- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 35(2); 2011 > Article
-
Original ArticleThe Association of Unintentional Changes in Weight, Body Composition, and Homeostasis Model Assessment Index with Glycemic Progression in Non-Diabetic Healthy Subjects
- Eun-Jung Rhee1, Ji-Hun Choi1, Seung-Hyun Yoo1, Ji-Cheol Bae1, Won-Jun Kim1, Eun-Suk Choi1, Se Eun Park1, Cheol-Young Park1, Seok Won Park2, Ki-Won Oh1, Sung-Woo Park1, Sun-Woo Kim1, Won-Young Lee1
-
Diabetes & Metabolism Journal 2011;35(2):138-148.
DOI: https://doi.org/10.4093/dmj.2011.35.2.138
Published online: April 30, 2011
1Department of Endocrinology and Metabolism, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
2Department of Internal Medicine, CHA University, Pocheon, Korea.
- Corresponding author: Won-Young Lee. Department of Endocrinology and Metabolism, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, 78 Saemunan-gil, Jongno-gu, Seoul 110-746, Korea. drlwy@hanmail.net
Copyright © 2011 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- We performed a retrospective longitudinal study on the effects of changes in weight, body composition, and homeostasis model assessment (HOMA) indices on glycemic progression in subjects without diabetes during a four-year follow-up period in a community cohort without intentional intervention.
-
Methods
- From 28,440 non-diabetic subjects who participated in a medical check-up program in 2004, data on anthropometric and metabolic parameters were obtained after four years in 2008. Body composition analyses were performed with a bioelectrical impedance analyzer. Skeletal muscle index (SMI, %) was calculated with lean mass/weight×100. Subjects were divided into three groups according to weight change status in four years: weight loss (≤-5.0%), stable weight (-5.0 to 5.0%), weight gain (≥5.0%). Progressors were defined as the subjects who progressed to impaired fasting glucose or diabetes.
-
Results
- Progressors showed worse baseline metabolic profiles compared with non-progressors. In logistic regression analyses, the increase in changes of HOMA-insulin resistance (HOMA-IR) in four years presented higher odds ratios for glycemic progression compared with other changes during that period. Among the components of body composition, a change in waist-hip ratio was the strongest predictor, and SMI change in four years was a significant negative predictor for glycemic progression. Changes in HOMA β-cell function in four years was a negative predictor for glycemic progression.
-
Conclusion
- Increased interval changes in HOMA-IR, weight gain and waist-hip ratio was associated with glycemic progression during a four-year period without intentional intervention in non-diabetic Korean subjects.
- Weight change is one of the most important markers that strongly reflects the effectiveness of interventions on lifestyle changes in prevention of type 2 diabetes. In the lifestyle intervention group of the Diabetes Prevention Program, weight loss was the dominant predictor of reduced diabetes risk, with a 16% reduction observed for every kilogram of weight loss during 3.2-year follow-up [1]. In addition to weight loss, changes in body composition may influence diabetes risk [2]. With regard to body-fat distribution, lifestyle interventions have led to a reduced diabetes risk, in parallel with reductions preferentially in visceral fat as well as subcutaneous fat and total body fat [3]. Furthermore, few studies report the association of skeletal muscle loss with glycemic status [4,5].
- Homeostasis model assessment-insulin resistance (HOMA-IR) is frequently used as a marker for insulin sensitivity, and HOMA β-cell function is the index of insulin secretory function derived from fasting plasma glucose and insulin concentrations [6,7]. Although the predictability of these markers for future development of type 2 diabetes was suggested in previous studies [8-11], few studies were performed in non-diabetic subjects examining their role as the predictor for future glycemic progression.
- Although there are studies reporting the effects of weight change, body composition, and insulin function on the future development of diabetes, most of the previous studies results are from the intervention studies, and have not examined what occurs in response to natural changes in weight status. Furthermore, there are some studies suggesting that purposeful weight loss may not be beneficial and may even be detrimental in patients with cardiovascular diseases [12,13]. Although we know that weight loss affects the progression of diabetes, we do not have a clear answer as to how the weight change would affect the body composition and insulin function, and prevent glycemic progression in subjects without diabetes.
- We hypothesized that weight increases would have a positive correlation with glycemic progression, and that increase in fat mass and decrease in muscle mass would affect glycemic progression. Furthermore, insulin resistance and decreased insulin secretory function assessed by HOMA indices would have deleterious effects on glycemic progression. Therefore, we designed a prospective study to observe the changes in weight, body composition and HOMA indices during a four year period, and analyzed how the interval changes in these parameters affected glycemic progression in association with weight change in Korean subjects without diabetes.
