- Skip Navigation
- Skip to contents

- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 47(2); 2023 > Article
-
ReviewBasic Research Multiple Roles of Sirtuin 6 in Adipose Tissue Inflammation
-
Eun Ju Bae1
 , Byung-Hyun Park2
, Byung-Hyun Park2
-
Diabetes & Metabolism Journal 2023;47(2):164-172.
DOI: https://doi.org/10.4093/dmj.2022.0270
Published online: January 12, 2023
1School of Pharmacy, Chonbuk National University, Jeonju, Korea
2Department of Biochemistry and Research Institute for Endocrine Sciences, Chonbuk National University Medical School, Jeonju, Korea
-
Corresponding authors: Eun Ju Bae
 School of Pharmacy, Chonbuk National University, 20 Geonji-ro, Deokjin-gu, Jeonju 54907, Korea E-mail: ejbae7@jbnu.ac.kr
School of Pharmacy, Chonbuk National University, 20 Geonji-ro, Deokjin-gu, Jeonju 54907, Korea E-mail: ejbae7@jbnu.ac.kr -
Byung-Hyun Park
 Department of Biochemistry and Research Institute for Endocrine Sciences, Chonbuk National University Medical School, 20 Geonji-ro, Deokjin-gu, Jeonju 54907, Korea E-mail: bhpark@jbnu.ac.kr
Department of Biochemistry and Research Institute for Endocrine Sciences, Chonbuk National University Medical School, 20 Geonji-ro, Deokjin-gu, Jeonju 54907, Korea E-mail: bhpark@jbnu.ac.kr
Copyright © 2023 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Adipose tissue (AT) inflammation is strongly associated with obesity-induced insulin resistance. When subjected to metabolic stress, adipocytes become inflamed and secrete a plethora of cytokines and chemokines, which recruit circulating immune cells to AT. Although sirtuin 6 (Sirt6) is known to control genomic stabilization, aging, and cellular metabolism, it is now understood to also play a pivotal role in the regulation of AT inflammation. Sirt6 protein levels are reduced in the AT of obese humans and animals and increased by weight loss. In this review, we summarize the potential mechanism of AT inflammation caused by impaired action of Sirt6 from the immune cells’ point of view. We first describe the properties and functions of immune cells in obese AT, with an emphasis on discrete macrophage subpopulations which are central to AT inflammation. We then highlight data that links Sirt6 to functional phenotypes of AT inflammation. Importantly, we discuss in detail the effects of Sirt6 deficiency in adipocytes, macrophages, and eosinophils on insulin resistance or AT browning. In our closing perspectives, we discuss emerging issues in this field that require further investigation.
- Keywords: Adipose tissue; Eosinophils; Inflammation; Macrophages
- Insulin resistance is the common underlying feature of obesity-related metabolic diseases. A number of mechanisms linking obesity to insulin resistance have been suggested and comprehensively reviewed elsewhere [1-5], and the main cause of insulin resistance is now considered to be chronic low-grade inflammation in obese adipose tissue (AT) [6]. Studies have demonstrated that individuals who are obese and insulin-resistant exhibit elevated levels of proinflammatory cytokines such as tumor necrosis factor β (TNFβ), interleukin 1β (IL-1β), and IL-6 [7]. Proinflammatory cytokines can interfere with insulin signaling via multiple mechanisms, such as serine phosphorylation of insulin receptor substrate 1 (IRS-1) [8], reduction of cytokine signaling 3 (SOCS-3) expression [9], or decrease in transcriptional activity of peroxisome proliferator-activated receptor γ (PPARγ) [10]. However, it is not clear whether AT inflammation is a cause or consequence of insulin resistance [11]. Using an adipocyte-specific insulin-resistant mouse model (mTORC2 knockout mice), Shimobayashi et al. [12] found that insulin resistance in adipocytes precedes and causes AT inflammation by recruiting monocytes and proinflammatory macrophages.
- In obese AT, adipocytes undergo hypertrophy and secrete a number of proinflammatory cytokines and chemokines, which activate and attract immune cells. The composition of immune cells that constitute the stromal vascular cell (SVC) fraction of ATs dramatically changes as adiposity increases [13]. In lean AT, eosinophils, type 2 innate lymphoid cells, T helper 2 (Th2) cells, and regulatory T cells are the predominant types of cells [14]. As obesity progresses, the loss of the aforementioned cells is coupled with the infiltration of macrophages, neutrophils, mast cells, T helper 1 cells, and natural killer (NK) cells into AT [14,15]. Adipose tissue macrophages (ATMs) are the most common immune cells in AT. ATMs are conventionally classified as CD11b+ CD11c+ M1 (classically activated) or CD11b+ CD11c− M2 (alternatively activated) macrophages. Classically activated macrophages express proinflammatory genes (e.g., TNFα and inducible nitric oxide synthase) and alternatively activated macrophages express anti-inflammatory cytokines (e.g., IL-10 and transforming growth factor β) [16]. The number and subtypes of ATMs greatly differ depending on the degree of obesity. For example, ATMs comprise less than 10% of all AT cells in lean mice; however, this percentage increases to 50% in extremely obese mice [17]. M2-type macrophages are found in abundance in lean AT while M1-type macrophages are predominant in obese AT. It is now generally accepted that ATMs shift from the M2-to-M1 state during the development of obesity, thereby contributing to chronic low-grade inflammation and insulin resistance [18]. Diverse types of ATMs are also observed between visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) or between brown adipose tissue (BAT) and white adipose tissue (WAT) [17,18].
