- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Ahead-of print > Article
-
Original ArticleDrug/Regimen Pioglitazone as Add-on THERAPY in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Dapagliflozin and Metformin: Double-Blind, Randomized, Placebo-Controlled Trial
-
Ji Hye Heo1
 , Kyung Ah Han2, Jun Hwa Hong3, Hyun-Ae Seo4, Eun-Gyoung Hong5, Jae Myung Yu6, Hye Seung Jung7, Bong-Soo Cha8
, Kyung Ah Han2, Jun Hwa Hong3, Hyun-Ae Seo4, Eun-Gyoung Hong5, Jae Myung Yu6, Hye Seung Jung7, Bong-Soo Cha8
-
DOI: https://doi.org/10.4093/dmj.2023.0314
Published online: February 2, 2024
- 1,307 Views
- 133 Download
1Division of Endocrinology and Metabolism, Department of Internal Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea
2Division of Endocrinology and Metabolism, Department of Internal Medicine, Nowon Eulji Medical Center, Eulji University School of Medicine, Seoul, Korea
3Division of Endocrinology, Department of Internal Medicine, Daejeon Eulji Medical Center, Eulji University School of Medicine, Daejeon, Korea
4Division of Endocrinology and Metabolism, Department of Internal Medicine, Daegu Fatima Hospital, Daegu, Korea
5Division of Endocrinology and Metabolism, Department of Internal Medicine, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Korea
6Division of Endocrinology and Metabolism, Department of Internal Medicine, Hallym University Kangnam Sacred Heart Hospital, College of Medicine, Hallym University, Seoul, Korea
7Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
8Division of Endocrinology and Metabolism, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- Corresponding author: Bong-Soo Cha Division of Endocrinology and Metabolism, Department of Internal Medicine, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea E-mail: BSCHA@yuhs.ac
Copyright © 2024 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- This study assessed the efficacy and safety of triple therapy with pioglitazone 15 mg add-on versus placebo in patients with type 2 diabetes mellitus (T2DM) inadequately controlled with metformin and dapagliflozin.
-
Methods
- In this multicenter, double-blind, randomized, phase 3 study, patients with T2DM with an inadequate response to treatment with metformin (≥1,000 mg/day) plus dapagliflozin (10 mg/day) were randomized to receive additional pioglitazone 15 mg/day (n=125) or placebo (n=125) for 24 weeks. The primary endpoint was the change in glycosylated hemoglobin (HbA1c) levels from baseline to week 24 (ClinicalTrials.gov identifier: NCT05101135).
-
Results
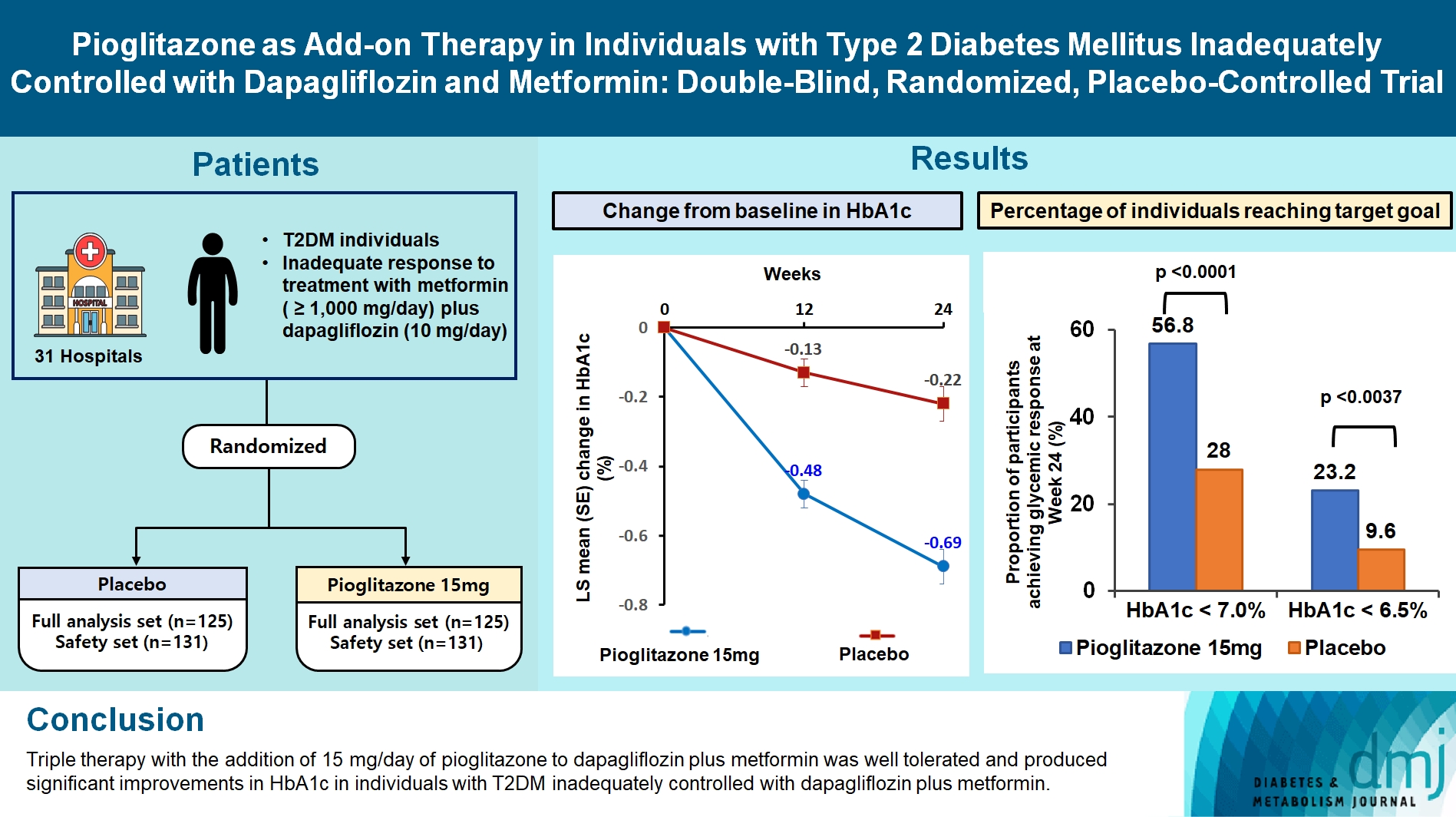
- At week 24, the adjusted mean change from baseline in HbA1c level compared with placebo was significantly greater with pioglitazone treatment (–0.47%; 95% confidence interval, –0.61 to –0.33; P<0.0001). A greater proportion of patients achieved HbA1c <7% or <6.5% at week 24 with pioglitazone compared to placebo as add-on to 10 mg dapagliflozin and metformin (56.8% vs. 28% for HbA1c <7%, and 23.2% vs. 9.6% for HbA1c <6.5%; P<0.0001 for all). The addition of pioglitazone also significantly improved triglyceride, highdensity lipoprotein cholesterol levels, and homeostatic model assessment of insulin resistance levels, while placebo did not. The incidence of treatment-emergent adverse events was similar between the groups, and the incidence of fluid retention-related side effects by pioglitazone was low (1.5%).
