- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 47(6); 2023 > Article
-
ReviewPathophysiology Immune-Checkpoint Inhibitors-Induced Type 1 Diabetes Mellitus: From Its Molecular Mechanisms to Clinical Practice
-
Yun Kyung Cho1,2
 , Chang Hee Jung1,2
, Chang Hee Jung1,2
-
Diabetes & Metabolism Journal 2023;47(6):757-766.
DOI: https://doi.org/10.4093/dmj.2023.0072
Published online: July 24, 2023
1Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
2Asan Diabetes Center, Asan Medical Center, Seoul, Korea
-
Corresponding author: Chang Hee Jung
 Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea E-mail: chjung0204@gmail.com
Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea E-mail: chjung0204@gmail.com
Copyright © 2023 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- With the increasing use of immune-checkpoint inhibitors (ICIs), such as anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and anti-programmed cell death-1 (PD-1), for the treatment of malignancies, cases of ICI-induced type 1 diabetes mellitus (ICI-T1DM) have been reported globally. This review focuses on the features and pathogenesis of this disease. T1DM is an immune-related adverse event that occurs following the administration of anti-PD-1 or anti-programmed death ligand-1 (PD-L1) alone or in combination with anti-CTLA-4. More than half of the reported cases presented as abrupt-onset diabetic ketoacidosis. The primary mechanism of ICI-T1DM is T-cell stimulation, which results from the loss of interaction between PD-1 and PD-L1 in pancreatic islet. The similarities and differences between ICI-T1DM and classical T1DM may provide insights into this disease entity. ICI-T1DM is a rare but often life-threatening medical emergency that healthcare professionals and patients need to be aware of. Early detection of and screening for this disease is imperative. At present, the only known treatment for ICI-T1DM is insulin injection. Further research into the mechanisms and risk factors associated with ICI-T1DM development may contribute to a better understanding of this disease entity and the identification of possible preventive strategies.
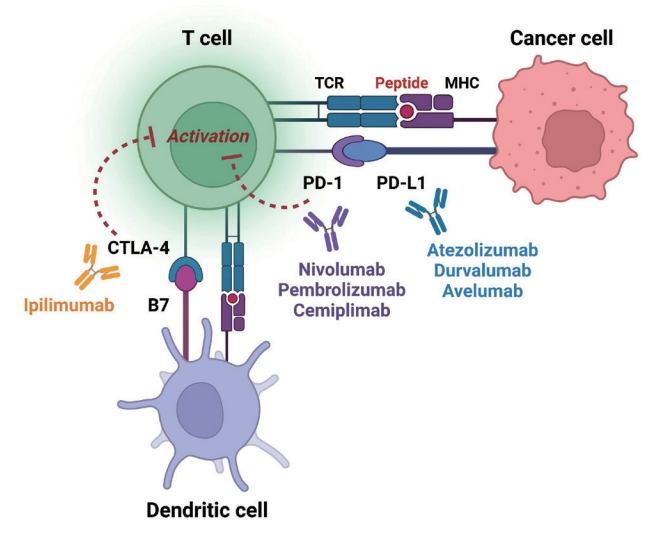
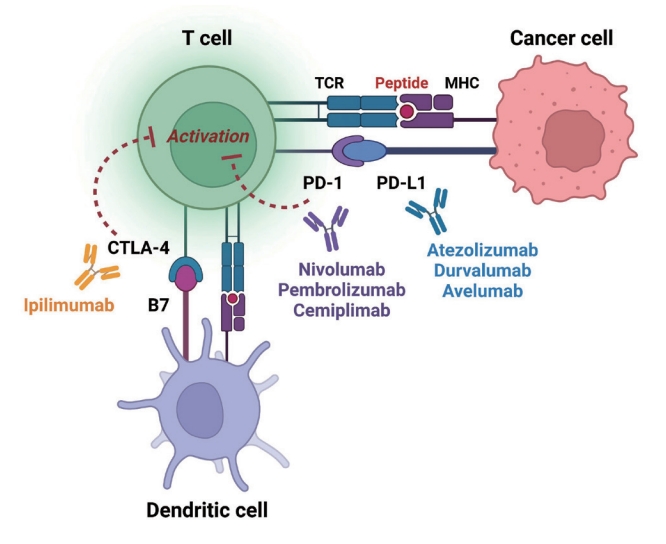
- Immunotherapy using immune-checkpoint inhibitors (ICIs) has improved cancer management and has become a cornerstone of cancer treatment over the past decade [1]. The successful use of ICIs has improved our understanding of the human immune system and cancer treatments. In 2011, ipilimumab, the first ICI targeting anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), was approved for use in cancer treatment [1]. Thereafter, several ICIs have been developed and authorized for use; the inhibitory immune checkpoints targeted by ICIs include CTLA-4, programmed cell death-1 (PD-1), and programmed death ligand-1 (PD-L1) [1,2]. In various clinical settings, ICIs are administered alone or in combination with other ICI classes or cytotoxic chemotherapy for 17 malignancies (Table 1) [2,3]. Recently, durable responses to these treatments have been reported, which led to their persistent use. Thus, characterization of the long-term physiological adverse effects of ICI treatment is important [4]. The mechanism of action of ICIs is mainly based on the inhibition of the physiological brake of immune activation; thus, they inevitably exert off-target effects that cause immune-related adverse events (irAEs) in various organs or tissues. This distinct entity of ICI-related adverse events prompts physicians and oncologists to provide immediate and appropriate treatment to patients developing irAEs due to the use of ICIs.
- Immune checkpoints are receptors expressed by immune cells that enable dynamic control of immune homeostasis [5]. The attachment of PD-1 to PD-L1 on activated T-cells causes T-cell exhaustion, which is characterized by diminished effector function (cytotoxicity or cytokine production) and weak immunological responses to stimuli [6,7]. Tumor cells use this relationship to induce immune tolerance [8]. CTLA-4 is increased on the surface of activated T-cells to avoid overstimulation of T-cell receptors [9,10]. CTLA-4 competes with CD28, a T-cell receptor co-stimulatory receptor, for the binding between B7-1 and B7-2, which suppresses CD28-mediated T-cell activation [8,11]. The oncogenic and immunosuppressive nature of the tumor microenvironment is characterized by PD-L1 overexpression in cancer cells as well as PD-1 and CTLA-4 overexpression in T-cells [12]. Collectively, the inhibition of these molecules results in an immune-mediated antitumor response (Fig. 1) [11].
