- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 44(4); 2020 > Article
-
Original ArticleDrug/Regimen Switching to Once-Daily Insulin Degludec/Insulin Aspart from Basal Insulin Improves Postprandial Glycemia in Patients with Type 2 Diabetes Mellitus: Randomized Controlled Trial
-
Kyu Yong Cho1,2
 , Akinobu Nakamura1, Chiho Oba-Yamamoto1, Kazuhisa Tsuchida1, Shingo Yanagiya1, Naoki Manda3, Yoshio Kurihara4, Shin Aoki5, Tatsuya Atsumi1, Hideaki Miyoshi1,6
, Akinobu Nakamura1, Chiho Oba-Yamamoto1, Kazuhisa Tsuchida1, Shingo Yanagiya1, Naoki Manda3, Yoshio Kurihara4, Shin Aoki5, Tatsuya Atsumi1, Hideaki Miyoshi1,6
-
Diabetes & Metabolism Journal 2020;44(4):532-541.
DOI: https://doi.org/10.4093/dmj.2019.0093
Published online: November 22, 2019
1Department of Rheumatology, Endocrinology and Nephrology, Faculty of Medicine and Graduate School of Medicine, Hokkaido University, Sapporo, Japan.
2Clinical Research and Medical Innovation Center, Hokkaido University Hospital, Sapporo, Japan.
3Manda Memorial Hospital, Sapporo, Japan.
4Kurihara Clinic, Sapporo, Japan.
5Aoki Clinic, Sapporo, Japan.
6Division of Diabetes and Obesity, Faculty of Medicine and Graduate School of Medicine, Hokkaido University, Sapporo, Japan.
- Corresponding author: Hideaki Miyoshi. Division of Diabetes and Obesity, Faculty of Medicine and Graduate School of Medicine, Hokkaido University, N15 W7, Kita-ku, Sapporo 060-8638, Japan. hmiyoshi@med.hokudai.ac.jp
Copyright © 2020 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- To explore the efficacy and safety of switching from once-daily basal insulin therapy to once-daily pre-meal injection insulin degludec/insulin aspart (IDegAsp) with respect to the glycemic control of participants with type 2 diabetes mellitus (T2DM).
-
Methods
- In this multicenter, open-label, prospective, randomized, parallel-group comparison trial, participants on basal insulin therapy were switched to IDegAsp (IDegAsp group; n=30) or continued basal insulin (Basal group; n=29). The primary endpoint was the superiority of IDegAsp in causing changes in the daily blood glucose profile, especially post-prandial blood glucose concentration after 12 weeks.
-
Results
- Blood glucose concentrations after dinner and before bedtime were lower in the IDegAsp group, and the improvement in blood glucose before bedtime was significantly greater in the IDegAsp group than in the Basal group at 12 weeks (−1.7±3.0 mmol/L vs. 0.3±2.1 mmol/L, P<0.05). Intriguingly, glycemic control after breakfast was not improved by IDegAsp injection before breakfast, in contrast to the favorable effect of injection before dinner on blood glucose after dinner. Glycosylated hemoglobin significantly decreased only in the IDegAsp group (58 to 55 mmol/mol, P<0.05). Changes in daily insulin dose, body mass, and recorded adverse effects, including hypoglycemia, were comparable between groups.
-
Conclusion
- IDegAsp was more effective than basal insulin at reducing blood glucose after dinner and before bedtime, but did not increase the incidence of hypoglycemia. Switching from basal insulin to IDegAsp does not increase the burden on the patient and positively impacts glycemic control in patients with T2DM.
- Type 2 diabetes mellitus (T2DM) is characterized by increasing insulin resistance and a gradual decline in β-cell function that in many instances eventually necessitates treatment with exogenous insulin injection to maintain glycemic control [12]. However, due to fear of hypoglycemia, weight gain, the practical difficulties associated with the use of injection devices, and the requirement to titrate insulin dose, it is challenging for patients and physicians to initiate or intensify insulin therapy [3].
- Once-daily basal insulin injection is an optimal initiating method with a relatively low frequency of hypoglycemic events, and is widely used worldwide [4]. It is also an ideal method because the frequency of injections required is low and the algorithm used to titrate the dose is relatively simple. The consensus statement by the American Diabetes Association and the European Association for the Study of Diabetes recommends once-daily basal insulin injection therapy as the first step in the introduction of insulin for the treatment of T2DM [5]. This approach mimics physiologic insulin secretion by adding insulin boluses in a sequential manner (evolving from basal insulin injection to basal-plus-one bolus to basal-plus-two boluses to basal-bolus therapy), but the increase in the frequency of injections adds an additional burden for patients.