- Aging has a similar pathophysiology to obesity, and is also associated with the development of chronic low-grade inflammation within AT [19]. There is strong clinical evidence that the risk of obesity increases with age [20,21]. Thus, obesity is linked not only to aging-associated metabolic diseases but also to aging itself [22,23]. However, the molecular mechanism by which obesity accelerates the aging process, or vice versa, is not well understood. In this review, we discuss the specific effects of sirtuins in the maintenance of AT homeostasis in the context of AT inflammation. Given the emerging therapeutic potential of sirtuin 6 (Sirt6) in metabolic diseases and the advances in the development of small molecule Sirt6 modulators (i.e., activators and inhibitors), we particularly paid attention to Sirt6. The current review presents the suppressive effects of Sirt6 on AT inflammation and its clinical significance.
INTRODUCTION
- Sirtuin is a nicotinamide adenine dinucleotide (NAD)+-dependent protein/histone deacetylase that has been highly conserved during evolution from bacteria to mammals [24]. Mammals have seven sirtuins (from Sirt1 to Sirt7) which all possess highly conserved NAD+-binding and catalytic domains, but differ in their physiological functions and subcellular localization [25]. Depending on their isotype, sirtuins can act as a protein/histone deacetylase, a mono-adenosine diphosphate (ADP)-ribosyltransferase, a fatty deacylase, or as a combination of these three (Table 1) [26-41]. Through enzyme dependent- or independent-actions, sirtuins can epigenetically modulate gene transcription and modify protein function via posttranslational regulation. Thus, sirtuins alter the expression level and activity of proteins; mostly enzymes and transcription factors. Since all members of the sirtuin family are ubiquitously expressed in various tissues and play roles in regulating energy metabolism, albeit some more directly than others, we examine their function in detail with a focus on WAT.
- Sirt1
- Sirt1, the most extensively studied sirtuin family member, is known for its ability to extend lifespan and for its role in intracellular energy sensor [27]. When mice are starved, Sirt1 in WAT is induced to deacetylate and inactivate PPARγ, thereby repressing the expression of adipogenesis markers [28]. In differentiated adipocytes, Sirt1 promotes the binding of forkhead box protein O1 (FoxO1) and CCAAT enhancer-binding protein alpha (C/EBPα) to the adiponectin gene promoter, and increases the expression of adiponectin, an insulin sensitizing hormone [42]. Transgenic overexpression of Sirt1 or treatment with resveratrol, a polyphenol that activates Sirt1, has been shown to reduce weight gain and metabolic derangements in high-fat diet (HFD)-fed mice [43-46]. Conversely, genetic ablation of Sirt1 in adipocytes leads to increased adiposity and insulin resistance [47]. These metabolic effects of Sirt1 in adipose tissue may be related to its anti-inflammatory properties. Adipocyte-specific Sirt1 ablation recruits macrophages, specifically M1-type macrophages, into adipose tissues in HFD-fed mice [26,48]. Mechanistically, Sirt1 in adipocytes deacetylates nuclear factor of activated T cells 1 (NFATc1) and enhances the binding of NFATc1 to IL-4 promoter, which facilitates M2-type macrophage polarization [48]. In the same study, the authors found that Sirt1 suppresses C-C motif chemokine ligand 2 (CCL2, also known as MCP-1) production in adipocytes, which in turn inhibits the recruitment of macrophages into adipose tissues.
- To determine the role of Sirt1 in myeloid cells, Schug et al. [29] generated myeloid cell-specific Sirt1 knockout mice, provided them with a HFD, and observed a high degree of infiltration of macrophages into WAT and systemic insulin resistance. In a previous study, we also detected adipose tissue inflammation and metabolic derangement in myeloid cell-specific Sirt1 knockout mice [49]. When an HFD is consumed, Sirt1 ablation in myeloid cells enhances hyperacetylation of focal adhesion kinase, resulting in increased migration of macrophages into adipose tissue.
- Sirt2–5 and Sirt7
- Sirt2 is primarily found in the cytoplasm, but it can migrate to the nucleus in a cell cycle-dependent manner [50]. A number of proteins such as α-tubulin [50], FoxO1 [30,51], FoxO3a [52], peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α) [53], Par-3 [54], p300 [55], p65 [56], and mitogen activated protein kinase phosphatase-1 [57] have been identified as deacetylation substrates of Sirt2. Studies have shown that Sirt2 expression is elevated in WAT of calorierestricted mice, where it deacetylates FoxO3a and reduces the amount of cellular reactive oxygen species [52]. Sirt2 deacetylates FoxO1 to repress the transcriptional activity of PPARγ, thereby suppressing adipogenesis [30]. Sirt2 also promotes oxidative catabolism in adipocytes by deacetylating PGC-1α, leading to an inverse correlation between adipose Sirt2 expression and obesity in human subjects [53].