- Immunological stimulation that exacerbates the severity of irAEs may be accompanied by an anticancer immune response. This specific ICI mechanism is supported by a small but reproducible positive connection between therapeutic responses and the incidence of irAEs [13-15]. Although the precise mechanisms underlying irAEs are unknown, they are assumed to be “bystander effects” of activated T-cells and are consistent with the mechanism of action of ICIs [13,16,17]. However, some non-antitumor pathways, such as those involving microbiota, viruses, or tissue-specific factors, are thought to cause irAEs [18-23]. Thus, “one-size-fits-all” mechanistic explanations become unlikely. The irAEs are most likely a result from factors that are or are not related to tumor. Chronic irAEs involving the endocrine system were the first to be reported, occurring in 15% to 40% of patients receiving ICIs [5]. Recently, ICI-induced type 1 diabetes mellitus (ICI-T1DM) has been proposed as a clinically significant and urgent irAE of ICIs. Herein, we aimed to review published articles on ICI-T1DM and experimental studies and discuss its mechanisms and pathogenesis as well as its distinct clinical characteristics.
INTRODUCTION
- Role of PD-1/PD-L1 in classical T1DM
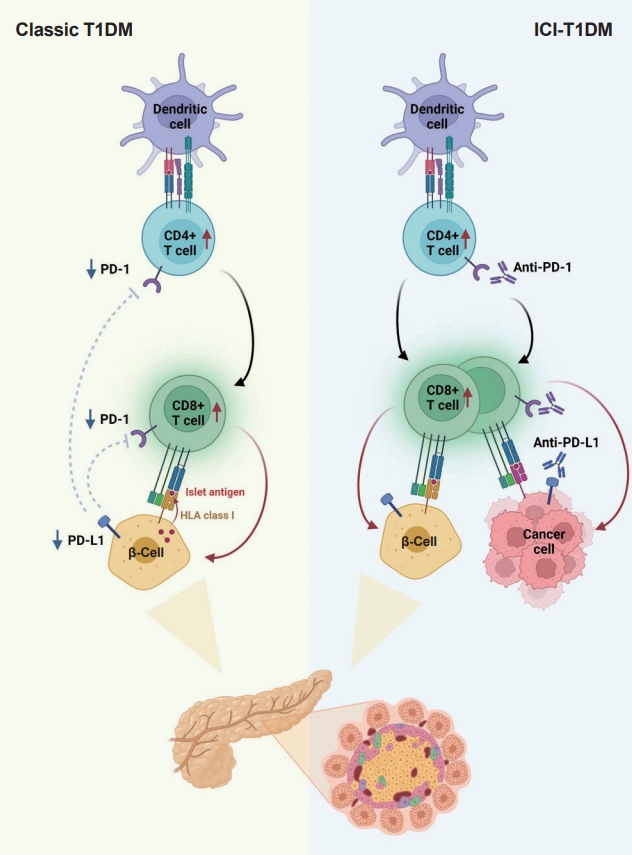
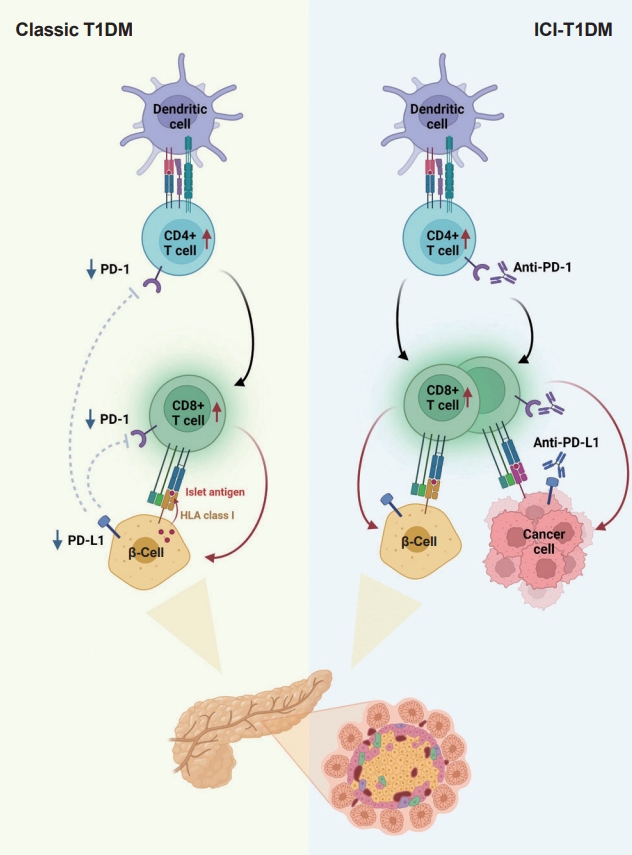
- T1DM is a chronic autoimmune disease triggered by both genetic and environmental factors, in which inappropriate hyperactivity in immune system causes destruction and dysfunction of insulin-producing β-cells [24,25]. Recently, teplizumab, a humanized anti-CD3 monoclonal antibody, was firstly approved for clinical use to delay the onset of T1DM, supporting the notion that T1DM is an immune disease and immunomodulation prior to clinical disease onset may be beneficial [26,27]. Human leukocyte antigen (HLA) antigens were first associated with insulin-dependent diabetes in 1973 [28], which established the relationship between the immune system and T1DM for the first time. Thereafter, genome-wide association studies have proven that HLA genes (particularly HLA class II loci) account for up to 50% of the genetic risk of T1DM, suggesting that the selective presentation of certain autoantigen peptides plays a role in T1DM pathogenesis [29-32]. Most T1DM cases are caused by immune-mediated death of pancreatic insulin-producing cells during an inflammatory process involving many immune cell types, including T lymphocytes [33]. Macrophages and dendritic cells enter the islets and migrate to the pancreatic lymph nodes, where they use antigen presentation to attract naive CD4+ T lymphocytes. These antigen-presenting cells (APCs) express β-cell antigens on major histocompatibility complex (MHC) class II molecules, causing CD4+ T lymphocytes to become activated. Th1 CD4+ T-cells interact with APCs, and the produced cytokines and free radicals drive CD8+ T-cell migration toward the islets. CD4+ and CD8+ T lymphocytes are the ultimate culprits of pancreatic β-cell death [34,35]. The immune system has been involved in the etiology of T1DM as immunotherapeutic therapies have demonstrated transient benefit in slowing down disease progression [36]. In other words, the T1DM response is analogous to effective anticancer immunity generated by immunotherapeutic suppression of PD-1 or its ligand, PD-L1, which otherwise controls autoimmune reactions. Other immunological and autoimmune responses, particularly those against pancreatic islets, may be generated by the use of ICIs in addition to anticancer immunity.
