Pharmacological and Nonpharmacological Treatments for Painful Diabetic Peripheral Neuropathy
Article information
Abstract
Diabetic peripheral neuropathy (DPN) is one of the most prevalent chronic complications of diabetes. The lifetime prevalence of DPN is thought to be >50%, and 15%–25% of patients with diabetes experience neuropathic pain, referred to as “painful DPN.” Appropriate treatment of painful DPN is important because this pain contributes to a poor quality of life by causing sleep disturbance, anxiety, and depression. The basic principle for the management of painful DPN is to control hyperglycemia and other modifiable risk factors, but these may be insufficient for preventing or improving DPN. Because there is no promising diseasemodifying medication for DPN, the pain itself needs to be managed when treating painful DPN. Drugs for neuropathic pain, such as gabapentinoids, serotonin–norepinephrine reuptake inhibitors, tricyclic antidepressants, alpha-lipoic acid, sodium channel blockers, and topical capsaicin, are used for the management of painful DPN. The U.S. Food and Drug Administration (FDA) has approved pregabalin, duloxetine, tapentadol, and the 8% capsaicin patch as drugs for the treatment of painful DPN. Recently, spinal cord stimulation using electrical stimulation is approved by the FDA for the treatment for painful DPN. This review describes the currently available pharmacological and nonpharmacological treatments for painful DPN.
INTRODUCTION
Diabetic peripheral neuropathy (DPN) is one of the most prevalent chronic complications of diabetes, and its prevalence has been reported to vary widely [1]. The risk of DPN increases with age, and the lifetime prevalence may be >50%. Around 15% to 25% of patients with diabetes experienced neuropathic pain or painful DPN [2], although many are not aware of the presence of this complication. A nationwide survey of DPN in Germany showed that 61.5% and 81.1% of patients with painful and painless DPN were undiagnosed previously [3]. A similar situation may be present in other countries. Because painful DPN contributes to poor quality of life by causing sleep disturbance, anxiety, and depression [4], this chronic complication of diabetes requires further study.
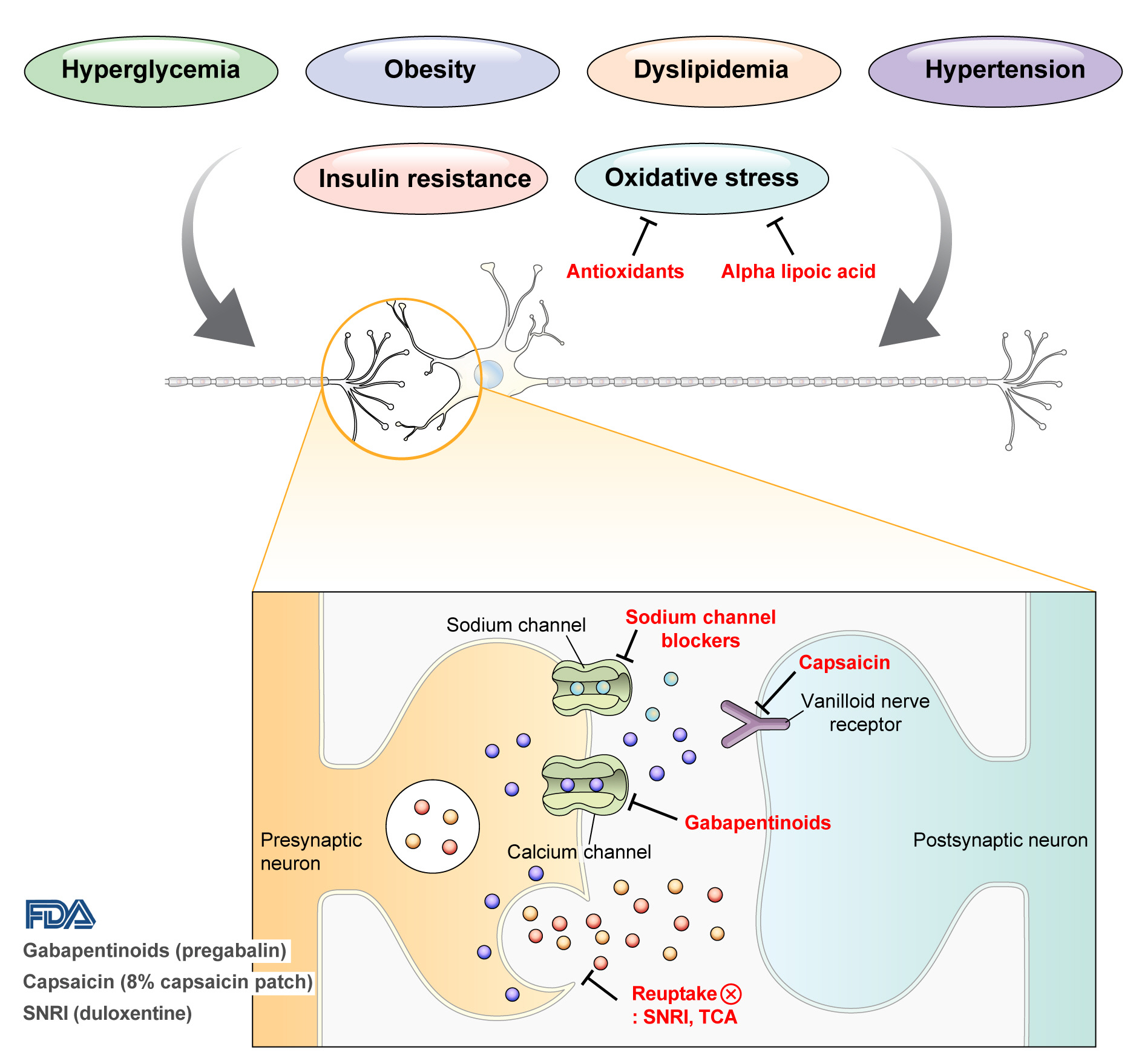