-
Conclusion
- Triple therapy with the addition of 15 mg/day of pioglitazone to dapagliflozin plus metformin was well tolerated and produced significant improvements in HbA1c in patients with T2DM inadequately controlled with dapagliflozin plus metformin.
- • This study evaluated pioglitazone add-on in T2DM with metformin and dapagliflozin.
- • Pioglitazone significantly improved glycemic parameters with metformin and dapagliflozin.
- • Adding pioglitazone improved lipid profile and insulin resistance compared to placebo.
- • No significant adverse effects were observed with pioglitazone addition.
Highlights
- Type 2 diabetes mellitus (T2DM) is characterized by the progressive deterioration of pancreatic β-cell function and worsening of insulin resistance [1]. Maintaining normoglycemia is essential to prevent chronic complications associated with T2DM. Consequently, as glycemic status worsens in T2DM, most people with T2DM require combination treatment with various antihyperglycemic agents over time [2]. Considering the various pathophysiological factors contributing to hyperglycemia, a combination of antihyperglycemic agents with different mechanisms of action is necessary to achieve effective glycemic control and prevent chronic complications of diabetes [3].
- Among the various treatment options, sodium-glucose cotransporter 2 (SGLT2) inhibitors have emerged as the standard of care for patients with T2DM and established atherosclerotic cardiovascular disease (ASCVD), congestive heart failure (CHF), or chronic kidney disease (CKD), as recommended by international treatment guidelines [4,5]. As many people with T2DM are at high risk of cardiorenal diseases, SGLT2 inhibitor and metformin dual combination therapy is likely to become the most used therapeutic approach in clinical practice. Nevertheless, when these combinations fail to maintain adequate glycemic control, the addition of other antihyperglycemic agents becomes necessary.
- Thiazolidinedione (TZD), an insulin sensitizing agent, demonstrates potent glycemic control without the risk of hypoglycemia. Recent clinical trials have also shown its cardioprotective and cerebroprotective potential [6,7]. However, side effects such as peripheral edema, weight gain, and increased risk of CHF due to volume expansion limit the use of TZD in clinical practice [8]. In this context, the plasma contraction of SGLT2 inhibitors may offset the fluid retention-related side effects of TZDs [9]. However, no clinical trials have investigated the efficacy of TZD as a third add-on antihyperglycemic agent in patients with T2DM inadequately controlled with a regimen comprising metformin plus an SGLT2 inhibitor. Therefore, the present study evaluated the efficacy and safety of pioglitazone in Korean patients with T2DM who did not achieve adequate glycemic control with metformin and dapagliflozin.
INTRODUCTION
- Study design and study participants
- This multicenter, randomized, double-blind, placebo-controlled phase 3 study was conducted at 31 sites in Korea between October 2021 and September 2022 (ClinicalTrials.gov identifier: NCT05101135). The main study design consisted of an 8-week single-blind treatment period, a 2-week run-in period before randomization, and a 24-week double-blind treatment period (Supplementary Fig. 1). Patients who agreed to participate could elect to participate in an additional 24-week open-label extension study (between May 2022 and March 2023). During the extension period, 15 mg/day of pioglitazone was administered to both groups in an open-label manner for 24 weeks from the day of visit 7 (week 24) to the day before visit 9 (week 48), Our study participants were patients ≥19 years of age with T2DM and a body mass index ≤45 kg/m2. The details of the inclusion and exclusion criteria are presented in Supplementary Table 1. Patients with inadequate glycemic control (glycosylated hemoglobin [HbA1c] 7.5% to 11.0%) after at least 8 weeks of stable-dose metformin monotherapy (≥1,000 mg/day) were considered for screening. Additionally, patients treated with a combination of metformin and other oral hypoglycemic agents (OHAs) were also screened if their HbA1c levels were within the eligible range (7.0% to 11%) after at least 8 weeks of washout from OHAs other than metformin. During the prescreening period, a stabilization period (≥1,000 mg/day titrated metformin plus 10 mg/day dapagliflozin for 8 weeks) was required for patients who were under metformin monotherapy (≥1,000 mg/day) or metformin (≥1,000 mg/day) plus other OHAs. Finally, patients with T2DM with HbA1c 7.0% to 11.0% at screening and taking stable metformin (≥1,000 mg/day) and dapagliflozin 10 mg/day for ≥8 weeks before screening were included. During the run-in period, patients took placebo tablets orally once daily in addition to the ongoing metformin plus dapagliflozin treatment. Patients with compliance rates for each of the placebo, metformin, and dapagliflozin between 70% and 120% and meeting the eligibility criteria were randomized either to the 15 mg dose of pioglitazone (JT-001, Jeil Pharmaceutical Co. Ltd., Seoul, Korea) or placebo groups in a 1:1 ratio. Throughout the study, the metformin (≥1,000 mg/day) and dapagliflozin (10 mg/day) regimens were maintained without change, along with ongoing diet and exercise routines. Follow-up assessments were made at 12-week intervals (weeks 12 and 24). Diet modification and exercise programme were maintained without change throughout the study period.