- Sirt3, Sirt4, and Sirt5 are mitochondrial sirtuins, and their role in obesity and AT inflammation has yet to be elucidated. Sirt3 is highly expressed in BAT and regulates adaptive thermogenesis [31]. Caloric restriction increases Sirt3 expression in both WAT and BAT, where it acts as a critical regulator of lipid homeostasis processes such as fatty acid β-oxidation and electron transport in mitochondria [58]. Global Sirt3 knockout mice display insulin resistance and obesity following suppression of mitochondrial fatty acid oxidation [59]. However, conflicting findings have been reported. In a recent study conducted by Porter et al. [60], adipocyte-specific Sirt3 knockout mice fed an HFD exhibited adipose tissue with normal mitochondrial function and showed no significant changes in whole-body metabolism. This study suggests that Sirt3 in adipocytes does not influence mitochondrial function or cause obesity-induced systemic metabolic complications. Sirt4 has an important role in regulating the proliferation and differentiation of preadipocytes [33]. In contrast to Sirt3, Sirt4 inhibits fatty acid β-oxidation and promotes de novo lipogenesis [32]. Sirt5 is expressed at higher levels in BAT than WAT, and is required for brown adipocyte differentiation. Sirt5 knockout mice exhibit reduced browning capacity in SAT and show cold intolerance [34].
- Sirt7, a nuclear sirtuin, is likely the least studied sirtuin. Sirt7 inhibits autodeacetylation of Sirt1, restricts catalytic activity of Sirt1, and facilitates adipogenic differentiation [40]. In addition, Sirt7 promotes lipogenesis in adipocytes by deacetylating PPARγ2 [41].
PLEIOTROPIC NATURE OF SIRTUIN ON OBESITY AND INSULIN RESISTANCE
- Sirt6 is localized in the nucleus and was first identified as an ADP-ribosyltranferase enzyme [61]. Subsequent studies have shown that Sirt6 deacetylates histone H3 at lysine 9 [62], lysine 18 [63], and lysine 56 [64] residues, resulting in a compact chromatin structure. Sirt6 expression is induced in adipose tissue by a calorie-restricted diet or weight loss [35,65], whereas its expression is suppressed in diet-induced obese c57BL/6 mice [35,38], genetically obese db/db mice [39], and overweight humans [66]. Sirt6-deficient mice exhibit severe hypoglycemia, loss of subcutaneous fat, and abnormalities associated with aging, which eventually cause premature death at approximately 4 weeks of age [67]. In contrast, transgenic overexpression of Sirt6 results in less accumulation of triglyceride (TG) in visceral fat, as Sirt6 suppresses the expression of angiopoietin-like 4, which is known to inhibit diglyceride acyltransferase activity [68]. For further information about the role of Sirt6 in adipocytes and adipose-infiltrated immune cells, we refer to recent publications by our and other groups.
- Adipocyte Sirt6
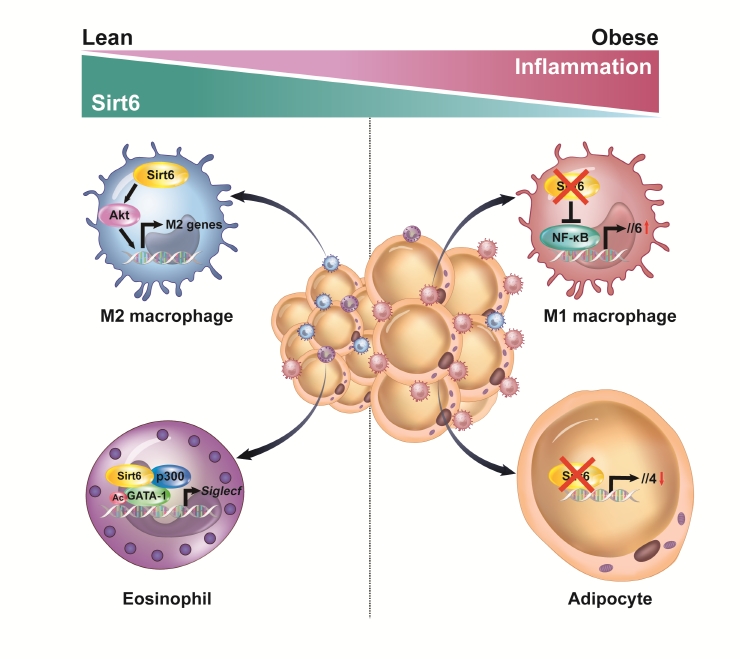
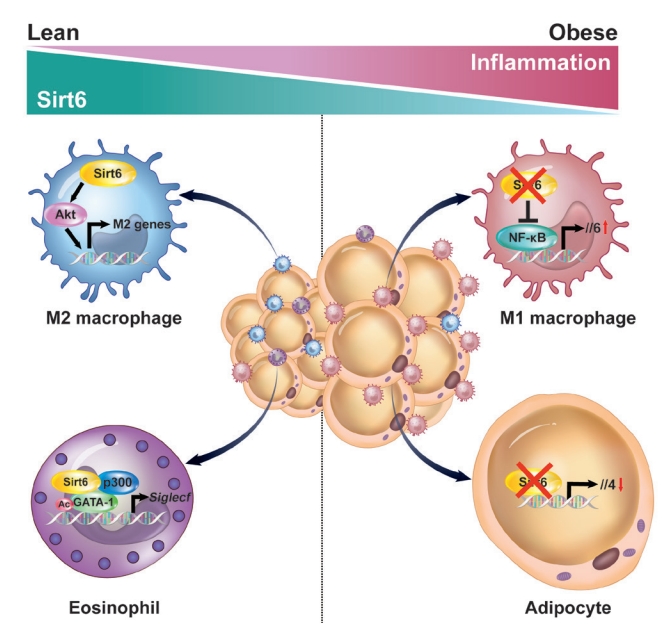
- Sirt6’s main function in adipocytes is to regulate lipid metabolism and prevent the development of inflammation. When a HFD is consumed, adiponectin (Adipoq)-Cre-mediated Sirt6 deficiency accelerates weight gain due to impaired lipolytic activity [35]. On a molecular level, Sirt6 deficiency increases FoxO1 acetylation, facilitates the nuclear export of FoxO1 to cytoplasm, and reduces its effect on adipose TG lipase induction. In a study that used fatty acid binding protein 4 (Fabp4)-Cre-mediated Sirt6 deficient mice, similar phenotypes were observed. Significant increases in body weight, fat mass, adipose tissue inflammation, and insulin resistance were observed in Sirt6 knockout mice as compared to wild-type control mice [38]. Although the Fabp4-Cre mouse line may be predisposed to a target gene recombination in certain endothelial cells and macrophages, the two studies mentioned above clearly indicate that Sirt6 inhibits maintenance of white adipocytes. Brown adipocytes are also affected by Sirt6. Sirt6 increases the thermogenic function of BAT and the browning of SAT by recruiting phospho-activating transcription factor 2 to the promoter of PGC-1α gene [39]. Our studies using Adipoq-Cre-mediated Sirt6 knockout mice highlight that the pathogenesis