
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 39(1); 2015 > Article
-
Original ArticleOthers Economic Impact of Combining Metformin with Dipeptidyl Peptidase-4 Inhibitors in Diabetic Patients with Renal Impairment in Spanish Patients
- Antoni Sicras-Mainar1, Ruth Navarro-Artieda2
-
Diabetes & Metabolism Journal 2015;39(1):74-81.
DOI: https://doi.org/10.4093/dmj.2015.39.1.74
Published online: February 16, 2015
1Management Planning, Badalona Serveis Assistencials SA, Barcelona, Spain.
2Medical Documentation, Hospital Germans Trias i Pujol, Barcelona, Spain.
- Corresponding author: Antoni Sicras-Mainar. Management Planning, Badalona Serveis Assistencials SA, C. Gaietà Soler, 6-8 entlo, 08911 Badalona, Barcelona, Spain. asicras@bsa.cat
Copyright © 2015 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- To evaluate resource use and health costs due to the combination of metformin and dipeptidyl peptidase-4 (DPP-4) inhibitors in patients with diabetes and renal impairment in routine clinical practice.
-
Methods
- An observational, retrospective study was performed. Patients aged ≥30 years treated with metformin who initiated a second oral antidiabetic treatment in 2009 to 2010 were included. Two groups of patients were analysed: metformin+DPP-4 inhibitors and other oral antidiabetics. The main measures were: compliance, persistence, metabolic control (glycosylated hemoglobin< 7%) and complications (hypoglycemia, cardiovascular events) and total costs. Patients were followed up for 2 years.
-
Results
- We included 395 patients, mean age 70.2 years, 56.5% male: 135 patients received metformin+DPP-4 inhibitors and 260 patients received metformin+other oral antidiabetics. Patients receiving DPP-4 inhibitors showed better compliance (66.0% vs. 60.1%), persistence (57.6% vs. 50.0%), and metabolic control (63.9% vs. 57.3%), respectively, compared with those receiving other oral antidiabetics (P<0.05), and also had a lower rate of hypoglycemia (20.0% vs. 47.7%) and lower total costs (€ 2,486 vs. € 3,002), P=0.001.
-
Conclusion
- Despite the limitations of the study, patients with renal impairment treated with DPP-4 inhibitors had better metabolic control, lower rates (association) of hypoglycaemia, and lower health costs for the Spanish national health system.
- Type 2 diabetes mellitus (T2DM) is a highly-prevalent disease that results in high morbidity, resulting in a high consumption of health resources [1]. Diabetic nephropathy is a complication that affects 25% to 40% of T2DM patients during the disease course and is considered as a marker of poor prognosis [2]. The prevalence of microalbuminuria, macroalbuminuria, and reduced glomerular filtration rate (GFR) is 20%, 7%, and 12% [3]. Complications related to renal impairment (RI) become more important with decreasing glomerular filtration [4]. In patients with diabetic nephropathy, these complications require a multifactorial approach ranging from the prevention of complications (metabolic control) to the prevention of nephrotoxicity [1,3,5].
- Metformin is recommended as the first therapeutic choice in these patients, together with dietary and lifestyle measures and when metabolic control is not achieved, the addition of a second drug in combination therapy is recommended [1,2]. The most frequent acute complication of diabetes is hypoglycaemia, especially in patients treated with insulin and/or sulfonylureas [1,6]. Dipeptidyl peptidase-4 (DPP-4) inhibitors have an advantage over traditional secretagogues in that they significantly reduce hypoglycaemia, since their insulin secretion stimulating mechanism is glucose-dependent [7,8].
- Some clinical trials have demonstrated the efficacy and safety of DPP-4 inhibitors in patients with renal failure [9,10]. The available evidence in routine clinical practice on the clinical and economic effects of therapy in these patients is limited, and therefore this study may be relevant. The aim of the study was to describe the use of resources and health costs resulting from the combination of metformin and DPP-4 inhibitors in patients with T2DM and RI followed up for 2 years. The secondary objectives were to determine adherence, metabolic control, hypoglycaemia, and macrovascular complications.
