
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Drug/Regimen
- Efficacy and Safety of IDegAsp in a Real-World Korean Population with Type 2 Diabetes Mellitus
- Shinae Kang, Yu-Bae Ahn, Tae Keun Oh, Won-Young Lee, Sung Wan Chun, Boram Bae, Amine Dahaoui, Jin Sook Jeong, Sungeun Jung, Hak Chul Jang
- Received August 24, 2023 Accepted November 22, 2023 Published online February 27, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0297 [Epub ahead of print]
- 689 View
- 49 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
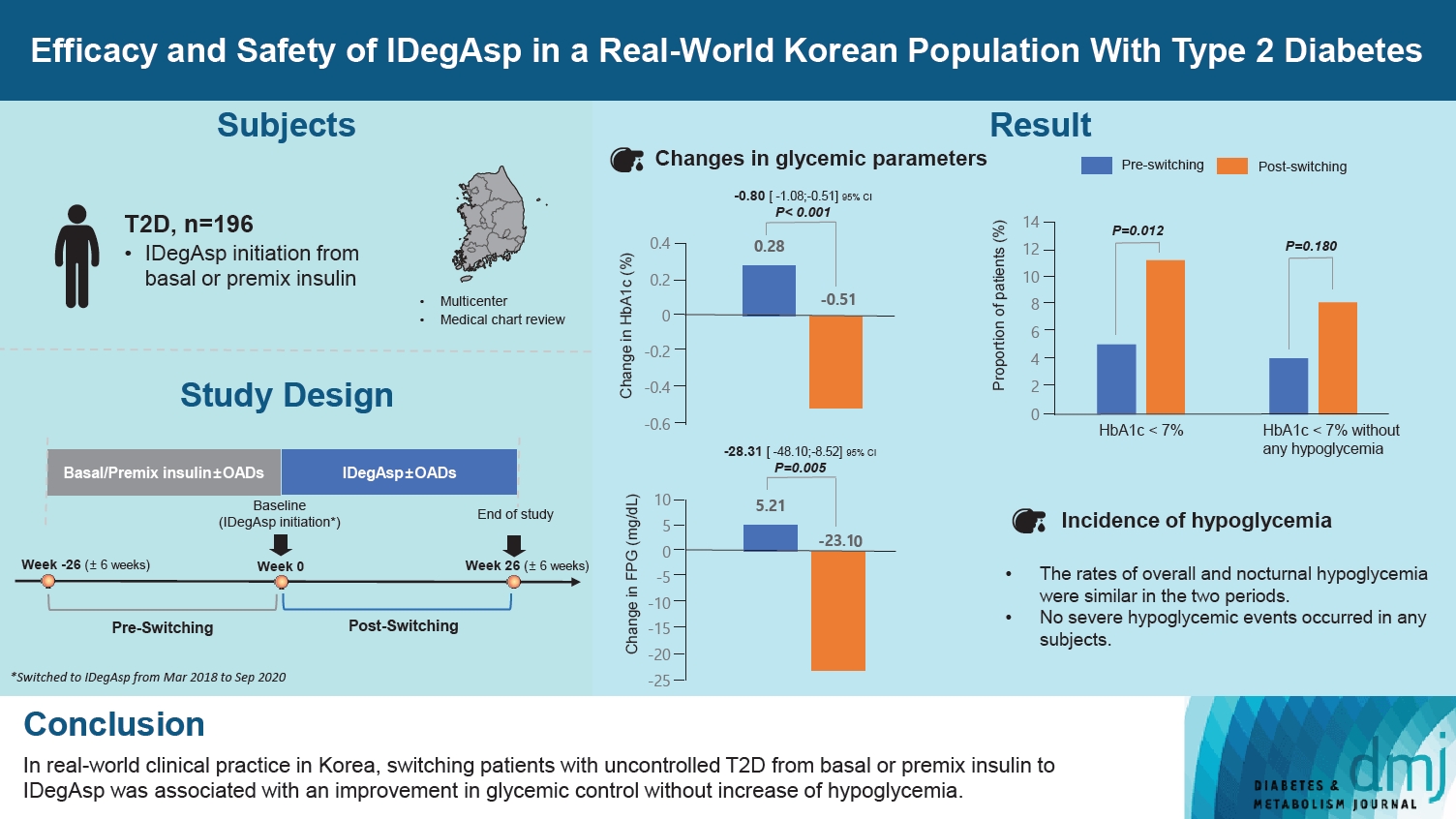
This study investigated the real-world efficacy and safety of insulin degludec/insulin aspart (IDegAsp) in Korean adults with type 2 diabetes mellitus (T2DM), whose insulin treatment was switched to IDegAsp.
