
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Genetics
- The rs2304256 Polymorphism in TYK2 Gene Is Associated with Protection for Type 1 Diabetes Mellitus
- Felipe Mateus Pellenz, Cristine Dieter, Guilherme Coutinho Kullmann Duarte, Luís Henrique Canani, Bianca Marmontel de Souza, Daisy Crispim
- Diabetes Metab J. 2021;45(6):899-908. Published online May 24, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0194
- 4,777 View
- 157 Download
- 2 Web of Science
- 3 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub 
- Background
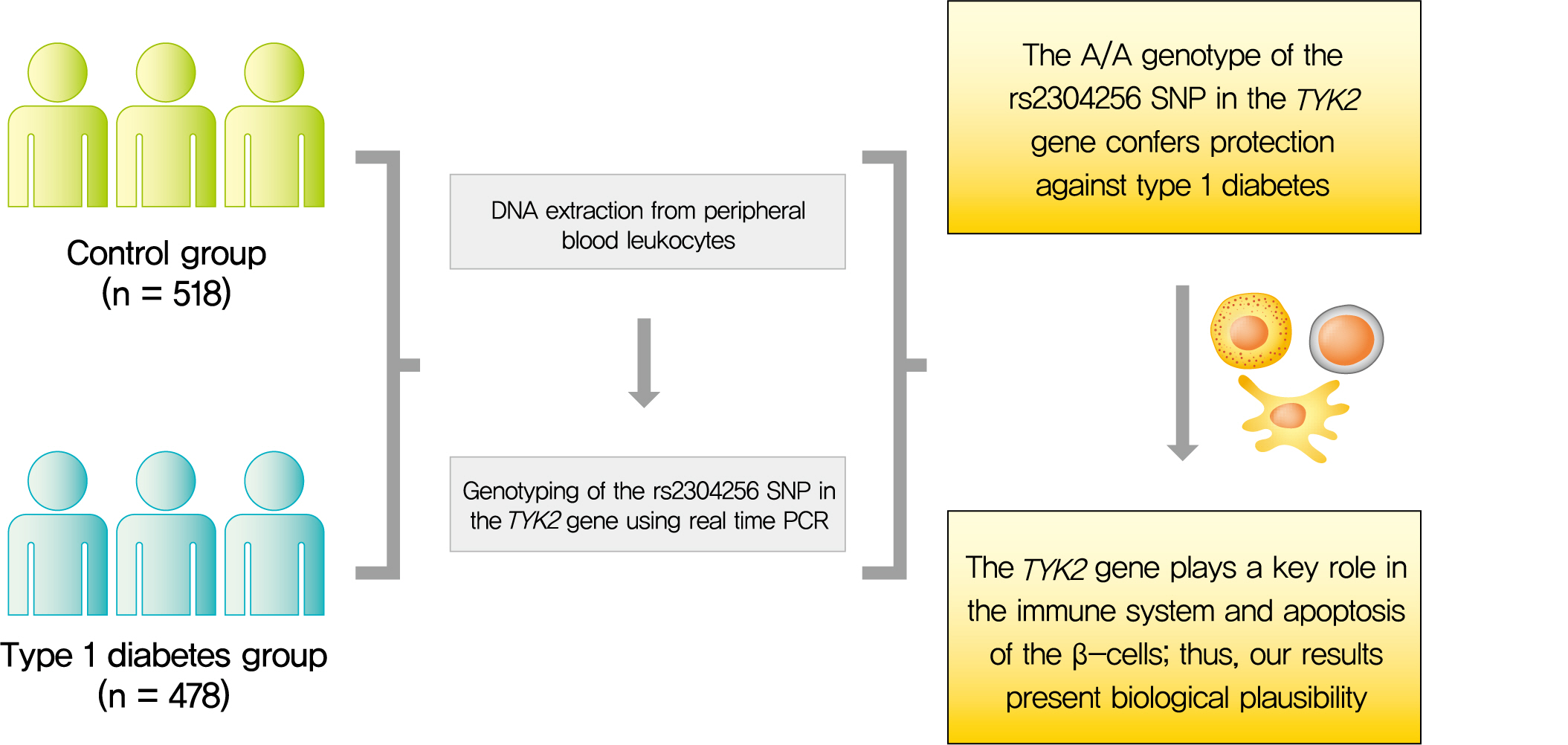
Tyrosine kinase 2 (TYK2) is a candidate gene for type 1 diabetes mellitus (T1DM) since it plays an important role in regulating apoptotic and pro-inflammatory pathways in pancreatic β-cells through modulation of the type I interferon signaling pathway. The rs2304256 single nucleotide polymorphism (SNP) in TYK2 gene has been associated with protection for different autoimmune diseases. However, to date, only two studies have evaluated the association between this SNP and T1DM, with discordant results. This study thus aimed to investigate the association between the TYK2 rs2304256 SNP and T1DM in a Southern Brazilian population.
