- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 47(6); 2023 > Article
-
ReviewComplications Pharmacological and Nonpharmacological Treatments for Painful Diabetic Peripheral Neuropathy
-
Han Na Jang1
 , Tae Jung Oh1,2
, Tae Jung Oh1,2
-
Diabetes & Metabolism Journal 2023;47(6):743-756.
DOI: https://doi.org/10.4093/dmj.2023.0018
Published online: September 6, 2023
1Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
-
Corresponding author: Tae Jung Oh
 Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, 82 Gumi-ro 173beon-gil, Bundang-gu, Seongnam 13620, Korea E-mail: ohtjmd@gmail.com
Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, 82 Gumi-ro 173beon-gil, Bundang-gu, Seongnam 13620, Korea E-mail: ohtjmd@gmail.com
Copyright © 2023 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Diabetic peripheral neuropathy (DPN) is one of the most prevalent chronic complications of diabetes. The lifetime prevalence of DPN is thought to be >50%, and 15%–25% of patients with diabetes experience neuropathic pain, referred to as “painful DPN.” Appropriate treatment of painful DPN is important because this pain contributes to a poor quality of life by causing sleep disturbance, anxiety, and depression. The basic principle for the management of painful DPN is to control hyperglycemia and other modifiable risk factors, but these may be insufficient for preventing or improving DPN. Because there is no promising diseasemodifying medication for DPN, the pain itself needs to be managed when treating painful DPN. Drugs for neuropathic pain, such as gabapentinoids, serotonin–norepinephrine reuptake inhibitors, tricyclic antidepressants, alpha-lipoic acid, sodium channel blockers, and topical capsaicin, are used for the management of painful DPN. The U.S. Food and Drug Administration (FDA) has approved pregabalin, duloxetine, tapentadol, and the 8% capsaicin patch as drugs for the treatment of painful DPN. Recently, spinal cord stimulation using electrical stimulation is approved by the FDA for the treatment for painful DPN. This review describes the currently available pharmacological and nonpharmacological treatments for painful DPN.
- Diabetic peripheral neuropathy (DPN) is one of the most prevalent chronic complications of diabetes, and its prevalence has been reported to vary widely [1]. The risk of DPN increases with age, and the lifetime prevalence may be >50%. Around 15% to 25% of patients with diabetes experienced neuropathic pain or painful DPN [2], although many are not aware of the presence of this complication. A nationwide survey of DPN in Germany showed that 61.5% and 81.1% of patients with painful and painless DPN were undiagnosed previously [3]. A similar situation may be present in other countries. Because painful DPN contributes to poor quality of life by causing sleep disturbance, anxiety, and depression [4], this chronic complication of diabetes requires further study.
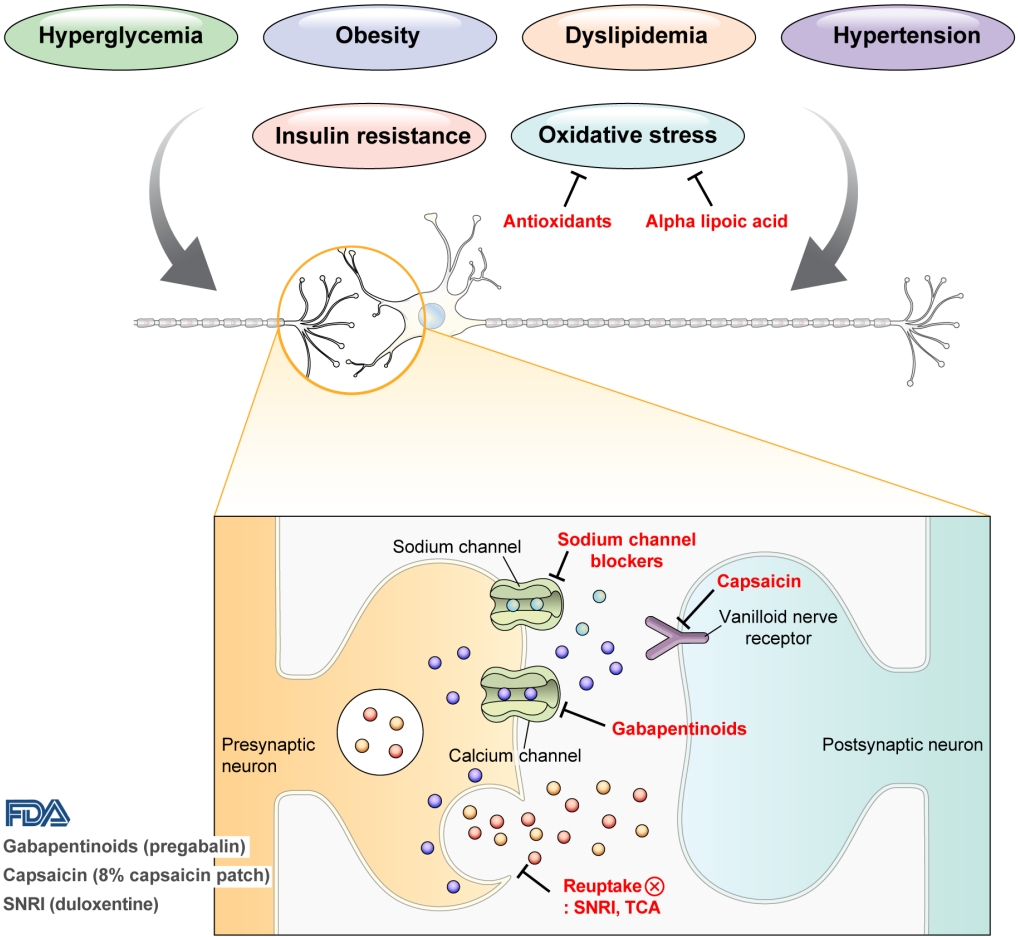
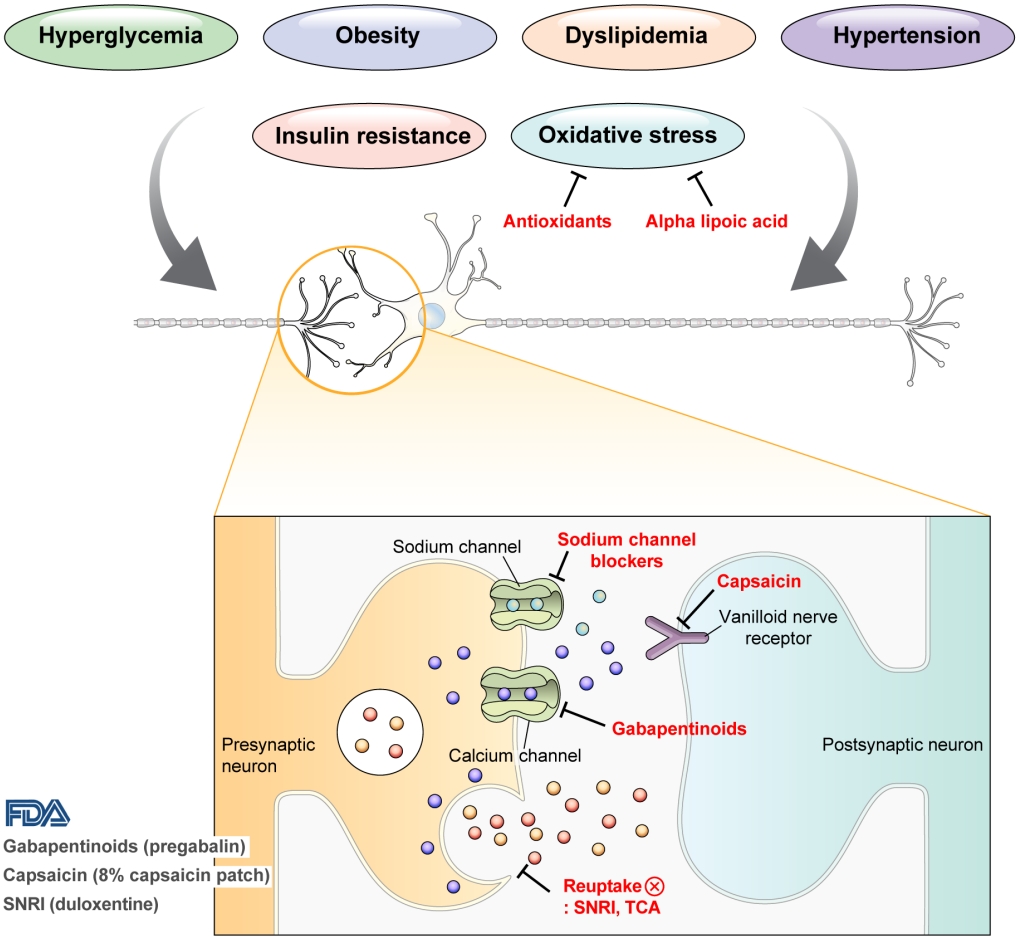
- DPN is one of the microvascular complications of diabetes and shares pathophysiologic mechanisms of glucose-mediated vascular damage with other complications [5]. However, glycemic control per se cannot fully prevent or improve DPN, especially in patients with type 2 diabetes mellitus (T2DM) [6-8]. Metabolic abnormalities other than hyperglycemia such as obesity, dyslipidemia, and hypertension were found to be important risk factors of DPN (Fig. 1) [9,10]. For example, obesity and visceral fat accumulation showed positive association with DPN, and insulin resistance can be involved in this pathophysiology observed in T2DM [11]. More specifically, in the neurologic system, insulin resistance could lead to impaired glucose uptake by Schwann cell, which in turn can cause axonal energy deficit [12]. Furthermore, obesity can be a risk factor for peripheral neuropathy even in the absence of diabetes [13], and features of DPN can be observed in subjects with prediabetes [14]. However, it is unclear whether the pathophysiology and risk factors differ between painful and painless DPN. Female sex [2], obesity [3], and higher dermal nerve fiber regeneration [15] have been suggested as risk factors for painful DPN compared with painless DPN. From a treatment perspective, there is no promising disease-modifying medication for DPN. Therefore, the pain must be managed carefully in patients with painful DPN. In this review, we focus on the pharmacological and nonpharmacological treatments for painful DPN and ways to optimize their effects by reducing the risk of treatment-related complications.
