- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 42(1); 2018 > Article
-
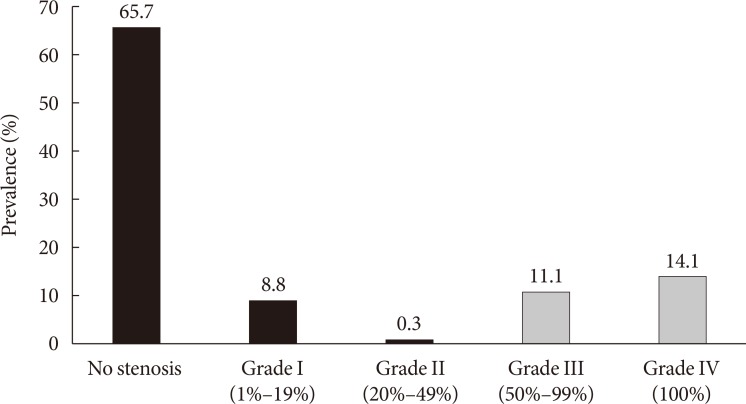
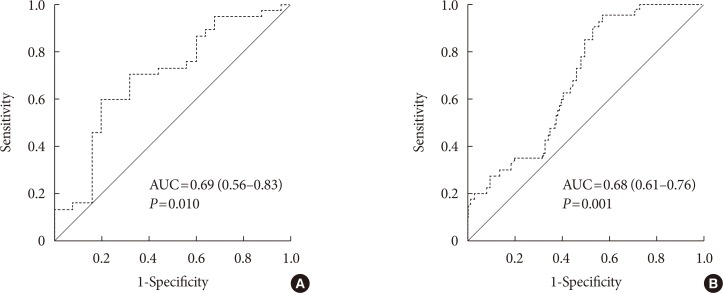
Original ArticleComplications Color Doppler Ultrasonography Is a Useful Tool for Diagnosis of Peripheral Artery Disease in Type 2 Diabetes Mellitus Patients with Ankle-Brachial Index 0.91 to 1.40
-
Kyu Yeon Hur1, Ji Eun Jun1, Young Ju Choi2, Yong-ho Lee3, Dae Jung Kim4, Seok Won Park5, Byung Wook Huh2, Eun Jig Lee3, Sun-Ha Jee6, Kap Bum Huh2, Sung Hee Choi7

-
Diabetes & Metabolism Journal 2018;42(1):63-73.
DOI: https://doi.org/10.4093/dmj.2018.42.1.63
Published online: February 23, 2018
1Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
2Huh's Diabetes Center and 21st Century Diabetes and Vascular Research Institute, Seoul, Korea.
3Division of Endocrinology and Metabolism, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
4Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, Korea.
5Department of Internal Medicine, CHA University School of Medicine, Seongnam, Korea.
6Department of Epidemiology and Health Promotion, Institute for Health Promotion, Yonsei University Graduate School of Public Health, Seoul, Korea.
7Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- Corresponding author: Sung Hee Choi. Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, 82 Gumi-ro 173beon-gil, Bundang-gu, Seongnam 13620, Korea. drshchoi@snu.ac.kr
- *Kyu Yeon Hur and Ji Eun Jun contributed equally to this study as first authors.