INTRODUCTION
- Study population
- We designed a retrospective longitudinal study to investigate the role of baseline and changes in body weight and components of body composition on glycemic progression during a four-year follow-up period in participants in a medical health checkup program in the Health Promotion Center at Kangbuk Samsung Hospital, Sungkyunkwan University, Seoul, Korea. The purpose of the medical health checkup program is to promote health of the employees through regular health checkups and early detection of existing diseases, if any. Most of the examinees are the employees of various industrial companies around the country and their family members. The cost of the medical examinations of the employees and their family members are largely paid by their employers, and a considerable proportion of the examinees repeat the exam annually or biannually. We took advantage of this opportunity to conduct a follow-up study.
- Among 30,108 subjects who participated in the medical checkup program in 2004, we excluded subjects who had a self-reported history of diabetes or fasting blood glucose level ≥126 mg/dL (n=645), history of malignancy (n=126), heart disease (n=112), cerebrovascular disease (n=22), abnormal serum free T4 concentration (n=176) or who had missing data for the analyses (n=1,150). These specific exclusions resulted in the final study population of 28,440 subjects (mean age, 39 years; range, 19 to 86 years) who were selected for the follow-up study after four years (Fig. 1). Ethics approval for study protocol and analysis of the data was obtained from the Institutional Review Board of Kangbuk Samsung Hospital.
- At baseline, all subjects were divided into two groups by fasting blood glucose, normoglycemia (<100 mg/dL) and impaired fasting glucose (IFG; ≥100 mg/dL). After four years, development of diabetes was defined by fasting blood glucose ≥126 mg/dL or self-reported diagnosis of diabetes. Glucose progression was defined by the following criteria: converted from normoglycemia to IFG, from normoglycemia to diabetes (≥126 mg/dL) or from IFG to diabetes after four years (progressor).
- Laboratory measurements
- Height, weight, systolic and diastolic blood pressures were measured in duplicate and the results were averaged. The blood pressures were taken with a standardized sphygmomanometer after at least 5 minutes of rest, according to the Hypertension Detection and Follow-up Program protocol [14]. The body mass index (BMI) was calculated by dividing the weight (kg) by the height (m) squared.
- After 12 hours of fasting, fasting blood glucose, total cholesterol, triglyceride, high density lipoprotein cholesterol (HDL-C), and low density lipoprotein cholesterol (LDL-C) levels were checked. The hexokinase method (Advia 1650 Autoanalyzer; Bayer Diagnostics, Leverkusen, Germany) was used to measure blood glucose levels and an enzymatic colorimetric test was used to measure total cholesterol and triglyceride levels. The selective inhibition method was used to measure the level of HDL-C and a homogeneous enzymatic calorimetric test was used to measure the level of LDL-C. Serum insulin concentration was measured with an immunoradiometric assay (INS-IRMA; Biosource, Nivelles, Belgium), with intra- and inter-assay coefficients of variation of 1.6 to 2.2% and 6.1 to 6.5%, respectively.
- Percent weight change (%) was calculated by the change in weight in four years of follow-up divided by baseline weight in 2004. Subjects were divided into three groups according to the percent weight change from baseline; weight loss group (≤-5.0%), stable weight group (-5.0 to 5.0%), weight gain (≥5.0%).
- Body composition analyses by bioelectrical impedance analyses (BIAs)
- Body composition measurements of the subjects were carried out by segmental bioelectric impedance, using eight tractile electrodes according to the manufacturer's instructions (InBody 3.0; Biospace, Seoul, Korea). Lean mass (kg), fat mass (kg), percent fat mass (%) and waist-hip ratio (WHR), as a marker of abdominal obesity, were measured. As muscle mass is strongly correlated with weight, the effect of weight was adjusted with the calculation of skeletal muscle mass (SMI, %) using the following formula [15]: SMI=lean mass/weight×100.