- To ensure the safety of the study participants, other OHA prescriptions, except for SGLT2 inhibitors or TZD, were permitted as rescue therapy for participants with fasting plasma glucose (FPG) values >270 mg/dL from baseline to 6 weeks, >240 mg/dL from 6 to 12 weeks, >200 mg/dL or HbA1c >8% from 12 to 24 weeks, and participants for whom the investigator was deemed it necessary to receive a rescue drug.
- This study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. The clinical trial protocols were approved by the Institutional Review Board of Severance Hospital (IRB approval No. 4-2021-0995). Before the initiation of any study-related assessments, including prescreening procedures, written informed consent was obtained from all patients.
- Endpoints and assessments
- The primary endpoint was the mean change in HbA1c from baseline after 24 weeks of double-blind treatment with 15 mg/day of pioglitazone versus a placebo add-on to dapagliflozin (10 mg/day) plus metformin (≥1,000 mg/day). The secondary endpoints were the mean change in HbA1c from baseline at 12 weeks, mean change in FPG from baseline at 12 and 24 weeks, and the proportion of patients achieving a therapeutic glycemic response, defined as HbA1c <7.0% and <6.5%, at 24 weeks. Other endpoints included changes in lipid profiles, homeostatic model assessment of β-cell function (HOMA-β) and insulin resistance (HOMA-IR) [10], blood pressure, body weight from baseline, and proportion of patients who need rescue therapy at 12 and 24 weeks. Blood samples were analyzed for efficacy parameters in a central laboratory. As safety endpoints, treatment-emergent adverse events (TEAEs) and results of complete blood count, serum chemistry, and 12-lead electrocardiogram were evaluated.
- Statistical analysis
- This study used a statistical superiority design to verify the superiority of 15 mg/day of pioglitazone over the placebo in terms of HbA1c reduction. Assuming a mean HbA1c reduction relative to placebo at week 24 of 0.39% with a standard deviation of 0.99%, based on previous clinical trials, a sample size of 102 per group was calculated to provide a statistical power of 80% at a significance level of 5%. Expecting a dropout rate of 20%, we aimed to recruit 128 participants per group. The full analysis set (FAS) consisted of patients who were exposed to at least one dose of the study drug and had at least one HbA1c result after randomization. The per-protocol set (PPS) consisted of patients who completed the 24-week treatment period without major protocol deviations among those included in the FAS. Although the main analysis set for efficacy evaluation was the FAS, efficacy analyses were repeated in the PPS. The safety analysis set included patients who received at least one dose of the study drug after randomization and had post-randomization safety follow-up data.
- Continuous variables were presented as descriptive statistics at each time point, and the significance of the changes from baseline within a group was tested using paired t-test or Wilcoxon signed-rank test. Between-group differences in continuous variables were tested using analysis of covariance (ANCOVA) with baseline values and randomization stratification factors as covariates. Changes from baseline for these endpoints are presented as adjusted mean changes and between-group differences with least square mean differences in changes were tested by ANCOVA. Categorical variables were summed as frequency (%) and tested for between-group differences using chi-square or Fisher’s exact tests. The between-group differences in the percentages of patients achieving various glycemic responses were evaluated based on the odds ratios and 95% confidence interval (CI), calculated using logistic regression analysis. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Statistical significance was set at P<0.05.
METHODS
- Baseline characteristics of the study participants
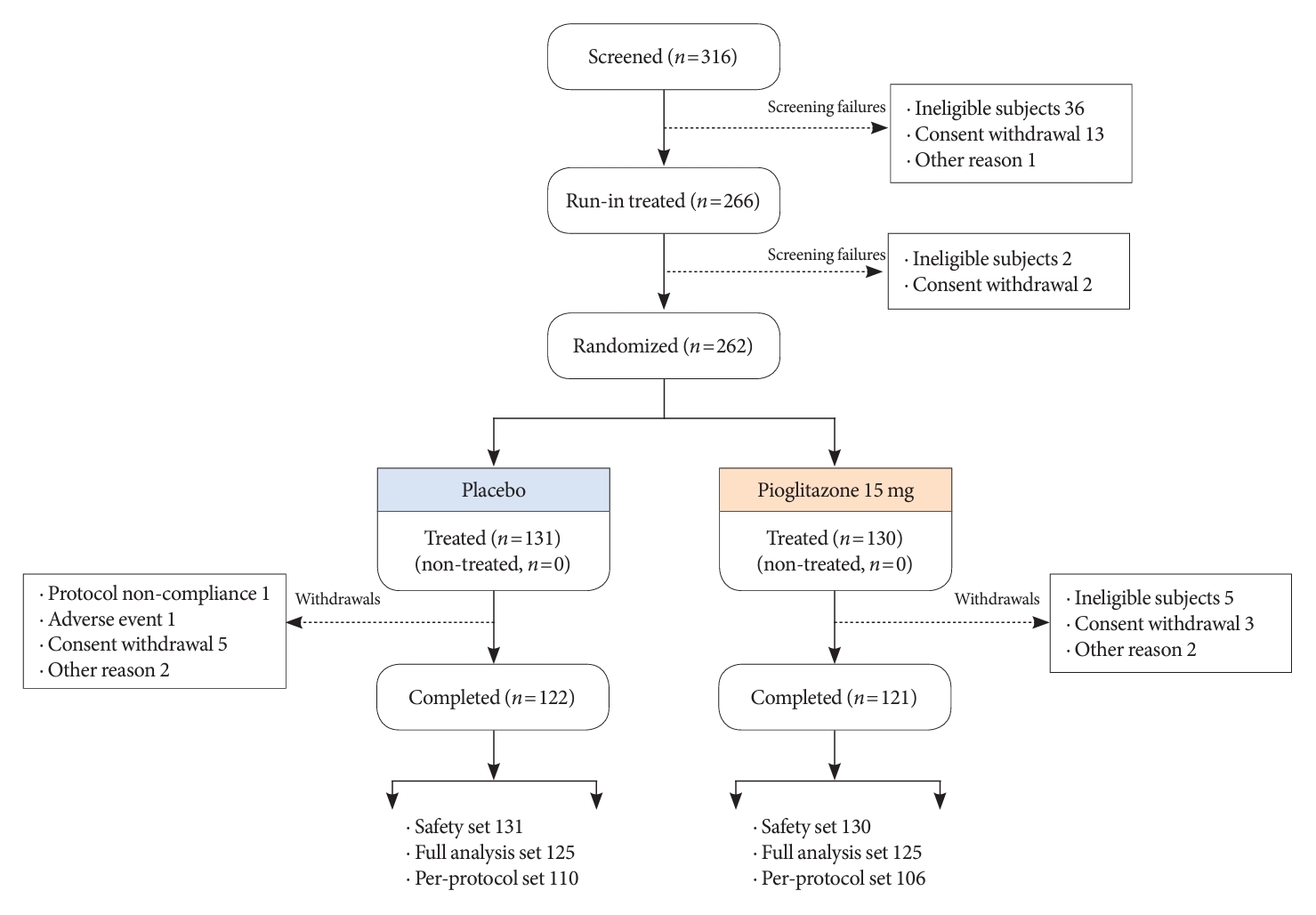
- Among the 316 patients screened for the study, 262 were randomized to one of the two treatment groups (131 to the 15 mg/day pioglitazone group and 131 to the placebo group), and 250 completed the study (125 in the 15 mg/day pioglitazone group and 125 to the placebo group). The numbers and reasons for screening failures and early withdrawal from the study are presented in Fig. 1. Patient demographics and baseline characteristics were balanced between the two treatment groups in the FAS (Table 1). The mean age of the participants was 57.2 years. The mean duration of T2DM was about 9.1 years, and the mean HbA1c level was 7.69%±0.67%.