of adipose tissue inflammation is affected by Sirt6. These mice displayed increased macrophage infiltration in VAT, mainly of M1-type macrophages, and obvious increases of body weight, fat mass, and systemic insulin resistance, even after being fed a normal chow diet [36]. In the same mouse model, Sirt6 induced the expression of Th2 cytokine IL-4 in adipocytes, which in turn promoted M2-type macrophage polarization (Fig. 1). Combined with our recent study showing that adipocytes require Sirt6 for metabolic adaptation to intermittent fasting [37], these studies demonstrate that Sirt6 stimulates lipolysis, enhances adipose tissue browning, ameliorates adipose tissue inflammation, and thereby improves insulin action in peripheral tissues.
- Macrophage Sirt6
- Hematopoietic stem cells give rise to two different types of blood cells; myeloid lineage cells and lymphoid lineage cells. The former cells include erythrocytes, monocytes, macrophages, neutrophils, eosinophils, basophils, and platelets. The latter cells include lymphocytes and NK cells. Lymphocyte Sirt6 interacts with and deacetylates GATA binding protein 3 (GATA-3), inhibits Th2 immune responses, and suppresses allergic airway inflammation induced by ovalbumin or house dust mice [69]. At the time of writing, no information regarding the role of lymphocyte Sirt6 in adipose tissue inflammation has been reported. However, increased infiltration of lymphocytes has been detected in obese adipose tissues [14,15], warranting future research on the potential role of lymphocyte Sirt6 in adipose tissue inflammation and systemic insulin resistance.
- We have investigated the influence of Sirt6 on various inflammatory diseases, including adipose tissue inflammation, with a focus on macrophage polarization. Sirt6 levels are noticeably reduced in VAT after HFD feeding [70]. Myeloid Sirt6 knockout mice exhibit increased proinflammatory M1-type macrophage infiltration in VAT and a concomitant reduction of the M2-type macrophages [70,71]. Mechanistically, a Sirt6 deficiency activates nuclear factor-κB (NF-κB), produces IL-6, and activates the signal transducer and activator of transcription 3 (STAT3), which ultimately polarizes bone marrow cells toward M1-type macrophages (Fig. 1). Similarly, in an experimental rheumatoid arthritis model, myeloid Sirt6 deficiency increased the infiltration of M1-type macrophages in joint tissues [72]. Sirt6 also affects M2-type macrophage polarization in myeloid cells. Under IL-4-stimulated M2 polarization conditions, Sirt6 activates the phosphoinositide 3-kinases (PI3K)-Akt pathway and increases the expression of M2 marker genes [73]. In a full-thickness excisional lesion model, M2 type macrophage infiltration was markedly suppressed in myeloid Sirt6 knockout mice, resulting in prolonged inflammation in the wound site and delayed wound closure.
- Eosinophil Sirt6
- Eosinophils are also important cells in adipose tissue inflammation. Eosinophils differentiate from CD34+ progenitor cells in bone marrow under the influence of a variety of cytokines. Of these cytokines, IL-5 secreted by type 2 innate lymphoid cell (ILC2) cells is essential to support the growth, activation, and survival of eosinophils [74]. GATA transcription factors are crucial in eosinophil lineage commitment and differentiation [75]. We recently reported that Sirt6 is an essential factor in eosinophil differentiation via cooperation with GATA-1, which, to our knowledge, is the only report describing the role of eosinophil Sirt6 in the biology of adipose tissue [76]. Importantly, as Sirt6 simply acts as a scaffolding protein that recruits p300 acetyltransferase and GATA-1 in eosinophils, its deacetylase activity is not required for the expression of GATA-1 target genes (Fig. 1). Indeed, mice with a myeloid Sirt6 deficiency display reduced M2-type macrophage content in SAT and impairment of SAT beiging after exposure to cold temperatures. These results are consistent with previous studies which found that adaptive thermogenesis requires production of Th2 cytokines, including IL-4 by eosinophils, which leads to M2 macrophage polarization and browning of white adipocytes [77-79].