- The protein PD-L1 exists in a variety of hematological and parenchymal cells, including pancreatic islet cells [37]. Transgenic overexpression of PD-L1 in pancreatic β-cells protected mice from developing diabetes; the comparison of PD-L1 transgenic mice with littermate controls led to the findings of reduced degree of insulitis, delayed disease onset, and dramatically reduced T1DM incidence [38]. The frequency of PD-L1+ cells and pancreatic surface expression of PD-L1 rapidly increased in nonobese diabetic (NOD) mice as they aged and developed diabetes, and infiltration of T-cells was significantly associated with increased cellular PD-L1 expression [39]. This could be explained by the fact that elevated PD-L1 expression in pancreatic β-cells is a protective mechanism designed to prevent the formation of self-reactive T-cells [39].
- Experimental and clinical research has suggested that the alteration in PD-1/PD-L1 pathway is one of major pathogenesis in the development of autoimmune T1DM [34]. Ansari et al. [40] found that PD-1 or PD-L1 blocking caused diabetes in pre-diabetic NOD mice, whereas CTLA-4 inhibition caused diabetes only in neonates. Pauken et al. [41] demonstrated that in the absence of PD-1, CD4+ cells penetrated deeply into the islet core, which transformed peri-insulitis into damaging insulitis. This study suggested a concept wherein PD-1 regulates islet-reactive CD4+ T-cells in a cell-intrinsic manner by restricting proliferation and limiting pancreatic infiltration, and eventually prevents diabetes [41].
- Tsutsumi et al. [42] conducted the first clinical study demonstrating that people with T1DM exhibited low PD-1 expression levels on activated T-cells. The researchers observed that in T1DM patients, the PD-1 expression levels in the peripheral CD4+ T-cells were significantly lower than those of healthy controls, indicating that decreased PD-1 gene expression in CD4+ T-cells may be related to the development of classic T1DM [42]. Contrarily, despite previous reports of elevated blood CTLA-4 levels in autoimmune disorders, the serum CTLA-4 levels did not significantly differ between T1DM patients and non-diabetic teenagers [43]. Colli et al. [44] employed immunofluorescence to examine the expression of PD-L1 in T1DM donor samples and found that PD-L1 was present in both young and older individuals with T1DM; however, it was absent in insulin-deficient islets where β-cells had been killed. Interestingly, a Chinese cohort study found that the partial remission (or honeymoon) phase of T1DM is accompanied by restoration of the PD-1/PD-L1 expression in peripheral CD4+ and CD8+ T-cells. These data indicate that the PD-1/PD-L1 pathway could be a viable target for therapeutics aimed at extending this stage, highlighting the significance of this pathway in T1DM etiology [45]. As a result, it can be hypothesized that the decreased PD-1 expression of T-cells stimulates autoreactive T-cells that infiltrate pancreatic islet cells, leading to the development of T1DM [34,46].
- ICI-T1DM: proposed pathogenesis
- Consistent with the proposed association between PD-1/PD-L1 and T1DM, ICIs which aimed to enhance antitumor immunity by inhibiting PD-1/PD-L1 pathway could result in the unintentional adverse effect including T1DM, due to the loss of indispensible immune regulation [47,48]. In theory, PD-1 inhibition through PD-1 or PD-L1 pharmacological inhibition increases pancreatic β-cell infiltration and death through activated autoreactive T-cells [24]. A recent study demonstrated that activated autoreactive T-cells respond to PD-1 inhibition by releasing interferons (IFNs), which activate monocyte-derived macrophages [49]. These T-cells use nitric oxide to kill pancreatic β-cells, which leads to insulin deficiency and ICI-T1DM. An examination of a patient’s pancreatic pathology revealed an increase in CD8+ T-cells relative to CD4+ T-cells as well as the absence of macrophages [50]. Another study found evidence of pancreatic inflammation, including pancreatic shrinkage, increased pancreatic enzyme levels, and peri-islet lymphocytic infiltration, in a patient who died with ICI-T1DM [51]. In the same study, anti-PD-L1, but not anti-CTLA-4, caused rapid development of diabetes in the NOD mouse model [51]. When treated with anti-PD-L1, cytolytic IFN-γ+CD8+ T-cells infiltrated islets and IFN-γ and tumor necrosis factor α (TNF-α) induced transcriptional changes suggesting dedifferentiation in pancreatic β-cells [51]. In vitro, IFN increases checkpoint ligand expression and activates apoptotic pathways in human β-cells, and in NOD mice, treatment with anti-PD-L1, anti-IFN, and anti-TNF prevented ICI-T1DM [51].