- Major glycemic endpoints
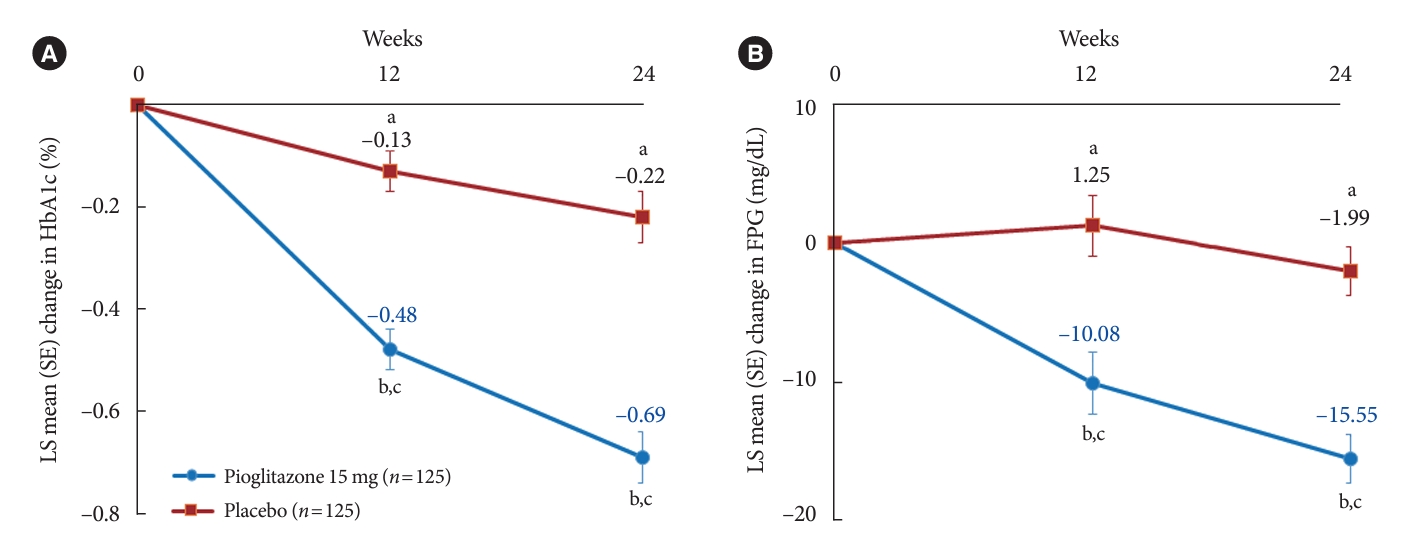
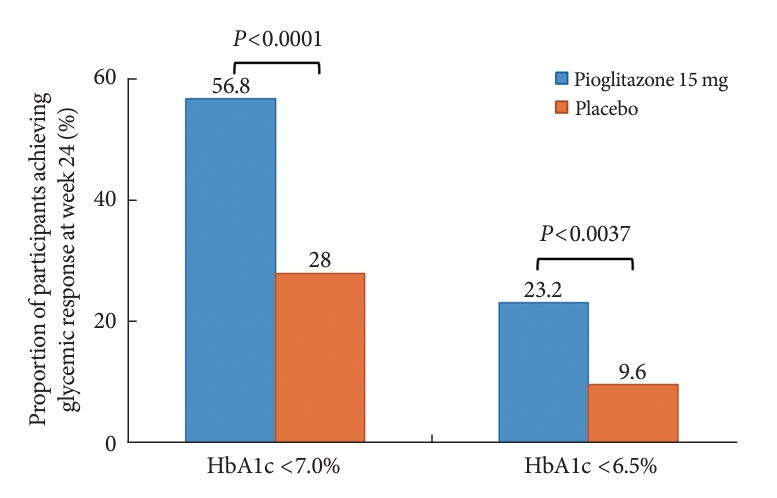
- The changes in glycemic parameters in both groups are presented in Table 2. At week 24, the adjusted mean change from baseline in HbA1c compared with placebo (placebo-15 mg/day pioglitazone) was significantly greater with pioglitazone treatment (–0.47%; 95% CI, –0.61 to –0.33; P<0.0001). Compared with placebo, a significant reduction in HbA1c was observed for 15 mg/day pioglitazone at all measurement points from week 12 to week 24 (P<0.0001) (Fig. 2). FPG levels also decreased significantly from baseline in the pioglitazone group at weeks 12 and 24 (P<0.0001). The placebo-adjusted mean changes in FPG at weeks 12 and 24 were –11.33 mg/dL (95% CI, –16.85 to –5.81) and –13.57 mg/dL (95% CI, –17.93 to –9.21), respectively. In the extended study, pioglitazone treatment resulted in a sustained decrease in HbA1c and FPG levels until week 48. The changes in HbA1c from baseline to week 48 were –0.74%±0.59% in the pioglitazone/pioglitazone group and –0.74%±0.60% in the placebo/pioglitazone group. A greater proportion of patients administered 15 mg/day pioglitazone achieved HbA1c <7% or <6.5% at week 24 compared to those who received placebo as add-on to dapagliflozin (10 mg) and metformin (56.8% vs. 28% for HbA1c <7%, P<0.0001; and 23.2% vs. 9.6% for HbA1c <6.5%, P<0.0037) (Fig. 3). These significant findings for the HbA1c and FPG endpoints were also confirmed by analyses in the PPS.