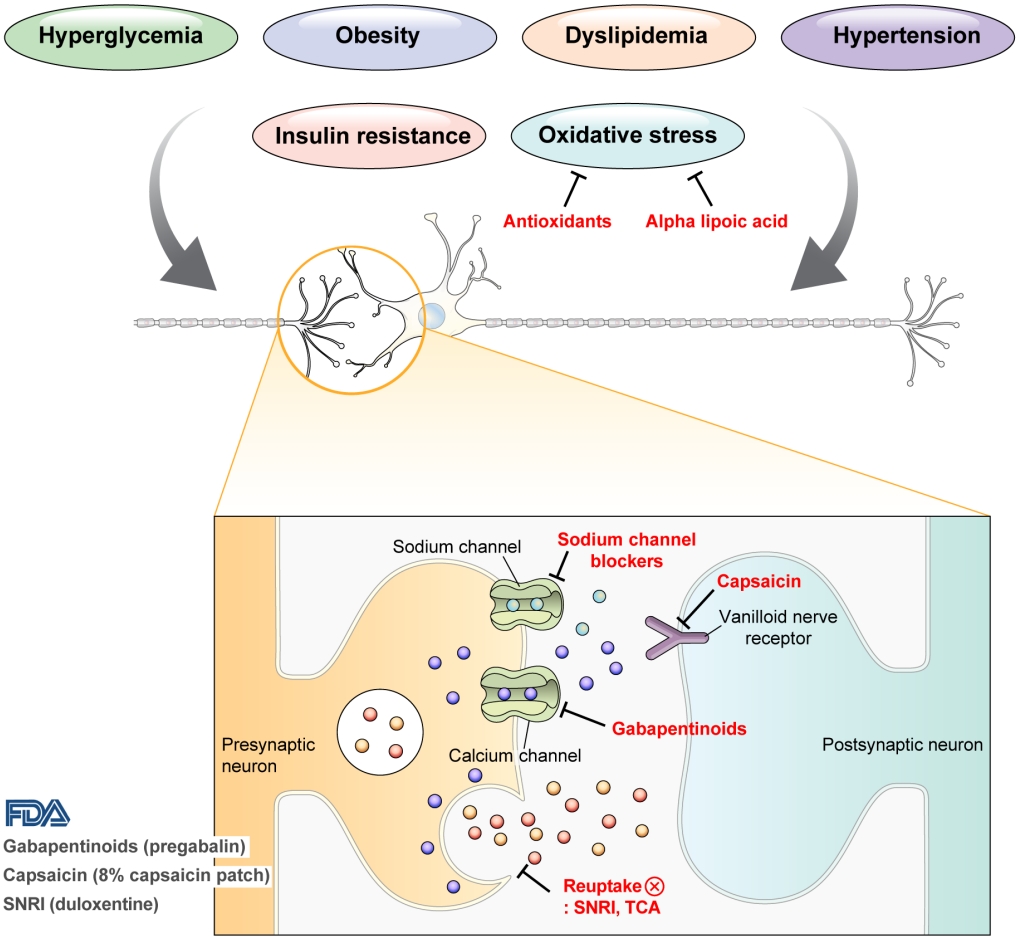
DPN is one of the microvascular complications of diabetes and shares pathophysiologic mechanisms of glucose-mediated vascular damage with other complications [5]. However, glycemic control per se cannot fully prevent or improve DPN, especially in patients with type 2 diabetes mellitus (T2DM) [6-8]. Metabolic abnormalities other than hyperglycemia such as obesity, dyslipidemia, and hypertension were found to be important risk factors of DPN (Fig. 1) [9,10]. For example, obesity and visceral fat accumulation showed positive association with DPN, and insulin resistance can be involved in this pathophysiology observed in T2DM [11]. More specifically, in the neurologic system, insulin resistance could lead to impaired glucose uptake by Schwann cell, which in turn can cause axonal energy deficit [12]. Furthermore, obesity can be a risk factor for peripheral neuropathy even in the absence of diabetes [13], and features of DPN can be observed in subjects with prediabetes [14]. However, it is unclear whether the pathophysiology and risk factors differ between painful and painless DPN. Female sex [2], obesity [3], and higher dermal nerve fiber regeneration [15] have been suggested as risk factors for painful DPN compared with painless DPN. From a treatment perspective, there is no promising disease-modifying medication for DPN. Therefore, the pain must be managed carefully in patients with painful DPN. In this review, we focus on the pharmacological and nonpharmacological treatments for painful DPN and ways to optimize their effects by reducing the risk of treatment-related complications.
RISK FACTOR MANAGEMENT
Intensive lifestyle intervention aiming to weight loss has been tested whether it was effective to mitigate metabolic risk factors for DPN. Look Action for Health in Diabetes (AHEAD) study showed a significant decrease in Michigan Neuropathy Screening Instrument (MNSI)-questionnaire score along with weight loss, but this study did not show a significant difference in MNSI-physical examination [16]. A database of primary care electronic records from United Kingdom showed that bariatric surgery decreased diabetes-related foot disease (adjusted hazard ratio of 0.61) [17], but we still need more data including standard methods to evaluate DPN. There are also some evidence that exercise could improve nerve function [18] and intraepidermal nerve fiber density [19]. In conclusion, multifactorial risk management of DPN might be beneficial to DPN, but large-scale intervention with DPN as a primary outcome are still necessary to test the efficacy of such interventions.