- A variety of studies, including systematic reviews and meta-analyses of trials comparing basal insulin therapy and twice a day premixed insulin therapy, have concluded that there is a higher risk of hypoglycemia with premixed insulin while the final glycosylated hemoglobin (HbA1c) is lower [6]. The products in the early phases of premixed insulins, including Neutral Protamine Hagedorn (NPH) or Neutral Protamine Lispro (NPL) are shorter-acting than basal insulin analogues; therefore, they do not effectively mimic physiologic basal insulin secretion, which can be approximated by basal insulin analogues in the higher extent. Moreover, resuspension is required before the injection of the early premixed insulin products, ensuring accurate and repeatable dosing from an insulin cartridge would be difficult [7].
- Insulin degludec/insulin aspart (IDegAsp) is a new combination insulin consisting of 70% long-acting basal insulin degludec (IDeg) and 30% rapid-acting prandial insulin aspart (IAsp), in which each component maintains its independent characteristics and there is no interaction between them [89]. Accordingly, IDegAsp is convenient for “step-up” therapy (i.e., changing to basal-plus-one bolus therapy from basal insulin therapy) without the need for an increase in injection frequency. IDeg, as the basal component of IDegAsp, has a longer duration of action and flatter profile than those of the early products of basal insulins, such as NPH, NPL, glargine, and detemir, resulting in fewer hypoglycemic episodes and less day-to-day variability [1011]. Use of IDegAsp has been reported to be associated with lower fasting blood glucose and frequency of hypoglycemia than other premixed insulin preparations [12]. Moreover, once-daily IDegAsp showed a significantly greater effect on reducing HbA1c in a phase 3 study, compared with once-daily insulin glargine (IGlr), without inducing more frequent hypoglycemia [13]. However, there have been few studies of the efficacy or safety of switching to IDegAsp in participants with T2DM that were being treated with basal insulin analogues. It would thus be useful to determine whether once-daily IDegAsp injection therapy can improve glycemic control, especially postprandial hyperglycemia, if participants are changed from once-daily long-acting basal insulin injection therapy in a clinical setting. To investigate the efficacy and safety of the switching to IDegAsp, we carried out a prospective, randomized, parallel-group comparison trial.
INTRODUCTION
- Trial population
- The inclusion criteria were as follows: Japanese adults with T2DM; aged 20 to 80 years; HbA1c 46 to 75 mmol/mol (6.0% to 9.0%); who had been treated with basal insulin (IDeg or IGlr) for ≥12 weeks before enrollment. Subjects who had a known or suspected allergy to trial products, unstable diabetic retinopathy, severe liver dysfunction or renal failure, and women who were pregnant or lactating, were excluded. Subjects with low insulin secretion (fasting plasma C-peptide ≤0.5 ng/mL) were also excluded. All informed consent was obtained from the subjects.
- Trial design and procedures
- A 12-week, multicenter, open-label, prospective, randomized, parallel-group comparison, treat-to-target study was conducted at nine sites in Hokkaido, Japan. Screening commenced in January 2017 and recruitment was completed by November 2017. The protocol for this research project has been approved by a suitably constituted Ethics Committee of the institution and it conforms to the provisions of the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013), Committee of Hokkaido University Hospital (approval No. 016-0228). The study was registered with the University Hospital Medical Information Network (UMIN) Center (UMIN000025199) before enrollment.
- Participants were assigned randomly (1:1) to continue administering once-daily basal insulin (Basal group) or to switch from once-daily basal insulin to once-daily IDegAsp (IDegAsp group). Randomization and allocation of participants were undertaken by a specialized center independent of the medical institutions involved. Participants were allocated such that body mass index, HbA1c, and the identity of the basal insulin used (IDeg or IGlr) did not differ between the groups. Although the initial dose of IDegAsp used was the same as for the basal insulin, it could be reduced at the discretion of the physician when there was a risk of hypoglycemia. IDeg or IGlr continued to be administered at the same time as before the study commenced, whereas IDegAsp was administered just before the largest meal of the day, in accordance with the manufacturer's instructions. The insulin titration method was as follows: the initial dosage of IDegAsp was identical to the previous basal insulin dosage or was slightly increased to contain an identical amount of the degludec component. Then, it was titrated based on self-measured blood glucose (SMBG) measurements made before breakfast, to achieve a target blood glucose level of 5.5 to 7.2 mmol/L. Concomitant medications were continued at the same dose from enrollment until the end of the study. However, when the risk of hypoglycemia was suspected to be high, the dose of any concomitant medication could be reduced at the discretion of the physician. Four instances of seven-point SMBG profiles (measurements made before and 2 hours after the start of breakfast, lunch, and dinner, and prior to bedtime) were carried out at before (two times) and during the treatment period of the study (two times); the average of the SMBG profiles at each period was used for the evaluation. A hypoglycaemic event was counted if blood glucose was confirmed to be <3.9 mmol/L by SMBG measurement or if assistance was required.