INTRODUCTION
- Intensive lifestyle intervention aiming to weight loss has been tested whether it was effective to mitigate metabolic risk factors for DPN. Look Action for Health in Diabetes (AHEAD) study showed a significant decrease in Michigan Neuropathy Screening Instrument (MNSI)-questionnaire score along with weight loss, but this study did not show a significant difference in MNSI-physical examination [16]. A database of primary care electronic records from United Kingdom showed that bariatric surgery decreased diabetes-related foot disease (adjusted hazard ratio of 0.61) [17], but we still need more data including standard methods to evaluate DPN. There are also some evidence that exercise could improve nerve function [18] and intraepidermal nerve fiber density [19]. In conclusion, multifactorial risk management of DPN might be beneficial to DPN, but large-scale intervention with DPN as a primary outcome are still necessary to test the efficacy of such interventions.
RISK FACTOR MANAGEMENT
- Pain arising from DPN is categorized as neuropathic pain that is a direct consequence of a lesion or disease in the somatosensory nervous system and may occur without tissue damage [20]. In this context, drugs for neuropathic pain may be useful for treating painful DPN (Table 1). The U.S. Food and Drug Administration (FDA) has approved pregabalin, duloxetine, and tapentadol for painful DPN [21] and has recently approved the 8% capsaicin patch (Fig. 1). According to Korea’s National Health Insurance Service-National Sample Cohort data, the proportion of pharmacological treatment in DPN ranged from 66.5% to 69.0%, of which monotherapy accounted for 91.6% and dual combination therapy accounted for 8.1%. In monotherapy, thioctic acid was most used, followed by anti-convulsive agents, tricyclic antidepressant (TCA). In combination therapy, the combination of anti-convulsive agent and TCA was most used, followed by the combination of TCA and thioctic acid [22]. In this section, we review both FDA approved and not approved but widely used drugs, and their combination treatment strategies for the management of painful DPN.
PHARMACOLOGICAL TREATMENTS
- Gabapentinoids
- Gabapentinoids reduce neuropathic pain by depressing dorsal horn sensitivity through multiple mechanisms. They inhibit neurotransmitter release by binding to the α2δ subunit of calcium voltagegated channels. They also inhibit movement of the α2δ subunit from dorsal root ganglion neurons to central terminals in the dorsal horn, which disrupts the reuse of the α2δ subunit from endosomal compartments. Gabapentinoids inhibit thrombospodin-mediated processes and stimulate the uptake of glutamate by excitatory amino acid transporters. In addition, the mode of action of gabapentinoids has been suggested to involve inhibition of descending serotonergic facilitation, stimulation of descending inhibition, anti-inflammatory actions, and influence on the affective component of pain [23].
- Gabapentin was first approved by the FDA as an anticonvulsant in 1993. It has been reported to be effective in alleviating pain and sleep interference associated with DPN [24] and has been approved for the treatment of peripheral neuropathic pain such as postherpetic neuralgia and DPN in the United Kingdom and Australia; however, the USA has approved gabapentin only for the treatment of postherpetic neuralgia [24-26]. In a phase II study of 165 patients who experienced painful DPN for 1 to 5 years and had a pain score of ≥40 mm on the Short-Form McGill Pain Questionnaire visual analogue scale (VAS), the daily pain score at the study end point was significantly lower in patients treated with gabapentin than in those treated with placebo for 8 weeks; the scores decreased from 6.4 to 3.9 in the gabapentin-treated group and from 6.5 to 5.1 in the placebo group (P<0.001) [24]. In another study of 147 patients with painful DPN and an average daily pain (ADP) score ≥4, the ADP score decreased more in patients treated with gabapentin extended-release formula than in those treated with placebo (gabapentin, −2.76; placebo, −1.38; P=0.001) [26]. In a study that compared gabapentin with amitriptyline, gabapentin was more effective in relieving pain caused by DPN [27].
- Pregabalin is a potent and selective ligand for the α2δ subunit of the voltage-gated calcium channel and has been approved for the treatment of painful DPN, postherpetic neuralgia, and spinal cord injury in the USA, Europe, and Canada [1,28]. In a multicenter study of 146 patients with painful DPN for 1 to 5 years and a pain score of ≥40 mm on the Short-Form McGill Pain Questionnaire or an ADP score of ≥4 on an 11-point pain numerical rating scale (NRS), pregabalin significantly reduced the mean pain score compared with placebo (pregabalin, from 6.5 to 4.0; placebo, from 6.1 to 5.3; P<0.0001) [29]. In a study of 338 patients with a 1 to 5-year history of DPN and an average weekly pain score of ≥4 on an 11-point NRS, pregabalin at 300 or 600 mg/day significantly reduced the mean pain score compared with placebo (difference from placebo: 300 mg/day, −1.26; 600 mg/day, −1.45; P=0.0001) [30]. In addition, pregabalin not only relieved pain associated with DPN and but also improved sleep interference and the profile of mood states scores in patients with DPN [29-31]. By contrast, some studies have reported that pregabalin is not more effective than placebo for improving DPN [32,33]. The possible explanations for the lack of effect of pregabalin on painful DPN include the use of low-dose pregabalin, a placebo response in people with painful DPN, or a carryover effect. However, in meta-analysis of studies of patients with painful DPN, pregabalin was more effective than placebo in improving neuropathic pain associated with DPN (change in least squares [LSs] mean change in mean pain score: –0.56 to –1.26) [34-36].
- Mirogabalin is a novel selective oral ligand for the α2δ subunit and is known to have a slower dissociation rate from the α2δ subunit than pregabalin. Mirogabalin has been shown to improve painful DPN in phase II and III trials [37-39]. In a phase II study, mirogabalin and pregabalin were administered to 452 adults with painful DPN for more than 6 months, and the LS mean difference in the change in ADP score from the baseline was evaluated. The effects of pregabalin and placebo were similar after the 3-week treatment, but mirogabalin at a dose of 15, 20, or 30 mg/day was significantly better than placebo in inducing a change in ADP score (–0.94, –0.88, and –1.01, respectively) [37]. In a phase III study of 834 Asian patients, a mirogabalin dose of 15, 20, or 30 mg/day reduced the ADP score by –1.34, –1.47, and –1.81, respectively; this pattern suggests that mirogabalin relieved DPN pain in a dose-dependent manner [39].
- The side effects reported for gabapentinoids include dizziness, somnolence, gastrointestinal complaints, sedation, ataxia, peripheral edema, headache, and postural hypotension, etc. [34,37,40]. Because of these side effects, caution is needed when prescribing this drug, especially in elderly patients. Furthermore, gabapentin and pregabalin are excreted primarily unchanged in the urine, renal dose adjustment is required [41]. The recommended maximal daily dose of gabapentin is 1,500 mg in people with grade 3 chronic kidney disease (CKD), 700 mg in those with grade 4 CKD, 300 mg in those with grade 5 CKD, and 100 to 300 mg after dialysis in dialysis patients. For pregabalin, the recommended maximal daily doses are 300, 150, and 75 mg for people with grade 3, grade 4, and grade 5 CKD, respectively, and 75 to 150 mg after dialysis for dialysis patients [42].
