
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
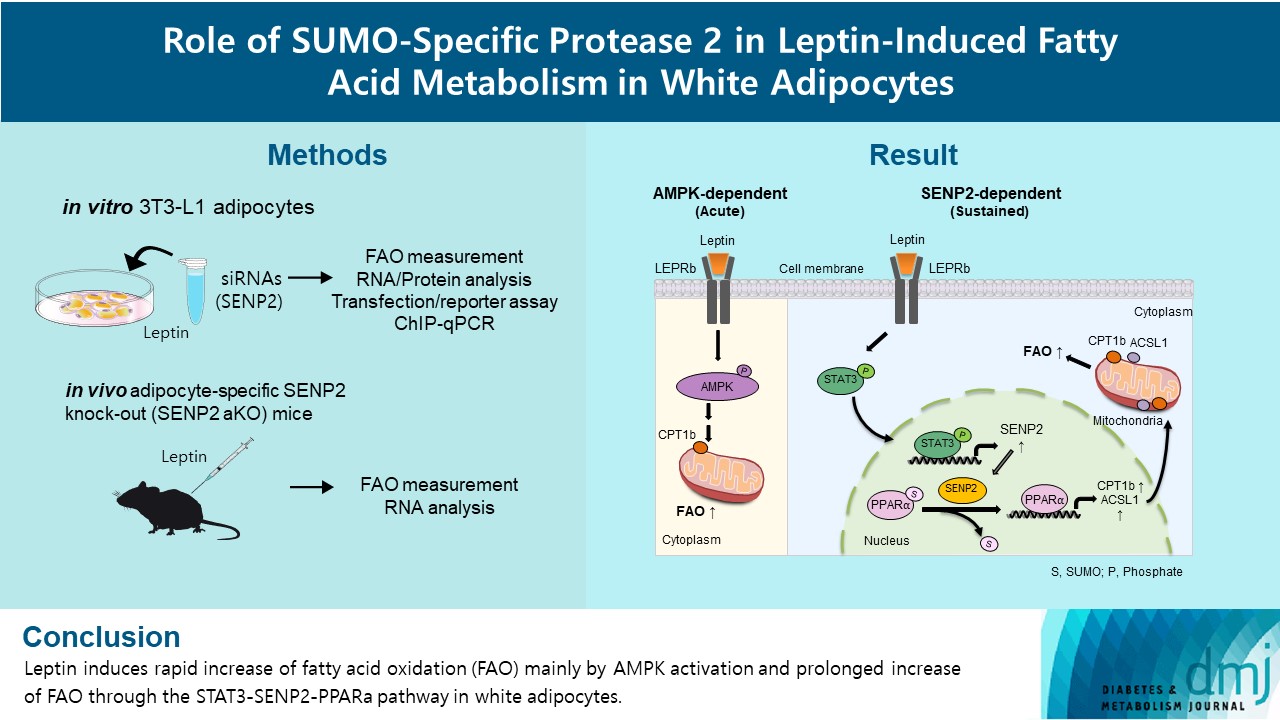
- Role of SUMO-Specific Protease 2 in Leptin-Induced Fatty Acid Metabolism in White Adipocytes
- Praise Chanmee Kim, Ji Seon Lee, Sung Soo Chung, Kyong Soo Park
- Diabetes Metab J. 2023;47(3):382-393. Published online March 6, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0156
- 3,194 View
- 158 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Leptin is a 16-kDa fat-derived hormone with a primary role in controlling adipose tissue levels. Leptin increases fatty acid oxidation (FAO) acutely through adenosine monophosphate-activated protein kinase (AMPK) and on delay through the SUMO-specific protease 2 (SENP2)–peroxisome proliferator-activated receptor δ/γ (PPARδ/γ) pathway in skeletal muscle. Leptin also directly increases FAO and decreases lipogenesis in adipocytes; however, the mechanism behind these effects remains unknown. Here, we investigated the role of SENP2 in the regulation of fatty acid metabolism by leptin in adipocytes and white adipose tissues.