- Endpoints and assessments
- The primary endpoint was the superiority of daily blood glucose profile using seven-point SMBG measurement, particularly that in the postprandial blood glucose concentration in the IDegAsp group. Secondary endpoints were change from baseline in HbA1c, body mass, insulin dose, and other clinical parameters at 12 weeks, as well as the occurrence of adverse effects, including hypoglycemic events.
- Statistical analyses
- The sample size of this study was calculated using data from the phase 3 trial of IDegAsp [13], in which the efficacy of once-daily IDegAsp was compared with that of once-daily IGlr in insulin-naïve participants with T2DM. It was determined that 28 participants (30 participants, including a 10% drop-out estimate) were required in each group to detect a significant difference, with 80% power and a statistical significance level of 5%, using the assumption that switching from IDeg or IGlr to IDegAsp would improve postprandial blood glucose by a mean 3.2 mmol/L, with a standard deviation (SD) of 4.1 mmol/L.
- Data are reported as mean±SD, median (range), or number (%). Differences in baseline characteristics between the two groups were evaluated using the unpaired t-test or the Mann-Whitney U test for continuous variables, and the chi-square test or Fisher's exact test for categorical variables. The Kolmogorov-Smirnov test was used to test for the normality of continuous variables. The relationship between continuous variables was evaluated using Spearman rank-order correlation analysis. Data were analyzed using JMP Pro v14.0.0 (SAS Institute, Cary, NC, USA). A value of P<0.05 was considered to represent statistical significance.
METHODS
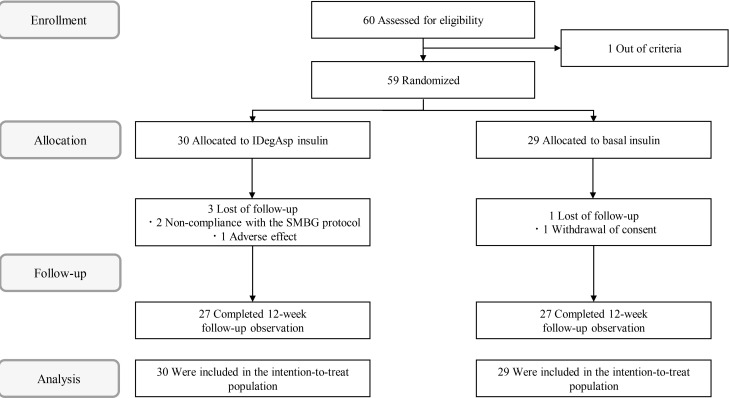
- A total of 60 participants were initially enrolled in the study, but one was subsequently excluded according to the entry criteria. Each participant was randomly assigned to either the IDegAsp group (n=30) or the Basal group (n=29) (Fig. 1). Four participants withdrew from the study prior to its completion. The reasons for non-completion were withdrawal of consent (n=1), non-compliance with the SMBG protocol (n=2), and the adverse effect of hot flushes (n=1). No major differences in baseline characteristics were identified between the groups (Table 1). There were 34 participants (57.6%) over the age of 65. In the IDegAsp group, there were 14 participants (46.7%) administering IDeg and 16 (53.3%) administering IGlr at baseline, and the Basal group contained 12 participants (41.4%) using IDeg and 17 (58.6%) using IGlr. There were no significant differences in the baseline characteristics of the two groups.