- Other clinical outcomes
- We observed no significant between-group differences in total cholesterol and low-density lipoprotein cholesterol levels; however, the placebo-adjusted mean changes in high-density lipoprotein cholesterol (HDL-C) (3.67 mg/dL, P<0.0001) and triglycerides (–16.01 mg/dL, P=0.0098) differed significantly between two groups at week 24 (Table 2). The changes in systolic or diastolic blood pressure did not differ significantly between the groups.
- At week 24, the pioglitazone group showed a significant decrease in HOMA-IR relative to the placebo (placebo-adjusted mean change in HOMA-IR: –0.78; 95% CI, –1.11 to –0.45; P< 0.0001). However, the mean percentage changes in HOMA-β from baseline at week 24 were small and similar between the two treatment groups. The body weight increased significantly in the pioglitazone group compared to the placebo group over the 24-week treatment period. The placebo-adjusted mean change in body weight at week 24 was 2.86 kg (95% CI, 2.26 to 3.45). During the 24-week treatment period, rescue therapy was administered to two patients (1.6%) in the placebo group and no patients in the pioglitazone group.
- Adverse events
- A summary of the TEAEs is presented in Table 3. A total of 27 (20.61%) of patients receiving 15 mg/day of pioglitazone experienced 40 TEAEs (mild, 35; moderate, 5; severe, 0), while 27 (20.77%) of patients receiving placebo experienced 43 TEAEs (mild, 35; moderate, 7; severe, 1). Among the TEAEs, two (1.5%) and one (0.8%) case was evaluated as adverse drug reactions (ADRs) in the 15 mg/day pioglitazone and placebo groups, respectively. Five serious adverse events were reported in the 15 mg/day pioglitazone group (diverticulitis, otitis media, pneumonia, intervertebral disc protrusion, and diabetic foot), while three were reported in the placebo group (osteoarthritis, meniscus injury, and Bell’s palsy), none of which were considered ADRs. Hypoglycemic episodes were infrequent, and no cases of major hypoglycemia occurred during the study period.
RESULTS
- This phase 3 trial evaluated the efficacy and safety of pioglitazone 15 mg compared with placebo as an add-on therapy in patients with T2DM who did not achieve glycemic control with dapagliflozin (10 mg/day) and metformin (>1,000 mg/day). Treatment with 15 mg/day of pioglitazone as a third therapy for 24 weeks resulted in significantly greater reductions in HbA1c and FPG levels compared to placebo. In particular, the proportions of patients achieving glycemic targets of HbA1c <7% or <6.5% at 24 weeks with pioglitazone were more than double those for placebo added to dapagliflozin and metformin. The proportion of patients achieving the glycemic target of HbA1c <7% with 15 mg/day of pioglitazone in this study (56.8%) was much higher than previously observed for saxagliptin as triple therapy with dapagliflozin plus metformin (35.6%) [11] or linagliptin as triple therapy with empagliflozin (25 mg/day) plus metformin (36.0%) [12]. Moreover, the addition of 15 mg/day of pioglitazone to dapagliflozin and metformin significantly improved triglyceride and HDL-C levels, which are often abnormal in people with diabetes, and had a beneficial effect on HOMA-IR compared with placebo.
- Globally, only approximately 50% of people with diabetes have achieved the target HbA1c level of <7% [13,14]. Therefore, the development of novel treatment strategies is essential to help patients achieve personalized glycemic goals while reducing the risk of hypoglycemia and side effects. Failure to escalate treatment for uncontrolled glucose levels exposes patients to prolonged periods of hyperglycemia, which is a common problem in the management of diabetes. According to clinical diabetes treatment guidelines, most patients begin treatment with metformin and then gradually add other OHAs as glycemic control worsens. Among the various OHAs, SGLT2 inhibitors are recommended as one of the most desirable addon therapy options to metformin monotherapy in patients with inadequate glycemic control [4]. SGLT2 inhibitors are highly recommended as the preferred agents over other OHAs for individuals with ASCVD, CHF, CKD, and overweight or obesity [4,15]. Therefore, dual therapy with metformin and SGLT2 inhibitors is expected to become increasingly common in patients with T2DM who are at high risk of other chronic metabolic diseases. Although some clinical trials have added a dipeptidyl peptidase-4 (DPP4) inhibitor to a combination of metformin and an SGLT2 inhibitor, few studies have evaluated the efficacy and safety of adding pioglitazone to metformin and SGLT2 inhibitors.
- The addition of TZD to an SGLT2 inhibitor provides a synergistic and complementary approach that targets multiple pathological defects present in T2DM. Specifically, pioglitazone, a peroxisome proliferator-activated receptor gamma agonist, improves insulin sensitivity in muscle, liver, and adipose tissue, thereby improving glycemic control by sensitizing these tissues to insulin. Additionally, pioglitazone can preserve pancreatic β-cell function, collectively contributing to its efficacy in the management of T2DM [16]. In contrast, SGLT2 inhibitors have a unique glucose-lowering mechanism involving increased renal glucose excretion, which offers the advantage of reducing hyperglycemia independent of β-cell function and circulating insulin levels [17]. The combination of pioglitazone and an SGLT2 inhibitor was beneficial for sustained glycemic control in previous studies [18,19] and the effectiveness of SGLT2 inhibitors as an add-on to pioglitazone±metformin therapy has been demonstrated. However, with recent guideline changes, the dual combination of metformin and an SGLT2 inhibitor has become more common in clinical practice than the combination of metformin and pioglitazone. However, few studies have examined the effectiveness of pioglitazone as a third OHA when combined with metformin and an SGLT2 inhibitor. To our knowledge, this is the first randomized clinical trial to show the effectiveness and safety of 15 mg/day of pioglitazone as a third add-on drug in patients with T2DM who have not achieved target glycemic levels despite the dual combination of metformin and dapagliflozin.