- Assessment of insulin resistance and insulin secretion by homeostasis model assessment (HOMA) indices
- HOMA-IR and HOMA β-cell were calculated according the following formula [6]:
- Statistical analysis
- Data are expressed as the number or proportion of the subjects (%) and means with standard deviation. To see the differences in metabolic parameters at baseline between those who progressed or did not progress, baseline parameters were compared between the progressor and non-progressor with Student's t-test. To see how the changes in weight would affect metabolic parameters in four years, mean values of parameters were compared in three groups divided according to weight change status at baseline and after four years by a one-way ANOVA test. To compare the effects of interval changes of each parameter on glycemic progression, logistic regression analyses by backward method were performed with glycemic progression to analyze the effects of interval changes in various body composition components and HOMA indices on glycemic progression in four years. The proportions of progressors were compared according to the tertiles groups of interval changes in each parameter with a chi-square test. A P value <0.05 was considered as statistically significant. Statistical analyses were performed using the SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
METHODS
- The clinical characteristics of the participants at baseline and after four years
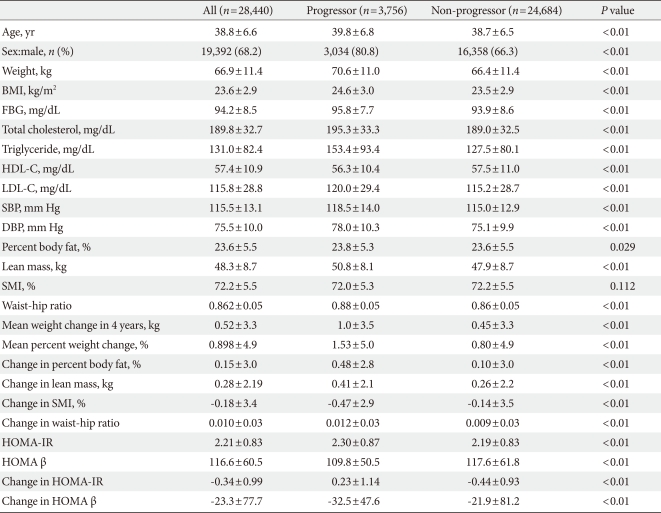
- Baseline characteristics of the participants are presented in Table 1. All baseline parameters were metabolically worse in progressors compared with non-progressors, except for SMI (Table 1). Although the baseline mean values for SMI were not significantly different between the two groups, progressors exhibited a significantly greater decline in SMI compared with non-progressors (P<0.01). Progressors displayed a significantly higher baseline HOMA-IR and a lower baseline HOMA β-cell function compared with non-progressors (Table 1). In addition, progressors had a significantly greater increase in HOMA-IR and greater decrease in HOMA β-cell function during the four-year follow-up compared with non-progressors (Table 1).
- At baseline in 2004, 6,991 subjects (24.6%) had IFG and after four years, 7,167 subjects (25.2%) had IFG, and 432 subjects (1.5%) developed diabetes mellitus. Regarding glycemic progression, in four years, 3,756 subjects (13.2%) progressed to a worse glucose tolerant status, that is, from normoglycemia to IFG, from IFG to diabetes or normoglycemia to diabetes.
- Comparisons of the baseline mean values of parameters according to the three groups of weight change status during the four-year follow-up
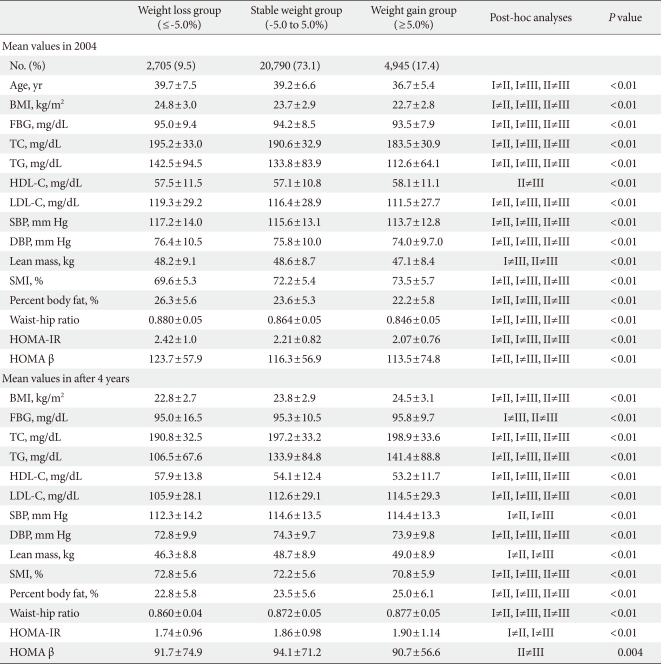
- When the participants were divided into three groups according to weight change status after four years, subjects in the weight gain group were the youngest and displayed the lowest BMI, therefore all the parameters were metabolically better than the subjects in the stable weight and weight loss groups (Table 2). However, after four years, although the weight gain group was the youngest group, all of the metabolic parameters worsened compared with the weight loss and stable weight groups (Table 2).
- Although the baseline HOMA-IR value was the lowest in weight gain group, after four years, the weight gain group demonstrated the highest HOMA-IR value among the three groups, and the weight loss group significantly demonstrated the lowest HOMA-IR value compared with the other two groups (Table 2). For HOMA β-cell function, although the weight gain group presented the lowest HOMA β-cell function at baseline, after four years, the weight gain group exhibited the lowest value and the stable weight group exhibited the highest value for HOMA β cell function (Table 2).