- Serotonin–norepinephrine reuptake inhibitors
- Neurotransmitters such as serotonin and norepinephrine are involved in the descending inhibitory nociceptive pathway. Serotonin–norepinephrine reuptake inhibitors (SNRIs) inhibit reuptake of serotonin and norepinephrine, which increases descending inhibition and reduces pain associated with DPN [43]. Among the available SNRIs, duloxetine is recommended for the initial symptomatic treatment of painful DPN, and venlafaxine is also used for the treatment of painful DPN [1]. In Korea, duloxetine is currently available, but venlafaxine is not.
- Duloxetine, a potent SNRI, reduced neuropathic pain in an animal model and significantly improved painful physical symptoms associated with depression in a study of patients with major depressive disorder [44,45]. Duloxetine is therefore expected to be effective for treating painful DPN. In a study that compared the effects of duloxetine and placebo in 457 patients with a score of ≥3 on the MNSI, duloxetine at a dose of 60 or 120 mg/day improved pain from the first week of administration and was continuously effective throughout the study compared with placebo. The differences in ADP scores compared with placebo were −1.17 (95% confidence interval [CI], −1.84 to −0.50) for 60 mg/day and −1.45 (95% CI, −2.13 to −0.78) for 120 mg/day [46]. Meta-analysis of studies that compared duloxetine and placebo concluded that duloxetine is effective in improving painful DPN [47,48].
- Another SNRI, venlafaxine, has also been shown to be effective in improving painful DPN by reducing VAS pain intensity by 50% after 6 weeks of administration in a study of 244 patients with painful DPN (P<0.001 vs. placebo) [49]. In studies that have compared the effects of venlafaxine and other drugs, venlafaxine was as effective as imipramine and carbamazepine for improving pain associated with DPN [50,51].
- Nausea, somnolence, dizziness, constipation, and loss of appetite have been reported as common side effects of duloxetine and venlafaxine, but they are considered relatively safe drugs to use [46,49].
- Tricyclic antidepressants
- TCAs are thought to relieve pain by inhibiting the reuptake of noradrenaline and serotonin in presynaptic neurons and by antagonizing N-methyl-D-aspartate receptors, which mediate hyperalgesia and allodynia [52-54]. TCAs include tertiary amines such as imipramine and amitriptyline, and secondary amines such as desipramine and nortriptyline; secondary amines are thought to be better tolerated than tertiary amines [55].
- A small (n=29) randomized, crossover study using “active” placebo, which mimics the side effects of amitriptyline, showed the superiority of amitriptyline in relieving pain associated with DPN compared with placebo [56]. In later, larger studies, amitriptyline was used as active comparator to gabapentin, pregabalin, and duloxetine, and was shown to have similar effects in relieving DPN pain as these other drugs [57-59].
- Despite their promising effects and low cost, TCAs should be prescribed cautiously because of the side effects such as dry mouth, constipation, urinary retention, and orthostatic hypotension. TCAs are contraindicated in patients with glaucoma and cardiac conduction disturbances [55,60].
- Alpha-lipoic acid
- Increased oxidative stress caused by free radical formation and defects in antioxidant defense related to hyperglycemia is thought to cause endoneurial hypoxia and nerve dysfunction, which contribute to DPN [61]. Preclinical studies have shown that administration of the antioxidant alpha-lipoic acid reduces neurovascular abnormalities associated with DPN [62-65].
- In a clinical study, intravenous alpha-lipoic acid and placebo were administered for 3 weeks to 328 patients with symptomatic DPN and intravenous alpha-lipoic acid induced significant improvement in pain associated with DPN compared with placebo. The total symptom score in the feet decreased by –4.5±3.7 (–58.6%) points in the group treated with alpha-lipoic acid at a dose of 1,200 mg (P=0.003) and by –5.0±4.1 (–63.5%) points at a dose of 600 mg (P<0.001) [66]. By contrast, a comparison of the effects of oral alpha-lipoic acid and placebo given for 7 months after intravenous alpha-lipoic acid administration for 3 weeks in 509 patients found no significant difference in total symptom score between alpha-lipoic acid and placebo [67]. High doses may be necessary to see the effects of alpha-lipoic acid on painful DPN, but it has not received FDA approval for DPN treatment. Nevertheless, alpha-lipoic acid is the most often prescribed agent as a monotherapy or as a combination therapy in Korea [22].
- Sodium channel blockers
- Carbamazepine, which is used in the treatment of simple and complex partial seizures of epilepsy and generalized convulsions, inhibits the secretion of neurotransmitters such as glutamine by blocking presynaptic voltage-sensitive sodium channels in the central nervous system (CNS) [68]. In a study that compared the effects of carbamazepine, venlafaxine, and pregabalin in 257 patients with clinically definite DPN, carbamazepine significantly decreased the VAS score (from 74.5 to 39.6, P=0.0001) and showed similar effects as venlafaxine and pregabalin in improving scores for sleep, mood, and work interference [51].
- Oxcarbazepine, a derivative of carbamazepine that has better safety and tolerability, has also been reported to be effective in improving pain associated with DPN [69,70]. In a meta-analysis, the percentage of patients with DPN whose pain was “improved” or “very much improved” was 45.9% in those treated with oxcarbazepine and 30.1% in those treated with placebo. However, the quality of evidence to support the effectiveness of oxcarbazepine for treating DPN was very low and the serious adverse effects occurred more frequently in oxcarbazepine than placebo (8.3% vs. 2.5%) [71]. In this regard, oxcarbazepine should be prescribed carefully in patients with DPN.
- Topical capsaicin
- Capsaicin is an alkaloid extracted from red chili peppers and reduces painful stimuli to the CNS by removing substance P from vanilloid nerve receptors [72]. Therefore, capsaicin was expected to be effective for treating painful DPN. Topical capsaicin formulations in the form of 0.025% capsaicin gel or 0.075% capsaicin lotion were not found to be superior to placebo in relieving painful DPN [73,74]. However, in a study in which an 8% capsaicin patch or placebo patch was applied to painful areas of the feet for 30 minutes in 369 patients with painful DPN, the percentage reduction in ADP score from the baseline to weeks 2 to 8 was significantly higher for the 8% capsaicin patch than placebo (–27.4% vs. –20.9%, P=0.025) [75]. Accordingly, in 2020, the FDA approved the 8% capsaicin patch for the treatment of neuropathic pain associated with DPN of the feet in adults [76]. Application site reactions such as a burning sensation and application site pain have been reported as side effects of capsaicin patch [73-75]. In Korea, only the lower concentrated topical capsaicin is available.
- Others
- Antioxidant supplementations such as benfotiamine, a fat-soluble derivative of thiamine, and gamma linolenic acid, an omega-6 fatty acid, were used in some countries for DPN treatment. Even though these drugs have potential mechanism to prevent or treat DPN [77,78] and very low chance to induce side effects, but large-scale randomized controlled trials to show its efficacy on DPN are scarce. Aldose reductase inhibitor has been developed as a promising drug because it targeted a rate-limiting enzyme of polyol pathway. However, it could not address symptomatic improvement of DPN in clinical trials, but it only improved nerve conduction velocity [79,80]. Another pharmacological agent to be used for painful DPN is opioid drug such as tapentadol and tramadol. However, considering lack of longterm treatment data and side effects [81] this class of drug is hardly recommended for the treatment of DPN [82].
MONOTHERAPY
- Considering that pain relief of DPN is not sufficient in monotherapy because of the difficulty increasing the dose given the side effects of the drugs, combination or sequential therapy has been attempted.
- One study compared the effects of high-dose duloxetine, pregabalin, and duloxetine with pregabalin combination therapy in 343 patients with a MNSI score ≥3 and who did not show improvement in pain with duloxetine or pregabalin monotherapy. The combination therapy relieved pain at rates similar to those for high-dose monotherapy as measured by the change in average pain on the Brief Pain Inventory Modified Short Form (mean change: combination, –2.35; high-dose monotherapy, –2.16; P=0.370) [83]. In studies that compared TCA monotherapy, gabapentinoid monotherapy, and their combination in patients with neuropathic pain including painful DPN, combination therapy was more effective than monotherapy [84,85]. Another study compared the effects of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin in 130 patients with painful DPN with a NRS score ≥4. The NRS score for all drug combination groups decreased from 6.6±1.5 to 3.3±1.8, and magnitude of the decrease in NRS did not differ between the drug combination groups. However, the magnitude of the decrease in NRS score was larger for the combination therapy than monotherapy [86].