- Clinically, T1DM does not develop in all patients receiving ICI treatment; only a very small proportion, approximately 1% of patients, develop ICI-T1DM. Additionally, the ICI-T1DM occurs several weeks to months after the initiation of ICI, suggesting that this immune-related type of diabetes may require sequential events. The events required for ICI-T1DM are not yet established, but the two-hit hypothesis, which involves an increase in PD-L1 expression in stressed β-cells and PD-L1/PD-1 blockade, is a possible explanation [52]. Notably, in NOD mice, PD-L1 expression in β-cells correlated with increased age and immune infiltrate in the islets, with diabetic mice displaying the highest PD-L1 levels [39,53]. Moreover, an adaptive immune response within the islets seems to be critical for the up-regulation of PD-L1 [39,53]. In the human pancreas, PD-L1 expression in islets was present in autoantibody-positive T1DM patients at greater levels than type 2 diabetes mellitus (T2DM) patients and healthy controls [39]. Thus, an increase in PD-L1 expression in the pancreas is likely indicative of an attempt to subdue an inflammatory response, which could be the first step in developing ICI-T1DM. Following the increase in PD-L1, particularly in certain patients, exposure to ICI may be the second and final trigger for the development of T1DM.
- In summary, ICIs that target the PD-1/PD-L1 pathway induce transcriptional changes in cells and immunological infiltrates, which may lead to the development of diabetes [51]. Other immunological and autoimmune responses, particularly those against pancreatic islets, may be generated by the use of ICIs in addition to anticancer immunity. Fig. 2 depicts the similarities and differences between classic T1DM and ICI-T1DM.
PATHOGENESIS OF ICI-T1DM
- Epidemiology of ICI-T1DM
- In the past decade, the number of reported ICI-T1DM cases has consistently and significantly increased [47,54-60]. Generally, the incidence rate of T1DM following ICI administration is expected to be 1% [59,61,62]. In detail, the incidence rates of T1DM caused by nivolumab (anti-PD-1), pembrolizumab (anti-PD-1), durvalumab (anti-PD-L1), and atezolizumab (anti-PD-L1) were reported to be 3.5%, 2.3%, 0.3%, and 0.7%, respectively [63]. Among ICIs, anti-PD-1 and anti-PD-L1 are the most possible causes of T1DM. Although anti-CTLA-4 antibody monotherapy is rarely associated with T1DM development, the combination of anti-CTLA-4 with those against PD-1 or PD-L1 increases the risk of ICI-T1DM [58].
- Clinical characteristics of ICI-T1DM from previous reports
- Based on previous reports, we summarized some clinical features of ICI-T1DM. The most common agent that causes ICI-T1DM is anti-PD-1, and rapid β-cell destruction and insulin deficiency occur as early as 5 days following ICI administration and up to several months following ICI discontinuation [59,64]. Median time to diagnosis of ICI-T1DM found to vary significantly across different studies, ranging from 7 to 25 weeks [52, 65,66]. The rapid onset of this disease causes fulminant presentation, including diabetic ketoacidosis (DKA), in 40% to 76% of the affected patients [47,59,64,65,67-69]. Majority of the patients report overt insulin insufficiency and low C-peptide levels (0.3 ng/mL in 63.4% of patients) [54]. Anti-glutamic acid decarboxylase autoantibodies were detected in 43% (positive total islet autoantibody was observed in 20% to 71% of patients) of patients and increased pancreatic enzyme in some [54]. In most cases, insulin deficiency is irreversible; thus, majority of the patients eventually need lifelong insulin therapy [47,59,67]. Limited information is available regarding ethnic disparities in the incidence of ICI-T1DM. A review published in 2019 including a total of 90 cases with ICI-T1DM reported that individuals of Asian ethnicity accounted for 15% of the cases [48]. A more recent cohort study which will be elaborated on in the following paragraph, revealed that race/ethnicity (categorized as White, Black, Asian, and Hispanic) did not significantly affect the risk of developing ICI-T1DM [58]. The patients’ clinical features are summarized in Table 2.
- Recently, a study on the incidence and characteristics of ICI-T1DM in a large de-identified cohort of patients was published in the United States [58]. The authors investigated patient/treatment factors related to the incidence of ICI-T1DM, which developed in 261 (0.86%) of 30,337 patients. The combined use of anti-CTLA-4 with anti-PD-1 or anti-PD-L1 (hazard ratio [HR], 1.62; 95% confidence interval [CI], 1.15 to 2.26), young age (HR, 1.19 for every 5-year drop; 95% CI, 1.13 to 1.25), and preexisting diabetes (HR, 4.48; 95% CI, 3.45 to 5.83) were related to an increased risk of ICI-T1DM [58]. Although the risk of ICI-T1DM is low (0.86%), over 30% of patients with ICI-T1DM present with major acute complications, such as DKA or pancreatitis. Therefore, clinicians should educate high-risk patients regarding the dangers of ICI-T1DM and symptoms of hyperglycemia, such as polydipsia and polyuria. Early detection of and intervention for ICI-T1DM, such as glucose level monitoring and insulin administration, are extremely important.
- A recent longitudinal trajectory analysis, which employed a tertiary care hospital database from Korea, indicated that ICI therapy is linked to a higher risk of developing incident diabetes than conventional chemotherapy, through a propensity matching analysis [70]. However, the study did not differentiate between T1DM and T2DM. In terms of ICI-T1DM, Hong et al. [71] reported four cases of ICI-T1DM, all of whom presented with ICI-induced DKA. Three cases were associated with the use of pembrolizumab, while one was caused by atezolizumab use [71]. Further studies with a sufficient number of participants should be conducted to estimate the risk of ICI-T1DM and identify predictive factors associated with this disease in Korea.
- Current treatment guidelines on ICI-T1DM
- The American Diabetes Association (ADA) guidelines for 2022 describe ICI-induced diabetes mellitus as a unique type of T1DM, an immune-mediated diabetes [72]. Immunotherapy, particularly ICIs, has resulted in unanticipated negative side effects, such as immune system activation, which led to the development of autoimmune diseases. T1DM can occur with DKA and low or undetectable C-peptide levels as markers of endogenous cellular activity [73,74]. Less than half of these patients have T1DM-associated autoantibodies, indicating a different pathobiology. This irAE occurs in less than 1% of patients receiving ICIs but is more common in those receiving medicines that block the PD-1/PD-L1 pathway, either alone or in combination with other ICIs [47]. Although the incidence of other high-risk HLA alleles is equivalent to that in the general population [47], 76% of patients are at a high-risk of the HLA-DR4 variant. To date, family history or autoantibodies cannot predict the risk; thus, all healthcare practitioners who administer these medications should be aware of this adverse effect and appropriately inform the patients. The American Association of Clinical Endocrinology mentions diabetes associated with ICIs as one of common forms of secondary diabetes; the guideline recommended that patients on ICI therapy should be monitored closely for early detection and therapy for hyperglycemia and the long-term development of diabetes [75]. A Consensus Report by the ADA and the European Association for the Study of Diabetes also briefly address that the development of profound insulin deficiency associated with the use of ICIs is an emerging issue in the management of T1DM in adults [76]. However, a universal consensus for this specific type of illness has not yet been reached. Additional epidemiological facts and evidence will provide a context for this distinct type of T1DM.