INTRODUCTION
- Design and study population
- We carried out an observational, longitudinal multicentre retrospective study through review of computerized medical records of outpatients and inpatients treated with metformin. The study population consisted of patients assigned to six primary care centres managed by Badalona Serveis Assistencials SA. Information on health resources used was obtained from two reference hospitals: Hospital Municipal de Badalona and Hospital Germans Trias y Pujol, Badalona. The population assigned to these centres is mostly urban, with middle-low socioeconomic status, and predominantly industrial occupations.
- Inclusion and exclusion criteria
- We included all patients who started a second antidiabetic treatment in 2009 and 2010 and fulfilled the following conditions: (1) age ≥30 years; (2) diagnosis of T2DM and RI at least 12 months before the study date; (3) patients who regularly followed (≥1 medical visit/year) the cardiovascular risk protocol/guidelines of the participating centres; (4) patients currently treated with metformin as the first therapeutic option (monotherapy); and (5) patients in whom follow-up was guaranteed. Patients transferring out to other municipalities or regions were excluded. Patients on dialysis or with GFR <30 mL/min were excluded. There were two study groups: (1) patients treated with metformin+DPP-4 inhibitors and (2) patients treated with metformin+other oral antidiabetics. Patients were followed for 24 months, which was considered as a sufficient time to assess the complications and health costs arising from these therapies.
- Diagnosis of type 2 diabetes and renal impairment
- The diagnosis of T2DM was obtained from the International Classification of Primary Care (ICPC-2, code T90) [11] and the International Classification of Diseases (ICD-9-CM, code 250). RI (estimated GFR, Modification of Diet in Renal Disease [MDRD]) was defined as deterioration in renal function (GFR: ≥30 mL/min/1.73 m2; stage 1 to 3). The last available readings were considered. Baseline data on microvascular complications (diabetic retinopathy, diabetic neuropathy) were obtained.
- Sociodemographic and comorbidity variables
- The variables studied were age, sex, length of evolution of T2DM, and RI (from diagnosis), as well as detailed personal history (Table 1).The general comorbidity summary variables used for each patient treated were: (1) the Charlson comorbidity index [12], which is used as a proxy of the severity of the health status and (2) the individual causality index, obtained from the Adjusted Clinical Groups (ACG), which is a patient classification system based on iso-resource use [13]. The ACG application provides resource utilisation bands (RUBs), with each patient, according to general morbidity, placed in one of five mutually-exclusive categories.
- Treatment compliance and persistence and metabolic control
- Information was collected on the following oral antidiabetics according to the Anatomical Therapeutic Chemical Classification System [14]: (1) metformin (A10BA*); (2) insulin release stimulators: sulfonylureas (A10BB*) and glinides (A10BX*); (3) glitazones (A10BG*); (4) DPP-4 inhibitors (A10BH*) in monotherapy or in combination (A10BD*). We did not include patients receiving α-glucosidase inhibitors due to the insufficient sample size. Compliance during the study period was calculated by dividing the total number of tablets dispensed by those recommended or prescribed. Treatment persistence was defined as the time, measured in months, without abandoning the initial treatment or with no change to another medication for at least 30 days after the initial prescription. Metabolic control was defined as levels of glycosylated hemoglobin (HbA1c) <7% [1].
- Macrovascular complications and cardiovascular events
- Macrovascular complications and cardiovascular events (CVEs) collected included: (1) heart disease, including cardiac ischemia, acute myocardial infarction, and heart failure, as defined by the diagnostic criteria of the World Health Organization; (2) cerebrovascular disease, including stroke (ischemic or haemorrhagic; according to the American Heart Association) and transient ischemic attack; and (3) peripheral arterial disease (all types). The cumulative incidence rate was defined as the proportion of patients who developed the complication (number of new cases during the study period). All cases of symptomatic hypoglycemia were identified during follow-up (according to medical judgment, the record of hypoglycemia was obtained in regular follow-up visits).
- Resource use and model of health costs
- Direct healthcare costs were defined as costs related to medical care. The unit costs used in the study in 2011 (EUR). The tariffs were obtained from the cost analytical accounting, except for medications whose cost was obtained using the retail price [15]. Description, tariff: medical visits, primary health care €23.19, hospitalization visits €104.41, emergency room €117.53; hospitalization (1 day) €320.90; laboratory tests €22.30, conventional radiology €18.50, and diagnostic/therapeutic tests €37.12.