REGULATION OF ADIPOSE TISSUE INFLAMMATION BY SIRTUIN 6
- Once largely recognized as an anti-aging molecule with the ability to repair DNA, the results of a number of research studies support the notion that Sirt6 prevents adipose tissue inflammation by modifying cellular processes in adipocytes and the surrounding immune cells.
- Numerous studies have emphasized the importance of metabolic homeostasis and plasticity in healthy aging. In cases of obesity, a number of innate and adaptive immune cells infiltrate into adipose tissue and cause inflammation. Notably, adipocytes act as key regulatory cells and control adipose tissue inflammation by providing an antigen to immune cells and by secreting a plethora of adipocytokines. Although the precise mechanisms involved in adipose tissue inflammation have not been fully elucidated, it is clear that a cross-talk between adipocytes and immune cells, or among immune cells, determines the immunometabolic phenotype of adipose tissue.
- Experimental results accumulated over the course of a decade indicate the importance of Sirt6 as a regulator of adipose tissue inflammation, which involves the secretion of anti-inflammatory adipocytokines and transcriptional regulation of immune cell maturation. Nevertheless, several questions remain to be addressed in the future. First, the functional role of Sirt6 in several innate and adaptive immune cells, other than macrophages, is poorly understood. Second, although it is evident that Sirt6 is present in both adipocytes and SVCs, transcriptional regulatory networks and intracellular signaling pathways that lead to functional interplay among the cells which compose adipose tissue have not been identified. Third, the therapeutic role of Sirt6 specific modulators (activators and inhibitors) in metabolic diseases including obesity and diabetes need to be understood to a better extent, despite the fact that several compounds have already been identified [80,81]. However, Sirt6 is known to promote the development of certain types of cancer [82]. To increase the therapeutic efficacy of Sirt6 modulators and minimize their side effects when treating adipose diseases, adipose tissue-specific drug delivery or gene therapy should be considered. Suitable answers to the above questions will bring us one step closer to a comprehensive treatment for adipose tissue inflammation and insulin resistance.
CONCLUSIONS AND FUTURE PERSPECTIVES
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING
This work was supported by grants from the Medical Research Center Program (2017R1A5A2015061) and from the Basic Science Research Program (2021R1A2B5B02001462) through the National Research Foundation (NRF), which is funded by the Korean government (MSIP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
NOTES
-
Acknowledgements
- The authors would like to thank the Writing Center at Chonbuk National University for its skilled proofreading service.
| Sirtuin | Localization | Enzymatic activity | Biological effects | Reference |
|---|---|---|---|---|
| Sirt1 | Nucleus, cytoplasm | Deacetylase | Intracellular energy sensor, adipogenesis, adipose tissue inflammation | [26-29] |
| Sirt2 | Cytoplasm, nucleus | Deacetylase | Adipogenesis | [30] |
| Sirt3 | Mitochondria | Deacetylase | Adaptive thermogenesis | [31] |
| Sirt4 | Mitochondria | ADP-ribosyltransferase | Adipogenesis, lipogenesis | [32,33] |
| Sirt5 | Mitochondria | Deacetylase, demalonlylase, desuccinylase, deglutarylase | Adaptive thermogenesis | [34] |
| Sirt6 | Nucleus | Deacetylase, long chain fatty acyl deacylase, ADP-ribosyltransferase | Lipolysis, adipose tissue inflammation, adaptive thermogenesis | [35-39] |
| Sirt7 | Nucleus | Deacetylase | Adipogenesis, lipogenesis | [40,41] |
- 1. Tong Y, Xu S, Huang L, Chen C. Obesity and insulin resistance: pathophysiology and treatment. Drug Discov Today 2022;27:822-30.ArticlePubMed
- 2. Capurso C, Capurso A. From excess adiposity to insulin resistance: the role of free fatty acids. Vascul Pharmacol 2012;57:91-7.ArticlePubMed
- 3. Assimacopoulos-Jeannet F. Fat storage in pancreas and in insulin-sensitive tissues in pathogenesis of type 2 diabetes. Int J Obes Relat Metab Disord 2004;28 Suppl 4:S53-7.PubMed
- 4. Stenvers DJ, Scheer FA, Schrauwen P, la Fleur SE, Kalsbeek A. Circadian clocks and insulin resistance. Nat Rev Endocrinol 2019;15:75-89.ArticlePubMedPDF
- 5. Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol 2016;12:15-28.ArticlePubMedPDF