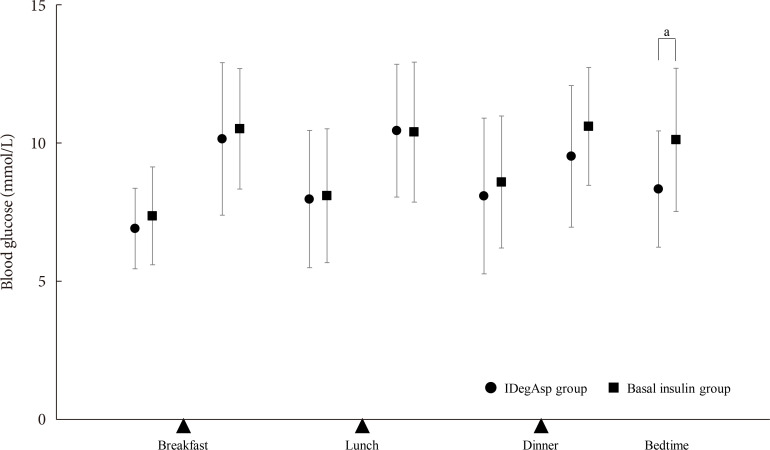
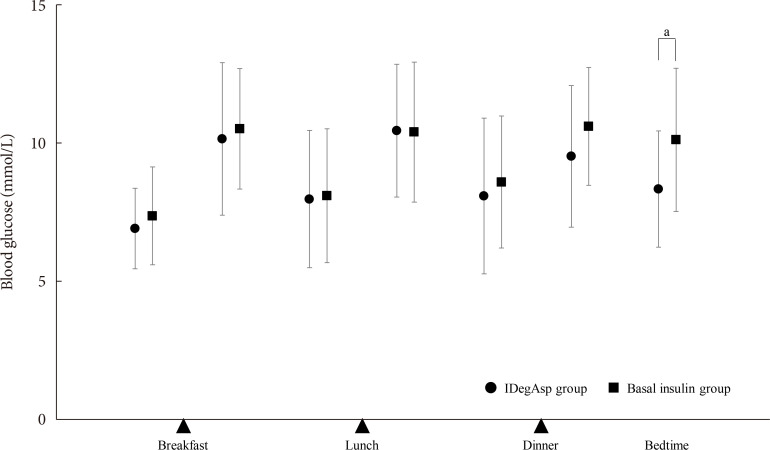
- Regarding the primary endpoint of blood glucose concentration measured using SMBG, the changes in total increase of postprandial blood glucose during the study were −0.8±3.1 mmol/L in the IDegAsp group and 0.1±3.1 mmol/L in the Basal group; these values were significantly different between groups (P= 0.04). In particular, blood glucose significantly decreased in the IDegAsp group, both after dinner (from 11.2±2.5 to 9.5±2.6 mmol/L, P<0.05) and at bedtime (from 10.1±2.4 to 8.4±2.1 mmol/L, P<0.01) (Table 2). Moreover, the reduction in blood glucose before bedtime in the IDegAsp group was significantly greater than that in the Basal group (P=0.02) (Fig. 2). The proportion of participants achieving their glycaemic target before breakfast was similar between the groups (53.3% in the IDegAsp group and 51.7% in the Basal group). Injection timing was evenly allocated between the groups at baseline (Table 1). In the IDegAsp group, the participants changed their injection timing to just before their main meal (breakfast, lunch, or dinner), following the instructions (n=9 [30.0%], n=3 [10.0%], and n=18 [60.0%], respectively). There were improvements in glycaemic control after dinner (11.2±2.5 to 9.5±1.8 mmol/L) and at bedtime (10.1±2.4 to 8.4±2.1 mmol/L) in the subgroup who injected IDegAsp before dinner (n=18). Intriguingly, no improvement in glycemia was detected after breakfast in the subgroup who injected IDegAsp before breakfast (10.3±2.3 to 9.8±5.1 mmol/L, n=9) (Table 3). In the IDegAsp group, 10 participants (55.6%) in the “before dinner injection” subgroup and four participants (44.4%) in the “before breakfast injection” subgroup achieved fasting blood glucose levels within the target range. The change of IDegAsp dose was from 13.5 to 13.7 units in the “before dinner injection” subgroup, whereas it was from 10.8 to 11.1 units in the “before breakfast injection” subgroup. The changes in HbA1c in IDegAsp group were 63 mmol/mol (7.6%±0.6%) to 59 mmol/mol (7.2%±0.6%) in before dinner subgroup and 58 mmol/mol (7.5%±0.7%) to 55 mmol/mol (7.3%±0.6%) in before breakfast subgroup. There were no significant differences between two subgroups although the decreasing tendency was observed in before dinner subgroup (P=0.07). There was no difference in blood glucose between participants who had previously used IDeg or IGlr as their basal insulin preparation (data not shown). The change in daily insulin dose was comparable between the groups (IDegAsp, 11.9±6.4 to 13.7±8.9 units; Basal, 13.0±5.4 to 13.7±6.9 units; P=0.94) (Table 3).