- Determinants of glycemic progression after adjustment for multiple metabolic parameters, including weight change, body composition, and HOMA indices
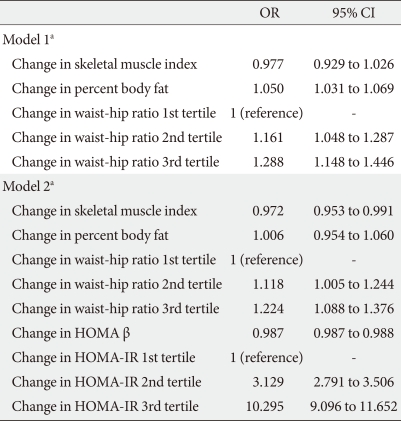
- When logistic regression analyses were performed with glycemic progression as the dependent variable, the odds ratio (OR) for glycemic progression increased 1.389 and 2.29 times in the stable weight and weight gain groups, respectively, compared with the weight loss group after adjustment for confounding variables (Table 3). When changes in HOMA indices were included in the model, the increment in the OR for glycemic progression observed in the stable weight and weight gain groups was attenuated to 1.203 and 1.765, respectively. In addition, changes in HOMA β-cell function produced a negative correlation with glycemic progression, and changes in HOMA-IR significantly produced a positive correlation with glycemic progression, with an OR of 3.153 and 10.468 in 2nd and 3rd tertile groups of changes in HOMA-IR compared with the 1st tertile group (Table 3).
- When changes in components of body composition were included in the model, changes in WHR showed the most significant association with glycemic progression, and the highest tertile for WHR change exhibited a 1.29-fold increased risk for glycemic progression compared with the lowest tertile (Table 4). When changes in HOMA indices were included in the model, changes in HOMA-IR produced the highest OR for glycemic progression after adjustment for other body composition components with an OR of 10.295 in the highest tertile of HOMA-IR compared with the lowest tertile (Table 4).
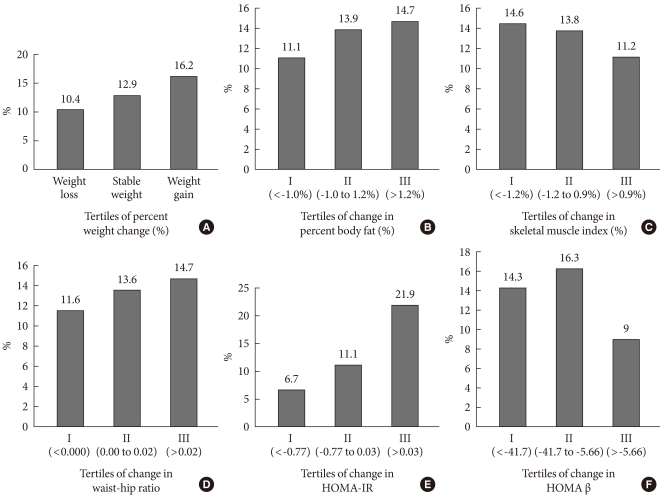
- Comparisons of the proportion of progressors according to the interval changes in multiple components during the four-year follow-up
- When the proportion of progressors was compared according to the interval changes in individual components, the proportion of progressors increased as the tertiles of weight change status increased from the weight loss to the weight gain groups (Fig. 2). The proportion of progressors significantly increased as the tertiles of changes of percent body fat and WHR increased from the 1st to 3rd tertiles, and as the tertiles of changes of SMI decreased from the 3rd to 1st tertiles (Fig. 2). For HOMA indices, the proportion of progressors increased as the changes of HOMA-IR increased from 1st to 3rd tertiles, and the proportion of progressors was significantly lower in 3rd tertile group, which demonstrated the smallest change in HOMA β-cell function compared with the 1st and 2nd tertiles (Fig. 2). These comparisons were all statistically significant (P<0.05).
RESULTS
- In this study, our results indicated that increased weight gain was the significant predictor for glycemic progression in subjects without diabetes mellitus at baseline during a four-year follow-up in an observational cohort without intentional intervention. Among the components of body composition assessed by the BIA method, increase in WHR was the strongest effector for future glycemic progression. However, when HOMA-IR was included in the same model, an increase in HOMA-IR predicted a higher risk for glycemic progression compared with increase in weight gain or WHR. HOMA β-cell function produced a negative correlation with glycemic progression. For the changes in each parameter in four years, changes in weight, percent body fat and WHR demonstrated a significant positive correlation with the proportion of progressors. Although it was attenuated after adjustment for confounding factors, SMI demonstrated a significantly negative association with glycemic progression.