- Confidentiality of information and statistical analysis
- The study was classified by the Spanish Agency for Medicines and Health Products (Non-Post Licensing Study) and was subsequently approved by the Ethics Committee for Clinical Research of the Hospital Universitari Germans Trias i Pujol, Badalona. A descriptive analysis was made, and the 95% confidence intervals (CI) calculated. The normality of the distribution of quantitative variables was verified using the Kolmogorov-Smirnov test. In the bivariate analysis, analysis of variance, the chi-square test, Pearson linear correlation, and the Mann-Whitney-Wilcoxon non-parametric test were used. A logistic regression analysis was performed to determine the variables associated with patients receiving DPP-4 inhibitors using an enter procedure (statistic: Wald). Costs were compared according to the recommendations of Thompson and Barber [16] using analysis of covariance (ANCOVA), with sex, age, RUB, the Charlson index, and the time of evolution since diagnosis of T2DM and RI as covariates.
METHODS
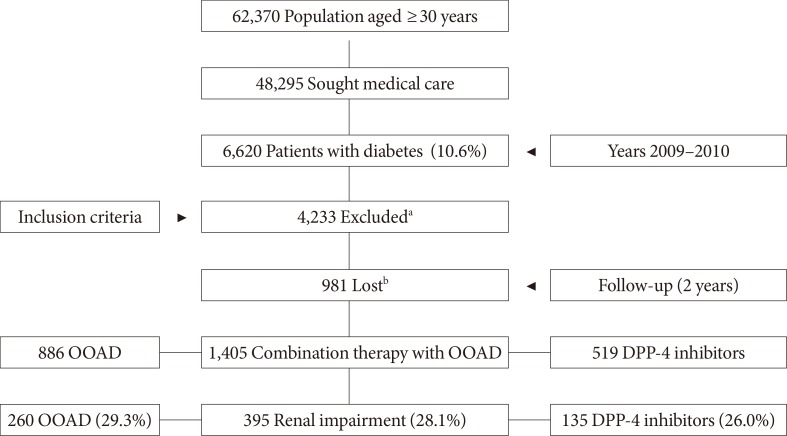
- The study population consisted of 62,370 patients aged ≥30 years. Of these, 48,295 sought medical attention and 6,620 were diagnosed with T2DM (prevalence, 10.6%; 95% CI, 10.4 to 10.8) (Fig. 1). A total of 395 patients with RI treated with oral antidiabetic agents (combination therapy with metformin) were included. Of which 34.2% (n=135) were receiving DPP-4 inhibitors (vildagliptin, 69.2%) and 65.8% other oral antidiabetics (n=260, 88.8% sulfonylureas and 11.2% glitazones).
- The baseline characteristics of the study patients according to treatment received are shown in Table 1. The mean age was 70.2 years (standard deviation 10.4) and 56.5% were male. Patients receiving DPP-4 inhibitors had better compliance (66.0% vs. 60.1%, P<0.05) and treatment persistence (57.6% vs. 50.0%, P<0.05), respectively (Table 2). Overall, the average dose of metformin at baseline was 1,362±363 and 944±238 mgr after the follow-up period. No statistically significant differences between the two study groups.
- There was an acceptable correlation between the degree of compliance and the time of treatment persistence in months (P<0.001, r=0.449). Metabolic control (HbA1c <7%) of T2DM at the end of follow-up was greater in patients receiving DPP-4 inhibitors (63.9% vs. 57.3% respectively, P<0.05). In the logistic model, patients treated with DPP-4 inhibitors were associated with better adherence (odds ratio [OR], 1.4; 95% CI, 1.1 to 1.7; P=0.021), treatment persistence (OR, 1.2; 95% CI, 1.0 to 1.6; P=0.044), and metabolic control (OR, 1.2; 95% CI, 1.0 to 1.6; P=0.035).