Methods
This was a multicenter, retrospective, observational study comprising two 26-week treatment periods, before and after switching to IDegAsp, respectively. Korean adults with uncontrolled T2DM treated with basal or premix insulin (±oral antidiabetic drugs) were enrolled. The primary objective was to compare the degree of glycosylated hemoglobin (HbA1c) change in each 26-week observation period. The analyses included changes in HbA1c, fasting plasma glucose (FPG), body weight, proportion of participants achieving HbA1c <7.0%, hypoglycemic events, and total daily insulin dose (ClinicalTrials.gov, number NCT04656106).
Results
In total, 196 adults (mean age, 65.95 years; mean T2DM duration, 18.99 years) were analyzed. The change in both HbA1c and FPG were significantly different between the pre-switching and the post-switching period (0.28% vs. –0.51%, P<0.001; 5.21 mg/dL vs. –23.10 mg/dL, P=0.005), respectively. After switching, the rate of achieving HbA1c <7.0% was significantly improved (5.10% at baseline vs. 11.22% with IDegAsp, P=0.012). No significant differences (before vs. after switching) were observed in body weight change, and total daily insulin dose. The rates of overall and severe hypoglycemia were similar in the two periods.
Conclusion
In real-world clinical practice in Korea, the change of insulin regimen to IDegAsp was associated with an improvement in glycemic control without increase of hypoglycemia, supporting the use of IDegAsp for patients with T2DM uncontrolled with basal or premix insulin.
- Drug/Regimen
- Switching to Once-Daily Insulin Degludec/Insulin Aspart from Basal Insulin Improves Postprandial Glycemia in Patients with Type 2 Diabetes Mellitus: Randomized Controlled Trial
- Kyu Yong Cho, Akinobu Nakamura, Chiho Oba-Yamamoto, Kazuhisa Tsuchida, Shingo Yanagiya, Naoki Manda, Yoshio Kurihara, Shin Aoki, Tatsuya Atsumi, Hideaki Miyoshi
- Diabetes Metab J. 2020;44(4):532-541. Published online November 22, 2019
- DOI: https://doi.org/10.4093/dmj.2019.0093
- 5,614 View
- 157 Download
- 7 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
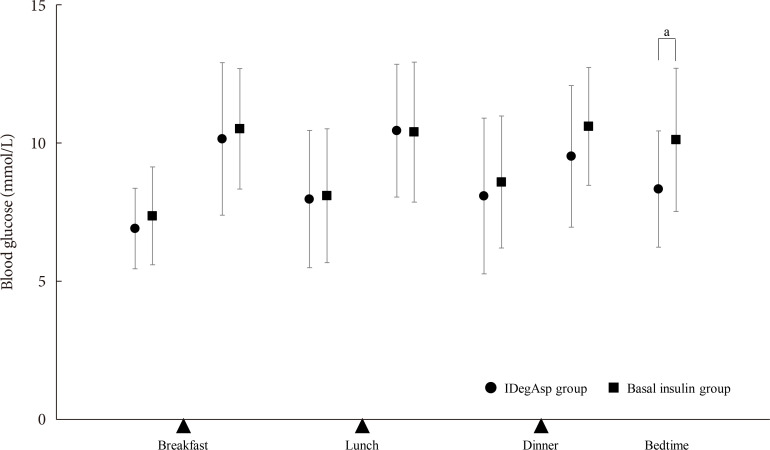
ePub Background To explore the efficacy and safety of switching from once-daily basal insulin therapy to once-daily pre-meal injection insulin degludec/insulin aspart (IDegAsp) with respect to the glycemic control of participants with type 2 diabetes mellitus (T2DM).