COMBINATION OR SEQUENTIAL THERAPY
- Neurostimulation
- Transcutaneous electrical nerve stimulation (TENS) is a noninvasive method of neuromodulation using cutaneous adhesive electrodes to apply pulsed electrical stimulation that stimulates A-beta fibers with the goal of indirectly inhibiting nociceptive transmission in the spinal cord dorsal horn [87-89]. TENS also contributes to pain relief by inducing the release of endogenous opioids [87,90]. Electrical stimulation applied in TENS can be modified in terms of frequency (stimulation rate), intensity and duration: Low-frequency TENS is defined as ≤10 Hz and often used at higher intensities inducing motor contraction, while high-frequency TENS is defined as ranging up to 50 or 100 Hz and above and used at lower intensities [91-93]. When low-frequency TENS was applied to 19 patients suffering from mild-to-moderate symptomatic DPN, the new total symptom score (NTSS-6) VAS decreased significantly compared to placebo [94]. When traditional TENS (80 Hz) or acupuncture-like TENS (2 Hz) was applied to five patients suffering from DPN, significant pain relief occurred in all five patients, but there was no placebo in this study [95]. In addition, in a study comparing micro-TENS (2 Hz) and placebo in 41 diabetic patients, micro-TENS did not show significant improvement in pain relief compared to placebo [90]. No specific adverse reactions were reported in patients treated with TENS in the studies [90,95].
- Spinal cord stimulation (SCS) is a treatment for chronic pain that uses electrical stimulation of the dorsal columns of the spinal cord. SCS includes low-frequency SCS (frequency 10–100 Hz, pulse width 100–1,000 ms, and amplitude 1–10 mA) that applies paresthesia-based stimulation and high-frequency SCS (frequency 1–10 kHz, pulse width 30–150 ms, and amplitude 1–5 mA) that applies stimulation below the paresthesia threshold [96]. Although the mechanisms of action of SCS have not been clearly identified, it is thought to involve both spinal and supraspinal effects and has been shown to be effective for the treatment of various neuropathic pain such as failed back surgery syndrome and complex regional pain syndrome [97-100].
- In a study that compared the effects of SCS and placebo for 6 months in 60 patients with DPN refractory to medical therapy, the VAS score decreased significantly only in the SCS group (Table 2) [101]. Similarly, in a study that analyzed the effect of SCS in 36 patients whose DPN did not improve with conventional therapy, the percentages of patients who achieved ≥50% pain relief were 59% in the SCS group and 7% in the best medical treatment group; these findings indicate that SCS was more effective for DPN pain relief [102]. In a study that analyzed the effect of high-frequency SCS on 216 patients with DPN refractory to medical therapy and a VAS score ≥5, the percentages of patients who achieved ≥50% pain relief were 79% in the SCS group and 5% in the conventional medical management group (P<0.001) [103]. The FDA approved SCS devices for the treatment of painful DPN in 2022.
- The side effects reported for SCS include infection and wound dehiscence; one patient who complained of postdural puncture headache experienced a lethal subdural hematoma 3 days after the procedure [101-104].
NONPHARMACOLOGICAL TREATMENTS
- Appropriate treatment of DPN is crucial because DPN impairs the quality of life and can adversely affect the employment of patients with diabetes. Drugs such as gabapentinoids, SNRIs, TCAs, alpha-lipoic acid, sodium channel blockers, and topical capsaicin are used for the treatment of DPN. However, these drugs are ineffective for some patients or can be difficult to use because of side effects. Generally, the pain relief and side effects appear early after treatment, and initial reevaluation is critical. These drugs should not be used to treat painless DPN because they do not provide benefits for these patients. The most beneficial therapeutic approach to treating uncontrolled painful DPN that does not respond to monotherapy is combination therapy, 8% capsaicin patch, or SCS. Given the lack of drugs that target the pathophysiology of DPN, new drugs are needed for the treatment of pain associated with DPN.
CONCLUSIONS
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (No. NRF-2020R1C1C1013766).
NOTES
-
Acknowledgements
- None
| Class | Drugs | Mechanisms | RCTs | Outcomes for mean pain score | Dose | Effects | Adverse effects | FDA approval | Available in Korea |
|---|---|---|---|---|---|---|---|---|---|
| Gabapentinoids | Pregabalin | Inhibition of neurotransmitter release through binding to the α2δ subunit of calcium voltagegated channels | Rosenstock et al. (2004) [29] | NRPS: 6.5→4.0 (pregabalin) vs. 6.1→5.3 (placebo) | Initial 150 mg/day (max. 300 mg/day) | Pain relief in painful DPN, postherpetic neuralgia, fibromyalgia, and spinal cord injury | Dizziness, somnolence, dry mouth, edema, blurred vision, weight gain, thinking abnormal | Painful DPN, postherpetic neuralgia, partial onset seizures, fibromyalgia, neuropathic pain associated with spinal cord injury | Yes |
| Lesser et al. (2004) [30] | Difference at endpoint: −1.26 (300 mg/day); −1.45 (600 mg/day) | ||||||||
| Gabapentin | Backonja et al. (1998) [24] | 11–point Likert scale: 6.4→3.9 (gabapentin) vs. 6.5→5.1 (placebo) | Initial 300 mg/ day (max. 1,800 mg/day) | Pain relief in painful DPN and postherpetic neuralgia | Dizziness, somnolence, peripheral edema | Postherpetic neuralgia, partial onset seizures | Yes | ||
| Sandercock et al. (2009) [26] | Difference in ADP score at endpoint: –2.76 (gabapentin extended-release) vs. –1.38 (placebo) | ||||||||
| Mirogabalin | Vinik et al. (2014) [37] | Difference in ADP score at endpoint: –0.94 (15 mg/day); –0.88 (20 mg/day); –1.01 (30 mg/day) | Initial 10 mg/day (max. 30 mg/day) | Pain relief in painful DPN and postherpetic neuralgia | Dizziness, somnolence, peripheral edema | None | No | ||
| Merante et al. (2017) [38] | “Much improved” or “better” for the PGIC: 49.1% (5 mg/day); 54.5% (10 mg/day); 48.0% (15 mg/day); 48.1% (20 mg/day); 50.0% (30 mg/day) vs. 31.1% (placebo) | ||||||||
| Baba et al. (2019) [39] | Difference in ADP score at endpoint: –1.81 (mirogabalin 30 mg/day) vs. –1.31 (placebo) | ||||||||
| SNRIs | Duloxetine | Inhibition of reuptake of serotonin and norepinephrine | Goldstein et al. (2005) [46] | Difference from placebo at endpoint: –1.17 (95% CI, –1.84 to –0.50) (60 mg/day); –1.45 (95% CI, –2.13 to –0.78) (120 mg/day) | 60 mg/day | Pain relief in painful DPN, fibromyalgia and chronic musculoskeletal pain | Nausea, dry mouth, somnolence, fatigue, constipation, decreased appetite, hyperhidrosis | MDD, GAD, painful DPN, fibromyalgia, chronic musculoskeletal pain | Yes |
| Venlafaxine | Rowbotham et al. (2004) [49] | Reduction in VAS-PI at endpoint: 50% (150–225 mg) vs. 27% (placebo) | Initial 37.5 mg/day (max. 225 mg/day) | Pain relief in painful DPN | Nausea, somnolence, dry mouth, sweating, abnormal ejaculation, anorexia, constipation, erectile dysfunction, libido decreased | MDD, anxiety disorder, panic disorder | No | ||
| TCA | Amitriptyline | Inhibition of reuptake of noradrenaline and serotonin in presynaptic neurons and antagonizing N-methyl-D-aspartate receptors | Max et al. (1987) [56] | Pain relief: 23/29 (amitriptyline) vs. 1/29 (placebo) | Initial 75 mg/day (max. 150 mg/day) | Pain relief in painful DPN, neuropathic pain and fibromyalgia | Dry mouth, somnolence, dizziness, constipation, weight gain | Depression | Yes |
| Alphalipoic acid | Thiotic acid | Antioxidant | Ziegler et al. (1995) [66] | Decrease in total symptom score at endpoint: –58.6% (1,200 mg/day); –63.5% (600 mg/day); –38.4% (placebo) | 600 mg/day | Pain relief in painful DPN | Headache, hearburn, nausea, vomiting | None | Yes |
| Sodium channel blockers | Carbamazepine | Inhibition of the secretion of neurotransmitters by blocking presynaptic voltage-sensitive sodium channels | Razazian et al. (2014) [51] | VAS score: 74.5→39.6 (carbamazepine) vs. 82.3→33.4 (pregabalin) vs. 74.5→46.6 (venlafaxine) | Initial 200 mg/day (max. 800 mg/day) | Pain relief in neuropathic pain | Dizziness, somnolence, unsteadiness, nausea, vomiting | Epilepsy, trigeminal neuralgia | Yes |
| Oxcarbazepine | Dogra et al. (2005) [69] | >50% reduction in VAS score at endpoint: 35.2% (oxcarbazepine) vs. 18.4% (placebo) | Initial 600 mg/day (max. 2,400 mg/day) | Pain relief in neuropathic pain | Dizziness, somnolence, diplopia, fatigue, nausea, vomiting, ataxia, abnormal vision, headache, nystagmus, tremor, abnormal gait | Partial seizures | |||
| Topical agent | Capsaicin 8% patch | Removal of substance P from vanilloid nerve receptors | Simpson et al. (2017) [75] | Difference in ADP score at endpoint: –27.4% (capsaicin 8% patch) vs. –20.9% (placebo) | Max. 4 patches for 30 min- utes | Relief of neuropathic pain associated with postherpetic neuralgia and DPN | Site erythema, application site pain, application site pruritus | Postherpetic neuralgia, painful DPN of the feet | No |
RCT, randomized controlled trial; FDA, U.S. Food and Drug Administration; NRPS, numeric pain rating scale; DPN, diabetic peripheral neuropathy; ADP, average daily pain; PGIC, patient global impression of change; SNRI, serotonin–norepinephrine reuptake inhibitor; CI, confidence interval; MDD, major depressive disorder; GAD, generalized anxiety disorder; VAS-PI, VAS pain intensity; TCA, tricyclic antidepressant; VAS, visual analogue scale.