- The treatment for ICI-T1DM is not different from that of classical T1DM; most patients require multiple insulin injections. However, the optimal glycemic target could be different from that of classical T1DM because ICI-T1DM patients have cancer and decreased life expectancy. Although there are no specific guidelines for ICI-T1DM, insulin therapy is necessary in ICI-T1DM patients, and clinicians should treat them as patients with T1DM and cancer, considering the risks and benefits of the treatment.
EPIDEMIOLOGY, CLINICAL CHARACTERISTICS, AND CURRENT GUIDELINES OF ICI-T1DM
- The use of ICIs that target PD-1/PD-L1 and CTLA-4 has improved cancer management; however, these medications can also cause autoimmune issues, such as ICI-T1DM, which occurs predominantly with PD-1 suppression. Patients with cancer who receive ICIs (anti-PD-1, anti-PD-L1, or anti-CTLA-4 antibodies) for the reduction of immune regulation and initiation of an immune response against tumor tissue are at risk of developing adverse effects, including acute T1DM, due to immune regulation loss combined with the activation of naive autoreactive T-cells. Thus, patients receiving ICIs, particularly PD-1/PD-L1 inhibitors, are advised to regularly monitor their glucose levels and check for early signs and symptoms of diabetes.
CONCLUSIONS
-
CONFLICTS OF INTEREST
Chang Hee Jung has been associate editor of the Diabetes & Metabolism Journal since 2022. He was not involved in the review process of this article. Otherwise, there was no conflict of interest.
-
FUNDING
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (grant numbers: NRF- 2020R1A2C1101977: Chang Hee Jung).
NOTES
-
Acknowledgements
- None
CTLA-4, cytotoxic T lymphocyte-associated antigen 4; CRC, colorectal cancer; HCC, hepatocellular carcinoma; NSCLC, non-small cell lung cancer; RCC, renal cell carcinoma; PD-1, programmed cell death-1; BCC, basal cell carcinoma; CSCC, cutaneous squamous cell carcinoma; SCC, squamous cell carcinoma; HL, Hodgkin lymphoma; HNSCC, head and neck squamous cell carcinoma; BC, breast cancer; MCC, Merkel cell carcinoma; MSI, microsatellite instability; MMR, mismatch repair; TMB, tumor mutational burden; SCLC, small cell lung cancer; PD-L1, programmed death ligand-1.
- 1. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun 2020;11:3801.ArticlePubMedPMCPDF
- 2. Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open 2019;2:e192535.ArticlePubMedPMC
- 3. Vilgelm AE, Johnson DB, Richmond A. Combinatorial approach to cancer immunotherapy: strength in numbers. J Leukoc Biol 2016;100:275-90.ArticlePubMedPMCPDF
- 4. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol 2019;5:1411-20.ArticlePubMedPMC
- 5. Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol 2022;19:254-67.ArticlePubMedPMCPDF
- 6. Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, et al. Defining ‘T cell exhaustion’. Nat Rev Immunol 2019;19:665-74.ArticlePubMedPMCPDF
- 7. Philip M, Fairchild L, Sun L, Horste EL, Camara S, Shakiba M, et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 2017;545:452-6.ArticlePubMedPMCPDF
- 8. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 2018;8:1069-86.ArticlePubMedPDF
- 9. Barton BM, Xu R, Wherry EJ, Porrett PM. Pregnancy promotes tolerance to future offspring by programming selective dysfunction in long-lived maternal T cells. J Leukoc Biol 2017;101:975-87.ArticlePubMedPDF
- 10. Shim YJ, Khedraki R, Dhar J, Fan R, Dvorina N, Valujskikh A, et al. Early T cell infiltration is modulated by programed cell death-1 protein and its ligand (PD-1/PD-L1) interactions in murine kidney transplants. Kidney Int 2020;98:897-905.ArticlePubMedPMC
- 11. Marin-Acevedo JA, Kimbrough EO, Lou Y. Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol 2021;14:45.ArticlePubMedPMCPDF
- 12. Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol 2018;8:86.ArticlePubMedPMC
- 13. Das S, Johnson DB. Immune-related adverse events and antitumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 2019;7:306.ArticlePubMedPMCPDF
- 14. Quach HT, Dewan AK, Davis EJ, Ancell KK, Fan R, Ye F, et al. Association of anti-programmed cell death 1 cutaneous toxic effects with outcomes in patients with advanced melanoma. JAMA Oncol 2019;5:906-8.ArticlePubMedPMC
- 15. Eggermont AM, Kicinski M, Blank CU, Mandala M, Long GV, Atkinson V, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol 2020;6:519-27.ArticlePubMedPMC
- 16. Yoest JM. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: a short review. Immunotargets Ther 2017;6:73-82.ArticlePubMedPMCPDF
- 17. Passat T, Touchefeu Y, Gervois N, Jarry A, Bossard C, Bennouna J. Physiopathological mechanisms of immune-related adverse events induced by anti-CTLA-4, anti-PD-1 and anti-PD-L1 antibodies in cancer treatment. Bull Cancer 2018;105:1033-41.PubMed
- 18. Johnson DB, McDonnell WJ, Gonzalez-Ericsson PI, Al-Rohil RN, Mobley BC, Salem JE, et al. A case report of clonal EBV-like memory CD4+ T cell activation in fatal checkpoint inhibitor-induced encephalitis. Nat Med 2019;25:1243-50.ArticlePubMedPMCPDF
- 19. Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun 2016;7:10391.ArticlePubMedPMCPDF
- 20. Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med 2014 6:230.ra45.Article