- 6. Kim J, Lee J. Role of obesity-induced inflammation in the development of insulin resistance and type 2 diabetes: history of the research and remaining questions. Ann Pediatr Endocrinol Metab 2021;26:1-13.ArticlePubMedPMCPDF
- 7. Reinehr T. Inflammatory markers in children and adolescents with type 2 diabetes mellitus. Clin Chim Acta 2019;496:100-7.ArticlePubMed
- 8. Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 1996;271:665-8.ArticlePubMed
- 9. Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol 2004;24:5434-46.ArticlePubMedPMCPDF
- 10. Ye J. Regulation of PPARgamma function by TNF-alpha. Biochem Biophys Res Commun 2008;374:405-8.PubMedPMC
- 11. Bluher M. Adipose tissue inflammation: a cause or consequence of obesity-related insulin resistance? Clin Sci (Lond) 2016;130:1603-14.ArticlePubMedPDF
- 12. Shimobayashi M, Albert V, Woelnerhanssen B, Frei IC, Weissenberger D, Meyer-Gerspach AC, et al. Insulin resistance causes inflammation in adipose tissue. J Clin Invest 2018;128:1538-50.ArticlePubMedPMC
- 13. Li Y, Yun K, Mu R. A review on the biology and properties of adipose tissue macrophages involved in adipose tissue physiological and pathophysiological processes. Lipids Health Dis 2020;19:164.ArticlePubMedPMCPDF
- 14. Michailidou Z, Gomez-Salazar M, Alexaki VI. Innate immune cells in the adipose tissue in health and metabolic disease. J Innate Immun 2022;14:4-30.ArticlePubMedPMCPDF
- 15. Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunology 2018;155:407-17.ArticlePubMedPMCPDF
- 16. Giron-Ulloa A, Gonzalez-Dominguez E, Klimek RS, PatinoMartinez E, Vargas-Ayala G, Segovia-Gamboa NC, et al. Specific macrophage subsets accumulate in human subcutaneous and omental fat depots during obesity. Immunol Cell Biol 2020;98:868-82.ArticlePubMedPDF
- 17. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796-808.ArticlePubMedPMC
- 18. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007;117:175-84.ArticlePubMedPMC
- 19. Trim W, Turner JE, Thompson D. Parallels in immunometabolic adipose tissue dysfunction with ageing and obesity. Front Immunol 2018;9:169.ArticlePubMedPMC
- 20. Villareal DT, Apovian CM, Kushner RF, Klein S; American Society for Nutrition; NAASO, The Obesity Society. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res 2005;13:1849-63.ArticlePubMedPDF
- 21. Canning KL, Brown RE, Jamnik VK, Kuk JL. Relationship between obesity and obesity-related morbidities weakens with aging. J Gerontol A Biol Sci Med Sci 2014;69:87-92.ArticlePubMed
- 22. Tzanetakou IP, Katsilambros NL, Benetos A, Mikhailidis DP, Perrea DN. “Is obesity linked to aging?”: adipose tissue and the role of telomeres. Ageing Res Rev 2012;11:220-9.PubMed
- 23. Santos AL, Sinha S. Obesity and aging: molecular mechanisms and therapeutic approaches. Ageing Res Rev 2021;67:101268.ArticlePubMed
- 24. Greiss S, Gartner A. Sirtuin/Sir2 phylogeny, evolutionary considerations and structural conservation. Mol Cells 2009;28:407-15.ArticlePubMedPMCPDF
- 25. Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun 2000;273:793-8.ArticlePubMed
- 26. Gillum MP, Kotas ME, Erion DM, Kursawe R, Chatterjee P, Nead KT, et al. SirT1 regulates adipose tissue inflammation. Diabetes 2011;60:3235-45.ArticlePubMedPMCPDF
- 27. Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature 2006;444:868-74.ArticlePubMedPDF
- 28. Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 2004;429:771-6.ArticlePubMedPMCPDF
- 29. Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB, et al. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol 2010;30:4712-21.ArticlePubMedPMCPDF
- 30. Wang F, Tong Q. SIRT2 suppresses adipocyte differentiation by deacetylating FOXO1 and enhancing FOXO1’s repressive interaction with PPARgamma. Mol Biol Cell 2009;20:801-8.PubMedPMC
- 31. Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem 2005;280:13560-7.ArticlePubMed
- 32. Laurent G, German NJ, Saha AK, de Boer VC, Davies M, Koves TR, et al. SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Mol Cell 2013;50:686-98.ArticlePubMedPMC
- 33. Zaganjor E, Yoon H, Spinelli JB, Nunn ER, Laurent G, Keskinidis P, et al. SIRT4 is an early regulator of branched-chain amino acid catabolism that promotes adipogenesis. Cell Rep 2021;36:109345.ArticlePubMedPMC
- 34. Shuai L, Zhang LN, Li BH, Tang CL, Wu LY, Li J, et al. SIRT5 regulates brown adipocyte differentiation and browning of subcutaneous white adipose tissue. Diabetes 2019;68:1449-61.ArticlePubMed