Methods
This case-control study comprised 478 patients with T1DM and 518 non-diabetic subjects. The rs2304256 (C/A) SNP was genotyped by real-time polymerase chain reaction technique using TaqMan minor groove binder (MGB) probes.
Results
Genotype and allele frequencies of the rs2304256 SNP differed between T1DM patients and non-diabetic subjects (P<0.0001 and P=0.001, respectively). Furthermore, the A allele was associated with protection against T1DM under recessive (odds ratio [OR], 0.482; 95% confidence interval [CI], 0.288 to 0.806) and additive (OR, 0.470; 95% CI, 0.278 to 0.794) inheritance models, adjusting for human leukocyte antigen (HLA) DR/DQ genotypes, gender, and ethnicity.
Conclusion
The A/A genotype of TYK2 rs2304256 SNP is associated with protection against T1DM in a Southern Brazilian population. -
Citations
Citations to this article as recorded by- Associations of genetic variants within TYK2 with pulmonary tuberculosis among Chinese population
Mingwu Zhang, Zhengwei Liu, Yelei Zhu, Kunyang Wu, Lin Zhou, Ying Peng, Junhang Pan, Bin Chen, Xiaomeng Wang, Songhua Chen
Molecular Genetics & Genomic Medicine.2024;[Epub] CrossRef - Host genetic variants associated with COVID-19 reconsidered in a Slovak cohort
Maria Skerenova, Michal Cibulka, Zuzana Dankova, Veronika Holubekova, Zuzana Kolkova, Vincent Lucansky, Dana Dvorska, Andrea Kapinova, Michaela Krivosova, Martin Petras, Eva Baranovicova, Ivana Baranova, Elena Novakova, Peter Liptak, Peter Banovcin, Anna
Advances in Medical Sciences.2024; 69(1): 198. CrossRef - Cross-Domain Text Mining of Pathophysiological Processes Associated with Diabetic Kidney Disease
Krutika Patidar, Jennifer H. Deng, Cassie S. Mitchell, Ashlee N. Ford Versypt
International Journal of Molecular Sciences.2024; 25(8): 4503. CrossRef
- Associations of genetic variants within TYK2 with pulmonary tuberculosis among Chinese population
- Type 1 Diabetes
- Differential Profile of Plasma Circular RNAs in Type 1 Diabetes Mellitus
- Yangyang Li, Ying Zhou, Minghui Zhao, Jing Zou, Yuxiao Zhu, Xuewen Yuan, Qianqi Liu, Hanqing Cai, Cong-Qiu Chu, Yu Liu
- Diabetes Metab J. 2020;44(6):854-865. Published online July 13, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0151

- 6,202 View
- 131 Download
- 19 Web of Science
- 19 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub Background No currently available biomarkers or treatment regimens fully meet therapeutic needs of type 1 diabetes mellitus (T1DM). Circular RNA (circRNA) is a recently identified class of stable noncoding RNA that have been documented as potential biomarkers for various diseases. Our objective was to identify and analyze plasma circRNAs altered in T1DM.