- In our study, during the four-year follow-up period in non-diabetic participants in a regular health check-up program in a university hospital, compared with the subjects who lost more than 5% of their initial weight, subjects who gained more than 5% of their initial weight in four years showed 2.3-fold increased risk for glycemic progression, confirming the deleterious effects of weight gain on diabetes risk and glycemic progression, in line with the results from previous intervention studies. In addition, the mean percent weight change in all the participants in this study was relatively smaller than expected, about 0.89% in four years. In contrast with previous intervention studies in which 5 to 7% weight change was used as the target for intervention [16,17], the results of our study could be interpreted in aspects relatively closer to the real-practice setting. As an observational study, only 9.5% of the participants lost 5% of their initial weight in four years without intervention. In the recently published Look AHEAD (Action for Health in Diabetes) study performed in diabetes patients, while the lifestyle intervention group lost 8.6% of their initial weight at one year on average, the education control group showed only 0.7% weight loss [18]. From these results and our current study results this suggests that in the general adult population without any lifestyle intervention, the range of weight change is not great, and therefore, a very small change in weight could impact the metabolic status.
- In this study, an interval increase in HOMA-IR was the most significant predictor for glycemic progression compared with weight change or visceral obesity assessed by WHR. In the Mexico City study, in which HOMA indices were assessed in 1,449 Mexican subjects without diabetes, after 3.5 years, the development of diabetes was associated with lower HOMA % sensitivity at baseline [19]. Although cross-sectional studies have been performed on the association of HOMA-indices with risk for diabetes or studies performed in subjects with type 2 diabetes [9,10], longitudinal studies assessing the association of baseline and the changes of HOMA indices with glycemic progression in a large number of Asian subjects are scarce. This study was the first study that assessed HOMA indices longitudinally in a large number of Asian subjects and analyzed their association with glycemic progression in non-diabetic subjects.
- In addition to weight change, changes in body fat distribution may influence diabetes risk. It has been shown that a relatively minor loss of body weight, which was accompanied by a major reduction in visceral fat mass and liver fat content, was associated with improved insulin sensitivity [20]. Visceral fat is known to be metabolically more active compared to non-visceral adipose tissue and to be a major source for free fatty acids (FFA) and adipokine production [21,22]. Moreover, visceral fat perturbs metabolism by exposing the liver to a high concentration of FFAs, causing steatohepatitis and hepatic insulin resistance. In Diabetes Prevention Program, decreased diabetes risk by lifestyle intervention was associated with reductions in body weight, BMI, and central body fat distribution after adjustment for age and self-reported ethnicity [3]. In our study, increased abdominal obesity assessed by WHR and measured by BIA produced a significant linear association with increased glycemic progression in subjects with no history of diabetes. In contrast, percent body fat change had a weaker effect on glycemic progression when weight change and HOMA indices were included in the model, suggesting that it is not the simple increment of fat amount, but where the fat is deposited, which carries more importance in glycemic progression.
- Age-related loss of skeletal muscle mass results in decreased skeletal muscle strength, and increased morbidity and mortality among the elderly [23,24]. Skeletal muscle is the main target for glucose use and insulin activity; therefore, this tissue may be important for glucose metabolism and could be an original target to treat metabolic disorders, such as insulin resistance, impaired glucose tolerance and type 2 diabetes [25,26]. In a recent report from the Health, Aging, and Body Composition Study (Health ABC Study), older adults with type 2 diabetes exhibited excessive loss of appendicular lean mass and trunk fat mass compared with non-diabetic subjects [5]. In our study, subjects with a high baseline SMI and lower timely decrease in SMI (lean mass adjusted by weight), demonstrated reduced glycemic progression compared with the subjects with a higher decrease in SMI. Furthermore, the progressors showed a significantly larger decrease in SMI in four years of follow-up compared with non-progressors, although there was no difference between the baseline SMI values between those two groups, emphasizing the importance of changes in muscle mass in glycemic progression. However, this significance was attenuated when all the components of body composition were included in the same model, suggesting that other components of body composition might be more important in glucose metabolism. Nonetheless, this study was to our knowledge, the first study to report and compare the effect of muscle mass on glycemic progression in subjects without diabetes.
- This study has some limitations. First, diabetes development was not selected as the target end-point in the analyses. The reason that we chose glycemic progression as the primary end point, not the development of diabetes itself, was because the overall incidence of diabetes in this cohort during the four-year period of observation, was relatively low compared with other intervention studies [16,17]. The reason for the low incidence of diabetes could be due to 'healthy worker effect' in that the study participants who obeyed the company strategy and participated in the regular exam might be mentally and physically healthier than those subjects that did not. Another reason could be the social status of the participants in our study cohort, in that most of the participants were either the employee or his or her family members of the large industrial companies that could afford the annual or biennial health check-up exams that the government recommends. Second, the diagnosis of diabetes mellitus or prediabetes was made by only one episode of high fasting glucose level or self-report of diabetes in the health check-up program, not oral glucose tolerance test (OGTT). However, in the studies managing large-scale health data, performing OGTT would be not feasible and not cost-effective [27]. Third, the use of the BIA method, to analyze body composition, could have biased the results, as the accuracy of this method has been debated. However, for the analysis of the body composition, BIA has been shown to have good correlation with dual-energy X-ray absorptiometry [28,29]. Fourth, personal history for medication, physical activity, smoking and alcohol drinking was not available for the analyses. In particular, the absence of the effect of physical activity could have bias in the analyses. However, there is still debate on whether being fat or not fit is important on glycemic progression [30]. Despite the above mentioned limitations, this was the first large-scale study performed in an Asian population to observe the effects of changes in weight and body composition on glycemic progression with the longitudinal assessment of HOMA indices, during a four year follow-up period in subjects without history of diabetes.
