- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 44(1); 2020 > Article
-
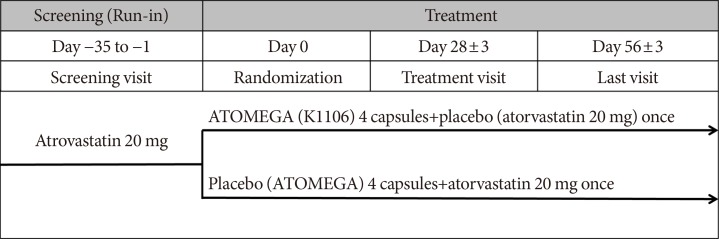
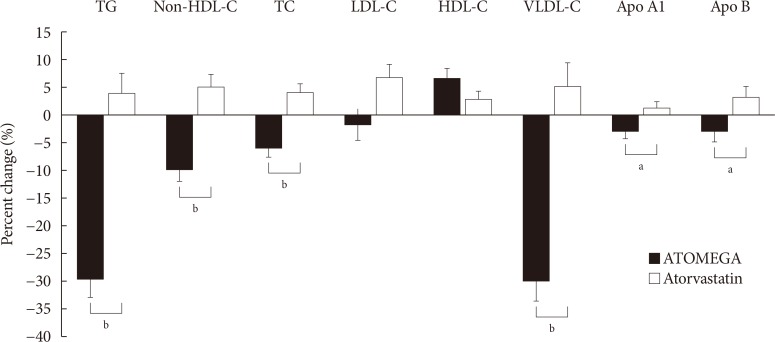
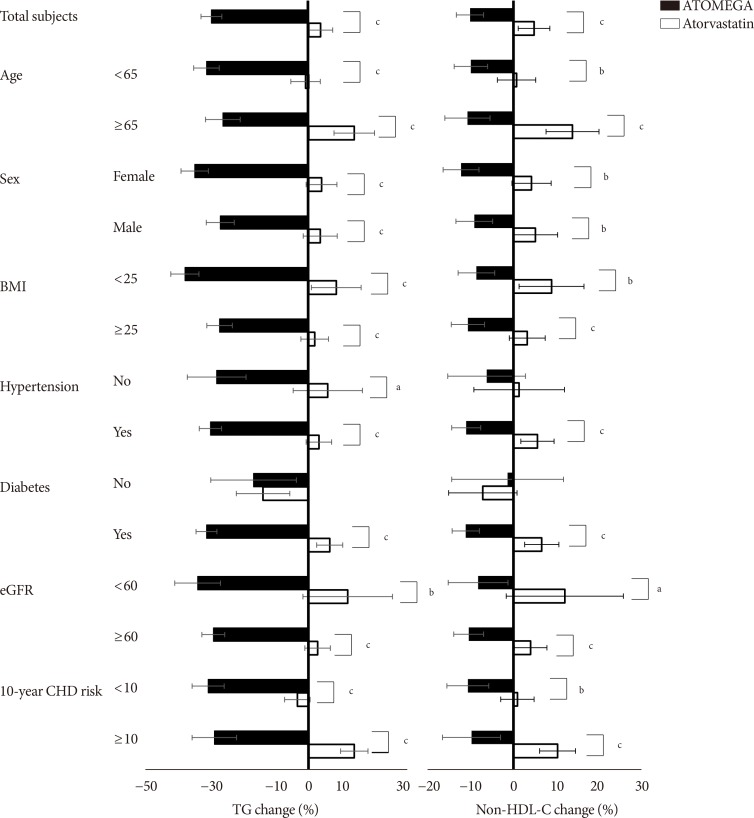
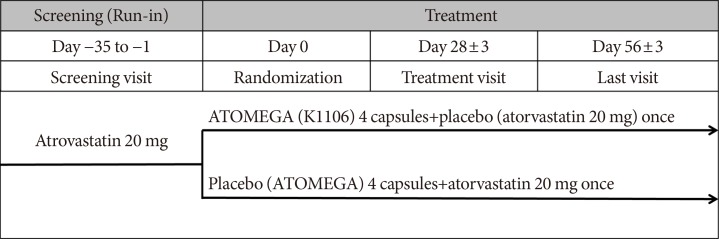
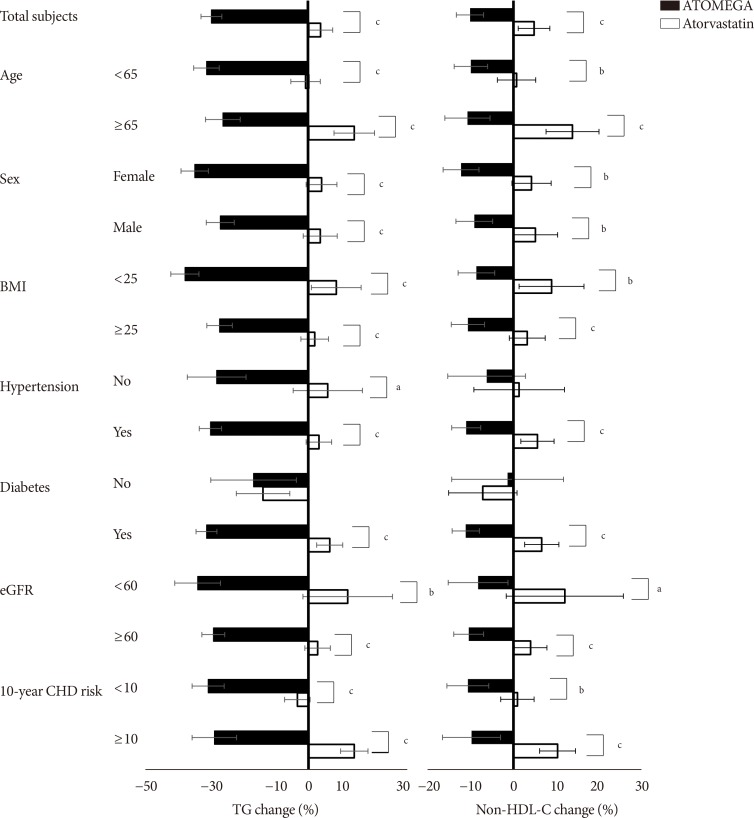
Original ArticleDrug/Regimen Efficacy and Safety of Omega-3 Fatty Acids in Patients Treated with Statins for Residual Hypertriglyceridemia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
-
Ji Eun Jun1
 , In-Kyung Jeong1
, In-Kyung Jeong1 , Jae Myung Yu2, Sung Rae Kim3, In Kye Lee4, Kyung-Ah Han5, Sung Hee Choi6, Soo-Kyung Kim7, Hyeong Kyu Park8, Ji-Oh Mok9, Yong-ho Lee10, Hyuk-Sang Kwon11, So Hun Kim12, Ho-Cheol Kang13, Sang Ah Lee14, Chang Beom Lee15, Kyung Mook Choi16, Sung-Ho Her17, Won Yong Shin18, Mi-Seung Shin19, Hyo-Suk Ahn20, Seung Ho Kang21, Jin-Man Cho22, Sang-Ho Jo23, Tae-Joon Cha24, Seok Yeon Kim25, Kyung Heon Won25, Dong-Bin Kim26, Jae Hyuk Lee27, Moon-Kyu Lee28
, Jae Myung Yu2, Sung Rae Kim3, In Kye Lee4, Kyung-Ah Han5, Sung Hee Choi6, Soo-Kyung Kim7, Hyeong Kyu Park8, Ji-Oh Mok9, Yong-ho Lee10, Hyuk-Sang Kwon11, So Hun Kim12, Ho-Cheol Kang13, Sang Ah Lee14, Chang Beom Lee15, Kyung Mook Choi16, Sung-Ho Her17, Won Yong Shin18, Mi-Seung Shin19, Hyo-Suk Ahn20, Seung Ho Kang21, Jin-Man Cho22, Sang-Ho Jo23, Tae-Joon Cha24, Seok Yeon Kim25, Kyung Heon Won25, Dong-Bin Kim26, Jae Hyuk Lee27, Moon-Kyu Lee28
-
Diabetes & Metabolism Journal 2020;44(1):78-90.
DOI: https://doi.org/10.4093/dmj.2018.0265
Published online: June 20, 2019
1Department of Endocrinology and Metabolism, Kyung Hee University Hospital at Gangdong, Kyung Hee University School of Medicine, Seoul, Korea.
2Division of Endocrinology and Metabolism, Department of Internal Medicine, Hallym University Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
3Division of Endocrinology and Metabolism, Department of Internal Medicine, Bucheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Bucheon, Korea.
4Department of Endocrinology and Metabolism of Internal Medicine, Kyungpook National University Hospital, School of Medicine, Kyungpook National University, Daegu, Korea.
5Division of Endocrinology and Metabolism, Department of Internal Medicine, Nowon Eulji Medical Center, Eulji University School of Medicine, Seoul, Korea.
6Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
7Division of Endocrinology and Metabolism, Department of Internal Medicine, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea.
8Division of Endocrinology and Metabolism, Department of Internal Medicine, Soonchunhyang University Seoul Hospital, Soonchunhyang University College of Medicine, Seoul, Korea.
9Division of Endocrinology and Metabolism, Department of Internal Medicine, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea.
10Division of Endocrinology and Metabolism, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
11Division of Endocrinology and Metabolism, Department of Internal Medicine, Yeouido St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
12Division of Endocrinology and Metabolism Department of Internal Medicine, Inha University Hospital, Inha University School of Medicine, Incheon, Korea.
13 Division of Endocrinology and Metabolism, Department of Internal Medicine, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Hwasun, Korea.