Methods We used microarray to screen differentially expressed plasma circRNAs in patients with new onset T1DM (
n =3) and age-/gender-matched healthy controls (n =3). Then, we selected six candidates with highest fold-change and validated them by quantitative real-time polymerase chain reaction in independent human cohort samples (n =12). Bioinformatic tools were adopted to predict putative microRNAs (miRNAs) sponged by these validated circRNAs and their downstream messenger RNAs (mRNAs). Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were performed to gain further insights into T1DM pathogenesis.Results We identified 68 differentially expressed circRNAs, with 61 and seven being up- and downregulated respectively. Four of the six selected candidates were successfully validated. Curations of their predicted interacting miRNAs revealed critical roles in inflammation and pathogenesis of autoimmune disorders. Functional relations were visualized by a circRNA-miRNA-mRNA network. GO and KEGG analyses identified multiple inflammation-related processes that could be potentially associated with T1DM pathogenesis, including cytokine-cytokine receptor interaction, inflammatory mediator regulation of transient receptor potential channels and leukocyte activation involved in immune response.
Conclusion Our study report, for the first time, a profile of differentially expressed plasma circRNAs in new onset T1DM. Further
in silico annotations and bioinformatics analyses supported future application of circRNAs as novel biomarkers of T1DM.-
Citations
Citations to this article as recorded by- Non-coding RNAs and exosomal non-coding RNAs in diabetic retinopathy: A narrative review
Yuhong Zhong, Juan Xia, Li Liao, Mohammad Reza Momeni
International Journal of Biological Macromolecules.2024; 259: 128182. CrossRef - Circular RNAs: Potential biomarkers and therapeutic targets for autoimmune diseases
Ren-Jie Zhao, Wan-Ying Zhang, Xing-Xing Fan
Heliyon.2024; 10(1): e23694. CrossRef - Research progress of circular RNA molecules in aging and age-related diseases
Zhidan Zhang, Yuling Huang, AYao Guo, Lina Yang
Ageing Research Reviews.2023; 87: 101913. CrossRef - CircRNAs and RNA-Binding Proteins Involved in the Pathogenesis of Cancers or Central Nervous System Disorders
Yuka Ikeda, Sae Morikawa, Moeka Nakashima, Sayuri Yoshikawa, Kurumi Taniguchi, Haruka Sawamura, Naoko Suga, Ai Tsuji, Satoru Matsuda
Non-Coding RNA.2023; 9(2): 23. CrossRef - Decrypting the circular RNAs does a favor for us: Understanding, diagnosing and treating diabetes mellitus and its complications
Zi Li, Yuanyuan Ren, Ziwei Lv, Man Li, Yujia Li, Xiaobin Fan, Yuyan Xiong, Lu Qian
Biomedicine & Pharmacotherapy.2023; 168: 115744. CrossRef - Circular RNA PIP5K1A Promotes Glucose and Lipid Metabolism Disorders and Inflammation in Type 2 Diabetes Mellitus
Ge Song, YiQian Zhang, YiHua Jiang, Huan Zhang, Wen Gu, Xiu Xu, Jing Yao, ZhengFang Chen
Molecular Biotechnology.2023;[Epub] CrossRef - Hsa_circRNA_405498 and hsa_circRNA_100033 Serve as Potential Biomarkers for Differential Diagnosis of Type 1 Diabetes
Ziwei Zhang, Shuoming Luo, Zilin Xiao, Wenfeng Yin, Xiajie Shi, Hongzhi Chen, Zhiguo Xie, Zhenqi Liu, Xia Li, Zhiguang Zhou
The Journal of Clinical Endocrinology & Metabolism.2023;[Epub] CrossRef - Circular RNA PIP5K1A act as microRNA-552-3p sponge to regulates inflammation, oxidative damage in glucolipotoxicity-induced pancreatic INS-1 β-cells via Janus kinase 1
Lei Ren
Bioengineered.2022; 13(3): 5724. CrossRef - Circular RNAs in diabetes mellitus and its complications
Wenqi Fan, Haipeng Pang, Zhiguo Xie, Gan Huang, Zhiguang Zhou
Frontiers in Endocrinology.2022;[Epub] CrossRef - Type 1 Diabetes Mellitus-Related circRNAs Regulate CD4+ T Cell Functions