PHARMACOLOGICAL TREATMENTS
Pain arising from DPN is categorized as neuropathic pain that is a direct consequence of a lesion or disease in the somatosensory nervous system and may occur without tissue damage [20]. In this context, drugs for neuropathic pain may be useful for treating painful DPN (Table 1). The U.S. Food and Drug Administration (FDA) has approved pregabalin, duloxetine, and tapentadol for painful DPN [21] and has recently approved the 8% capsaicin patch (Fig. 1). According to Korea’s National Health Insurance Service-National Sample Cohort data, the proportion of pharmacological treatment in DPN ranged from 66.5% to 69.0%, of which monotherapy accounted for 91.6% and dual combination therapy accounted for 8.1%. In monotherapy, thioctic acid was most used, followed by anti-convulsive agents, tricyclic antidepressant (TCA). In combination therapy, the combination of anti-convulsive agent and TCA was most used, followed by the combination of TCA and thioctic acid [22]. In this section, we review both FDA approved and not approved but widely used drugs, and their combination treatment strategies for the management of painful DPN.
MONOTHERAPY
Gabapentinoids
Gabapentinoids reduce neuropathic pain by depressing dorsal horn sensitivity through multiple mechanisms. They inhibit neurotransmitter release by binding to the α2δ subunit of calcium voltagegated channels. They also inhibit movement of the α2δ subunit from dorsal root ganglion neurons to central terminals in the dorsal horn, which disrupts the reuse of the α2δ subunit from endosomal compartments. Gabapentinoids inhibit thrombospodin-mediated processes and stimulate the uptake of glutamate by excitatory amino acid transporters. In addition, the mode of action of gabapentinoids has been suggested to involve inhibition of descending serotonergic facilitation, stimulation of descending inhibition, anti-inflammatory actions, and influence on the affective component of pain [23].