Methods In this multicenter, open-label, prospective, randomized, parallel-group comparison trial, participants on basal insulin therapy were switched to IDegAsp (IDegAsp group;
n =30) or continued basal insulin (Basal group;n =29). The primary endpoint was the superiority of IDegAsp in causing changes in the daily blood glucose profile, especially post-prandial blood glucose concentration after 12 weeks.Results Blood glucose concentrations after dinner and before bedtime were lower in the IDegAsp group, and the improvement in blood glucose before bedtime was significantly greater in the IDegAsp group than in the Basal group at 12 weeks (−1.7±3.0 mmol/L vs. 0.3±2.1 mmol/L,
P <0.05). Intriguingly, glycemic control after breakfast was not improved by IDegAsp injection before breakfast, in contrast to the favorable effect of injection before dinner on blood glucose after dinner. Glycosylated hemoglobin significantly decreased only in the IDegAsp group (58 to 55 mmol/mol,P <0.05). Changes in daily insulin dose, body mass, and recorded adverse effects, including hypoglycemia, were comparable between groups.Conclusion IDegAsp was more effective than basal insulin at reducing blood glucose after dinner and before bedtime, but did not increase the incidence of hypoglycemia. Switching from basal insulin to IDegAsp does not increase the burden on the patient and positively impacts glycemic control in patients with T2DM.
-
Citations
Citations to this article as recorded by- Glycaemic outcomes in hospital with IDegAsp versus BIAsp30 premixed insulins
Joshua R. Walt, Julie Loughran, Spiros Fourlanos, Rahul D. Barmanray, Jasmine Zhu, Suresh Varadarajan, Mervyn Kyi
Internal Medicine Journal.2024;[Epub] CrossRef - Low fasting glucose‐to‐estimated average glucose ratio was associated with superior response to insulin degludec/aspart compared with basal insulin in patients with type 2 diabetes
Han Na Jang, Ye Seul Yang, Tae Jung Oh, Bo Kyung Koo, Seong Ok Lee, Kyong Soo Park, Hak Chul Jang, Hye Seung Jung
Journal of Diabetes Investigation.2022; 13(1): 85. CrossRef - Comparing time to intensification between insulin degludec/insulin aspart and insulin glargine: A single-center experience from India
Rajiv Kovil
Journal of Diabetology.2022; 13(2): 171. CrossRef - Use of Insulin Degludec/Insulin Aspart in the Management of Diabetes Mellitus: Expert Panel Recommendations on Appropriate Practice Patterns
Tevfik Demir, Serap Turan, Kursad Unluhizarci, Oya Topaloglu, Tufan Tukek, Dilek Gogas Yavuz
Frontiers in Endocrinology.2021;[Epub] CrossRef - Pharmacoeconomic comparison of the second generation insulin analogs and insulins on their base
I. N. Dyakov, S. K. Zyryanov
Kachestvennaya Klinicheskaya Praktika = Good Clinical Practice.2021; 20(1): 4. CrossRef - Efficacy and Safety of Insulin Degludec/Insulin Aspart Compared with a Conventional Premixed Insulin or Basal Insulin: A Meta-Analysis
Shinje Moon, Hye-Soo Chung, Yoon-Jung Kim, Jae-Myung Yu, Woo-Ju Jeong, Jiwon Park, Chang-Myung Oh
Metabolites.2021; 11(9): 639. CrossRef - Insulin therapy in diabetic kidney disease
Yan Liu, Chanyue Zhao, Xiaofen Xiong, Ming Yang, Lin Sun
Diabetic Nephropathy.2021; 1(2): 67. CrossRef - Indirect comparison of efficacy and safety of insulin glargine/lixisenatide and insulin degludec/insulin aspart in type 2 diabetes patients not controlled on basal insulin
Anwar Ali Jammah
Primary Care Diabetes.2020;[Epub] CrossRef
- Glycaemic outcomes in hospital with IDegAsp versus BIAsp30 premixed insulins

 KDA
KDA
 First
First Prev
Prev