- In conclusion, without intentional intervention, change in body weight, even in relatively narrow range of a four year period of observation, conferred increased risk for glycemic progression to IFG or diabetes in adult subjects without a history of diabetes. Furthermore, increased HOMA-IR during the follow-up period had the most significant predictive power for glycemic progression. Among the components of body composition, increase in abdominal obesity assessed by WHR demonstrated the most significant association with glycemic progression, although the influence was not bigger than increased HOMA-IR. Changes in muscle mass showed a significant positive correlation with glycemic progression, although these effects could not overcome the deleterious effects of increase in visceral fat or HOMA-IR. These findings confirm that earlier intervention in lifestyle change to ultimately prevent weight gain and insulin resistance might be the most effective way for the prevention of the worldwide epidemic of diabetes mellitus.
DISCUSSION
-
Acknowledgements
- This work was supported by the Samsung grant, #SBRI C-A8-223-1.
ACKNOWLEDGMENT
- 1. Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-Sunyer X, Regensteiner J, Venditti B, Wylie-Rosett J. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29:2102-2107. ArticlePubMedPMCPDF
- 2. Kim JY, Jee JH, Kim HJ, Lee BW, Chung YJ, Chung JH, Min YK, Lee MS, Lee MK, Kim KW. Effects of aging and obesity on insulin secretion and sensitivity. J Korean Diabetes Assoc 2005;29:39-47.
- 3. Fujimoto WY, Jablonski KA, Bray GA, Kriska A, Barrett-Connor E, Haffner S, Hanson R, Hill JO, Hubbard V, Stamm E, Pi-Sunyer FX. Diabetes Prevention Program Research Group. Body size and shape changes and the risk of diabetes in the Diabetes Prevention Program. Diabetes 2007;56:1680-1685. ArticlePubMedPMCPDF
- 4. Park SW, Goodpaster BH, Strotmeyer ES, Kuller LH, Broudeau R, Kammerer C, deRekeneire N, Harris TB, Schwartz AV, Tylavsky FA, Cho YW, Newman AB. Health, Aging, and Body Composition Study. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care 2007;30:1507-1512. PubMed
- 5. Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, de Rekeneire N, Harris TB, Kritchevsky S, Tylavsky FA, Nevitt M, Cho YW, Newman AB. Health, Aging, and Body Composition Study. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009;32:1993-1997. ArticlePubMedPMCPDF
- 6. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412-419. ArticlePubMedPDF
- 7. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487-1495. ArticlePubMedPDF
- 8. U.K. Prospective Diabetes Study Group. U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes 1995;44:1249-1258. ArticlePubMed
- 9. Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes Care 1997;20:1087-1092. ArticlePubMedPDF
- 10. Matsumoto K, Miyake S, Yano M, Ueki Y, Yamaguchi Y, Akazawa S, Tominaga Y. Glucose tolerance, insulin secretion, and insulin sensitivity in nonobese and obese Japanese subjects. Diabetes Care 1997;20:1562-1568. ArticlePubMedPDF
- 11. Levy J, Atkinson AB, Bell PM, McCance DR, Hadden DR. Beta-cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus: the 10-year follow-up of the Belfast Diet Study. Diabet Med 1998;15:290-296. ArticlePubMed
- 12. Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol 2001;38:789-795. ArticlePubMed
- 13. Allison DB, Zannolli R, Faith MS, Heo M, Pietrobelli A, VanItallie TB, Pi-Sunyer FX, Heymsfield SB. Weight loss increases and fat loss decreases all-cause mortality rate: results from two independent cohort studies. Int J Obes Relat Metab Disord 1999;23:603-611. ArticlePubMedPDF
- 14. Curb JD, Ford C, Hawkins CM, Smith EO, Zimbaldi N, Carter B, Cooper C. A coordinating center in a clinical trial: the Hypertension Detection and Followup Program. Control Clin Trials 1983;4:171-186. ArticlePubMed
- 15. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889-896. ArticlePubMed
- 16. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343-1350. ArticlePubMed
- 17. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-403. ArticlePubMedPMC
- 18. Look AHEAD Research Group. Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, Curtis JM, Espeland MA, Foreyt JP, Graves K, Haffner SM, Harrison B, Hill JO, Horton ES, Jakicic J, Jeffery RW, Johnson KC, Kahn S, Kelley DE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montgomery B, Nathan DM, Patricio J, Peters A, Redmon JB, Reeves RS, Ryan DH, Safford M, van Dorsten B, Wadden TA, Wagenknecht L, Wesche-Thobaben J, Wing RR, Yanovski SZ. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007;30:1374-1383. PubMed
- 19. Haffner SM, Kennedy E, Gonzalez C, Stern MP, Miettinen H. A prospective analysis of the HOMA model. The Mexico City Diabetes Study. Diabetes Care 1996;19:1138-1141. ArticlePubMedPDF
- 20. Tiikkainen M, Bergholm R, Vehkavaara S, Rissanen A, Hakkinen AM, Tamminen M, Teramo K, Yki-Jarvinen H. Effects of identical weight loss on body composition and features of insulin resistance in obese women with high and low liver fat content. Diabetes 2003;52:701-707. ArticlePubMedPDF
- 21. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000;21:697-738. ArticlePubMed
- 22. Kim HK, Park KG, Kim MK, Jang YY, Kim SY, Jung ED, Kim HS, Do JH, Lee IK. Comparison of the relationship of leptin to metabolic parameters between premenopausal normal weight and obese women. J Korean Diabetes Assoc 2005;29:223-230.