| Study | Intervention | Control treatment | Study population; follow-up duration | Outcomes (mean±SD or mean [95% CI] or number [%]): Intervention vs. Control (P value) |
|---|---|---|---|---|
| De Vos et al. (2014) [101] | LF-SCS | Medical therapy | 40 (intervention) vs. 20 (control); 6 months | Percentages of patients with 50% pain reduction: 25 (60%) vs. 1 (5%) (P<0.001) |
| VAS: 73±16→31±28 vs. 67±18→67±21 (P<0.001) | ||||
| MPQ NWC-T: 13±5→8±7 vs. 13±3→13±4 (P<0.01) | ||||
| MPQ PRI-T: 27±13→15±14 vs. 24±9→26±10 (P<0.01) | ||||
| MPQ QoL: 16±5→8±7 vs. 15±6→14±6 (P<0.001) | ||||
| EQ-5D VAS: 50±19→61±22 vs. 46±17→41±20 (P<0.01) | ||||
| PGIC pain: 29 (73%) vs. 3 (17%) (P<0.01) | ||||
| Satisfaction with treatment: 8/10 vs. 4/10 (P<0.001) | ||||
| Slangen et al. (2014) [102] | LF-SCS | Medical therapy | 22 (intervention) vs. 14 (control); 6 months | Percentage of patients with 50% pain reduction (day): 9 (41%) vs. 0 (0%) (P<0.001) |
| Percentages of patients with 50% pain reduction (night): 8 (36%) vs. 1 (7%) (P<0.01) | ||||
| NRS during the day: –3.1 points vs. no change (P<0.001) | ||||
| NRS during the night: –2.4 points vs. –0.9 points (P<0.003) | ||||
| EQ-5D Utility score: 0.25±031→0.50±0.33 vs. 0.33±0.32→0.33±0.29 (NS) | ||||
| EQ-5D Current health: 53.9±18.5→57.6±24.3 vs. 54.6±16.7→56.5±14.2 (NS) | ||||
| PGIC for pain: 12 (55%) vs. 0 (0%) (P<0.001) | ||||
| PGIC for sleep: 8 (36%) vs. 0 (5%) (P<0.05) | ||||
| Treatment successa: 13 (59%) vs. 1 (7%) (P<0.01) | ||||
| Petersen et al. (2021) [103] | HF-SCS | Medical therapy | 113 (intervention) vs. 103 (control); 6 months | Combination of 50% pain reduction and no deterioration on neurological examination: 75 (79%) vs. 5 (5%) (P<0.001) |
| Percentages of patients with 50% pain reduction: 74 (85%) vs. 5 (5%) (P<0.001) | ||||
| VAS: 7.6 [7.3–7.9]→1.7 [1.3–2.1] vs. 7.0 [6.7–7.3]→6.9 [6.5–7.3] (P<0.001) | ||||
| Percentages of patients with VAS ≤3: 53 (60%) vs. 1 (1%) (P<0.001) | ||||
| EQ-5D-5L index: 0.130±0.159 vs. –0.031±0.127 (P<0.001) | ||||
| EQ-5D-5L health VAS: 15.9±21.6 vs. –1.7±23.0 (P<0.001) |
SD, standard deviation; CI, confidence interval; LF, low-frequency; SCS, spinal cord stimulation; VAS, visual analogue scale; MPQ, McGill Pain Questionnaire; NWC-T, the total number of words chosen from the McGill Pain Questionnaire; PRI-T, pain rating index; QoL, quality of life; EQ-5D, EuroQoL-5D; PGIC, patient global impression of change; NRS, numeric rating scale; NS, not statistically significant; HF, high-frequency.
a Treatment success means ≥50% reduction in pain intensity during the daytime or nighttime or an improvement in pain and sleep of ≥6 in the PGIC score.
- 1. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017;40:136-54.ArticlePubMedPMCPDF
- 2. Shillo P, Sloan G, Greig M, Hunt L, Selvarajah D, Elliott J, et al. Painful and painless diabetic neuropathies: what is the difference? Curr Diab Rep 2019;19:32.ArticlePubMedPMCPDF
- 3. Ziegler D, Landgraf R, Lobmann R, Reiners K, Rett K, Schnell O, et al. Painful and painless neuropathies are distinct and largely undiagnosed entities in subjects participating in an educational initiative (PROTECT study). Diabetes Res Clin Pract 2018;139:147-54.ArticlePubMed
- 4. Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, et al. Neuropathic pain. Nat Rev Dis Primers 2017;3:17002.ArticlePubMedPMCPDF
- 5. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813-20.ArticlePubMedPDF
- 6. Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419-30.ArticlePubMedPMC
- 7. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129-39.ArticlePubMed
- 8. Ang L, Jaiswal M, Martin C, Pop-Busui R. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep 2014;14:528.ArticlePubMedPMCPDF
- 9. Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol 2012;11:521-34.ArticlePubMedPMC
- 10. Callaghan BC, Xia R, Reynolds E, Banerjee M, Rothberg AE, Burant CF, et al. Association between metabolic syndrome components and polyneuropathy in an obese population. JAMA Neurol 2016;73:1468-76.ArticlePubMedPMC
- 11. Oh TJ, Lee JE, Choi SH, Jang HC. Association between body fat and diabetic peripheral neuropathy in middle-aged adults with type 2 diabetes mellitus: a preliminary report. J Obes Metab Syndr 2019;28:112-7.ArticlePubMedPMC
- 12. Elafros MA, Andersen H, Bennett DL, Savelieff MG, Viswanathan V, Callaghan BC, et al. Towards prevention of diabetic peripheral neuropathy: clinical presentation, pathogenesis, and new treatments. Lancet Neurol 2022;21:922-36.ArticlePubMedPMC
- 13. Kim K, Oh TJ, Park YS, Chang W, Cho HC, Lee J, et al. Association between fat mass or fat fibrotic gene expression and polyneuropathy in subjects with obesity: a Korean Metabolic Bariatric Surgery Cohort. Front Endocrinol (Lausanne) 2022;13:881093.ArticlePubMedPMC
- 14. Stino AM, Smith AG. Peripheral neuropathy in prediabetes and the metabolic syndrome. J Diabetes Investig 2017;8:646-55.ArticlePubMedPMCPDF
- 15. Bonhof GJ, Strom A, Puttgen S, Ringel B, Bruggemann J, Bodis K, et al. Patterns of cutaneous nerve fibre loss and regeneration in type 2 diabetes with painful and painless polyneuropathy. Diabetologia 2017;60:2495-503.ArticlePubMedPDF
- 16. Look AHEAD Research Group. Effects of a long-term lifestyle modification programme on peripheral neuropathy in overweight or obese adults with type 2 diabetes: the Look AHEAD study. Diabetologia 2017;60:980-8.ArticlePubMedPMCPDF
- 17. Singh P, Adderley N, Subramanian A, Gokhale K, Singhal R, Toulis KA, et al. The impact of bariatric surgery on incident microvascular complications in patients with type 2 diabetes: a matched controlled population-based retrospective cohort study. Diabetes Care 2021;44:116-24.ArticlePubMedPMCPDF
- 18. Balducci S, Iacobellis G, Parisi L, Di Biase N, Calandriello E, Leonetti F, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications 2006;20:216-23.ArticlePubMed
- 19. Singleton JR, Marcus RL, Jackson JE, Lessard MK, Graham TE, Smith AG. Exercise increases cutaneous nerve density in diabetic patients without neuropathy. Ann Clin Transl Neurol 2014;1:844-9.ArticlePubMedPMCPDF