Copyright © 2018 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Effectiveness of bedside investigations to diagnose peripheral artery disease among people with diabetes mellitus: A systematic review
Vivienne Chuter, Nicolaas Schaper, Joseph Mills, Robert Hinchliffe, David Russell, Nobuyoshi Azuma, Christian‐Alexander Behrendt, Edward J. Boyko, Michael S. Conte, Misty Humphries, Lee Kirksey, Katharine C. McGinigle, Sigrid Nikol, Joakim Nordanstig, Vin
Diabetes/Metabolism Research and Reviews.2024;[Epub] CrossRef - The intersocietal IWGDF, ESVS, SVS guidelines on peripheral artery disease in people with diabetes and a foot ulcer
Robert Fitridge, Vivienne Chuter, Joseph Mills, Robert Hinchliffe, Nobuyoshi Azuma, Christian‐Alexander Behrendt, Edward J. Boyko, Michael S. Conte, Misty Humphries, Lee Kirksey, Katharine C. McGinigle, Sigrid Nikol, Joakim Nordanstig, Vincent Rowe, David
Diabetes/Metabolism Research and Reviews.2024;[Epub] CrossRef - Diabetic Foot Complications in Saudi Arabia: A Retrospective Study
Sherif M Zaki, Dina S El Karsh, Tuleen M Faden, Leen T Almghamsi, Joud O Fathaldin, Omar A Alhazmi
Cureus.2024;[Epub] CrossRef - Role of Color Doppler Ultrasound to Evaluate the Lower Limb Deep Venous Thrombosis in Diabetic Patients
Muhammad Ahmad Raza, Abdul Rauf, Bushra Akmal Khan, Muhammad Asad Alam, Laamia Altuf, Aftab Alloudin, Saman Fatima
Pakistan Journal of Health Sciences.2024; : 03. CrossRef - Risk factors of peripheral occlusive arterial disease in patients with diabetic retinopathy due to type 2 diabetes
Milos Maksimovic
Srpski arhiv za celokupno lekarstvo.2024; 152(1-2): 50. CrossRef - Comparison of Color Doppler Ultrasound and Ankle-Brachial Pressure Index Measurement in Peripheral Vascular Diseases
Shradha Gupta, Amit Mahajan, Anil Luther, Shubhra Rathore
Indian Journal of Surgery.2023; 85(S1): 177. CrossRef - Are portable ankle brachial pressure index measurement devices suitable for hypertension screening?
Justyna Janus, Jennifer K. Nicholls, Edward Pallett, Matthew Bown, Emma M. L. Chung, Mohamed Yacin Sikkandar
PLOS ONE.2023; 18(3): e0283281. CrossRef - Clinical characteristics and risk factors of lower extremity amputation in the diabetic inpatients with foot ulcers
Hongping Gong, Yan Ren, Zhenyi Li, Panpan Zha, Raju Bista, Yan Li, Dawei Chen, Yun Gao, Lihong Chen, Xingwu Ran, Chun Wang
Frontiers in Endocrinology.2023;[Epub] CrossRef - Diabetes mellitus and long-time outcomes of autovenous femoro-popliteal bypass
A. S. Artemova, M. A. Chernyavskiy
Diabetes mellitus.2023; 26(2): 182. CrossRef - Association between carotid ultrasonographic parameters and microvascular and macrovascular complications in diabetes: A systematic review and meta-analysis
Meimei Liao, Sen Chen, Ruiqiang Guo
Journal of Diabetes and its Complications.2023; 37(8): 108554. CrossRef - The intersocietal IWGDF, ESVS, SVS guidelines on peripheral artery disease in people with diabetes mellitus and a foot ulcer
Robert Fitridge, Vivienne Chuter, Joseph Mills, Robert Hinchliffe, Nobuyoshi Azuma, Christian-Alexander Behrendt, Edward J. Boyko, Michael S. Conte, Misty Humphries, Lee Kirksey, Katharine C. McGinigle, Sigrid Nikol, Joakim Nordanstig, Vincent Rowe, David
Journal of Vascular Surgery.2023; 78(5): 1101. CrossRef - Editor's Choice – The Intersocietal IWGDF, ESVS, SVS Guidelines on Peripheral Artery Disease in People With Diabetes Mellitus and a Foot Ulcer
Robert Fitridge, Vivienne Chuter, Joseph Mills, Robert Hinchliffe, Nobuyoshi Azuma, Christian-Alexander Behrendt, Edward J. Boyko, Michael S. Conte, Misty Humphries, Lee Kirksey, Katharine C. McGinigle, Sigrid Nikol, Joakim Nordanstig, Vincent Rowe, David