Jianni Chen, Guanfei Jia, Xue Lv, Shufa Li, Christos K. Kontos
BioMed Research International.2022; 2022: 1. CrossRef - An intriguing role of circular RNA in insulin resistance and endothelial dysfunction: the future perspectives
Monisha Prasad, Selvaraj Jayaraman, Vishnu Priya Veeraraghavan
Hypertension Research.2022; 45(11): 1843. CrossRef - Circular RNAs in Diabetic Nephropathy: Updates and Perspectives
Miao Liu, Junli Zhao
Aging and disease.2022; 13(5): 1365. CrossRef - CircRNAs: Key molecules in the prevention and treatment of ischemic stroke
Zeyu Liu, Yanhong Zhou, Jian Xia
Biomedicine & Pharmacotherapy.2022; 156: 113845. CrossRef - Pro-Inflammatory Cytokines Promote the Transcription of Circular RNAs in Human Pancreatic β Cells
Simranjeet Kaur, Caroline Frørup, Aashiq H. Mirza, Tina Fløyel, Reza Yarani, Maikel L. Colli, Jesper Johannesen, Joachim Størling, Decio L. Eizirik, Flemming Pociot
Non-Coding RNA.2022; 8(5): 69. CrossRef - Differential Expression and Bioinformatics Analysis of Plasma-Derived Exosomal circRNA in Type 1 Diabetes Mellitus
Haipeng Pang, Wenqi Fan, Xiajie Shi, Shuoming Luo, Yimeng Wang, Jian Lin, Yang Xiao, Xia Li, Gan Huang, Zhiguo Xie, Zhiguang Zhou, Jinhui Liu
Journal of Immunology Research.2022; 2022: 1. CrossRef - Circular RNAs in diabetes and its complications: Current knowledge and future prospects
Wenfeng Yin, Ziwei Zhang, Zilin Xiao, Xia Li, Shuoming Luo, Zhiguang Zhou
Frontiers in Genetics.2022;[Epub] CrossRef - Circular RNA in autoimmune diseases: special emphasis on regulation mechanism in RA and SLE
Yurong Huang, Qiuyun Xue, Chenglong Cheng, Yuting Wang, Xiao Wang, Jun Chang, Chenggui Miao
Journal of Pharmacy and Pharmacology.2022;[Epub] CrossRef - Emerging roles of circular RNAs in systemic lupus erythematosus
Xin Wang, Rui Ma, Weimin Shi, Zhouwei Wu, Yuling Shi
Molecular Therapy - Nucleic Acids.2021; 24: 212. CrossRef - Understanding Competitive Endogenous RNA Network Mechanism in Type 1 Diabetes Mellitus Using Computational and Bioinformatics Approaches
Xuanzi Yi, Xu Cheng
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2021; Volume 14: 3865. CrossRef
- Non-coding RNAs and exosomal non-coding RNAs in diabetic retinopathy: A narrative review
- Basic Research
- Histone Deacetylase 9: Its Role in the Pathogenesis of Diabetes and Other Chronic Diseases
- Siqi Hu, Eun-Hee Cho, Ji-Young Lee
- Diabetes Metab J. 2020;44(2):234-244. Published online March 24, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0243
- 6,410 View
- 161 Download
- 20 Web of Science
- 21 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader As a member of the class IIa histone deacetylases (HDACs), HDAC9 catalyzes the deacetylation of histones and transcription factors, commonly leading to the suppression of gene transcription. The activity of HDAC9 is regulated transcriptionally and post-translationally. HDAC9 is known to play an essential role in regulating myocyte and adipocyte differentiation and cardiac muscle development. Also, recent studies have suggested that HDAC9 is involved in the pathogenesis of chronic diseases, including cardiovascular diseases, osteoporosis, autoimmune disease, cancer, obesity, insulin resistance, and liver fibrosis. HDAC9 modulates the expression of genes related to the pathogenesis of chronic diseases by altering chromatin structure in their promotor region or reducing the transcriptional activity of their respective transcription factors. This review summarizes the current knowledge of the regulation of HDAC9 expression and activity. Also, the roles of HDAC9 in the pathogenesis of chronic diseases are discussed, along with potential underlying mechanisms.