- 35. Kuang J, Zhang Y, Liu Q, Shen J, Pu S, Cheng S, et al. Fat-specific Sirt6 ablation sensitizes mice to high-fat diet-induced obesity and insulin resistance by inhibiting lipolysis. Diabetes 2017;66:1159-71.ArticlePubMedPDF
- 36. Song MY, Kim SH, Ryoo GH, Kim MK, Cha HN, Park SY, et al. Adipose sirtuin 6 drives macrophage polarization toward M2 through IL-4 production and maintains systemic insulin sensitivity in mice and humans. Exp Mol Med 2019;51:1-10.ArticlePubMedPMCPDF
- 37. Wu D, Bang IH, Park BH, Bae EJ. Loss of Sirt6 in adipocytes impairs the ability of adipose tissue to adapt to intermittent fasting. Exp Mol Med 2021;53:1298-306.ArticlePubMedPMCPDF
- 38. Xiong X, Zhang C, Zhang Y, Fan R, Qian X, Dong XC. Fabp4-Cre-mediated Sirt6 deletion impairs adipose tissue function and metabolic homeostasis in mice. J Endocrinol 2017;233:307-14.ArticlePubMedPMC
- 39. Yao L, Cui X, Chen Q, Yang X, Fang F, Zhang J, et al. Cold-inducible SIRT6 regulates thermogenesis of brown and beige fat. Cell Rep 2017;20:641-54.ArticlePubMed
- 40. Fang J, Ianni A, Smolka C, Vakhrusheva O, Nolte H, Kruger M, et al. Sirt7 promotes adipogenesis in the mouse by inhibiting autocatalytic activation of Sirt1. Proc Natl Acad Sci U S A 2017;114:E8352-61.ArticlePubMedPMC
- 41. Akter F, Tsuyama T, Yoshizawa T, Sobuz SU, Yamagata K. SIRT7 regulates lipogenesis in adipocytes through deacetylation of PPARγ2. J Diabetes Investig 2021;12:1765-74.ArticlePubMedPMCPDF
- 42. Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem 2006;281:39915-24.PubMed
- 43. Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, et al. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab 2007;6:307-19.ArticlePubMed
- 44. Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A 2008;105:9793-8.ArticlePubMedPMC
- 45. Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab 2008;8:333-41.ArticlePubMedPMC
- 46. Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 2008;8:347-58.ArticlePubMed
- 47. Chalkiadaki A, Guarente L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab 2012;16:180-8.ArticlePubMedPMC
- 48. Hui X, Zhang M, Gu P, Li K, Gao Y, Wu D, et al. Adipocyte SIRT1 controls systemic insulin sensitivity by modulating macrophages in adipose tissue. EMBO Rep 2017;18:645-57.PubMedPMCPDF
- 49. Ka SO, Song MY, Bae EJ, Park BH. Myeloid SIRT1 regulates macrophage infiltration and insulin sensitivity in mice fed a high-fat diet. J Endocrinol 2015;224:109-18.ArticlePubMed
- 50. North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell 2003;11:437-44.ArticlePubMed
- 51. Li W, Zhang B, Tang J, Cao Q, Wu Y, Wu C, et al. Sirtuin 2, a mammalian homolog of yeast silent information regulator-2 longevity regulator, is an oligodendroglial protein that decelerates cell differentiation through deacetylating alpha-tubulin. J Neurosci 2007;27:2606-16.ArticlePubMedPMC
- 52. Wang F, Nguyen M, Qin FX, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell 2007;6:505-14.ArticlePubMed
- 53. Krishnan J, Danzer C, Simka T, Ukropec J, Walter KM, Kumpf S, et al. Dietary obesity-associated Hif1α activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD+ system. Genes Dev 2012;26:259-70.ArticlePubMedPMC
- 54. Beirowski B, Gustin J, Armour SM, Yamamoto H, Viader A, North BJ, et al. Sir-two-homolog 2 (Sirt2) modulates peripheral myelination through polarity protein Par-3/atypical protein kinase C (aPKC) signaling. Proc Natl Acad Sci U S A 2011;108:E952-61.ArticlePubMedPMC
- 55. Black JC, Mosley A, Kitada T, Washburn M, Carey M. The SIRT2 deacetylase regulates autoacetylation of p300. Mol Cell 2008;32:449-55.ArticlePubMedPMC
- 56. Rothgiesser KM, Erener S, Waibel S, Luscher B, Hottiger MO. SIRT2 regulates NF-κB dependent gene expression through deacetylation of p65 Lys310. J Cell Sci 2010;123(Pt 24):4251-8.ArticlePubMedPDF
- 57. Wang J, Koh HW, Zhou L, Bae UJ, Lee HS, Bang IH, et al. Sirtuin 2 aggravates postischemic liver injury by deacetylating mitogen-activated protein kinase phosphatase-1. Hepatology 2017;65:225-36.ArticlePubMedPMCPDF
- 58. Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 2010;464:121-5.ArticlePubMedPMCPDF
- 59. Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell 2011;44:177-90.ArticlePubMedPMC
- 60. Porter LC, Franczyk MP, Pietka T, Yamaguchi S, Lin JB, Sasaki Y, et al. NAD+-dependent deacetylase SIRT3 in adipocytes is dispensable for maintaining normal adipose tissue mitochondrial function and whole body metabolism. Am J Physiol Endocrinol Metab 2018;315:E520-30.ArticlePubMedPMC