- 21. Wei SC, Meijers WC, Axelrod ML, Anang NA, Screever EM, Wescott EC, et al. A genetic mouse model recapitulates immune checkpoint inhibitor-associated myocarditis and supports a mechanism-based therapeutic intervention. Cancer Discov 2021;11:614-25.ArticlePubMedPMCPDF
- 22. Luoma AM, Suo S, Williams HL, Sharova T, Sullivan K, Manos M, et al. Molecular pathways of colon inflammation induced by cancer immunotherapy. Cell 2020;182:655-71.ArticlePubMedPMC
- 23. Andrews MC, Duong CP, Gopalakrishnan V, Iebba V, Chen WS, Derosa L, et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat Med 2021;27:1432-41.ArticlePubMedPDF
- 24. Roep BO, Thomaidou S, van Tienhoven R, Zaldumbide A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat Rev Endocrinol 2021;17:150-61.ArticlePubMedPMCPDF
- 25. Nguyen C, Varney MD, Harrison LC, Morahan G. Definition of high-risk type 1 diabetes HLA-DR and HLA-DQ types using only three single nucleotide polymorphisms. Diabetes 2013;62:2135-40.ArticlePubMedPMCPDF
- 26. Herold KC, Bundy BN, Long SA, Bluestone JA, DiMeglio LA, Dufort MJ, et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 2019;381:603-13.ArticlePubMedPMC
- 27. Carvalho T. FDA approves first drug to delay type 1 diabetes. Nat Med 2023;29:280.ArticlePubMedPDF
- 28. Nerup J, Platz P, Andersen OO, Christy M, Lyngsoe J, Poulsen JE, et al. HL-A antigens and diabetes mellitus. Lancet 1974;2:864-6.ArticlePubMed
- 29. Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 2009;41:703-7.ArticlePubMedPMCPDF
- 30. van Lummel M, van Veelen PA, de Ru AH, Janssen GM, Pool J, Laban S, et al. Dendritic cells guide islet autoimmunity through a restricted and uniquely processed peptidome presented by high-risk HLA-DR. J Immunol 2016;196:3253-63.ArticlePubMedPDF
- 31. van Lummel M, van Veelen PA, de Ru AH, Pool J, Nikolic T, Laban S, et al. Discovery of a selective islet peptidome presented by the highest-risk HLA-DQ8trans molecule. Diabetes 2016;65:732-41.ArticlePubMedPDF
- 32. Kim KY, Kim MS, Lee YJ, Lee YA, Lee SY, Shin CH, et al. Glutamic acid decarboxylase and tyrosine phosphatase-related islet antigen-2 positivity among children and adolescents with diabetes in Korea. Diabetes Metab J 2022;46:948-52.ArticlePubMedPMCPDF
- 33. Vella A, Cooper JD, Lowe CE, Walker N, Nutland S, Widmer B, et al. Localization of a type 1 diabetes locus in the IL2RA/CD25 region by use of tag single-nucleotide polymorphisms. Am J Hum Genet 2005;76:773-9.ArticlePubMedPMC
- 34. Mourad D, Azar NS, Eid AA, Azar ST. Immune checkpoint inhibitor-induced diabetes mellitus: potential role of T cells in the underlying mechanism. Int J Mol Sci 2021;22:2093.ArticlePubMedPMC
- 35. Won TJ, Jung YJ, Kwon SJ, Lee YJ, Lee DI, Min H, et al. Forced expression of programmed death-1 gene on T cell decreased the incidence of type 1 diabetes. Arch Pharm Res 2010;33:1825-33.ArticlePubMedPDF
- 36. Roep BO, Wheeler DC, Peakman M. Antigen-based immune modulation therapy for type 1 diabetes: the era of precision medicine. Lancet Diabetes Endocrinol 2019;7:65-74.ArticlePubMed
- 37. Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol 2006;27:195-201.ArticlePubMed
- 38. Wang CJ, Chou FC, Chu CH, Wu JC, Lin SH, Chang DM, et al. Protective role of programmed death 1 ligand 1 (PD-L1) in nonobese diabetic mice: the paradox in transgenic models. Diabetes 2008;57:1861-9.PubMedPMC
- 39. Osum KC, Burrack AL, Martinov T, Sahli NL, Mitchell JS, Tucker CG, et al. Interferon-gamma drives programmed death-ligand 1 expression on islet β cells to limit T cell function during autoimmune diabetes. Sci Rep 2018;8:8295.ArticlePubMedPMCPDF
- 40. Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med 2003;198:63-9.ArticlePubMedPMCPDF
- 41. Pauken KE, Jenkins MK, Azuma M, Fife BT. PD-1, but not PD-L1, expressed by islet-reactive CD4+ T cells suppresses infiltration of the pancreas during type 1 diabetes. Diabetes 2013;62:2859-69.ArticlePubMedPMCPDF
- 42. Tsutsumi Y, Jie X, Ihara K, Nomura A, Kanemitsu S, Takada H, et al. Phenotypic and genetic analyses of T-cell-mediated immunoregulation in patients with type 1 diabetes. Diabet Med 2006;23:1145-50.ArticlePubMed
- 43. Al-Hakami A. Serum sCTLA-4 level is not associated with type 1 diabetes or the coexistence of autoantibodies in children and adolescent patients from the southern region of Saudi Arabia. Auto Immun Highlights 2020;11:19.ArticlePubMedPMCPDF
- 44. Colli ML, Hill JL, Marroqui L, Chaffey J, Dos Santos RS, Leete P, et al. PD-L1 is expressed in the islets of people with type 1 diabetes and is up-regulated by interferons-α and-γ via IRF1 induction. EBioMedicine 2018;36:367-75.ArticlePubMedPMC
- 45. Li X, Zhong T, Tang R, Wu C, Xie Y, Liu F, et al. PD-1 and PD-L1 expression in peripheral CD4/CD8+ T cells is restored in the partial remission phase in type 1 diabetes. J Clin Endocrinol Metab 2020;105:dgaa130.ArticlePubMedPDF
- 46. Kapke J, Shaheen Z, Kilari D, Knudson P, Wong S. Immune checkpoint inhibitor-associated type 1 diabetes mellitus: case series, review of the literature, and optimal management. Case Rep Oncol 2017;10:897-909.ArticlePubMedPMCPDF
- 47. Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes 2018;67:1471-80.ArticlePubMedPMCPDF