- 20. Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain 2020;161:1976-82.ArticlePubMedPMC
- 21. Yang M, Qian C, Liu Y. Suboptimal treatment of diabetic peripheral neuropathic pain in the United States. Pain Med 2015;16:2075-83.ArticlePubMed
- 22. Moon SS, Kim CH, Kang SM, Kim ES, Oh TJ, Yun JS, et al. Status of diabetic neuropathy in Korea: a National Health Insurance Service-National Sample Cohort analysis (2006 to 2015). Diabetes Metab J 2021;45:115-9.ArticlePubMedPMCPDF
- 23. Chincholkar M. Analgesic mechanisms of gabapentinoids and effects in experimental pain models: a narrative review. Br J Anaesth 2018;120:1315-34.ArticlePubMed
- 24. Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA 1998;280:1831-6.ArticlePubMed
- 25. Mathieson S, Lin CC, Underwood M, Eldabe S. Pregabalin and gabapentin for pain. BMJ 2020;369:m1315.ArticlePubMed
- 26. Sandercock D, Cramer M, Wu J, Chiang YK, Biton V, Heritier M. Gabapentin extended release for the treatment of painful diabetic peripheral neuropathy: efficacy and tolerability in a double-blind, randomized, controlled clinical trial. Diabetes Care 2009;32:e20.PubMedPMC
- 27. Dallocchio C, Buffa C, Mazzarello P, Chiroli S. Gabapentin vs. amitriptyline in painful diabetic neuropathy: an open-label pilot study. J Pain Symptom Manage 2000;20:280-5.PubMed
- 28. McClelland D, Evans RM, Barkworth L, Martin DJ, Scott RH. A study comparing the actions of gabapentin and pregabalin on the electrophysiological properties of cultured DRG neurones from neonatal rats. BMC Pharmacol 2004;4:14.ArticlePubMedPMC
- 29. Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain 2004;110:628-38.ArticlePubMed
- 30. Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology 2004;63:2104-10.ArticlePubMed
- 31. Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain 2005;6:253-60.ArticlePubMed
- 32. Huffman C, Stacey BR, Tuchman M, Burbridge C, Li C, Parsons B, et al. Efficacy and safety of pregabalin in the treatment of patients with painful diabetic peripheral neuropathy and pain on walking. Clin J Pain 2015;31:946-58.ArticlePubMed
- 33. Mu Y, Liu X, Li Q, Chen K, Liu Y, Lv X, et al. Efficacy and safety of pregabalin for painful diabetic peripheral neuropathy in a population of Chinese patients: a randomized placebo-controlled trial. J Diabetes 2018;10:256-65.ArticlePubMedPDF
- 34. Parsons B, Li C. The efficacy of pregabalin in patients with moderate and severe pain due to diabetic peripheral neuropathy. Curr Med Res Opin 2016;32:929-37.ArticlePubMed
- 35. Hurley RW, Lesley MR, Adams MC, Brummett CM, Wu CL. Pregabalin as a treatment for painful diabetic peripheral neuropathy: a meta-analysis. Reg Anesth Pain Med 2008;33:389-94.ArticlePubMed
- 36. Freeman R, Durso-Decruz E, Emir B. Efficacy, safety, and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: findings from seven randomized, controlled trials across a range of doses. Diabetes Care 2008;31:1448-54.PubMedPMC
- 37. Vinik A, Rosenstock J, Sharma U, Feins K, Hsu C, Merante D, et al. Efficacy and safety of mirogabalin (DS-5565) for the treatment of diabetic peripheral neuropathic pain: a randomized, double-blind, placebo- and active comparator-controlled, adaptive proof-of-concept phase 2 study. Diabetes Care 2014;37:3253-61.ArticlePubMedPDF
- 38. Merante D, Rosenstock J, Sharma U, Feins K, Hsu C, Vinik A, et al. Efficacy of mirogabalin (DS-5565) on patient-reported pain and sleep interference in patients with diabetic neuropathic pain: secondary outcomes of a phase II proof-of-concept study. Pain Med 2017;18:2198-207.ArticlePubMed
- 39. Baba M, Matsui N, Kuroha M, Wasaki Y, Ohwada S. Mirogabalin for the treatment of diabetic peripheral neuropathic pain: a randomized, double-blind, placebo-controlled phase III study in Asian patients. J Diabetes Investig 2019;10:1299-306.ArticlePubMedPMCPDF
- 40. Mellegers MA, Furlan AD, Mailis A. Gabapentin for neuropathic pain: systematic review of controlled and uncontrolled literature. Clin J Pain 2001;17:284-95.ArticlePubMed
- 41. Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet 2010;49:661-9.ArticlePubMed
- 42. Raouf M, Atkinson TJ, Crumb MW, Fudin J. Rational dosing of gabapentin and pregabalin in chronic kidney disease. J Pain Res 2017;10:275-8.ArticlePubMedPMCPDF
- 43. Bates D, Schultheis BC, Hanes MC, Jolly SM, Chakravarthy KV, Deer TR, et al. A comprehensive algorithm for management of neuropathic pain. Pain Med 2019;20(Suppl 1):S2-12.ArticlePubMedPMC
- 44. Iyengar S, Webster AA, Hemrick-Luecke SK, Xu JY, Simmons RM. Efficacy of duloxetine, a potent and balanced serotoninnorepinephrine reuptake inhibitor in persistent pain models in rats. J Pharmacol Exp Ther 2004;311:576-84.ArticlePubMed
- 45. Detke MJ, Lu Y, Goldstein DJ, Hayes JR, Demitrack MA. Duloxetine, 60 mg once daily, for major depressive disorder: a randomized double-blind placebo-controlled trial. J Clin Psychiatry 2002;63:308-15.ArticlePubMed
- 46. Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain 2005;116:109-18.ArticlePubMed
- 47. Rudroju N, Bansal D, Talakokkula ST, Gudala K, Hota D, Bhansali A, et al. Comparative efficacy and safety of six antidepressants and anticonvulsants in painful diabetic neuropathy: a network meta-analysis. Pain Physician 2013;16:E705-14.PubMed
- 48. Griebeler ML, Morey-Vargas OL, Brito JP, Tsapas A, Wang Z, Carranza Leon BG, et al. Pharmacologic interventions for painful diabetic neuropathy: an umbrella systematic review and comparative effectiveness network meta-analysis. Ann Intern Med 2014;161:639-49.ArticlePubMed
- 49. Rowbotham MC, Goli V, Kunz NR, Lei D. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain 2004;110:697-706.ArticlePubMed
- 50. Sindrup SH, Bach FW, Madsen C, Gram LF, Jensen TS. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology 2003;60:1284-9.ArticlePubMed
- 51. Razazian N, Baziyar M, Moradian N, Afshari D, Bostani A, Mahmoodi M. Evaluation of the efficacy and safety of pregabalin, venlafaxine, and carbamazepine in patients with painful diabetic peripheral neuropathy: a randomized, double-blind trial. Neurosciences (Riyadh) 2014;19:192-8.PubMedPMC