- Our findings demonstrated that the addition of pioglitazone to the existing regimen of metformin and dapagliflozin for 24 weeks resulted in significant improvements in all glycemic measures while maintaining a favorable safety profile. The degree of reduction in HbA1c levels in the pioglitazone group (vs. placebo, –0.47%; 95% CI, –0.61 to –0.33) was similar to results reported in other studies that assessed the efficacy of DPP4 inhibitors as a therapeutic option in combination with metformin and SGLT2 inhibitors [12,20]. Notably, although our study participants had slightly lower baseline HbA1c levels (7.7%) compared to those in the aforementioned studies (7.9% to 8.0%) [12,20], the efficacy of triple therapy with 15 mg/day pioglitazone added to dapagliflozin plus metformin was comparable. This resulted in a greater proportion of patients achieving their target HbA1c levels, highlighting the clinical implications of this treatment approach. Furthermore, the addition of pioglitazone to dual combination therapy did not result in any episodes of hypoglycemia during the 24-week study period. These findings suggest that the early initiation of pioglitazone as a third OHA may be beneficial for preventing treatment failure in patients with T2DM who have not reached their glycemic target. Our results confirmed that adding 15 mg/day of pioglitazone to combination therapy with metformin and dapagliflozin was complementary and addressed multiple pathological defects associated with T2DM [3,9]. This triple combination therapy led to a clinically relevant reduction in hyperglycemia in patients who failed to achieve adequate glycemic control with two OHAs while avoiding the burden of hypoglycemia.
- Pioglitazone, classified as a TZD, reduced the composite risk of all-cause mortality, nonfatal myocardial infarction, and stroke in patients with T2DM at high risk for ASCVD [6,21]. Our results also support the potential cardiovascular benefits of pioglitazone in improving dyslipidemia (diabetic dyslipidemia with low HDL-C and high triglyceride levels) [22,23] and insulin resistance, both of which are important contributors to cardiovascular disease. In recent clinical trials, SGLT2 inhibitors have consistently exhibited moderate cardiovascular and renal benefits in patients with T2DM [24]. Given the different mechanisms of action of pioglitazone and SGLT2 inhibitors, their combination may offer additional benefits in reducing the risk of cardiovascular disease, particularly in individuals with diabetes at an increased risk of cardiovascular events. Moreover, the combination of an SGLT2 inhibitor and pioglitazone may lead to a lower incidence of heart failure compared with pioglitazone monotherapy. This is because of the well-established ability of SGLT2 inhibitors to significantly reduce heart failure, as evidenced in large clinical trials [25-27]. Considering the potential synergistic effects and cardiovascular implications of this combination therapy, it is essential to conduct a well-designed, large-scale, randomized clinical trial. Such studies would be instrumental in determining the comprehensive cardiovascular effects of pioglitazone in combination with SGLT2 inhibitors.
- The triple therapy with 15 mg pioglitazone, in addition to dapagliflozin and metformin, was well tolerated. The proportions of patients who experienced adverse events were similar between the pioglitazone and placebo groups. ADRs were rare in both pioglitazone and placebo groups, although the incidence of generalized edema and exertional dyspnea was higher with pioglitazone than with placebo. Nevertheless, the observed prevalence of pioglitazone-induced peripheral edema in this study (0.8%) was relatively low compared to the reported rates of 3% to 5% [28]. Moreover, the addition of pioglitazone to metformin and dapagliflozin attenuated weight gain compared with the addition of pioglitazone without dapagliflozin, as observed in previous studies (1.1 kg vs. 2.0–4.3 kg) [29]. These findings suggest that the side effects of weight gain and fluid retention associated with pioglitazone may be counteracted by plasma contraction and weight loss induced by SGLT2 inhibitors. Despite their potent and sustained glycemic control, along with potential cardiovascular benefits over DPP4 inhibitors, TZDs, including pioglitazone, are under-prescribed in clinical practice owing to their side effects [13,30]. In this context, the combination of pioglitazone and an SGLT2 inhibitor could potentially mitigate the adverse effects of pioglitazone monotherapy, leading to a more proactive use of pioglitazone in clinical settings.
- Several study limitations should be considered when interpreting our findings. First, the relatively short follow-up duration precluded a comprehensive assessment of the long-term efficacy and safety of triple therapy with pioglitazone in combination with dapagliflozin and metformin. Nevertheless, in contrast to other clinical trials, we demonstrated the efficacy and safety of 15 mg/day of pioglitazone for at least 1 year when added to dapagliflozin and metformin in an additional 6-month extension study. Further studies with extended observation periods are required for additional insight into the sustained effects of this combination treatment. Second, this study administered only a low dose of pioglitazone (15 mg), which precluded our evaluation of the efficacy and safety of higher doses. Studies using different doses would be valuable for determining the optimal balance between therapeutic benefits and potential adverse events. Therefore, the forthcoming outcomes of a clinical trial (NCT05296044) examining the effects of 30 mg of pioglitazone (JT-003) are anticipated. Finally, the present study was conducted exclusively on Korean patients. Consequently, the generalizability of our findings to individuals of different ethnicities is limited. To establish broader applicability, similar studies must be conducted in diverse ethnic populations.
- In conclusion, the findings of this study indicated that the addition of 15 mg/day of pioglitazone to dapagliflozin and metformin in patients with T2DM who had not achieved target glycemic levels was well tolerated and led to significant reductions in HbA1c levels. This triple combination therapy showed a greater proportion of patients with T2DM achieving the target HbA1c (<7%) without an increased risk of hypoglycemia compared to dual therapy with dapagliflozin and metformin. Pioglitazone combined with metformin and dapagliflozin also showed favorable effects on insulin sensitivity and lipid profiles, both of which are important parameters for preventing cardiovascular disease. Therefore, pioglitazone may be a useful treatment option to improve glycemic control with a lower risk of hypoglycemia and less weight gain in patients with T2DM who have inadequate glycemic control with dapagliflozin and metformin. Further research with extended follow-up periods and diverse populations would help validate and expand these findings to provide a more comprehensive understanding of the potential advantages of this combination therapy.