- 23. Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 2005;60:324-333. ArticlePubMed
- 24. Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 2006;61:72-77. ArticlePubMed
- 25. DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988;37:667-687. PubMed
- 26. Shepherd PR, Kahn BB. Glucose transporters and insulin action--implications for insulin resistance and diabetes mellitus. N Engl J Med 1999;341:248-257. ArticlePubMed
- 27. Engelgau MM, Narayan KM, Herman WH. Screening for type 2 diabetes. Diabetes Care 2000;23:1563-1580. ArticlePubMedPDF
- 28. Svendsen OL, Haarbo J, Heitmann BL, Gotfredsen A, Christiansen C. Measurement of body fat in elderly subjects by dual-energy X-ray absorptiometry, bioelectrical impedance, and anthropometry. Am J Clin Nutr 1991;53:1117-1123. ArticlePubMed
- 29. Bolanowski M, Nilsson BE. Assessment of human body composition using dual-energy X-ray absorptiometry and bioelectrical impedance analysis. Med Sci Monit 2001;7:1029-1033. PubMed
- 30. Fogelholm M. Physical activity, fitness and fatness: relations to mortality, morbidity and disease risk factors. A systematic review. Obes Rev 2010;11:202-221. ArticlePubMed
REFERENCES

Values are presented as means±standard deviation or numbers (percent).
BMI, body mass index; FBG, fasting blood glucose; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; SMI, skeletal muscle index; HOMA, homeostasis model assessment; IR, insulin resistance.
Values are presented as means±standard deviation or numbers (percent).
BMI, body mass index; FBG, fasting blood glucose; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; SMI, skeletal muscle index; HOMA, homeostasis model assessment; IR, insulin resistance.
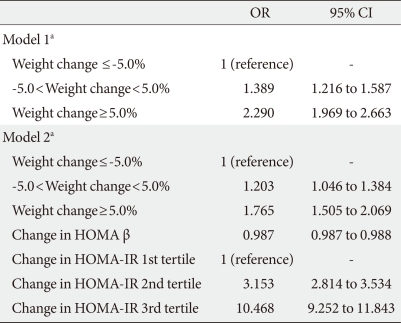
Figure & Data
References
Citations

- Increased Risk of Nonalcoholic Fatty Liver Disease in Individuals with High Weight Variability
Inha Jung, Dae-Jeong Koo, Mi Yeon Lee, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
Endocrinology and Metabolism.2021; 36(4): 845. CrossRef - Effects of nutritional supplementation on glucose metabolism and insulin function among people with HIV initiating ART
Hiwot Amare, Mette F. Olsen, Henrik Friis, Pernille Kæstel, Åse B. Andersen, Alemseged Abdissa, Daniel Yilma, Tsinuel Girma, Daniel Faurholt-Jepsen
BMC Nutrition.2021;[Epub] CrossRef - The ratio of estimated average glucose to fasting plasma glucose level as an indicator of insulin resistance in young adult diabetes
Jun Guo, Sisi Lei, Yu Zhou, Congqing Pan
Medicine.2020; 99(40): e22337. CrossRef - Reduced Skeletal Muscle Volume and Increased Skeletal Muscle Fat Deposition Characterize Diabetes in Individuals after Pancreatitis: A Magnetic Resonance Imaging Study
Andre E. Modesto, Juyeon Ko, Charlotte E. Stuart, Sakina H. Bharmal, Jaelim Cho, Maxim S. Petrov
Diseases.2020; 8(3): 25. CrossRef - Insulin resistance increases the risk of incident type 2 diabetes mellitus in patients with non‐alcoholic fatty liver disease