- 52. Boulton AJ. Management of diabetic peripheral neuropathy. Clin Diabetes 2005;23:9-15.ArticlePDF
- 53. Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med 1992;326:1250-6.ArticlePubMed
- 54. Ulugol A, Karadag HC, Tamer M, Firat Z, Aslantas A, Dokmeci I. Involvement of adenosine in the anti-allodynic effect of amitriptyline in streptozotocin-induced diabetic rats. Neurosci Lett 2002;328:129-32.ArticlePubMed
- 55. Khdour MR. Treatment of diabetic peripheral neuropathy: a review. J Pharm Pharmacol 2020;72:863-72.ArticlePubMedPDF
- 56. Max MB, Culnane M, Schafer SC, Gracely RH, Walther DJ, Smoller B, et al. Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology 1987;37:589-96.ArticlePubMed
- 57. Morello CM, Leckband SG, Stoner CP, Moorhouse DF, Sahagian GA. Randomized double-blind study comparing the efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathy pain. Arch Intern Med 1999;159:1931-7.ArticlePubMed
- 58. Bansal D, Bhansali A, Hota D, Chakrabarti A, Dutta P. Amitriptyline vs. pregabalin in painful diabetic neuropathy: a randomized double blind clinical trial. Diabet Med 2009;26:1019-26.ArticlePubMed
- 59. Kaur H, Hota D, Bhansali A, Dutta P, Bansal D, Chakrabarti A. A comparative evaluation of amitriptyline and duloxetine in painful diabetic neuropathy: a randomized, double-blind, cross-over clinical trial. Diabetes Care 2011;34:818-22.PubMedPMC
- 60. Benbouzid M, Gaveriaux-Ruff C, Yalcin I, Waltisperger E, Tessier LH, Muller A, et al. Delta-opioid receptors are critical for tricyclic antidepressant treatment of neuropathic allodynia. Biol Psychiatry 2008;63:633-6.ArticlePubMed
- 61. Low PA, Nickander KK, Tritschler HJ. The roles of oxidative stress and antioxidant treatment in experimental diabetic neuropathy. Diabetes 1997;46 Suppl 2:S38-42.ArticlePubMedPDF
- 62. Nagamatsu M, Nickander KK, Schmelzer JD, Raya A, Wittrock DA, Tritschler H, et al. Lipoic acid improves nerve blood flow, reduces oxidative stress, and improves distal nerve conduction in experimental diabetic neuropathy. Diabetes Care 1995;18:1160-7.ArticlePubMedPDF
- 63. Nickander KK, McPhee BR, Low PA, Tritschler H. Alpha-lipoic acid: antioxidant potency against lipid peroxidation of neural tissues in vitro and implications for diabetic neuropathy. Free Radic Biol Med 1996;21:631-9.ArticlePubMed
- 64. Garrett NE, Malcangio M, Dewhurst M, Tomlinson DR. alpha-Lipoic acid corrects neuropeptide deficits in diabetic rats via induction of trophic support. Neurosci Lett 1997;222:191-4.PubMed
- 65. Hounsom L, Horrobin DF, Tritschler H, Corder R, Tomlinson DR. A lipoic acid-gamma linolenic acid conjugate is effective against multiple indices of experimental diabetic neuropathy. Diabetologia 1998;41:839-43.ArticlePubMedPDF
- 66. Ziegler D, Hanefeld M, Ruhnau KJ, Meissner HP, Lobisch M, Schutte K, et al. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid: a 3-week multicentre randomized controlled trial (ALADIN Study). Diabetologia 1995;38:1425-33.ArticlePubMedPDF
- 67. Ziegler D, Hanefeld M, Ruhnau KJ, Hasche H, Lobisch M, Schutte K, et al. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a 7-month multicenter randomized controlled trial (ALADIN III Study). ALADIN III Study Group. Alpha-Lipoic Acid in Diabetic Neuropathy. Diabetes Care 1999;22:1296-301.ArticlePubMedPDF
- 68. Spiller HA, Carlisle RD. Status epilepticus after massive carbamazepine overdose. J Toxicol Clin Toxicol 2002;40:81-90.ArticlePubMed
- 69. Dogra S, Beydoun S, Mazzola J, Hopwood M, Wan Y. Oxcarbazepine in painful diabetic neuropathy: a randomized, placebo-controlled study. Eur J Pain 2005;9:543-54.ArticlePubMed
- 70. Erdemoglu AK, Varlibas A. Effectiveness of oxcarbazepine in symptomatic treatment of painful diabetic neuropathy. Neurol India 2006;54:173-7.PubMed
- 71. Zhou M, Chen N, He L, Yang M, Zhu C, Wu F. Oxcarbazepine for neuropathic pain. Cochrane Database Syst Rev 2017;12:CD007963.ArticlePubMedPMC
- 72. Cortright DN, Szallasi A. Biochemical pharmacology of the vanilloid receptor TRPV1: an update. Eur J Biochem 2004;271:1814-9.ArticlePubMed
- 73. Kulkantrakorn K, Lorsuwansiri C, Meesawatsom P. 0.025% Capsaicin gel for the treatment of painful diabetic neuropathy: a randomized, double-blind, crossover, placebo-controlled trial. Pain Pract 2013;13:497-503.ArticlePubMed
- 74. Kulkantrakorn K, Chomjit A, Sithinamsuwan P, Tharavanij T, Suwankanoknark J, Napunnaphat P. 0.075% Capsaicin lotion for the treatment of painful diabetic neuropathy: a randomized, double-blind, crossover, placebo-controlled trial. J Clin Neurosci 2019;62:174-9.ArticlePubMed
- 75. Simpson DM, Robinson-Papp J, Van J, Stoker M, Jacobs H, Snijder RJ, et al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: a randomized, double-blind, placebocontrolled study. J Pain 2017;18:42-53.ArticlePubMed
- 76. Abrams RMC, Pedowitz EJ, Simpson DM. A critical review of the capsaicin 8% patch for the treatment of neuropathic pain associated with diabetic peripheral neuropathy of the feet in adults. Expert Rev Neurother 2021;21:259-66.ArticlePubMed
- 77. Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med 2003;9:294-9.ArticlePubMedPDF
- 78. Coste T, Pierlovisi M, Leonardi J, Dufayet D, Gerbi A, Lafont H, et al. Beneficial effects of gamma linolenic acid supplementation on nerve conduction velocity, Na+, K+ ATPase activity, and membrane fatty acid composition in sciatic nerve of diabetic rats. J Nutr Biochem 1999;10:411-20.ArticlePubMed
- 79. Satoh J, Kohara N, Sekiguchi K, Yamaguchi Y. Effect of ranirestat on sensory and motor nerve function in Japanese patients with diabetic polyneuropathy: a randomized double-blind placebo-controlled study. J Diabetes Res 2016;2016:5383797.ArticlePubMedPMCPDF
- 80. Sekiguchi K, Kohara N, Baba M, Komori T, Naito Y, Imai T, et al. Aldose reductase inhibitor ranirestat significantly improves nerve conduction velocity in diabetic polyneuropathy: a randomized double-blind placebo-controlled study in Japan. J Diabetes Investig 2019;10:466-74.ArticlePubMedPMCPDF