- The HbA1c of the IDegAsp group significantly decreased from 58 mmol/mol (7.5%±0.6%) to 55 mmol/mol (7.3%± 0.6%) (P<0.05), but there was no significant difference in the change in HbA1c between the groups (P=0.11) (Table 4). There were no significant differences in the changes in body mass, blood pressure, liver function, renal function, or lipid concentrations between baseline and 12 weeks, or between the two groups.
- There were no severe adverse events during the study period. Twenty-four hypoglycemic episodes were recorded in the IDegAsp group and 26 in the Basal group during the 12 weeks of the trial (difference between groups, P=0.82). Few adverse events occurred in either group (Supplementary Table 1). One participant, who felt hot flushes, feared the development of hypoglycemia and dropped out of the study. However, it was not determined whether this was a hypoglycemic symptom, because SMBG was not being carried out at that time. Other adverse effects were similarly mild and resolved with appropriate support, such that no other participants dropped out of the trial. No participants had infections, dehydration, ketoacidosis, or cardiovascular events.
RESULTS
- This randomized controlled trial in a real-world clinical setting demonstrated that switching from once-daily basal insulin to once-daily IDegAsp comprises a useful step-up treatment modality that improves glycaemic control without the requirement for an increased insulin dose or with a greater risk of hypoglycemia. Seven-point SMBG revealed that trial participants administering once-daily basal insulin had relatively high blood glucose after dinner, and that IDegAsp significantly improved this. The relative contribution of the postprandial blood glucose level to HbA1c score is larger than that of the fasting blood glucose level at lower HbA1c [14], and a significant improvement in HbA1c only occurred in the IDegAsp group, in which the mean HbA1c level was <7.5%. Thus, this improvement was likely the result of the reduction in postprandial blood glucose level caused by the rapid-acting IAsp component of IDegAsp.
- Although there have been several comparison studies regarding the use of IDegAsp and IGlr in insulin-naive participants for the initiation of once-daily insulin [131516], there have only been two studies regarding the potential benefits of switching from basal insulin to IDegAsp [1718]. One of these reported that IDegAsp (n=10) was more effective than basal insulin at reducing postprandial plasma glucose, measured following a 2-hour meal test, after 4 weeks of administration [17]. Consistent with our findings, there was a significant improvement in post-meal plasma glucose concentration after switching from basal insulin analogues to IDegAsp. A noteworthy aspect of the present study was the low frequency of hypoglycemia, because of the gentle insulin dose adjustment in order to ensure safety; this is most important in real-world clinical settings. From this perspective, the present study differed from previous phase 3 trials or studies using intensive insulin titration protocols, which caused high frequencies (almost half of the participants) of hypoglycemia and serious adverse events [13].
- In the present study, injection of IDegAsp before breakfast did not significantly improve blood glucose at any time point, including after breakfast. Although one of the possible explanations is the small sample size of the subgroup (n=9), the mean improvement in this subgroup was smaller than that in the subgroup that administered IDegAsp before dinner (−0.5 mmol/L vs. −2.5 mmol/L, respectively). There were similar findings in phase 3 studies of once-daily IDegAsp versus IGlr in Japanese and western participants with T2DM, in which postprandial blood glucose decreased when IDegAsp was administered before dinner, but not before breakfast [1318]. In addition, another study of once-daily IDegAsp injection, in which the participants injected IDegAsp before breakfast (n=266, 52 weeks), demonstrated no improvement in postprandial blood glucose after breakfast. Moreover, there was significantly more hypoglycemia in the morning in the IDegAsp group than in the IGr group [15]. Taken together, these results highlight the importance of the timing of IDegAsp administration. IDegAsp injection before dinner seems to be more effective at improving postprandial blood glucose than the injection of this mixture before breakfast. A possible mechanism was identified in the previous study, which demonstrated more stable nocturnal glycemia and less hypoglycemia in European participants with insulin naive T2DM administering IDegAsp than in those administering IGlar. Plasma glucose concentration in the morning is influenced by many factors that are unrelated to the insulin absorption profile, such as meals, variation in meal times, and exercise patterns. At night, glycemia is minimally affected by these factors, meaning that there is a stronger influence of insulin type on the blood glucose profile [16]. Although IDegAsp once-daily before breakfast was effective in a prior study, it was determined with a 2-hour meal test and the dose of IDegAsp was increased by >60% for only 4 weeks in that study [17].