Gabapentin was first approved by the FDA as an anticonvulsant in 1993. It has been reported to be effective in alleviating pain and sleep interference associated with DPN [24] and has been approved for the treatment of peripheral neuropathic pain such as postherpetic neuralgia and DPN in the United Kingdom and Australia; however, the USA has approved gabapentin only for the treatment of postherpetic neuralgia [24-26]. In a phase II study of 165 patients who experienced painful DPN for 1 to 5 years and had a pain score of ≥40 mm on the Short-Form McGill Pain Questionnaire visual analogue scale (VAS), the daily pain score at the study end point was significantly lower in patients treated with gabapentin than in those treated with placebo for 8 weeks; the scores decreased from 6.4 to 3.9 in the gabapentin-treated group and from 6.5 to 5.1 in the placebo group (P<0.001) [24]. In another study of 147 patients with painful DPN and an average daily pain (ADP) score ≥4, the ADP score decreased more in patients treated with gabapentin extended-release formula than in those treated with placebo (gabapentin, −2.76; placebo, −1.38; P=0.001) [26]. In a study that compared gabapentin with amitriptyline, gabapentin was more effective in relieving pain caused by DPN [27].
Pregabalin is a potent and selective ligand for the α2δ subunit of the voltage-gated calcium channel and has been approved for the treatment of painful DPN, postherpetic neuralgia, and spinal cord injury in the USA, Europe, and Canada [1,28]. In a multicenter study of 146 patients with painful DPN for 1 to 5 years and a pain score of ≥40 mm on the Short-Form McGill Pain Questionnaire or an ADP score of ≥4 on an 11-point pain numerical rating scale (NRS), pregabalin significantly reduced the mean pain score compared with placebo (pregabalin, from 6.5 to 4.0; placebo, from 6.1 to 5.3; P<0.0001) [29]. In a study of 338 patients with a 1 to 5-year history of DPN and an average weekly pain score of ≥4 on an 11-point NRS, pregabalin at 300 or 600 mg/day significantly reduced the mean pain score compared with placebo (difference from placebo: 300 mg/day, −1.26; 600 mg/day, −1.45; P=0.0001) [30]. In addition, pregabalin not only relieved pain associated with DPN and but also improved sleep interference and the profile of mood states scores in patients with DPN [29-31]. By contrast, some studies have reported that pregabalin is not more effective than placebo for improving DPN [32,33]. The possible explanations for the lack of effect of pregabalin on painful DPN include the use of low-dose pregabalin, a placebo response in people with painful DPN, or a carryover effect. However, in meta-analysis of studies of patients with painful DPN, pregabalin was more effective than placebo in improving neuropathic pain associated with DPN (change in least squares [LSs] mean change in mean pain score: –0.56 to –1.26) [34-36].
Mirogabalin is a novel selective oral ligand for the α2δ subunit and is known to have a slower dissociation rate from the α2δ subunit than pregabalin. Mirogabalin has been shown to improve painful DPN in phase II and III trials [37-39]. In a phase II study, mirogabalin and pregabalin were administered to 452 adults with painful DPN for more than 6 months, and the LS mean difference in the change in ADP score from the baseline was evaluated. The effects of pregabalin and placebo were similar after the 3-week treatment, but mirogabalin at a dose of 15, 20, or 30 mg/day was significantly better than placebo in inducing a change in ADP score (–0.94, –0.88, and –1.01, respectively) [37]. In a phase III study of 834 Asian patients, a mirogabalin dose of 15, 20, or 30 mg/day reduced the ADP score by –1.34, –1.47, and –1.81, respectively; this pattern suggests that mirogabalin relieved DPN pain in a dose-dependent manner [39].