- Patients receiving DPP-4 inhibitors had fewer primary care medical visits (23.1 vs. 34.1, P<0.001) and hospital care visits (2.1 vs. 3.3, P=0.004) than those receiving other oral antidiabetics. The model of gross and adjusted costs (covariates) for the 2-year follow-up according to the study groups is shown in Table 3. The total cost of care for diabetic patients with RI was €1.1 million. The mean unit cost of patients receiving DPP-4 inhibitors was lower than that of those receiving other oral antidiabetics (€2,370 vs. €3,038, P<0.001). In the ANCOVA model adjusted for covariates, the corresponding sums were €2,486 (95% CI, 2,251 to 2,721) vs. €3,002 (95% CI, 2,835 to 3,170); P=0.001. These differences were reflected in all components of the health costs (primary and hospital care). Health costs were moderately correlated with age (r=0.665), general comorbidity (RUB, r=0.548, P<0.001), and the values of RI (MDRD, r=0.787, P<0.001).
- During the follow-up, the rate of new CVE was 9.9% (95% CI, 7.0 to 12.8), and was lower in patients receiving DPP-4 inhibitors compared with those receiving other oral antidiabetics (8.0% vs. 11.5%, P=0.122). Patients receiving DPP-4 inhibitors had a similar proportion of new cases of ischemic heart disease (2.8% vs. 4.2%, P=0.478) and stroke (5.1% vs. 7.3%, P=0.527) to those receiving other oral antidiabetics. The percentage of patients with hypoglycemia was 38.2%, and was lower in patients receiving DPP-4 inhibitors (20.0% vs. 47.7%, P<0.001).
RESULTS
- Our results show that diabetic patients with RI treated with the combination of metformin and DPP-4 inhibitors had better compliance and disease control than those treated with metformin and other oral antidiabetics, and that this was associated with lower rates of hypoglycemia and lower health costs in routine medical practice in a population setting. In Spain, there is little evidence of the evaluation of these measures in a single study, thus enhancing the value of our results.
- We did not include patients receiving insulin, because they have more advanced T2DM and/or greater genetic susceptibility, making it more difficult to measure adherence to drug treatment. The distribution in the two groups (comparability) was similar (around 22%); and therefore, in our opinion, did not affect the results. In addition, relatively few diabetic patients progressed to RI (Fig. 1). It is known that the risk of CVE in these patients increases as the rate of reduction in the GFR increases. Our results are consistent with the literature reviewed [2,3,4].
- At 2-year follow-up, patients receiving DPP-4 inhibitors were more closely associated with better treatment compliance/persistence, improved metabolic control, and less hypoglycaemia. Studies of compliance and persistence with oral agents are scarce and difficult to compare due to the different methods used to measure these factors. These studies show compliance rates of between 40% and 80% [17,18]. A review of 139 studies by Cramer et al. [19] found that, at 12 months, the rate of persistence with oral antidiabetics was 63% and compliance was 58%: the rates were similar in all therapeutic classes analysed. Jermendy et al. [20] found a persistence rate of 56% at 1 year per year in a series of patients receiving combination therapy with metformin and sulfonylureas. Although these results are consistent with ours, there is a slight superiority of DPP-4 inhibitors [21]. This may be randomly due to individual variability, but a plausible explanation could be a better safety and tolerability profile, which would result in lower rates of hypoglycemia, although more studies comparing antidiabetic drug use in combination therapy are required to enhance the consistency of these results. Furthermore, the results of some controlled trials suggest a possible cardioprotective effect of DPP-4 inhibitors, with a trend to a reduction in CVE [22,23]. It seems clear that the role of DPP-4 inhibitors in the therapeutic arsenal of T2DM is evolving rapidly, although long-term data to evaluate their effect on metabolic control are lacking [24,25].
- Patients receiving DPP-4 inhibitors also had lower associated health costs and a lesser use of health resources. The few existing studies show that the higher the compliance and metabolic control in these patients, the lower the risk of hospitalization. For example, a review by Breitscheidel et al. [26] concluded that improving compliance may result in a reduction in total healthcare costs in T2DM: in seven studies, compliance was inversely associated with healthcare costs, especially due to the lower cost resulting from fewer days of hospitalization. Overall, our findings are consistent with these results.