- There were several limitations in the current trial. It was a randomized controlled trial, but used an open-label design, which might have generated some reporting bias. The injection timing of IDegAsp exhibited variation because it was administered immediately prior to the largest meal of the day, in accordance with the manufacturer's instructions. The sample number at each meal was not uniform because of the instruction. At the end of this study, nearly half of the participants achieved fasting glucose levels in the target range. Although insulin dose was titrated to achieve a target fasting blood glucose level of 5.5 to 7.2 mmol/L before breakfast in this study, it could be reduced at the discretion of the physician when there was a risk of hypoglycemia. Because this study was performed in a standard clinical setting and included relatively older patients (mean age, 64.8 years), the physicians might have been reluctant to risk the onset of hypoglycemia. If strict titration were adopted in younger people, more improvement might be detected in glycemic control. In this study, there were fewer hypoglycaemic events, but uncounted undetected hypoglycemia might exist. The peak of postprandial blood glucose was not always at 2 hours after meals. Because postprandial hyperglycemia and asymptomatic hypoglycemia were not easy to detect by SMBG [19], and were more effectively detected by continuous glucose monitoring (CGM), further trials using CGM in real-world clinical settings should be planned in the future.
- Our findings show that IDegAsp reduces hyperglycemia after dinner and at bedtime without increasing the frequency of hypoglycaemic episodes or causing other adverse effects, when injected before dinner. Switching from once-daily basal insulin to once daily IDegAsp before the main meal of the day can be regarded as a positive step in the management of participants with inadequately controlled T2DM.
DISCUSSION
-
Acknowledgements
- The authors thank the participants, their families, and all the investigators involved in this study. We thank Mark Cleasby, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST: Akinobu Nakamura, Naoki Manda, Yoshio Kurihara, Tatsuya Atsumi, and Hideaki Miyoshi have received honoraria for lectures and received research funding from some organizations as described below. There was no financial support for this study.
Akinobu Nakamura has received research funding from Mitsubishi Tanabe Pharma Co., Ono Pharmaceutical Co. Ltd.; Naoki Manda has received honoraria for lectures from Ono Pharmaceutical Co. Ltd.; Yoshio Kurihara has received honoraria for lectures from Astellas Pharma Inc., AstraZeneca, Mitsubishi Tanabe Pharma Co. Ltd., MSD, Ono Pharmaceutical Co. Ltd., Sanofi, Shionogi & Co. Ltd., Taisho Toyama Pharmaceutical Co. Ltd., and Takeda Pharmaceutical Co. Ltd.; Tatsuya Atsumi has received honoraria for lectures from Mitsubishi Tanabe Pharma Co. Chugai Pharmaceutical Co. Ltd., Astellas Pharma Inc., Takeda Pharmaceutical Co. Ltd., Pfizer Inc., AbbVie Inc., Eisai Co. Ltd., Daiichi Sankyo Co. Ltd., Bristol-Myers Squibb Co., UCB Japan Co. Ltd., Eli Lilly Japan K.K., and has received research funding from Astellas Pharma Inc., Takeda Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Co., Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Pfizer Inc., and Alexion Inc. Hideaki Miyoshi has received honoraria for lectures from Astellas Pharma Inc., Dainippon Pharma Co., Eli Lilly, Mitsubishi Tanabe Pharma Co., MSD, Novartis Pharma, Novo Nordisk Pharma, Kowa Pharmaceutical Co. Ltd., Nippon Boehringer Ingelheim Co., Ono Pharmaceutical Co. Ltd., and Sanofi, and has received research funding from Astellas Pharma Inc., Daiichi Sankyo, Dainippon Pharma Co., Eli Lilly, Mitsubishi Tanabe Pharma Co., Novo Nordisk Pharma, Kowa Pharmaceutical Co., Abbott Japan Co., Nippon Boehringer Ingelheim Co., Ono Pharmaceutical Co. Ltd., and Taisho Toyama Pharmaceutical Co., Ltd.
The other authors declare no conflict of interest.