- 81. Xie J, Strauss VY, Martinez-Laguna D, Carbonell-Abella C, Diez-Perez A, Nogues X, et al. Association of tramadol vs codeine prescription dispensation with mortality and other adverse clinical outcomes. JAMA 2021;326:1504-15.ArticlePubMedPMC
- 82. Price R, Smith D, Franklin G, Gronseth G, Pignone M, David WS, et al. Oral and topical treatment of painful diabetic polyneuropathy: practice guideline update summary: report of the AAN Guideline Subcommittee. Neurology 2022;98:31-43.ArticlePubMed
- 83. Tesfaye S, Wilhelm S, Lledo A, Schacht A, Tolle T, Bouhassira D, et al. Duloxetine and pregabalin: high-dose monotherapy or their combination?: the “COMBO-DN study”: a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain 2013;154:2616-25.ArticlePubMed
- 84. Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet 2009;374:1252-61.ArticlePubMed
- 85. Holbech JV, Bach FW, Finnerup NB, Brosen K, Jensen TS, Sindrup SH. Imipramine and pregabalin combination for painful polyneuropathy: a randomized controlled trial. Pain 2015;156:958-66.PubMed
- 86. Tesfaye S, Sloan G, Petrie J, White D, Bradburn M, Julious S, et al. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind, randomised crossover trial. Lancet 2022;400:680-90.ArticlePubMedPMC
- 87. Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. J Pain 2003;4:109-21.ArticlePubMed
- 88. Dubinsky RM, Miyasaki J. Assessment: efficacy of transcutaneous electric nerve stimulation in the treatment of pain in neurologic disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2010;74:173-6.ArticlePubMed
- 89. Jin DM, Xu Y, Geng DF, Yan TB. Effect of transcutaneous electrical nerve stimulation on symptomatic diabetic peripheral neuropathy: a meta-analysis of randomized controlled trials. Diabetes Res Clin Pract 2010;89:10-5.ArticlePubMed
- 90. Gossrau G, Wahner M, Kuschke M, Konrad B, Reichmann H, Wiedemann B, et al. Microcurrent transcutaneous electric nerve stimulation in painful diabetic neuropathy: a randomized placebo-controlled study. Pain Med 2011;12:953-60.ArticlePubMed
- 91. Johnson MI, Bjordal JM. Transcutaneous electrical nerve stimulation for the management of painful conditions: focus on neuropathic pain. Expert Rev Neurother 2011;11:735-53.ArticlePubMed
- 92. Moran F, Leonard T, Hawthorne S, Hughes CM, McCrumGardner E, Johnson MI, et al. Hypoalgesia in response to transcutaneous electrical nerve stimulation (TENS) depends on stimulation intensity. J Pain 2011;12:929-35.ArticlePubMed
- 93. Walsh DM, Howe TE, Johnson MI, Sluka KA. Transcutaneous electrical nerve stimulation for acute pain. Cochrane Database Syst Rev 2009;2:CD006142.Article
- 94. Forst T, Nguyen M, Forst S, Disselhoff B, Pohlmann T, Pfutzner A. Impact of low frequency transcutaneous electrical nerve stimulation on symptomatic diabetic neuropathy using the new Salutaris device. Diabetes Nutr Metab 2004;17:163-8.PubMed
- 95. Upton GA, Tinley P, Al-Aubaidy H, Crawford R. The influence of transcutaneous electrical nerve stimulation parameters on the level of pain perceived by participants with painful diabetic neuropathy: a crossover study. Diabetes Metab Syndr 2017;11:113-8.ArticlePubMed
- 96. Katz N, Dworkin RH, North R, Thomson S, Eldabe S, Hayek SM, et al. Research design considerations for randomized controlled trials of spinal cord stimulation for pain: Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials/Institute of Neuromodulation/International Neuromodulation Society recommendations. Pain 2021;162:1935-56.ArticlePubMedPMC
- 97. Barchini J, Tchachaghian S, Shamaa F, Jabbur SJ, Meyerson BA, Song Z, et al. Spinal segmental and supraspinal mechanisms underlying the pain-relieving effects of spinal cord stimulation: an experimental study in a rat model of neuropathy. Neuroscience 2012;215:196-208.ArticlePubMed
- 98. Linderoth B, Foreman RD. Mechanisms of spinal cord stimulation in painful syndromes: role of animal models. Pain Med 2006;7(Suppl 1):S14-26.Article
- 99. Stancak A, Kozak J, Vrba I, Tintera J, Vrana J, Polacek H, et al. Functional magnetic resonance imaging of cerebral activation during spinal cord stimulation in failed back surgery syndrome patients. Eur J Pain 2008;12:137-48.ArticlePubMedPDF
- 100. Deer TR, Mekhail N, Provenzano D, Pope J, Krames E, Leong M, et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation 2014;17:515-50.ArticlePubMed
- 101. de Vos CC, Meier K, Zaalberg PB, Nijhuis HJ, Duyvendak W, Vesper J, et al. Spinal cord stimulation in patients with painful diabetic neuropathy: a multicentre randomized clinical trial. Pain 2014;155:2426-31.ArticlePubMed
- 102. Slangen R, Schaper NC, Faber CG, Joosten EA, Dirksen CD, van Dongen RT, et al. Spinal cord stimulation and pain relief in painful diabetic peripheral neuropathy: a prospective twocenter randomized controlled trial. Diabetes Care 2014;37:3016-24.ArticlePubMedPDF
- 103. Petersen EA, Stauss TG, Scowcroft JA, Brooks ES, White JL, Sills SM, et al. Effect of high-frequency (10-kHz) spinal cord stimulation in patients with painful diabetic neuropathy: a randomized clinical trial. JAMA Neurol 2021;78:687-98.PubMedPMC
- 104. Duarte RV, Nevitt S, Copley S, Maden M, de Vos CC, Taylor RS, et al. Systematic review and network meta-analysis of neurostimulation for painful diabetic neuropathy. Diabetes Care 2022;45:2466-75.ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

- J-2156, a small molecule somatostatin type 4 receptor agonist, alleviated hindpaw hypersensitivity in the streptozotocin-induced rat model of painful diabetic neuropathy but with a 2-fold decrease in potency at an advanced stage in the model, mimicking mo
A. Kuo, M. Z. Imam, R. Li, L. Lin, A. Raboczyj, A. E. Bohmer, J. R. Nicholson, L. Corradini, M. T. Smith
Frontiers in Pharmacology.2024;[Epub] CrossRef - The Chronic Wound–Related Pain Model
Kevin Woo
Clinics in Geriatric Medicine.2024;[Epub] CrossRef

 KDA
KDA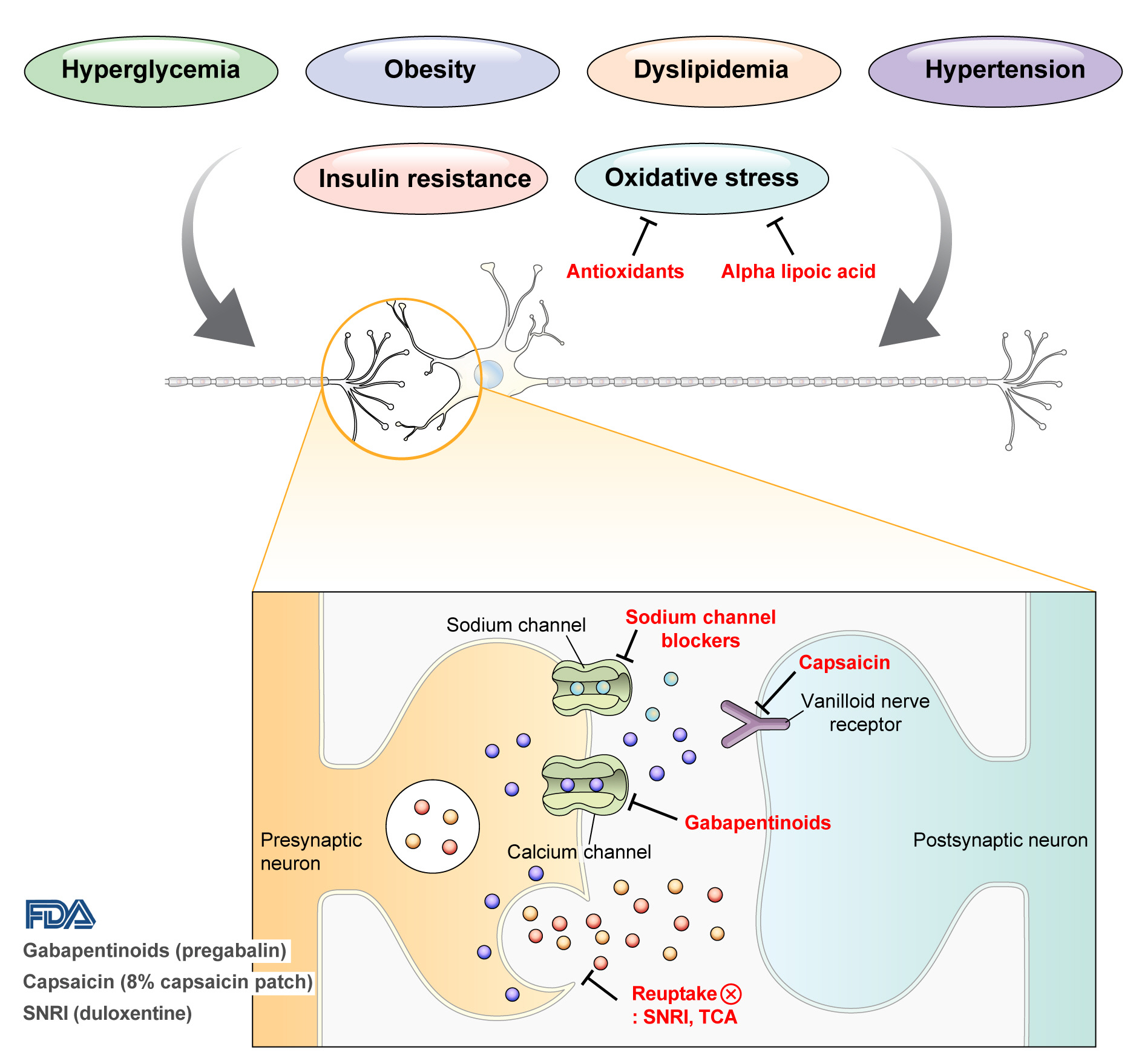
 PubReader
PubReader ePub Link
ePub Link Cite
Cite