- Our results show lower rates of CVE in patients treated with DPP-4 inhibitors, although the differences were not significant. Given the close relationship between some microangiopathies (principally nephropathy) and CVE, it is logical to consider that good metabolic control would positively affect this situation, but less intensely than the control of other risk factors such as dyslipidaemia and hypertension [22,23]. DDP-4 inhibitors in patients with RI showed a higher benefice. It is possible for the pharmacological characteristics of the drug; although the most consistent explanation is to present fewer complications and better metabolic control, circumstances related to a better use of health resources.
- The possible limitations of the study include the accurate diagnosis of T2DM, the possible bias in patient classification and the operational measurement of the costs attributable to the information system developed. This type of study design is subject to various types of bias (factors not taken into account such as the socioeconomic, cultural or educational levels, the pharmacological doses administered and the correctness of therapy, among others) which should be minimized. The main limitation of this study is the undoubted selection bias on the part of the attending physician when administering one or another drug; and therefore, the results should be interpreted with caution. Another limitation relates to the measurement of hypoglycemia, since only episodes in which the patient required medical care and this was documented were identified, leaving open the possibility of under-diagnosis of cases. Likewise, although good metabolic control is generally considered to be HbA1c <7%, this remains subject to discussion today, with current recommendations based on individualizing targets. Because of the low number of patients, no subanalysis was performed in the group of other oral antidiabetics (including sulfonylureas and glitazones), should be considered another limitation of the study.
- Our results should be replicated in other health institutions while awaiting the results of current clinical trials on the efficacy of DPP-4 inhibitors. Although our study had some limitations, diabetic patients with RI treated with DPP-4 inhibitors in combination with metformin had better metabolic control and lower rates of hypoglycaemia overall, resulting in lower health care costs for the Spanish National Health System.
DISCUSSION
- 1. Sinclair A, Morley JE, Rodriguez-Manas L, Paolisso G, Bayer T, Zeyfang A, Bourdel-Marchasson I, Vischer U, Woo J, Chapman I, Dunning T, Meneilly G, Rodriguez-Saldana J, Gutierrez Robledo LM, Cukierman-Yaffe T, Gadsby R, Schernthaner G, Lorig K. Diabetes mellitus in older people: position statement on behalf of the International Association of Gerontology and Geriatrics (IAGG), the European Diabetes Working Party for Older People (EDWPOP), and the International Task Force of Experts in Diabetes. J Am Med Dir Assoc 2012;13:497-502. ArticlePubMed
- 2. Van Buren PN, Toto R. Current update in the management of diabetic nephropathy. Curr Diabetes Rev 2013;9:62-77. ArticlePubMed
- 3. Arora MK, Singh UK. Molecular mechanisms in the pathogenesis of diabetic nephropathy: an update. Vascul Pharmacol 2013;58:259-271. ArticlePubMed
- 4. Tramonti G, Kanwar YS. Review and discussion of tubular biomarkers in the diagnosis and management of diabetic nephropathy. Endocrine 2013;43:494-503. ArticlePubMedPDF
- 5. Fagerudd J, Forsblom C, Pettersson-Fernholm K, Groop PH. FinnDiane Study Group. Implementation of guidelines for the prevention of diabetic nephropathy. Diabetes Care 2004;27:803-804. ArticlePubMedPDF
- 6. Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, Heller SR, Rodriguez H, Rosenzweig J, Vigersky R;. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. J Clin Endocrinol Metab 2013;98:1845-1859. ArticlePubMed
- 7. Stein SA, Lamos EM, Davis SN. A review of the efficacy and safety of oral antidiabetic drugs. Expert Opin Drug Saf 2013;12:153-175. ArticlePubMed
- 8. Green JB. The dipeptidyl peptidase-4 inhibitors in type 2 diabetes mellitus: cardiovascular safety. Postgrad Med 2012;124:54-61. ArticlePubMed
- 9. Kothny W, Shao Q, Groop PH, Lukashevich V. One-year safety, tolerability and efficacy of vildagliptin in patients with type 2 diabetes and moderate or severe renal impairment. Diabetes Obes Metab 2012;14:1032-1039. ArticlePubMed
- 10. Lukashevich V, Schweizer A, Foley JE, Dickinson S, Groop PH, Kothny W. Efficacy of vildagliptin in combination with insulin in patients with type 2 diabetes and severe renal impairment. Vasc Health Risk Manag 2013;9:21-28. ArticlePubMedPMC
- 11. Lamberts H, Wood M, Hofmans-Okkes I. The International classification of primary care in the European community: with a multi-language layer. Oxford: Oxford University Press; 1993.