Methods
The effects of leptin mediated by SENP2 on fatty acid metabolism were tested by siRNA-mediated knockdown in 3T3-L1 adipocytes. The role of SENP2 was confirmed in vivo using adipocyte-specific Senp2 knockout (Senp2-aKO) mice. We revealed the molecular mechanism involved in the leptin-induced transcriptional regulation of carnitine palmitoyl transferase 1b (Cpt1b) and long-chain acyl-coenzyme A synthetase 1 (Acsl1) using transfection/reporter assays and chromatin immunoprecipitation.
Results
SENP2 mediated the increased expression of FAO-associated enzymes, CPT1b and ACSL1, which peaked 24 hours after leptin treatment in adipocytes. In contrast, leptin stimulated FAO through AMPK during the initial several hours after treatment. In white adipose tissues, FAO and mRNA levels of Cpt1b and Acsl1 were increased by 2-fold 24 hours after leptin injection in control mice but not in Senp2-aKO mice. Leptin increased PPARα binding to the Cpt1b and Acsl1 promoters in adipocytes through SENP2.
Conclusion
These results suggest that the SENP2-PPARα pathway plays an important role in leptin-induced FAO in white adipocytes. -
Citations
Citations to this article as recorded by- Intermittent cold stimulation affects energy metabolism and improves stress resistance in broiler heart
Tingting Li, Haidong Wei, Shijie Zhang, Xiaotao Liu, Lu Xing, Yuanyuan Liu, Rixin Gong, Jianhong Li
Poultry Science.2024; 103(1): 103190. CrossRef
- Intermittent cold stimulation affects energy metabolism and improves stress resistance in broiler heart
- Basic Research
- Role of CRTC2 in Metabolic Homeostasis: Key Regulator of Whole-Body Energy Metabolism?
- Hye-Sook Han, Yongmin Kwon, Seung-Hoi Koo
- Diabetes Metab J. 2020;44(4):498-508. Published online March 5, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0200

- 6,942 View
- 163 Download
- 14 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Cyclic adenosine monophosphate (cAMP) signaling is critical for regulating metabolic homeostasis in mammals. In particular, transcriptional regulation by cAMP response element-binding protein (CREB) and its coactivator, CREB-regulated transcription coactivator (CRTC), is essential for controlling the expression of critical enzymes in the metabolic process, leading to more chronic changes in metabolic flux. Among the CRTC isoforms, CRTC2 is predominantly expressed in peripheral tissues and has been shown to be associated with various metabolic pathways in tissue-specific manners. While initial reports showed the physiological role of CRTC2 in regulating gluconeogenesis in the liver, recent studies have further delineated the role of this transcriptional coactivator in the regulation of glucose and lipid metabolism in various tissues, including the liver, pancreatic islets, endocrine tissues of the small intestines, and adipose tissues. In this review, we discuss recent studies that have utilized knockout mouse models to delineate the role of CRTC2 in the regulation of metabolic homeostasis.
-
Citations
Citations to this article as recorded by- Integration of genomic and transcriptomic data of inbred mouse models for polygenic obesity and leanness revealed “obese” and “lean” candidate alleles in polyadenylation signals
Martin Šimon, Špela Mikec, Nicholas M. Morton, Santosh S. Atanur, Simon Horvat, Tanja Kunej
Gene Reports.2024; 35: 101903. CrossRef - Mylabris phalerata induces the apoptosis and cell cycle delay in HCC, and potentiates the effect of sorafenib based on the molecular and network pharmacology approach