The side effects reported for gabapentinoids include dizziness, somnolence, gastrointestinal complaints, sedation, ataxia, peripheral edema, headache, and postural hypotension, etc. [34,37,40]. Because of these side effects, caution is needed when prescribing this drug, especially in elderly patients. Furthermore, gabapentin and pregabalin are excreted primarily unchanged in the urine, renal dose adjustment is required [41]. The recommended maximal daily dose of gabapentin is 1,500 mg in people with grade 3 chronic kidney disease (CKD), 700 mg in those with grade 4 CKD, 300 mg in those with grade 5 CKD, and 100 to 300 mg after dialysis in dialysis patients. For pregabalin, the recommended maximal daily doses are 300, 150, and 75 mg for people with grade 3, grade 4, and grade 5 CKD, respectively, and 75 to 150 mg after dialysis for dialysis patients [42].
Serotonin–norepinephrine reuptake inhibitors
Neurotransmitters such as serotonin and norepinephrine are involved in the descending inhibitory nociceptive pathway. Serotonin–norepinephrine reuptake inhibitors (SNRIs) inhibit reuptake of serotonin and norepinephrine, which increases descending inhibition and reduces pain associated with DPN [43]. Among the available SNRIs, duloxetine is recommended for the initial symptomatic treatment of painful DPN, and venlafaxine is also used for the treatment of painful DPN [1]. In Korea, duloxetine is currently available, but venlafaxine is not.
Duloxetine, a potent SNRI, reduced neuropathic pain in an animal model and significantly improved painful physical symptoms associated with depression in a study of patients with major depressive disorder [44,45]. Duloxetine is therefore expected to be effective for treating painful DPN. In a study that compared the effects of duloxetine and placebo in 457 patients with a score of ≥3 on the MNSI, duloxetine at a dose of 60 or 120 mg/day improved pain from the first week of administration and was continuously effective throughout the study compared with placebo. The differences in ADP scores compared with placebo were −1.17 (95% confidence interval [CI], −1.84 to −0.50) for 60 mg/day and −1.45 (95% CI, −2.13 to −0.78) for 120 mg/day [46]. Meta-analysis of studies that compared duloxetine and placebo concluded that duloxetine is effective in improving painful DPN [47,48].
Another SNRI, venlafaxine, has also been shown to be effective in improving painful DPN by reducing VAS pain intensity by 50% after 6 weeks of administration in a study of 244 patients with painful DPN (P<0.001 vs. placebo) [49]. In studies that have compared the effects of venlafaxine and other drugs, venlafaxine was as effective as imipramine and carbamazepine for improving pain associated with DPN [50,51].
Nausea, somnolence, dizziness, constipation, and loss of appetite have been reported as common side effects of duloxetine and venlafaxine, but they are considered relatively safe drugs to use [46,49].
Tricyclic antidepressants
TCAs are thought to relieve pain by inhibiting the reuptake of noradrenaline and serotonin in presynaptic neurons and by antagonizing N-methyl-D-aspartate receptors, which mediate hyperalgesia and allodynia [52-54]. TCAs include tertiary amines such as imipramine and amitriptyline, and secondary amines such as desipramine and nortriptyline; secondary amines are thought to be better tolerated than tertiary amines [55].
A small (n=29) randomized, crossover study using “active” placebo, which mimics the side effects of amitriptyline, showed the superiority of amitriptyline in relieving pain associated with DPN compared with placebo [56]. In later, larger studies, amitriptyline was used as active comparator to gabapentin, pregabalin, and duloxetine, and was shown to have similar effects in relieving DPN pain as these other drugs [57-59].
Despite their promising effects and low cost, TCAs should be prescribed cautiously because of the side effects such as dry mouth, constipation, urinary retention, and orthostatic hypotension. TCAs are contraindicated in patients with glaucoma and cardiac conduction disturbances [55,60].
Alpha-lipoic acid
Increased oxidative stress caused by free radical formation and defects in antioxidant defense related to hyperglycemia is thought to cause endoneurial hypoxia and nerve dysfunction, which contribute to DPN [61]. Preclinical studies have shown that administration of the antioxidant alpha-lipoic acid reduces neurovascular abnormalities associated with DPN [62-65].
In a clinical study, intravenous alpha-lipoic acid and placebo were administered for 3 weeks to 328 patients with symptomatic DPN and intravenous alpha-lipoic acid induced significant improvement in pain associated with DPN compared with placebo. The total symptom score in the feet decreased by –4.5±3.7 (–58.6%) points in the group treated with alpha-lipoic acid at a dose of 1,200 mg (P=0.003) and by –5.0±4.1 (–63.5%) points at a dose of 600 mg (P<0.001) [66]. By contrast, a comparison of the effects of oral alpha-lipoic acid and placebo given for 7 months after intravenous alpha-lipoic acid administration for 3 weeks in 509 patients found no significant difference in total symptom score between alpha-lipoic acid and placebo [67]. High doses may be necessary to see the effects of alpha-lipoic acid on painful DPN, but it has not received FDA approval for DPN treatment. Nevertheless, alpha-lipoic acid is the most often prescribed agent as a monotherapy or as a combination therapy in Korea [22].