-
Citations
Citations to this article as recorded by- Impact of housing temperature on adipose tissue HDAC9 expression and adipogenic differentiation in high fat‐fed mice
Samah Ahmadieh, Brandee Goo, Abdalrahman Zarzour, David Kim, Hong Shi, Praneet Veerapaneni, Ronnie Chouhaita, Nicole K. H. Yiew, Carla Dominguez Gonzalez, Akash Chakravartty, James Pennoyer, Nazeera Hassan, Tyler W. Benson, Mourad Ogbi, David J. Fulton, R
Obesity.2024; 32(1): 107. CrossRef - HDAC9 inhibition reduces skeletal muscle atrophy and enhances regeneration in mice with cigarette smoke-induced COPD
Guixian Zheng, Chao Li, Xiaoli Chen, Zhaohui Deng, Ting Xie, Zengyu Huo, Xinyan Wei, Yanbing Huang, Xia Zeng, Yu Luo, Jing Bai
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2024; 1870(3): 167023. CrossRef - Identification of HDAC9 and ARRDC4 as potential biomarkers and targets for treatment of type 2 diabetes
Jing Liu, Lingzhen Meng, Zhihong Liu, Ming Lu, Ruiying Wang
Scientific Reports.2024;[Epub] CrossRef - HDAC9 as a Privileged Target: Reviewing its Role in Different Diseases
and Structure-activity Relationships (SARs) of its Inhibitors
Totan Das, Samima Khatun, Tarun Jha, Shovanlal Gayen
Mini-Reviews in Medicinal Chemistry.2024; 24(7): 767. CrossRef - Targeting histone deacetylases for cancer therapy: Trends and challenges
Tao Liang, Fengli Wang, Reham M. Elhassan, Yongmei Cheng, Xiaolei Tang, Wengang Chen, Hao Fang, Xuben Hou
Acta Pharmaceutica Sinica B.2023; 13(6): 2425. CrossRef - Therapeutic approach of natural products that treat osteoporosis by targeting epigenetic modulation
Guokai Zhang, Zhenying Liu, Zihan Li, Bing Zhang, Pengyu Yao, Yun Qiao
Frontiers in Genetics.2023;[Epub] CrossRef - Research Progress on Histone Deacetylase Inhibitors
玉姜 汤
Hans Journal of Medicinal Chemistry.2023; 11(02): 116. CrossRef - HDAC9 Inhibition as a Novel Treatment for Stroke
Hugh S. Markus
Stroke.2023; 54(12): 3182. CrossRef - Histone deacetylase 9 exacerbates podocyte injury in hyperhomocysteinemia through epigenetic repression of Klotho
Min Liu, Yang Zhang, Ping Zhan, Wenjuan Sun, Chuanqiao Dong, Xiaohan Liu, Yujie Yang, Xiaojie Wang, Yusheng Xie, Chengjiang Gao, Huili Hu, Benkang Shi, Ziying Wang, Chun Guo, Fan Yi
Pharmacological Research.2023; 198: 107009. CrossRef - Molecular mechanism and therapeutic potential of HDAC9 in intervertebral disc degeneration
Ming Lei, Hui Lin, Deyao Shi, Pan Hong, Hui Song, Bomansaan Herman, Zhiwei Liao, Cao Yang
Cellular & Molecular Biology Letters.2023;[Epub] CrossRef - Interindividual variability in transgene mRNA and protein production following adeno-associated virus gene therapy for hemophilia A
Sylvia Fong, Bridget Yates, Choong-Ryoul Sihn, Aras N. Mattis, Nina Mitchell, Su Liu, Chris B. Russell, Benjamin Kim, Adebayo Lawal, Savita Rangarajan, Will Lester, Stuart Bunting, Glenn F. Pierce, K. John Pasi, Wing Yen Wong
Nature Medicine.2022; 28(4): 789. CrossRef - Active RhoA Exerts an Inhibitory Effect on the Homeostasis and Angiogenic Capacity of Human Endothelial Cells