Yuya Seko, Yoshio Sumida, Saiyu Tanaka, Kojiroh Mori, Hiroyoshi Taketani, Hiroshi Ishiba, Tasuku Hara, Akira Okajima, Atsushi Umemura, Taichiro Nishikawa, Kanji Yamaguchi, Michihisa Moriguchi, Kazuyuki Kanemasa, Kohichiroh Yasui, Shunsuke Imai, Keiji Shim
Hepatology Research.2018;[Epub] CrossRef - Use of Novel High-Protein Functional Food Products as Part of a Calorie-Restricted Diet to Reduce Insulin Resistance and Increase Lean Body Mass in Adults: A Randomized Controlled Trial
Carol Johnston, Barry Sears, Mary Perry, Jessica Knurick
Nutrients.2017; 9(11): 1182. CrossRef - Gender differences in the association between food insecurity and insulin resistance among U.S. adults: National Health and Nutrition Examination Survey, 2005–2010
Junxiu Liu, Yong-Moon Mark Park, Seth A. Berkowitz, Qingwei Hu, Kyungdo Han, Andrew Ortaglia, Robert E. McKeown, Angela D. Liese
Annals of Epidemiology.2015; 25(9): 643. CrossRef - 1,5-Anhydroglucitol Is Associated with Early-Phase Insulin Secretion in Chinese Patients with Newly Diagnosed Type 2 Diabetes Mellitus
Xiaojing Ma, Yaping Hao, Xiang Hu, Yuqi Luo, Zixuan Deng, Jian Zhou, Yuqian Bao, Weiping Jia
Diabetes Technology & Therapeutics.2015; 17(5): 320. CrossRef - Serum glycated albumin as a new glycemic marker in pediatric diabetes
Ji Woo Lee, Hyung Jin Kim, Young Se Kwon, Yong Hoon Jun, Soon Ki Kim, Jong Weon Choi, Ji Eun Lee
Annals of Pediatric Endocrinology & Metabolism.2013; 18(4): 208. CrossRef - The Association of Maximum Body Weight on the Development of Type 2 Diabetes and Microvascular Complications: MAXWEL Study
Soo Lim, Kyoung Min Kim, Min Joo Kim, Se Joon Woo, Sung Hee Choi, Kyong Soo Park, Hak Chul Jang, James B. Meigs, Deborah J. Wexler, Noel Christopher Barengo
PLoS ONE.2013; 8(12): e80525. CrossRef - Relative contributions of insulin resistance and β‐cell dysfunction to the development of Type 2 diabetes in Koreans
C.‐H. Kim, H.‐K. Kim, E. H. Kim, S. J. Bae, J.‐Y. Park
Diabetic Medicine.2013; 30(9): 1075. CrossRef - Body Mass Index and Waist Circumference According to Glucose Tolerance Status in Korea: The 2005 Korean Health and Nutrition Examination Survey
Hye Mi Kang, Dong-Jun Kim
Journal of Korean Medical Science.2012; 27(5): 518. CrossRef - The Relationship between β-cell Function and Nutrient Intakes in Korean Adult - Using 4thKorea National Health and Nutrition Examination Survey 2009 -
You Mi Lee, Hye Kyung Chung, Heejin Kimm, Sun Ha Jee
Korean Journal of Community Nutrition.2012; 17(2): 243. CrossRef - The ratio of glycated albumin to glycated haemoglobin correlates with insulin secretory function
Daham Kim, Kwang J. Kim, Ji H. Huh, Byung‐Wan Lee, Eun S. Kang, Bong S. Cha, Hyun C. Lee
Clinical Endocrinology.2012; 77(5): 679. CrossRef

 KDA
KDA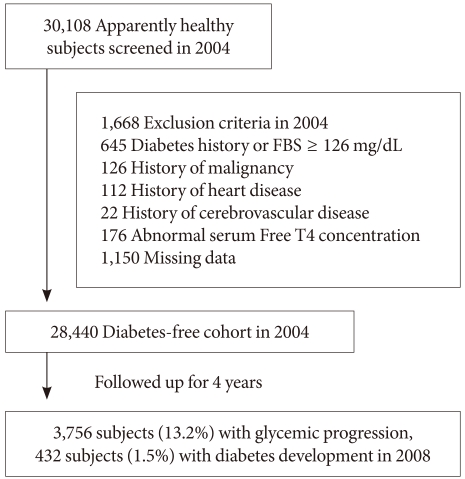
 PubReader
PubReader Cite
Cite