Young Woo Kim, Seon Been Bak, Su Youn Baek, Il Kon Kim, Won-Yung Lee, Un-Jung Yun, Kwang-Il Park
Molecular & Cellular Toxicology.2023; 19(4): 731. CrossRef - Emerging Role of SMILE in Liver Metabolism
Nanthini Sadasivam, Kamalakannan Radhakrishnan, Hueng-Sik Choi, Don-Kyu Kim
International Journal of Molecular Sciences.2023; 24(3): 2907. CrossRef - PIMT regulates hepatic gluconeogenesis in mice
Bandish Kapadia, Soma Behera, Sireesh T. Kumar, Tapan Shah, Rebecca Kristina Edwin, Phanithi Prakash Babu, Partha Chakrabarti, Kishore V.L. Parsa, Parimal Misra
iScience.2023; 26(3): 106120. CrossRef - Biological functions of CRTC2 and its role in metabolism-related diseases
Hong-Yu Zheng, Yan-Xia Wang, Kun Zhou, Hai-Lin Xie, Zhong Ren, Hui-Ting Liu, Yang-Shao Ou, Zhi-Xiang Zhou, Zhi-Sheng Jiang
Journal of Cell Communication and Signaling.2023; 17(3): 495. CrossRef - An insulin-regulated arrestin domain protein controls hepatic glucagon action
Sezin Dagdeviren, Megan F. Hoang, Mohsen Sarikhani, Vanessa Meier, Jake C. Benoit, Marinna C. Okawa, Veronika Y. Melnik, Elisabeth M. Ricci-Blair, Natalie Foot, Randall H. Friedline, Xiaodi Hu, Lauren A. Tauer, Arvind Srinivasan, Maxim B. Prigozhin, Sudha
Journal of Biological Chemistry.2023; 299(8): 105045. CrossRef - The Pleiotropic Face of CREB Family Transcription Factors
Md. Arifur Rahman Chowdhury, Jungeun An, Sangyun Jeong
Molecules and Cells.2023; 46(7): 399. CrossRef - It is a branched road to adipose tissue aging
N. Touitou, B. Lerrer, H. Y. Cohen
Nature Aging.2023; 3(8): 911. CrossRef - Impaired BCAA catabolism in adipose tissues promotes age-associated metabolic derangement
Hye-Sook Han, Eunyong Ahn, Eun Seo Park, Tom Huh, Seri Choi, Yongmin Kwon, Byeong Hun Choi, Jueun Lee, Yoon Ha Choi, Yujin L. Jeong, Gwang Bin Lee, Minji Kim, Je Kyung Seong, Hyun Mu Shin, Hang-Rae Kim, Myeong Hee Moon, Jong Kyoung Kim, Geum-Sook Hwang, S
Nature Aging.2023; 3(8): 982. CrossRef - Exploring the diagnostic value, prognostic value, and biological functions of NPC gene family members in hepatocellular carcinoma based on a multi-omics analysis
Keheng Chen, Xin Zhang, Huixin Peng, Fengdie Huang, Guangyu Sun, Qijiang Xu, Lusheng Liao, Zhiyong Xing, Yanping Zhong, Zhichao Fang, Meihua Liao, Shihua Luo, Wencheng Chen, Mingyou Dong
Functional & Integrative Genomics.2023;[Epub] CrossRef - MicroRNA regulation of AMPK in nonalcoholic fatty liver disease
Hao Sun, Jongsook Kim Kemper
Experimental & Molecular Medicine.2023; 55(9): 1974. CrossRef - Serine active site containing protein 1 depletion alters lipid metabolism and protects against high fat diet-induced obesity in mice
Miaomiao Du, Xueyun Li, Fangyi Xiao, Yinxu Fu, Yu Shi, Sihan Guo, Lifang Chen, Lu Shen, Lan Wang, Huang Cheng, Hao Li, Anran Xie, Yaping Zhou, Kaiqiang Yang, Hezhi Fang, Jianxin Lyu, Qiongya Zhao
Metabolism.2022; 134: 155244. CrossRef - cAMP Signaling in Cancer: A PKA-CREB and EPAC-Centric Approach
Muhammad Bilal Ahmed, Abdullah A. A. Alghamdi, Salman Ul Islam, Joon-Seok Lee, Young-Sup Lee
Cells.2022; 11(13): 2020. CrossRef - Hepatic Sam68 Regulates Systemic Glucose Homeostasis and Insulin Sensitivity
Aijun Qiao, Wenxia Ma, Ying Jiang, Chaoshan Han, Baolong Yan, Junlan Zhou, Gangjian Qin
International Journal of Molecular Sciences.2022; 23(19): 11469. CrossRef - The Role of Small Heterodimer Partner-Interacting Leucine Zipper
(SMILE) as a Transcriptional Corepressor in Hepatic Glucose and Lipid
Metabolism
Woo-Ram Park, Byungyoon Choi, Nanthini Sadasivam, Don-Kyu Kim
Trends in Agriculture & Life Sciences.2022; 60: 7. CrossRef - AMPK Localization: A Key to Differential Energy Regulation