Michael Hauke, Robert Eckenstaler, Anne Ripperger, Anna Ender, Heike Braun, Ralf A. Benndorf
Journal of the American Heart Association.2022;[Epub] CrossRef - HDAC9 Contributes to Serous Ovarian Cancer Progression through Regulating Epithelial–Mesenchymal Transition
Long Xu, Jian Wang, Buhan Liu, Jiaying Fu, Yuanxin Zhao, Sihang Yu, Luyan Shen, Xiaoyu Yan, Jing Su
Biomedicines.2022; 10(2): 374. CrossRef - Common protein-coding variants influence the racing phenotype in galloping racehorse breeds
Haige Han, Beatrice A. McGivney, Lucy Allen, Dongyi Bai, Leanne R. Corduff, Gantulga Davaakhuu, Jargalsaikhan Davaasambuu, Dulguun Dorjgotov, Thomas J. Hall, Andrew J. Hemmings, Amy R. Holtby, Tuyatsetseg Jambal, Badarch Jargalsaikhan, Uyasakh Jargalsaikh
Communications Biology.2022;[Epub] CrossRef - Proposed minimal essential co-expression and physical interaction networks involved in the development of cognition impairment in human mid and late life
Zahra Salehi, Masoud Arabfard, Omid Sadatpour, Mina Ohadi
Neurological Sciences.2021; 42(3): 951. CrossRef - Emerging roles of SIRT6 in human diseases and its modulators
Gang Liu, Haiying Chen, Hua Liu, Wenbo Zhang, Jia Zhou
Medicinal Research Reviews.2021; 41(2): 1089. CrossRef - Quis Custodiet Ipsos Custodes (Who Controls the Controllers)? Two Decades of Studies on HDAC9
Claudio Brancolini, Eros Di Giorgio, Luigi Formisano, Teresa Gagliano
Life.2021; 11(2): 90. CrossRef - circ_0003204 Regulates Cell Growth, Oxidative Stress, and Inflammation in ox-LDL-Induced Vascular Endothelial Cells via Regulating miR-942-5p/HDAC9 Axis
Huan Wan, Ting You, Wei Luo
Frontiers in Cardiovascular Medicine.2021;[Epub] CrossRef - Histone deacetylase (HDAC) 9: versatile biological functions and emerging roles in human cancer
Chun Yang, Stéphane Croteau, Pierre Hardy
Cellular Oncology.2021; 44(5): 997. CrossRef - Dual HDAC/BRD4 inhibitors against cancer
Negar Omidkhah, Farzin Hadizadeh, Razieh Ghodsi
Medicinal Chemistry Research.2021; 30(10): 1822. CrossRef - miR‐211‐5p is down‐regulated and a prognostic marker in bladder cancer
Weisheng Wang, Zhiming Liu, Xuegang Zhang, Junning Liu, Junqing Gui, Maorong Cui, Yong Li
The Journal of Gene Medicine.2020;[Epub] CrossRef
- Impact of housing temperature on adipose tissue HDAC9 expression and adipogenic differentiation in high fat‐fed mice
- Clinical Features and Causes of Endogenous Hyperinsulinemic Hypoglycemia in Korea
- Chang-Yun Woo, Ji Yun Jeong, Jung Eun Jang, Jaechan Leem, Chang Hee Jung, Eun Hee Koh, Woo Je Lee, Min-Seon Kim, Joong-Yeol Park, Jung Bok Lee, Ki-Up Lee
- Diabetes Metab J. 2015;39(2):126-131. Published online March 9, 2015
- DOI: https://doi.org/10.4093/dmj.2015.39.2.126
- 5,246 View
- 85 Download
- 22 Web of Science
- 19 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Endogenous hyperinsulinemic hypoglycemia (EHH) is characterized by an inappropriately high plasma insulin level, despite a low plasma glucose level. Most of the EHH cases are caused by insulinoma, whereas nesidioblastosis and insulin autoimmune syndrome (IAS) are relatively rare.