-
AUTHOR CONTRIBUTIONS:
NOTES
SUPPLEMENTARY MATERIALS
- 1. Butler PC, Meier JJ, Butler AE, Bhushan A. The replication of beta cells in normal physiology, in disease and for therapy. Nat Clin Pract Endocrinol Metab 2007;3:758-768. PubMedPDF
- 2. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140-149. ArticlePubMedPDF
- 3. Ishii H, Iwamoto Y, Tajima N. An exploration of barriers to insulin initiation for physicians in Japan: findings from the Diabetes Attitudes, Wishes And Needs (DAWN) JAPAN study. PLoS One 2012;7:e36361. ArticlePubMedPMC
- 4. Holman RR, Thorne KI, Farmer AJ, Davies MJ, Keenan JF, Paul S, Levy JC. 4-T Study Group. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 2007;357:1716-1730.ArticlePubMed
- 5. Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. American Diabetes Association. European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193-203. ArticlePubMedPMCPDF
- 6. Pontiroli AE, Miele L, Morabito A. Metabolic control and risk of hypoglycaemia during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta-analysis. Diabetes Obes Metab 2012;14:433-446. ArticlePubMed
- 7. Jehle PM, Micheler C, Jehle DR, Breitig D, Boehm BO. Inadequate suspension of neutral protamine Hagendorn (NPH) insulin in pens. Lancet 1999;354:1604-1607. ArticlePubMed
- 8. Haahr H, Fita EG, Heise T. A review of insulin degludec/insulin aspart: pharmacokinetic and pharmacodynamic properties and their implications in clinical use. Clin Pharmacokinet 2017;56:339-354. ArticlePubMedPDF
- 9. Niskanen L, Leiter LA, Franek E, Weng J, Damci T, Munoz-Torres M, Donnet JP, Endahl L, Skjoth TV, Vaag A. Comparison of a soluble co-formulation of insulin degludec/insulin aspart vs biphasic insulin aspart 30 in type 2 diabetes: a randomised trial. Eur J Endocrinol 2012;167:287-294. ArticlePubMedPMC
- 10. Wysham C, Bhargava A, Chaykin L, de la Rosa R, Handelsman Y, Troelsen LN, Kvist K, Norwood P. Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 2 diabetes: the SWITCH 2 randomized clinical trial. JAMA 2017;318:45-56. ArticlePubMedPMC
- 11. Heise T, Norskov M, Nosek L, Kaplan K, Famulla S, Haahr HL. Insulin degludec: lower day-to-day and within-day variability in pharmacodynamic response compared with insulin glargine 300 U/mL in type 1 diabetes. Diabetes Obes Metab 2017;19:1032-1039. ArticlePubMedPMCPDF
- 12. Fulcher GR, Christiansen JS, Bantwal G, Polaszewska-Muszynska M, Mersebach H, Andersen TH, Niskanen LK. BOOST: Intensify Premix I Investigators. Intensify Premix I Investigators. Comparison of insulin degludec/insulin aspart and biphasic insulin aspart 30 in uncontrolled, insulin-treated type 2 diabetes: a phase 3a, randomized, treat-to-target trial. Diabetes Care 2014;37:2084-2090. PubMed
- 13. Onishi Y, Ono Y, Rabol R, Endahl L, Nakamura S. Superior glycaemic control with once-daily insulin degludec/insulin aspart versus insulin glargine in Japanese adults with type 2 diabetes inadequately controlled with oral drugs: a randomized, controlled phase 3 trial. Diabetes Obes Metab 2013;15:826-832. ArticlePubMedPDF
- 14. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care 2003;26:881-885. PubMed
- 15. Kumar A, Franek E, Wise J, Niemeyer M, Mersebach H, Simo R. Efficacy and safety of once-daily insulin degludec/insulin aspart versus insulin glargine (U100) for 52 weeks in insulin-naive patients with type 2 diabetes: a randomized controlled trial. PLoS One 2016;11:e0163350. ArticlePubMedPMC
- 16. Liebl A, Davidson J, Mersebach H, Dykiel P, Tack CJ, Heise T. A novel insulin combination of insulin degludec and insulin aspart achieves a more stable overnight glucose profile than insulin glargine: results from continuous glucose monitoring in a proof-of-concept trial. J Diabetes Sci Technol 2013;7:1328-1336. ArticlePubMedPMCPDF
- 17. Nagai Y, Nishine A, Hashimoto E, Nakayama T, Sasaki Y, Murakami M, Ishii S, Kato H, Tanaka Y. Efficacy and safety of switching from basal insulin to once-daily insulin degludec/insulin aspart in Japanese patients with inadequately controlled type 2 diabetes: a 4-week, randomized, open-label, treat-to-target study. J Diabetes Investig 2017;9:567-572.ArticlePubMedPMCPDF
- 18. Kumar S, Jang HC, Demirag NG, Skjoth TV, Endahl L, Bode B. Efficacy and safety of once-daily insulin degludec/insulin aspart compared with once-daily insulin glargine in participants with type 2 diabetes: a randomized, treat-to-target study. Diabet Med 2017;34:180-188. ArticlePubMedPDF
- 19. Levy JC, Davies MJ, Holman RR. 4-T Study Group. Continuous glucose monitoring detected hypoglycaemia in the Treating to Target in Type 2 Diabetes Trial (4-T). Diabetes Res Clin Pract 2017;131:161-168. ArticlePubMed
REFERENCES
Study protocol flow diagram. IDegAsp, insulin degludec/insulin aspart; SMBG, self-measured blood glucose.