Qonita Afinanisa, Min Kyung Cho, Hyun-A Seong
International Journal of Molecular Sciences.2021; 22(20): 10921. CrossRef
- Integration of genomic and transcriptomic data of inbred mouse models for polygenic obesity and leanness revealed “obese” and “lean” candidate alleles in polyadenylation signals
- Pathophysiology
-
- Essential Role of Protein Arginine Methyltransferase 1 in Pancreas Development by Regulating Protein Stability of Neurogenin 3
- Kanghoon Lee, Hyunki Kim, Joonyub Lee, Chang-Myung Oh, Heein Song, Hyeongseok Kim, Seung-Hoi Koo, Junguee Lee, Ajin Lim, Hail Kim
- Diabetes Metab J. 2019;43(5):649-658. Published online April 8, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0232
- 5,194 View
- 70 Download
- 4 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Protein arginine methyltransferase 1 (PRMT1) is a major enzyme responsible for the formation of methylarginine in mammalian cells. Recent studies have revealed that PRMT1 plays important roles in the development of various tissues. However, its role in pancreas development has not yet been elucidated.
Methods Pancreatic progenitor cell-specific
Prmt1 knock-out (Prmt1 PKO) mice were generated and characterized for their metabolic and histological phenotypes and their levels ofNeurog3 gene expression and neurogenin 3 (NGN3) protein expression. Protein degradation assays were performed in mPAC cells.Results Prmt1 PKO mice showed growth retardation and a severely diabetic phenotype. The pancreatic size and β-cell mass were significantly reduced inPrmt1 PKO mice. Proliferation of progenitor cells during the secondary transition was decreased and endocrine cell differentiation was impaired. These defects in pancreas development could be attributed to the sustained expression of NGN3 in progenitor cells. Protein degradation assays in mPAC cells revealed that PRMT1 was required for the rapid degradation of NGN3.Conclusion PRMT1 critically contributes to pancreas development by destabilizing the NGN3 protein.
-
Citations
Citations to this article as recorded by- Arginine 65 methylation of Neurogenin 3 by PRMT1 is required for pancreatic endocrine development of hESCs
Gahyang Cho, Kwangbeom Hyun, Jieun Choi, Eunji Shin, Bumsoo Kim, Hail Kim, Jaehoon Kim, Yong-Mahn Han
Experimental & Molecular Medicine.2023; 55(7): 1506. CrossRef - Protein arginine methyltransferase 1 in the generation of immune megakaryocytes: A perspective review
Xinyang Zhao, Zechen Chong, Yabing Chen, X. Long Zheng, Qian-Fei Wang, Yueying Li
Journal of Biological Chemistry.2022; 298(11): 102517. CrossRef - Arginine 65 Methylation of Neurogenin 3 by PRMT1 Is Required for Pancreatic Endocrine Development of hESCs
Gahyang Cho, Kwangbeom Hyun, Jieun Choi, Eun Ji Shin, Bumsoo Kim, Hail Kim, Jaehoon Kim, Yong-Mahn Han
SSRN Electronic Journal .2022;[Epub] CrossRef - Protein Arginine Methyltransferase 1 Is Essential for the Meiosis of Male Germ Cells
Sahar Waseem, Sudeep Kumar, Kanghoon Lee, Byoung-Ha Yoon, Mirang Kim, Hail Kim, Keesook Lee
International Journal of Molecular Sciences.2021; 22(15): 7951. CrossRef - Proteome-Wide Alterations of Asymmetric Arginine Dimethylation Associated With Pancreatic Ductal Adenocarcinoma Pathogenesis
Meijin Wei, Chaochao Tan, Zhouqin Tang, Yingying Lian, Ying Huang, Yi Chen, Congwei Chen, Wen Zhou, Tao Cai, Jiliang Hu
Frontiers in Cell and Developmental Biology.2020;[Epub] CrossRef
- Arginine 65 methylation of Neurogenin 3 by PRMT1 is required for pancreatic endocrine development of hESCs