Methods To evaluate the relative frequencies of various causes of EHH in Korea, we retrospectively analyzed 84 patients who were diagnosed with EHH from 1998 to 2012 in a university hospital.
Results Among the 84 EHH patients, 74 patients (88%), five (6%), and five (6%) were diagnosed with insulinoma, nesidioblastosis or IAS, respectively. The most common clinical manifestation of EHH was neuroglycopenic symptoms. Symptom duration before diagnosis was 14.5 months (range, 1 to 120 months) for insulinoma, 1.0 months (range, 6 days to 7 months) for nesidioblastosis, and 2.0 months (range, 1 to 12 months) for IAS. One patient, who was diagnosed with nesidioblastosis in 2006, underwent distal pancreatectomy but was later determined to be positive for insulin autoantibodies. Except for one patient who was diagnosed in 2007, the remaining three patients with nesidioblastosis demonstrated severe hyperinsulinemia (157 to 2,719 µIU/mL), which suggests that these patients might have had IAS, rather than nesidioblastosis.
Conclusion The results of this study suggest that the prevalence of IAS may be higher in Korea than previously thought. Therefore, measurement of insulin autoantibody levels is warranted for EHH patients, especially in patients with very high plasma insulin levels.
-
Citations
Citations to this article as recorded by- Case report: Insulinomatosis: description of four sporadic cases and review of the literature
Delmar Muniz Lourenço, Maria Lucia Corrêa-Giannella, Sheila Aparecida Coelho Siqueira, Marcia Nery, Flavio Galvão Ribeiro, Elizangela Pereira de Souza Quedas, Manoel de Souza Rocha, Ramon Marcelino do Nascimento, Maria Adelaide Albergaria Pereira
Frontiers in Endocrinology.2024;[Epub] CrossRef - Insulin Autoimmune Syndrome: A Systematic Review
MingXu Lin, YuHua Chen, Jie Ning, Tatsuya Kin
International Journal of Endocrinology.2023; 2023: 1. CrossRef - Diffuse, Adult-Onset Nesidioblastosis/Non-Insulinoma Pancreatogenous Hypoglycemia Syndrome (NIPHS): Review of the Literature of a Rare Cause of Hyperinsulinemic Hypoglycemia
Martin Philipp Dieterle, Ayman Husari, Sophie Nicole Prozmann, Hendrik Wiethoff, Albrecht Stenzinger, Manuel Röhrich, Uwe Pfeiffer, Wolfgang Rüdiger Kießling, Helena Engel, Harald Sourij, Thorsten Steinberg, Pascal Tomakidi, Stefan Kopf, Julia Szendroedi
Biomedicines.2023; 11(6): 1732. CrossRef - An Uncommon Cause of Recurrent Presyncope, Dizziness, and Tachycardia: A Case Report of Diffuse, Adult-Onset Nesidioblastosis/Non-Insulinoma Pancreatogenous Hypoglycemia Syndrome (NIPHS)
Martin Philipp Dieterle, Ayman Husari, Sophie Nicole Prozmann, Hendrik Wiethoff, Albrecht Stenzinger, Manuel Röhrich, Uwe Pfeiffer, Wolfgang Rüdiger Kießling, Helena Engel, Harald Sourij, Thorsten Steinberg, Pascal Tomakidi, Stefan Kopf, Julia Szendroedi
Biomedicines.2023; 11(6): 1741. CrossRef - An Uncommon Case of Recurrent Hypoglycemic Episodes in a Healthy Non-diabetic Male: Insulin Autoimmune Syndrome
Kanwarpal K Dhaliwal, Gaurav Bector, Saurabh Arora, Amanpreet Singh, Sanjay Kalra
Cureus.2023;[Epub] CrossRef - The After-Dinner Dip
Caren G. Solomon, Gertrud L.G. Haverkamp, Richard G. Ijzerman, Jos Kooter, Yvonne H.M. Krul-Poel