DISCUSSION
SUPPLEMENTARY MATERIALS
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: J.H.H., K.A.H., J.H.H., H.A.S., E.G.H., J.M.Y., H.S.J., B.S.C.
Acquisition, analysis, or interpretation of data: J.H.H., K.A.H., J.H.H., H.A.S., E.G.H., J.M.Y., B.S.C.
Drafting the work or revising: J.H.H., K.A.H., J.H.H., H.A.S., E.G.H., J.M.Y.
Final approval of the manuscript: J.H.H., K.A.H., J.H.H., H. A.S., E.G.H., J.M.Y., H.S.J., B.S.C.
-
FUNDING
This study was sponsored by Jeil Pharmaceutical Co. Ltd., Seoul, South Korea. The sponsor participated in the study design, data management, and analysis.
NOTES
-
Acknowledgements
- The authors appreciate and acknowledge Dr. You-Cheol Hwang from Kyung Hee University College of Medicine (Seoul, Korea), Dr. Han Seok Choi from Dongguk University Ilsan Hospital (Ilsan, Korea), Dr. Hun-Sung Kim from Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea (Seoul, Korea), Dr. Chul Sik Kim from Yongin Severance Hospital, Yonsei University College of Medicine (Yongin, Korea), Dr. Gwanpyo Koh from Jeju National University College of Medicine (Jeju, Korea), Dr. Ho Sang Shon from Catholic University of Daegu School of Medicine (Daegu, Korea), Dr. SooKyung Kim from CHA University School of Medicine (Seongnam, Korea). Dr. Hae Jin Kim from Ajou University School of Medicine (Suwon, Korea), Dr. Park Ie Byung from Gachon University School of Medicine (Incheon, Korea), Dr. Nan Hee Kim from Korea University College of Medicine (Seoul, Korea), Dr. Hyuk-Sang Kwon from Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea (Seoul, Korea), Dr. Kyung Mook Choi from Korea University College of Medicine (Seoul, Korea), Dr. Sung Hee Choi from Seoul National University College of Medicine (Seoul, Korea), Dr. Chang Beom Lee from Hanyang University, (Seoul, Korea), Dr. Jong Chul Won from Inje University (Seoul, Korea), Dr. Hye Soon Kim from Keimyung University School of Medicine (Daegu, Korea), Hyun Jeong Jeon from Chungbuk National University (Cheongju, Korea), Dr. Su Kyoung Kwon from Kosin University College of Medicine (Busan, Korea), Dr. Hyeong-Kyu Park from Soonchunhyang University College of Medicine (Seoul, Korea), and Dr. Jung Hwan Park Hanyang University College of Medicine (Seoul, Korea) for their great efforts in recruiting patients and collecting study data.
Values are presented as mean±standard deviation or number (%).
BMI, body mass index; HbA1c, glycosylated hemoglobin; FPG, fasting plasma glucose; HOMA-IR, homeostatic model assessment of insulin resistance; HOMA-β, homeostatic model assessment of β-cell function; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; OHA, oral hypoglycemic agent.
Values are presented as mean±standard deviation unless otherwise indicated.
HbA1c, glycosylated hemoglobin; LS, least square; SE, standard error; CI, confidence interval; FPG, fasting plasma glucose; HOMA-IR, homeostasis model assessment of insulin resistance; HOMA-β, homeostasis model assessment of β-cell function; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
- 1. Kim JM, Kim SS, Kim JH, Kim MK, Kim TN, Lee SH, et al. Efficacy and safety of pioglitazone versus glimepiride after metformin and alogliptin combination therapy: a randomized, open-label, multicenter, parallel-controlled study. Diabetes Metab J 2020;44:67-77.ArticlePubMedPDF
- 2. Bae J, Huh JH, Lee M, Lee YH, Lee BW. Glycaemic control with add-on thiazolidinedione or a sodium-glucose co-transporter-2 inhibitor in patients with type 2 diabetes after the failure of an oral triple antidiabetic regimen: a 24-week, randomized controlled trial. Diabetes Obes Metab 2021;23:609-18.PubMed
- 3. Defronzo RA. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773-95.PubMedPMC
- 4. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care 2023;46(Suppl 1):S140-57.
- 5. Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022;45:2753-86.ArticlePubMedPMCPDF
- 6. Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279-89.PubMed
- 7. Viscoli CM, Kernan WN, Young LH. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016;375:704.
- 8. Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care 2004;27:256-63.ArticlePubMedPDF
- 9. van Baar MJ, van Ruiten CC, Muskiet MH, van Bloemendaal L, IJzerman RG, van Raalte DH. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care 2018;41:1543-56.ArticlePubMedPDF
- 10. Song Y, Manson JE, Tinker L, Howard BV, Kuller LH, Nathan L, et al. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: the Women’s Health Initiative Observational Study. Diabetes Care 2007;30:1747-52.ArticlePubMedPDF
- 11. Mathieu C, Ranetti AE, Li D, Ekholm E, Cook W, Hirshberg B, et al. Randomized, double-blind, phase 3 trial of triple therapy with dapagliflozin add-on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care 2015;38:2009-17.ArticlePubMedPDF
- 12. Tinahones FJ, Gallwitz B, Nordaby M, Gotz S, Maldonado-Lutomirsky M, Woerle HJ, et al. Linagliptin as add-on to empagliflozin and metformin in patients with type 2 diabetes: two 24-week randomized, double-blind, double-dummy, parallel-group trials. Diabetes Obes Metab 2017;19:266-74.ArticlePubMedPDF
- 13. Bae JH, Han KD, Ko SH, Yang YS, Choi JH, Choi KM, et al. Diabetes fact sheet in Korea 2021. Diabetes Metab J 2022;46:417-26.ArticlePubMedPMCPDF
- 14. Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med 2013;368:1613-24.ArticlePubMed
- 15. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 10. Cardiovascular disease and risk management: standards of care in diabetes-2023. Diabetes Care 2023;46(Suppl 1):S158-90.