- Glycosphingolipid Modification: Structural Diversity, Functional and Mechanistic Integration of Diabetes
- Tadashi Yamashita
- Diabetes Metab J. 2011;35(4):309-316. Published online August 31, 2011
- DOI: https://doi.org/10.4093/dmj.2011.35.4.309
- 2,920 View
- 34 Download
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Glycosphingolipids (GSLs) are present in all mammalian cell plasma membranes and intracellular membrane structures. They are especially concentrated in plasma membrane lipid domains that are specialized for cell signaling. Plasma membranes have typical structures called rafts and caveola domain structures, with large amounts of sphingolipids, cholesterol, and sphingomyelin. GSLs are usually observed in many organs ubiquitously. However, GSLs, including over 400 derivatives, participate in diverse cellular functions. Several studies indicate that GSLs might have an effect on signal transduction related to insulin receptors and epidermal growth factor receptors. GSLs may modulate immune responses by transmitting signals from the exterior to the interior of the cell. Guillain-Barré syndrome is one of the autoimmune disorders characterized by symmetrical weakness in the muscles of the legs. The targets of the immune response are thought to be gangliosides, which are one group of GSLs. Other GSLs may serve as second messengers in several signaling pathways that are important to cell survival or programmed cell death. In the search for clear evidence that GSLs may play critical roles in various biological functions, many researchers have made genetically engineered mice. Before the era of gene manipulation, spontaneous animal models or chemical-induced disease models were used.
-
Citations
Citations to this article as recorded by- Quantitative proteomics reveals Piccolo as a candidate serological correlate of recovery from Guillain-Barré syndrome
Lourdes Mateos-Hernández, Margarita Villar, Ernesto Doncel-Pérez, Marco Trevisan-Herraz, Ángel García-Forcada, Francisco Romero Ganuza, Jesús Vázquez, José de la Fuente
Oncotarget.2016; 7(46): 74582. CrossRef - Convergence and divergence of genetic and modular networks between diabetes and breast cancer
Xiaoxu Zhang, Yingying Zhang, Yanan Yu, Jun Liu, Ye Yuan, Yijun Zhao, Haixia Li, Jie Wang, Zhong Wang
Journal of Cellular and Molecular Medicine.2015; 19(5): 1094. CrossRef - Profiling over 1500 Lipids in Induced Lung Sputum and the Implications in Studying Lung Diseases
Ruben t’Kindt, Eef D. Telenga, Lucie Jorge, Antoon J. M. Van Oosterhout, Pat Sandra, Nick H. T. Ten Hacken, Koen Sandra
Analytical Chemistry.2015; 87(9): 4957. CrossRef - Untargeted Lipidomic Analysis in Chronic Obstructive Pulmonary Disease. Uncovering Sphingolipids
Eef D. Telenga, Roland F. Hoffmann, Ruben t’Kindt, Susan J. M. Hoonhorst, Brigitte W. M. Willemse, Antoon J. M. van Oosterhout, Irene H. Heijink, Maarten van den Berge, Lucie Jorge, Pat Sandra, Dirkje S. Postma, Koen Sandra, Nick H. T. ten Hacken
American Journal of Respiratory and Critical Care Medicine.2014; 190(2): 155. CrossRef - Immunological cell type characterization and Th1–Th17 cytokine production in a mouse model of Gaucher disease
Manoj Kumar Pandey, Reena Rani, Wujuan Zhang, Kenneth Setchell, Gregory A. Grabowski
Molecular Genetics and Metabolism.2012; 106(3): 310. CrossRef
- Quantitative proteomics reveals Piccolo as a candidate serological correlate of recovery from Guillain-Barré syndrome
- Effect of Mouse Type and Human Type of CpG Oligonucleotide Vaccination on Development of Diabetes in NOD Mice.