New England Journal of Medicine.2022; 386(22): 2130. CrossRef - Hirata's disease (insulin autoimmune syndrome) following envenomation by a common krait
Subramanian Senthilkumaran, Stephen W. Miller, Harry F. Williams, Ponniah Thirumalaikolundusubramanian, Sakthivel Vaiyapuri, Ketan Patel
Toxicon.2022; 219: 106923. CrossRef - Analysis of the clinical characteristics of insulin autoimmune syndrome induced by methimazole
Linli Sun, Weijin Fang, Dan Yi, Wei Sun, Chunjiang Wang
Journal of Clinical Pharmacy and Therapeutics.2021; 46(2): 470. CrossRef - Continuous glucose monitoring and Rituximab treatment in insulin autoimmune syndrome
Hiya Boro, Uttio Gupta, Charandeep Singh, Rakhi Malhotra, Rajesh Khadgawat
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2021; 15(6): 102294. CrossRef Insulin Autoimmune Syndrome (Hirata Disease): A Comprehensive Review Fifty Years After Its First Description
Daniele Cappellani, Enrico Macchia, Alberto Falorni, Piero Marchetti
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2020; Volume 13: 963. CrossRef- Nationwide survey of endogenous hyperinsulinemic hypoglycemia in Japan (2017–2018): Congenital hyperinsulinism, insulinoma, non‐insulinoma pancreatogenous hypoglycemia syndrome and insulin autoimmune syndrome (Hirata’s disease)
Yuki Yamada, Kana Kitayama, Maki Oyachi, Shinji Higuchi, Rie Kawakita, Yutaka Kanamori, Tohru Yorifuji
Journal of Diabetes Investigation.2020; 11(3): 554. CrossRef - Insulin Autoimmune Syndrome – A Case Series
Hiya Boro, Uttio Gupta, Charandeep Singh, Rakhi Malhotra, Rajesh Khadgawat
European Endocrinology.2020; 16(2): 168. CrossRef - Management of Insulin Autoimmune Hypoglycaemia: Single‐centre experience from Western India with systematic review of world literature
Muniraj Patel, Ravikumar Shah, Swati Ramteke‐Jadhav, Virendra Patil, Shivendra Kumar Patel, Anurag Lila, Nalini Shah, Tushar Bandgar
Clinical Endocrinology.2020; 92(5): 409. CrossRef - Is insulin intoxication still the perfect crime? Analysis and interpretation of postmortem insulin: review and perspectives in forensic toxicology
Charline Bottinelli, Nathalie Cartiser, Fabien Bévalot, Laurent Fanton, Jérôme Guitton
Critical Reviews in Toxicology.2020; 50(4): 324. CrossRef - Insulin autoimmune syndrome induced by exogenous insulin injection: a four-case series
Yimin Shen, Xiaoxiao Song, Yuezhong Ren
BMC Endocrine Disorders.2019;[Epub] CrossRef - An observational analysis of insulinoma from a single institution
S Shao, Z Zeng, S Hu
QJM: An International Journal of Medicine.2018; 111(4): 237. CrossRef - Anti-tuberculosis Treatment-Induced Insulin Autoimmune Syndrome
Jung Suk Han, Han Ju Moon, Jin Seo Kim, Hong Il Kim, Cheol Hyeon Kim, Min Joo Kim
The Ewha Medical Journal.2016; 39(4): 122. CrossRef - Spontaneous hypoglycemia: diagnostic evaluation and management
Leelavathy Kandaswamy, Rajeev Raghavan, Joseph M. Pappachan
Endocrine.2016; 53(1): 47. CrossRef - Hypoglycemia due to Insulin Autoimmune Syndrome: A rare cause not to be forgotten
Sarah Alam, Maaz Ozair, Jamal Ahmad
Journal of Clinical and Translational Endocrinology: Case Reports.2016; 2: 7. CrossRef
- Case report: Insulinomatosis: description of four sporadic cases and review of the literature

 KDA
KDA
 First
First Prev
Prev