- 16. Yki-Jarvinen H. Thiazolidinediones. N Engl J Med 2004;351:1106-18.ArticlePubMed
- 17. Nauck MA. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des Devel Ther 2014;8:1335-80.ArticlePubMedPMC
- 18. Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care 2012;35:1473-8.ArticlePubMedPMCPDF
- 19. Kovacs CS, Seshiah V, Swallow R, Jones R, Rattunde H, Woerle HJ, et al. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab 2014;16:147-58.ArticlePubMed
- 20. Matthaei S, Catrinoiu D, Celinski A, Ekholm E, Cook W, Hirshberg B, et al. Randomized, double-blind trial of triple therapy with saxagliptin add-on to dapagliflozin plus metformin in patients with type 2 diabetes. Diabetes Care 2015;38:2018-24.ArticlePubMedPDF
- 21. Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016;374:1321-31.ArticlePubMedPMC
- 22. Charpentier G, Halimi S; F-PIO-100 Study Investigators. Earlier triple therapy with pioglitazone in patients with type 2 diabetes. Diabetes Obes Metab 2009;11:844-54.ArticlePubMed
- 23. Yang G, Li L, Tang Y, Boden G. Short-term pioglitazone treatment prevents free fatty acid-induced hepatic insulin resistance in normal rats: possible role of the resistin and adiponectin. Biochem Biophys Res Commun 2006;339:1190-6.ArticlePubMed
- 24. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393:31-9.ArticlePubMed
- 25. McMurray JJ, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995-2008.PubMed
- 26. Peikert A, Martinez FA, Vaduganathan M, Claggett BL, Kulac IJ, Desai AS, et al. Efficacy and safety of dapagliflozin in heart failure with mildly reduced or preserved ejection fraction according to age: the DELIVER Trial. Circ Heart Fail 2022;15:e010080.ArticlePubMed
- 27. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413-24.PubMed
- 28. Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation 2003;108:2941-8.ArticlePubMed
- 29. Kushner RF, Sujak M. Prevention of weight gain in adult patients with type 2 diabetes treated with pioglitazone. Obesity (Silver Spring) 2009;17:1017-22.ArticlePubMedPDF
- 30. Iketani R, Imai S. Prescription trends for the antidiabetic agents used to treat type 2 diabetes mellitus in Japan from 2012-2020: a time-series analysis. Biol Pharm Bull 2023;46:592-8.ArticlePubMed
REFERENCES
Figure & Data
References
Citations


 KDA
KDA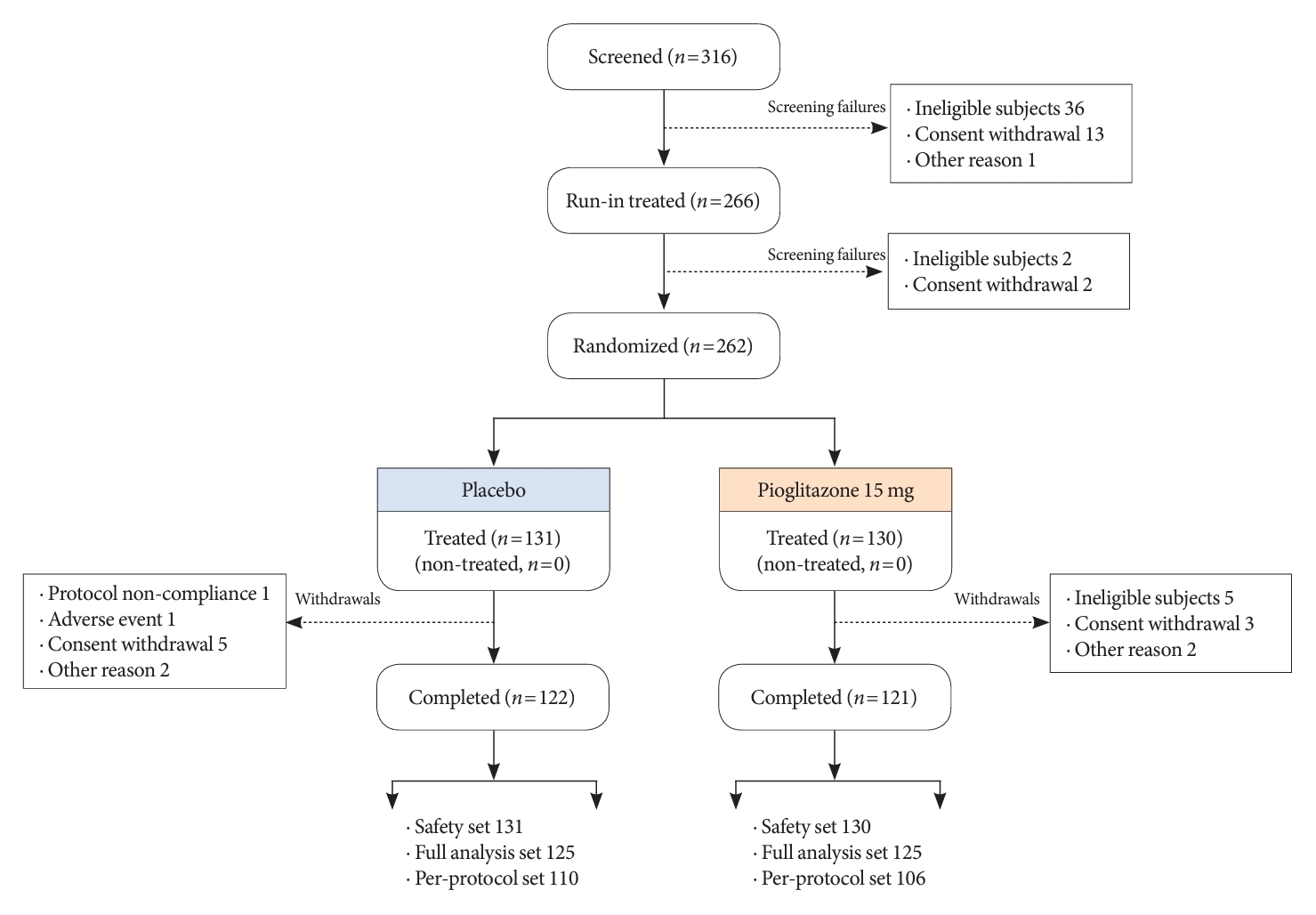
 PubReader
PubReader ePub Link
ePub Link Cite
Cite