- Byong Jun Lee, Soo Kie Kim, Eon Sub Park, Hyun Jin Jang, Hyun Chul Cho, Myung Sook Shim, Mi Jin Kim, Young Goo Shin, Choon Hee Chung
- Korean Diabetes J. 2002;26(6):451-459. Published online December 1, 2002
- 1,041 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Type 1 diabetes is autoimmune disease and the modulation of immune system could offer breakthrough to the disease. Unmethylated CpG motifs and their oligoneucleotide are potent immunostimulators that can rebalance autoimmune mechanism. To explore DNA based immunotherapy in type 1 diabetes, we vaccinated different types (mouse and human) of CpG ODN to NOD mice. METHODS: Forty 5 week-old female NOD mice were injected with 100 L (10 g) of mouse type CpG ODN or human type CpG ODN or 0.9% normal saline on inguinal area subcutaneously. Seven, 14, and 28 days later we injected to mice same dose of mouse type CpG ODN or human type CpG ODN or normal saline. Blood glucose was measured and mice were sacrificed when they were diabetic. Pancreata and serum were earned from sacrificed NOD mice to evaluate insulitis and insulin immunoassay. RESULTS: Though the final cumulative incidences of diabetes were not significantly different among groups, the tendency of delaying and suppressing the development of diabetes was observed in the early period of vaccination group of CpG ODN. Especially, mouse type CpG ODN was more effective for rodent species than human type CpG ODN. CONCLUSION: This result suggests that immunomodulation therapy using species- specific CpG motif may have a potential to control autoimmune process as well as dissecting T cell milieu in NOD mice.
- Distinct Pattern of GAD65 and GAD67 Gene Expression in the Pancreas of NOD Mouse.
- In Young Ko, Yup Kang
- Korean Diabetes J. 1997;21(3):243-253. Published online January 1, 2001
- 689 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Glutamic acid decarboxylase(GAD; EC 4.1.1.15), one of the major B-cell autoantigens in IDDM, is an enzyme which catalyzes the synthesis of major inhibitory neurotransmitter, r-aminobutyric acid (GARA), in the mammalian brain, pancreas and other organs. Two isoforms of GAD, GAD65 and GAD67, have been identified which differ in their intracellular localization. Autoantibodies to GAD have been detected several years before the clinical onset of IDDM, implicating GAD as a leading autoantigen which somehow correlated with the pathogenesis of IDDM. We have determined the characteristics of GAD isoform expression in the pancreas of NOD mouse, an animal model extensively employed in IDDM study, using RT-PCR and Southern blot methods. METHODS: Pancreas was obtained from female NOD mouse(neonate, 4, 8, 12, 16, 20 week-old) and age-matched female ICR mouse. Total cellular RNA was I.solated by acid guanidinium thiocyanate method and employed in the RT-PCR amplification using GAD65- and GAD67-specific primer designed in our laboratory. The PCR product was blotted onto the nylon membrane and subjected to Southern analysis using 32P-ATP labelled hybridization probe. RESULTS: In NOD pancreas, GAD67 was expressed six times higher than GAD65 at neonatal stage. Then, the expression was dramatically decreased from 4 weeks when the pancreatic insulitis begins to occur. After 12 weeks of age, both GAD67 and GAD65 expression was almost undetectable. However, in control ICR mouse, there were no significant differenees between GAD65 and GAD67 expression throughout the ages. And, the expression of both GAD65 and OAD67 was not decreased with ages in contrast to NOD mouse. CONCLUSION: In this experiment, we found that the expression of GAD isoforms in NOD mouse shows distinct pattern in comparison to that of control ICR mouse. The expression of GAD67 was significantly higher than GAD65 in neonatal NOD mouse while, in control ICR mouse, same level of GAD isoforrns expression was observed. This finding clearly suggested the possibility that the expression of GAD isoforms in diabetic NOD mouse is quite distinct and may somehow play a role in the pathogenesis of diabetes although the precise mechanism remains to be unveiled. In addition, our data also supported the hypothesis that expressional pattern, and, if possible, ' the etiophysiological function of GAD isoforms in NOD mouse pancreas may be quite different from that in human pancreas.

 KDA
KDA
 First
First Prev
Prev