- 48. de Filette JM, Pen JJ, Decoster L, Vissers T, Bravenboer B, Van der Auwera BJ, et al. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur J Endocrinol 2019;181:363-74.ArticlePubMedPMC
- 49. Hu H, Zakharov PN, Peterson OJ, Unanue ER. Cytocidal macrophages in symbiosis with CD4 and CD8 T cells cause acute diabetes following checkpoint blockade of PD-1 in NOD mice. Proc Natl Acad Sci U S A 2020;117:31319-30.ArticlePubMedPMC
- 50. Yoneda S, Imagawa A, Hosokawa Y, Baden MY, Kimura T, Uno S, et al. T-lymphocyte infiltration to islets in the pancreas of a patient who developed type 1 diabetes after administration of immune checkpoint inhibitors. Diabetes Care 2019;42:e116-8.ArticlePubMedPDF
- 51. Perdigoto AL, Deng S, Du KC, Kuchroo M, Burkhardt DB, Tong A, et al. Immune cells and their inflammatory mediators modify β cells and cause checkpoint inhibitor-induced diabetes. JCI Insight 2022;7:e156330.ArticlePubMedPMC
- 52. Quandt Z, Young A, Anderson M. Immune checkpoint inhibitor diabetes mellitus: a novel form of autoimmune diabetes. Clin Exp Immunol 2020;200:131-40.ArticlePubMedPMCPDF
- 53. Rui J, Deng S, Arazi A, Perdigoto AL, Liu Z, Herold KC. β Cells that resist immunological attack develop during progression of autoimmune diabetes in NOD mice. Cell Metab 2017;25:727-38.ArticlePubMedPMC
- 54. Tachibana M, Imagawa A. Type 1 diabetes related to immune checkpoint inhibitors. Best Pract Res Clin Endocrinol Metab 2022;36:101657.ArticlePubMed
- 55. Baden MY, Imagawa A, Abiru N, Awata T, Ikegami H, Uchigata Y, et al. Characteristics and clinical course of type 1 diabetes mellitus related to anti-programmed cell death-1 therapy. Diabetol Int 2018;10:58-66.ArticlePubMedPMCPDF
- 56. Lo Preiato V, Salvagni S, Ricci C, Ardizzoni A, Pagotto U, Pelusi C. Diabetes mellitus induced by immune checkpoint inhibitors: type 1 diabetes variant or new clinical entity?: review of the literature. Rev Endocr Metab Disord 2021;22:337-49.ArticlePubMedPDF
- 57. Kotwal A, Haddox C, Block M, Kudva YC. Immune checkpoint inhibitors: an emerging cause of insulin-dependent diabetes. BMJ Open Diabetes Res Care 2019;7:e000591.ArticlePubMedPMC
- 58. Chen X, Affinati AH, Lee Y, Turcu AF, Henry NL, Schiopu E, et al. Immune checkpoint inhibitors and risk of type 1 diabetes. Diabetes Care 2022;45:1170-6.ArticlePubMedPMCPDF
- 59. Akturk HK, Kahramangil D, Sarwal A, Hoffecker L, Murad MH, Michels AW. Immune checkpoint inhibitor-induced type 1 diabetes: a systematic review and meta-analysis. Diabet Med 2019;36:1075-81.ArticlePubMedPMCPDF
- 60. Zezza M, Kosinski C, Mekoguem C, Marino L, Chtioui H, Pitteloud N, et al. Combined immune checkpoint inhibitor therapy with nivolumab and ipilimumab causing acute-onset type 1 diabetes mellitus following a single administration: two case reports. BMC Endocr Disord 2019;19:144.ArticlePubMedPMCPDF
- 61. Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol 2019;5:1008-19.ArticlePubMedPMC
- 62. Marmura H, Tremblay PF, Getgood AM, Bryant DM. The knee injury and osteoarthritis outcome score does not have adequate structural validity for use with young, active patients with ACL tears. Clin Orthop Relat Res 2022;480:1342-50.ArticlePubMedPMC
- 63. Takada S, Hirokazu H, Yamagishi K, Hideki S, Masayuki E. Predictors of the onset of type 1 diabetes obtained from real-world data analysis in cancer patients treated with immune checkpoint inhibitors. Asian Pac J Cancer Prev 2020;21:1697-9.ArticlePubMedPMC
- 64. Wright JJ, Salem JE, Johnson DB, Lebrun-Vignes B, Stamatouli A, Thomas JW, et al. Increased reporting of immune checkpoint inhibitor-associated diabetes. Diabetes Care 2018;41:e150-1.ArticlePubMedPMCPDF
- 65. Liu J, Shi Y, Liu X, Zhang D, Zhang H, Chen M, et al. Clinical characteristics and outcomes of immune checkpoint inhibitor-induced diabetes mellitus. Transl Oncol 2022;24:101473.ArticlePubMedPMC
- 66. Qiu J, Luo S, Yin W, Guo K, Xiang Y, Li X, et al. Characterization of immune checkpoint inhibitor-associated fulminant type 1 diabetes associated with autoantibody status and ethnic origin. Front Immunol 2022;13:968798.ArticlePubMedPMC
- 67. Dougan M, Pietropaolo M. Time to dissect the autoimmune etiology of cancer antibody immunotherapy. J Clin Invest 2020;130:51-61.ArticlePubMedPMC
- 68. Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. Osaka IDDM Study Group. N Engl J Med 2000;342:301-7.ArticlePubMed
- 69. Shiba M, Inaba H, Ariyasu H, Kawai S, Inagaki Y, Matsuno S, et al. Fulminant type 1 diabetes mellitus accompanied by positive conversion of anti-insulin antibody after the administration of anti-CTLA-4 antibody following the discontinuation of anti-PD-1 antibody. Intern Med 2018;57:2029-34.ArticlePubMedPMC
- 70. Lee M, Jeong K, Park YR, Rhee Y. Increased risk of incident diabetes after therapy with immune checkpoint inhibitor compared with conventional chemotherapy: a longitudinal trajectory analysis using a tertiary care hospital database. Metabolism 2023;138:155311.ArticlePubMed
- 71. Hong AR, Yoon JH, Kim HK, Kang HC. Immune checkpoint inhibitor-induced diabetic ketoacidosis: a report of four cases and literature review. Front Endocrinol (Lausanne) 2020;11:14.ArticlePubMedPMC
- 72. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care 2023;46(Suppl 1):S19-40.