- 61. Liszt G, Ford E, Kurtev M, Guarente L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J Biol Chem 2005;280:21313-20.ArticlePubMed
- 62. Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell 2009;136:62-74.ArticlePubMedPMC
- 63. Tasselli L, Xi Y, Zheng W, Tennen RI, Odrowaz Z, Simeoni F, et al. SIRT6 deacetylates H3K18ac at pericentric chromatin to prevent mitotic errors and cellular senescence. Nat Struct Mol Biol 2016;23:434-40.ArticlePubMedPMCPDF
- 64. Michishita E, McCord RA, Boxer LD, Barber MF, Hong T, Gozani O, et al. Cell cycle-dependent deacetylation of telomeric histone H3 lysine K56 by human SIRT6. Cell Cycle 2009;8:2664-6.ArticlePubMedPMC
- 65. Moschen AR, Wieser V, Gerner RR, Bichler A, Enrich B, Moser P, et al. Adipose tissue and liver expression of SIRT1, 3, and 6 increase after extensive weight loss in morbid obesity. J Hepatol 2013;59:1315-22.ArticlePubMed
- 66. Martinez-Jimenez V, Cortez-Espinosa N, Rodriguez-Varela E, Vega-Cardenas M, Briones-Espinoza M, Ruiz-Rodriguez VM, et al. Altered levels of sirtuin genes (SIRT1, SIRT2, SIRT3 and SIRT6) and their target genes in adipose tissue from individual with obesity. Diabetes Metab Syndr 2019;13:582-9.ArticlePubMed
- 67. Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 2006;124:315-29.ArticlePubMed
- 68. Kanfi Y, Peshti V, Gil R, Naiman S, Nahum L, Levin E, et al. SIRT6 protects against pathological damage caused by diet-induced obesity. Aging Cell 2010;9:162-73.ArticlePubMed
- 69. Jang HY, Gu S, Lee SM, Park BH. Overexpression of sirtuin 6 suppresses allergic airway inflammation through deacetylation of GATA3. J Allergy Clin Immunol 2016;138:1452-5.ArticlePubMed
- 70. Lee Y, Ka SO, Cha HN, Chae YN, Kim MK, Park SY, et al. Myeloid sirtuin 6 deficiency causes insulin resistance in high-fat diet-fed mice by eliciting macrophage polarization toward an M1 phenotype. Diabetes 2017;66:2659-68.ArticlePubMedPDF
- 71. Giblin W, Lombard DB. Sirtuin 6 builds a wall against inflammation, trumping diabetes. Diabetes 2017;66:2535-7.ArticlePubMedPMCPDF
- 72. Woo SJ, Noh HS, Lee NY, Cheon YH, Yi SM, Jeon HM, et al. Myeloid sirtuin 6 deficiency accelerates experimental rheumatoid arthritis by enhancing macrophage activation and infiltration into synovium. EBioMedicine 2018;38:228-37.ArticlePubMedPMC
- 73. Koo JH, Jang HY, Lee Y, Moon YJ, Bae EJ, Yun SK, et al. Myeloid cell-specific sirtuin 6 deficiency delays wound healing in mice by modulating inflammation and macrophage phenotypes. Exp Mol Med 2019;51:1-10.ArticlePubMedPMCPDF
- 74. Iwasaki H, Mizuno S, Mayfield R, Shigematsu H, Arinobu Y, Seed B, et al. Identification of eosinophil lineage-committed progenitors in the murine bone marrow. J Exp Med 2005;201:1891-7.ArticlePubMedPMCPDF
- 75. Willebrand R, Voehringer D. Regulation of eosinophil development and survival. Curr Opin Hematol 2017;24:9-15.ArticlePubMed
- 76. Bang IH, Park D, Lee Y, Cho H, Park BH, Bae EJ. Sirtuin 6 promotes eosinophil differentiation by activating GATA-1 transcription factor. Aging Cell 2021;20:e13418.ArticlePubMedPMCPDF
- 77. Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014;157:1279-91.ArticlePubMedPMC
- 78. Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014;157:1292-308.ArticlePubMedPMC
- 79. Huang Z, Zhong L, Lee JT, Zhang J, Wu D, Geng L, et al. The FGF21-CCL11 axis mediates beiging of white adipose tissues by coupling sympathetic nervous system to type 2 immunity. Cell Metab 2017;26:493-508.ArticlePubMed
- 80. Raj S, Dsouza LA, Singh SP, Kanwal A. Sirt6 deacetylase: a potential key regulator in the prevention of obesity, diabetes and neurodegenerative disease. Front Pharmacol 2020;11:598326.ArticlePubMedPMC
- 81. Bae EJ. Sirtuin 6, a possible therapeutic target for type 2 diabetes. Arch Pharm Res 2017;40:1380-9.ArticlePubMedPDF
- 82. Fiorentino F, Carafa V, Favale G, Altucci L, Mai A, Rotili D. The two-faced role of SIRT6 in cancer. Cancers (Basel) 2021;13:1156.ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- The Role of Increased Expression of Sirtuin 6 in the Prevention of Premature Aging Pathomechanisms
Adrianna Dzidek, Olga Czerwińska-Ledwig, Małgorzata Żychowska, Wanda Pilch, Anna Piotrowska
International Journal of Molecular Sciences.2023; 24(11): 9655. CrossRef - Exploring the Influence of Age, Gender and Body Mass Index on Colorectal Cancer Location
Dorel Popovici, Cristian Stanisav, Sorin Saftescu, Serban Negru, Radu Dragomir, Daniel Ciurescu, Razvan Diaconescu
Medicina.2023; 59(8): 1399. CrossRef

 KDA
KDA
 PubReader
PubReader ePub Link
ePub Link Cite
Cite