Sodium channel blockers
Carbamazepine, which is used in the treatment of simple and complex partial seizures of epilepsy and generalized convulsions, inhibits the secretion of neurotransmitters such as glutamine by blocking presynaptic voltage-sensitive sodium channels in the central nervous system (CNS) [68]. In a study that compared the effects of carbamazepine, venlafaxine, and pregabalin in 257 patients with clinically definite DPN, carbamazepine significantly decreased the VAS score (from 74.5 to 39.6, P=0.0001) and showed similar effects as venlafaxine and pregabalin in improving scores for sleep, mood, and work interference [51].
Oxcarbazepine, a derivative of carbamazepine that has better safety and tolerability, has also been reported to be effective in improving pain associated with DPN [69,70]. In a meta-analysis, the percentage of patients with DPN whose pain was “improved” or “very much improved” was 45.9% in those treated with oxcarbazepine and 30.1% in those treated with placebo. However, the quality of evidence to support the effectiveness of oxcarbazepine for treating DPN was very low and the serious adverse effects occurred more frequently in oxcarbazepine than placebo (8.3% vs. 2.5%) [71]. In this regard, oxcarbazepine should be prescribed carefully in patients with DPN.
Topical capsaicin
Capsaicin is an alkaloid extracted from red chili peppers and reduces painful stimuli to the CNS by removing substance P from vanilloid nerve receptors [72]. Therefore, capsaicin was expected to be effective for treating painful DPN. Topical capsaicin formulations in the form of 0.025% capsaicin gel or 0.075% capsaicin lotion were not found to be superior to placebo in relieving painful DPN [73,74]. However, in a study in which an 8% capsaicin patch or placebo patch was applied to painful areas of the feet for 30 minutes in 369 patients with painful DPN, the percentage reduction in ADP score from the baseline to weeks 2 to 8 was significantly higher for the 8% capsaicin patch than placebo (–27.4% vs. –20.9%, P=0.025) [75]. Accordingly, in 2020, the FDA approved the 8% capsaicin patch for the treatment of neuropathic pain associated with DPN of the feet in adults [76]. Application site reactions such as a burning sensation and application site pain have been reported as side effects of capsaicin patch [73-75]. In Korea, only the lower concentrated topical capsaicin is available.
Others
Antioxidant supplementations such as benfotiamine, a fat-soluble derivative of thiamine, and gamma linolenic acid, an omega-6 fatty acid, were used in some countries for DPN treatment. Even though these drugs have potential mechanism to prevent or treat DPN [77,78] and very low chance to induce side effects, but large-scale randomized controlled trials to show its efficacy on DPN are scarce. Aldose reductase inhibitor has been developed as a promising drug because it targeted a rate-limiting enzyme of polyol pathway. However, it could not address symptomatic improvement of DPN in clinical trials, but it only improved nerve conduction velocity [79,80]. Another pharmacological agent to be used for painful DPN is opioid drug such as tapentadol and tramadol. However, considering lack of longterm treatment data and side effects [81] this class of drug is hardly recommended for the treatment of DPN [82].
COMBINATION OR SEQUENTIAL THERAPY
Considering that pain relief of DPN is not sufficient in monotherapy because of the difficulty increasing the dose given the side effects of the drugs, combination or sequential therapy has been attempted.
One study compared the effects of high-dose duloxetine, pregabalin, and duloxetine with pregabalin combination therapy in 343 patients with a MNSI score ≥3 and who did not show improvement in pain with duloxetine or pregabalin monotherapy. The combination therapy relieved pain at rates similar to those for high-dose monotherapy as measured by the change in average pain on the Brief Pain Inventory Modified Short Form (mean change: combination, –2.35; high-dose monotherapy, –2.16; P=0.370) [83]. In studies that compared TCA monotherapy, gabapentinoid monotherapy, and their combination in patients with neuropathic pain including painful DPN, combination therapy was more effective than monotherapy [84,85]. Another study compared the effects of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin in 130 patients with painful DPN with a NRS score ≥4. The NRS score for all drug combination groups decreased from 6.6±1.5 to 3.3±1.8, and magnitude of the decrease in NRS did not differ between the drug combination groups. However, the magnitude of the decrease in NRS score was larger for the combination therapy than monotherapy [86].