European Journal of Vascular and Endovascular Surgery.2023; 66(4): 454. CrossRef - Peripheral Artery Disease: A Comprehensive Updated Review
Garba Rimamskep Shamaki, Favour Markson, Demilade Soji-Ayoade, Chibuike Charles Agwuegbo, Michael Olaseni Bamgbose, Bob-Manuel Tamunoinemi
Current Problems in Cardiology.2022; 47(11): 101082. CrossRef - Reliability of bedside tests for diagnosing peripheral arterial disease in patients prone to medial arterial calcification: A systematic review
Jeroen J.W.M. Brouwers, Siem A. Willems, Lauren N. Goncalves, Jaap F. Hamming, Abbey Schepers
eClinicalMedicine.2022; 50: 101532. CrossRef - Comparative Doppler ultrasound findings of foot arteries in patients with type 2 diabetes mellitus and normoglycaemic patients
LatifatTunrayo Oduola-Owoo, AdekunleAyokunle Adeyomoye, OmodeleAbosede Olowoyeye, IfedayoAdeola Odeniyi, BukunmiMichael Idowu, BadmusBabatunde Oduola-Owoo, AdeniyiSunday Aderibigbe
Journal of West African College of Surgeons.2022; 12(1): 55. CrossRef - Reliability of Bedside Tests for Diagnosing Peripheral Arterial Disease in Patients Prone to Medial Arterial Calcification: A Systematic Review
Jeroen Brouwers, Siem Willems, Lauren Goncalves, Jaap Hamming, Abbey Schepers
SSRN Electronic Journal .2022;[Epub] CrossRef - Estimating the diagnostic accuracy of the ankle–brachial pressure index for detecting peripheral arterial disease in people with diabetes: A systematic review and meta‐analysis
V. H. Chuter, A. Searle, A. Barwick, J. Golledge, L. Leigh, C. Oldmeadow, B. Peterson, P. Tehan, S. M. Twigg
Diabetic Medicine.2021;[Epub] CrossRef - Prevalence of peripheral arterial disease and associated factors in people with type 2 diabetes
Pedro José da Silva Filho, Elaine Cristina Martinez Teodoro, Elaine Cristina Alves Pereira, Vania Cristina dos Reis Miranda
Fisioterapia em Movimento.2021;[Epub] CrossRef - Peripheral Arterial Disease and Its Associated Factors among Type 2 Diabetes Mellitus Patients at Debre Tabor General Hospital, Northwest Ethiopia
Yonas Akalu, Ambaye Birhan
Journal of Diabetes Research.2020; 2020: 1. CrossRef - Critical analysis and limitations of resting ankle-brachial index in the diagnosis of symptomatic peripheral arterial disease patients and the role of diabetes mellitus and chronic kidney disease
Ali F. AbuRahma, Elliot Adams, Joseph AbuRahma, Luis A. Mata, L. Scott Dean, Cristyn Caron, Jennifer Sloan
Journal of Vascular Surgery.2020; 71(3): 937. CrossRef - Association of Time in Range levels with Lower Extremity Arterial Disease in patients with type 2 diabetes
Jinfeng Li, Ya Li, Weiguo Ma, Yishan Liu, Xiaohong Yin, Chuanqing Xie, Jiao Bai, Min Zhang
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2020; 14(6): 2081. CrossRef - The accuracy of toe brachial index and ankle brachial index in the diagnosis of lower limb peripheral arterial disease: A systematic review and meta-analysis
Ángel Herraiz-Adillo, Iván Cavero-Redondo, Celia Álvarez-Bueno, Diana P. Pozuelo-Carrascosa, Montserrat Solera-Martínez
Atherosclerosis.2020; 315: 81. CrossRef - Exercise-induced calf muscle hyperemia: quantitative mapping with low-dose dynamic contrast enhanced magnetic resonance imaging
Jeff L. Zhang, Gwenael Layec, Christopher Hanrahan, Christopher C. Conlin, Corey Hart, Nan Hu, Lillian Khor, Michelle Mueller, Vivian S. Lee
American Journal of Physiology-Heart and Circulatory Physiology.2019; 316(1): H201. CrossRef - Comparison of different methods of ABI acquisition for detection of peripheral artery disease in diabetic patients
Miroslav Homza, Ondrej Machaczka, Martin Porzer, Milan Kozak, Jiri Plasek, David Sipula
Biomedical Papers.2019; 163(3): 227. CrossRef

 KDA
KDA


 PubReader
PubReader Cite
Cite