14Division of Endocrinology and Metabolism, Department of Internal Medicine, Jeju National University School of Medicine, Jeju, Korea.
15Department of Endocrinology and Metabolism, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Korea.
16Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea.
17Division of Cardiology, Department of Internal Medicine, Daejeon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Daejeon, Korea.
18Division of Cardiology, Department of Internal Medicine, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea.
19Division of Cardiology, Department of Internal Medicine, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea.
20Division of Cardiology, Department of Internal Medicine, Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Uijeongbu, Korea.
21Division of Cardiology, Department of Internal Medicine, Cheju Halla General Hospital, Jeju, Korea.
22Division of Cardiology, Department of Internal Medicine, Kyung Hee University Hospital at Gangdong, Kyung Hee University School of Medicine, Seoul, Korea.
23Division of Cardiology, Department of Internal Medicine, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea.
24Division of Cardiology, Department of Internal Medicine, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea.
25Division of Cardiology, Department of Internal Medicine, Seoul Medical Center, Seoul, Korea.
26Division of Cardiology, Department of Internal Medicine, St. Paul's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
27Division of Endocrinology, Department of Internal Medicine, Myongji Hospital, Goyang, Korea.
28Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- Corresponding author: Moon-Kyu Lee. Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea. leemk@skku.edu
- *Ji Eun Jun and In-Kyung Jeong contributed equally to this study as first authors.
Copyright © 2020 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Association Between Omega‐3 Fatty Acid Intake and Dyslipidemia: A Continuous Dose–Response Meta‐Analysis of Randomized Controlled Trials
Tianjiao Wang, Xin Zhang, Na Zhou, Yuxuan Shen, Biao Li, Bingshu E. Chen, Xinzhi Li
Journal of the American Heart Association.2023;[Epub] CrossRef - Nutraceutical support in the prevention and treatment of cardiovascular diseases
E. V. Gracheva, E. A. Starovoytova, E. S. Kulikov, N. A. Kirillova, S. V. Fedosenko, M. A. Balaganskaya, D. V. Kromka
Rational Pharmacotherapy in Cardiology.2023; 19(3): 298. CrossRef - Effect of coadministration of omega-3 fatty acids with glimepiride on glycemic control, lipid profile, irisin, and sirtuin-1 in type 2 diabetes mellitus patients: a randomized controlled trial
Rehab H. Werida, Aalaa Ramzy, Youssri Nassief Ebrahim, Maged Wasfy Helmy
BMC Endocrine Disorders.2023;[Epub] CrossRef - The Effect of Dietary Interventions on Hypertriglyceridemia: From Public Health to Molecular Nutrition Evidence
Karla Paulina Luna-Castillo, Xochitl Citlalli Olivares-Ochoa, Rocío Guadalupe Hernández-Ruiz, Iris Monserrat Llamas-Covarrubias, Saraí Citlalic Rodríguez-Reyes, Alejandra Betancourt-Núñez, Barbara Vizmanos, Erika Martínez-López, José Francisco Muñoz-Valle
Nutrients.2022; 14(5): 1104. CrossRef - The effect of omega-3 fatty acids and its combination with statins on lipid profile in patients with hypertriglyceridemia: A systematic review and meta-analysis of randomized controlled trials
Yunjiao Yang, Wen Deng, Yanmei Wang, Tongyi Li, Yiding Chen, Cong Long, Qing Wen, Yue Wu, Qiu Chen
Frontiers in Nutrition.2022;[Epub] CrossRef - Comparison of the Efficacy and Safety of Atorvastatin 40 mg/ω-3 Fatty Acids 4 g Fixed-dose Combination and Atorvastatin 40 mg Monotherapy in Hypertriglyceridemic Patients who Poorly Respond to Atorvastatin 40 mg Monotherapy: An 8-week, Multicenter, Random
Jong Shin Woo, Soon Jun Hong, Dong Hoon Cha, Kee Sik Kim, Moo Hyun Kim, Jun-Won Lee, Myung Ho Jeong, Jin-Ok Jeong, Jun-Hee Lee, Doo Soo Jeon, Eun Joo Cho, Soon Kil Kim, Jun Kwan, Chang Gyu Park, Hae Young Lee, Taek Jong Hong, Jinho Shin, Ho Joong Youn, Do
Clinical Therapeutics.2021; 43(8): 1419. CrossRef - All-Cause Mortality and Cardiovascular Death between Statins and Omega-3 Supplementation: A Meta-Analysis and Network Meta-Analysis from 55 Randomized Controlled Trials
Jeongseon Kim, Tung Hoang, Ji-Myung Kim, So Young Bu, Jeong-Hwa Choi, Eunju Park, Seung-Min Lee, Eunmi Park, Ji Yeon Min, In Seok Lee, So Young Youn, Jee-Young Yeon
Nutrients.2020; 12(10): 3203. CrossRef

 KDA
KDA

 PubReader
PubReader Cite
Cite