaling pathway in HepG2 and ASGR1-/- HepG2 cells. pInsRβ, phosphorylation of insulin receptor β.
Differentially expressed genes (DEGs) in asialoglycoprotein receptor 1 (Asgr1)-/- mice were enriched in the phosphoinositide 3-kinase (PI3K)-protein kinase B (AKT) signal pathway. The liver tissues of high-fat diet-fed wild type (WT) and Asgr1-/- mice were collected for RNA sequencing (n=2). (A) Gene set enrichment analyses of gene sets for PI3K-AKT signal pathway. (B) Heatmap shows hierarchical clustering of significant DEGs in Asgr1-/- mice compared to the WT mice. Values are column-scaled to show expression level. (C) Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of significant DEGs. NES, normalized enrichment score; PPAR, peroxisome proliferator-activated receptor; FoxO, Forkhead box O; MAPK, mitogen-activated protein kinase.
Glycogen metabolism in vitro. The relative mRNA expression level of glycogen synthase 2 (Gys2) and glucokinase (Gck) in liver tissues of mice (n=4). WT, wild type; Asgr1, asialoglycoprotein receptor 1.
Knockout asialoglycoprotein receptor 1 (ASGR1) had no effect on insulin signaling in HepG2 cells under normal culture condition. The Western blot analysis of the insulin-induced phosphorylation of insulin receptor β (pInsRβ) and protein kinase B (AKT) in HepG2 and ASGR1-/- HepG2 cells.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: X.Y., Y.X., Y.X.L.
Acquisition, analysis, or interpretation of data: X.Y., J.T., Y.W., Y.C., P.L., F.Y., M.T., A.S., Y.T.
Drafting the work or revising: X.Y., Y.X., Y.X.L.
Final approval of manuscripts: X.Y., J.T., Y.W., Y.C., P.L., F.Y., M.T., A.S., Y.T., Y.X., Y.X.L.
FUNDING
This work was supported by the National Key R&D Program of China (2019YFA0111300), China-New Zealand Joint Laboratory on Biomedicine and Health (2022YFE0210600), Guangdong Basic and Applied Research Foundation (2021A151522 0095), Guangzhou Science and technology planning project (202102021138), Guangzhou Science and Technology Program (2023B01J1004), Basic Research Project of Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Science (GIBHBRP23-01). The project was partially funded by the CAS Key Laboratory of Regenerative Biology, Guangdong Provincial Key Laboratory of Stem Cell and Regenerative Medicine, Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Science. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements
We are grateful to the animal center and instrument center of GIBH for technical support.