- 73. Smith CJ, Almodallal Y, Jatoi A. Rare adverse events with programmed death-1 and programmed death-1 ligand inhibitors: justification and rationale for a systematic review. Curr Oncol Rep 2021;23:86.PubMed
- 74. Zhao Z, Wang X, Bao XQ, Ning J, Shang M, Zhang D. Autoimmune polyendocrine syndrome induced by immune checkpoint inhibitors: a systematic review. Cancer Immunol Immunother 2021;70:1527-40.ArticlePubMedPDF
- 75. Blonde L, Umpierrez GE, Reddy SS, McGill JB, Berga SL, Bush M, et al. American Association of Clinical Endocrinology clinical practice guideline: developing a diabetes mellitus comprehensive care plan-2022 update. Endocr Pract 2022;28:923-1049.ArticlePubMedPMC
- 76. Holt RI, DeVries JH, Hess-Fischl A, Hirsch IB, Kirkman MS, Klupa T, et al. The management of type 1 diabetes in adults: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2021;64:2609-52.ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations

- Research Advances of Immune Checkpoint Inhibitors Related Endocrine Adverse Events
晶晶 王
Advances in Clinical Medicine.2024; 14(02): 2706. CrossRef - Immune checkpoint inhibitor‑associated diabetes mellitus in patients with HCC: Report of three cases and literature review
Gaocheng Wang, Jingjing Wang, Shuilin Dong, Zhanguo Zhang, Wanguang Zhang, Jianping Zhao
Experimental and Therapeutic Medicine.2024;[Epub] CrossRef

 KDA
KDA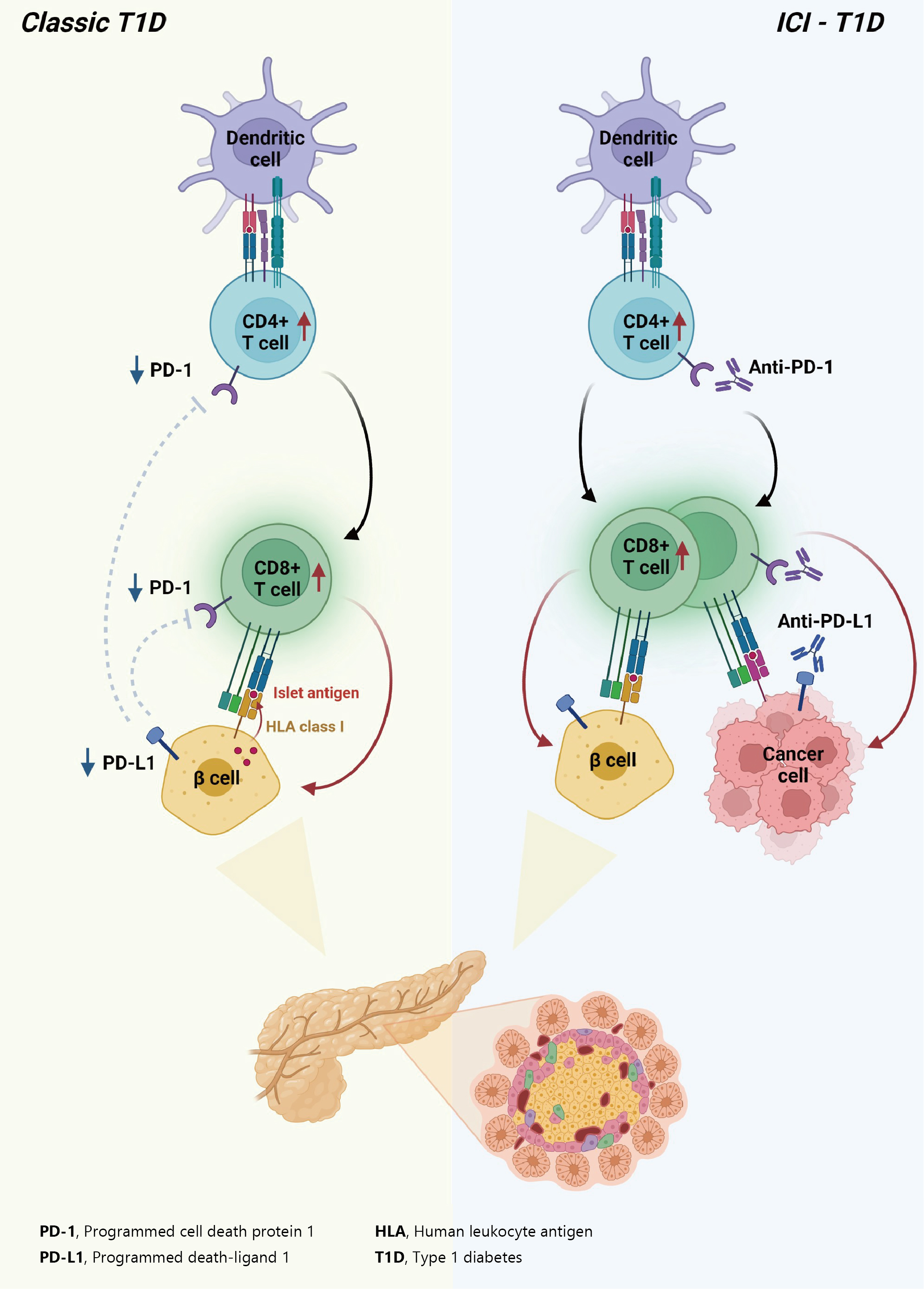
 PubReader
PubReader ePub Link
ePub Link Cite
Cite