NONPHARMACOLOGICAL TREATMENTS
Neurostimulation
Transcutaneous electrical nerve stimulation (TENS) is a noninvasive method of neuromodulation using cutaneous adhesive electrodes to apply pulsed electrical stimulation that stimulates A-beta fibers with the goal of indirectly inhibiting nociceptive transmission in the spinal cord dorsal horn [87-89]. TENS also contributes to pain relief by inducing the release of endogenous opioids [87,90]. Electrical stimulation applied in TENS can be modified in terms of frequency (stimulation rate), intensity and duration: Low-frequency TENS is defined as ≤10 Hz and often used at higher intensities inducing motor contraction, while high-frequency TENS is defined as ranging up to 50 or 100 Hz and above and used at lower intensities [91-93]. When low-frequency TENS was applied to 19 patients suffering from mild-to-moderate symptomatic DPN, the new total symptom score (NTSS-6) VAS decreased significantly compared to placebo [94]. When traditional TENS (80 Hz) or acupuncture-like TENS (2 Hz) was applied to five patients suffering from DPN, significant pain relief occurred in all five patients, but there was no placebo in this study [95]. In addition, in a study comparing micro-TENS (2 Hz) and placebo in 41 diabetic patients, micro-TENS did not show significant improvement in pain relief compared to placebo [90]. No specific adverse reactions were reported in patients treated with TENS in the studies [90,95].
Spinal cord stimulation (SCS) is a treatment for chronic pain that uses electrical stimulation of the dorsal columns of the spinal cord. SCS includes low-frequency SCS (frequency 10–100 Hz, pulse width 100–1,000 ms, and amplitude 1–10 mA) that applies paresthesia-based stimulation and high-frequency SCS (frequency 1–10 kHz, pulse width 30–150 ms, and amplitude 1–5 mA) that applies stimulation below the paresthesia threshold [96]. Although the mechanisms of action of SCS have not been clearly identified, it is thought to involve both spinal and supraspinal effects and has been shown to be effective for the treatment of various neuropathic pain such as failed back surgery syndrome and complex regional pain syndrome [97-100].
In a study that compared the effects of SCS and placebo for 6 months in 60 patients with DPN refractory to medical therapy, the VAS score decreased significantly only in the SCS group (Table 2) [101]. Similarly, in a study that analyzed the effect of SCS in 36 patients whose DPN did not improve with conventional therapy, the percentages of patients who achieved ≥50% pain relief were 59% in the SCS group and 7% in the best medical treatment group; these findings indicate that SCS was more effective for DPN pain relief [102]. In a study that analyzed the effect of high-frequency SCS on 216 patients with DPN refractory to medical therapy and a VAS score ≥5, the percentages of patients who achieved ≥50% pain relief were 79% in the SCS group and 5% in the conventional medical management group (P<0.001) [103]. The FDA approved SCS devices for the treatment of painful DPN in 2022.
The side effects reported for SCS include infection and wound dehiscence; one patient who complained of postdural puncture headache experienced a lethal subdural hematoma 3 days after the procedure [101-104].
CONCLUSIONS
Appropriate treatment of DPN is crucial because DPN impairs the quality of life and can adversely affect the employment of patients with diabetes. Drugs such as gabapentinoids, SNRIs, TCAs, alpha-lipoic acid, sodium channel blockers, and topical capsaicin are used for the treatment of DPN. However, these drugs are ineffective for some patients or can be difficult to use because of side effects. Generally, the pain relief and side effects appear early after treatment, and initial reevaluation is critical. These drugs should not be used to treat painless DPN because they do not provide benefits for these patients. The most beneficial therapeutic approach to treating uncontrolled painful DPN that does not respond to monotherapy is combination therapy, 8% capsaicin patch, or SCS. Given the lack of drugs that target the pathophysiology of DPN, new drugs are needed for the treatment of pain associated with DPN.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (No. NRF-2020R1C1C1013766).
Acknowledgements
None