Blood glucose concentrations at the end of the study. Seven-point self-monitoring of blood glucose was undertaken by participants in the insulin degludec/insulin aspart (IDegAsp) and Basal groups during the study periods. Data are mean±standard deviation. P values indicate comparisons between the IDegAsp and Basal groups. Unpaired t-test. aP<0.05.
Differences in clinical characteristics between the IDegAsp group and the Basal group
Comparison of the change in daily blood glucose concentrations
Changes in daily blood glucose concentrations in patients of the IDegAsp group who injected either before breakfast (n=9) or dinner (n=18)
Comparison of the effects of insulin type on other parameters
Values are presented as mean±standard deviation, median (25%–75% confidence interval), or number (%). P values refer to differences between the IDegAsp and Basal insulin groups.
IDegAsp, insulin degludec/insulin aspart; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycosylated hemoglobin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GTP, γ-glutamyl transpeptidase; ACR, albumin/creatinine ratio; UA, uric acid; TG, triglyceride; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol.
aP<0.05 between baseline and the end of the study, bP<0.01 between the two groups in the change during the study.
Figure & Data
References
Citations

- Glycaemic outcomes in hospital with IDegAsp versus BIAsp30 premixed insulins
Joshua R. Walt, Julie Loughran, Spiros Fourlanos, Rahul D. Barmanray, Jasmine Zhu, Suresh Varadarajan, Mervyn Kyi
Internal Medicine Journal.2024;[Epub] CrossRef - Low fasting glucose‐to‐estimated average glucose ratio was associated with superior response to insulin degludec/aspart compared with basal insulin in patients with type 2 diabetes
Han Na Jang, Ye Seul Yang, Tae Jung Oh, Bo Kyung Koo, Seong Ok Lee, Kyong Soo Park, Hak Chul Jang, Hye Seung Jung
Journal of Diabetes Investigation.2022; 13(1): 85. CrossRef - Comparing time to intensification between insulin degludec/insulin aspart and insulin glargine: A single-center experience from India
Rajiv Kovil
Journal of Diabetology.2022; 13(2): 171. CrossRef - Use of Insulin Degludec/Insulin Aspart in the Management of Diabetes Mellitus: Expert Panel Recommendations on Appropriate Practice Patterns
Tevfik Demir, Serap Turan, Kursad Unluhizarci, Oya Topaloglu, Tufan Tukek, Dilek Gogas Yavuz
Frontiers in Endocrinology.2021;[Epub] CrossRef - Pharmacoeconomic comparison of the second generation insulin analogs and insulins on their base
I. N. Dyakov, S. K. Zyryanov
Kachestvennaya Klinicheskaya Praktika = Good Clinical Practice.2021; 20(1): 4. CrossRef - Efficacy and Safety of Insulin Degludec/Insulin Aspart Compared with a Conventional Premixed Insulin or Basal Insulin: A Meta-Analysis
Shinje Moon, Hye-Soo Chung, Yoon-Jung Kim, Jae-Myung Yu, Woo-Ju Jeong, Jiwon Park, Chang-Myung Oh
Metabolites.2021; 11(9): 639. CrossRef - Insulin therapy in diabetic kidney disease
Yan Liu, Chanyue Zhao, Xiaofen Xiong, Ming Yang, Lin Sun
Diabetic Nephropathy.2021; 1(2): 67. CrossRef - Indirect comparison of efficacy and safety of insulin glargine/lixisenatide and insulin degludec/insulin aspart in type 2 diabetes patients not controlled on basal insulin
Anwar Ali Jammah
Primary Care Diabetes.2020;[Epub] CrossRef

 KDA
KDA PubReader
PubReader ePub Link
ePub Link Cite
Cite