- 12. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-383. ArticlePubMed
- 13. Weiner JP, Starfield BH, Steinwachs DM, Mumford LM. Development and application of a population-oriented measure of ambulatory care case-mix. Med Care 1991;29:452-472. ArticlePubMed
- 14. The Anatomical Therapeutic Chemical Classification System [Internet]. Oslo: World Health Organization; 1991. cited 2014 Nov 21. 1990 - Available from: http://www.who.int/.
- 15. Sicras Mainar A, Roldan Suarez C, Font Ramos B, Navarro Artieda R, Ibanez Nolla J. Clinical and economical consequences of the combination of metformin with dipeptidyl peptidase inhibitors in type 2 diabetes patients. Rev Clin Esp 2013;213:377-384. ArticlePubMed
- 16. Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed. BMJ 2000;320:1197-1200. ArticlePubMedPMC
- 17. Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Ther 2011;33:74-109. ArticlePubMed
- 18. Bailey CJ, Kodack M. Patient adherence to medication requirements for therapy of type 2 diabetes. Int J Clin Pract 2011;65:314-322. ArticlePubMed
- 19. Cramer JA, Benedict A, Muszbek N, Keskinaslan A, Khan ZM. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract 2008;62:76-87. ArticlePubMedPMC
- 20. Jermendy G, Wittmann I, Nagy L, Kiss Z, Rokszin G, Abonyi-Toth Z, Katona L, Paragh G, Karadi I, Merkely B. Persistence of initial oral antidiabetic treatment in patients with type 2 diabetes mellitus. Med Sci Monit 2012;18:CR72-CR77. ArticlePubMedPMC
- 21. Erlich DR, Slawson DC, Shaughnessy A. Diabetes update: new drugs to manage type 2 diabetes. FP Essent 2013;408:20-24. PubMed
- 22. Scheen AJ. Cardiovascular effects of dipeptidyl peptidase-4 inhibitors: from risk factors to clinical outcomes. Postgrad Med 2013;125:7-20. Article
- 23. Baetta R, Corsini A. Pharmacology of dipeptidyl peptidase-4 inhibitors: similarities and differences. Drugs 2011;71:1441-1467. ArticlePubMed
- 24. Guillausseau PJ. Impact of compliance with oral antihyperglycemic agents on health outcomes in type 2 diabetes mellitus: a focus on frequency of administration. Treat Endocrinol 2005;4:167-175. PubMed
- 25. Scheen AJ. DPP-4 inhibitors in the management of type 2 diabetes: a critical review of head-to-head trials. Diabetes Metab 2012;38:89-101. ArticlePubMed
- 26. Breitscheidel L, Stamenitis S, Dippel FW, Schoffski O. Economic impact of compliance to treatment with antidiabetes medication in type 2 diabetes mellitus: a review paper. J Med Econ 2010;13:8-15. ArticlePubMed
REFERENCES
Flow diagram of study patients. OOAD, other oral antidiabetics (including sulfonylureas and glitazones); DPP-4, dipeptidyl peptidase-4. aA total of 4,233 patients were excluded from the study: 978 received no drug treatment, 1,127 received other drug therapies (insulin: 663), 241 discontinued treatment, and 1,887 changed therapy during follow-up, bSix hundred fifty-five patients were lost to the study and 326 excluded for other reasons. The percentage distribution of patients who were excluded and lost was similar in the two study groups. Patients with renal impairment were 6.0% of diabetics, 16.5% of those treated and 28.1% of those receiving oral antidiabetic treatment.
Compliance, persistence, and metabolic control according to the study groups
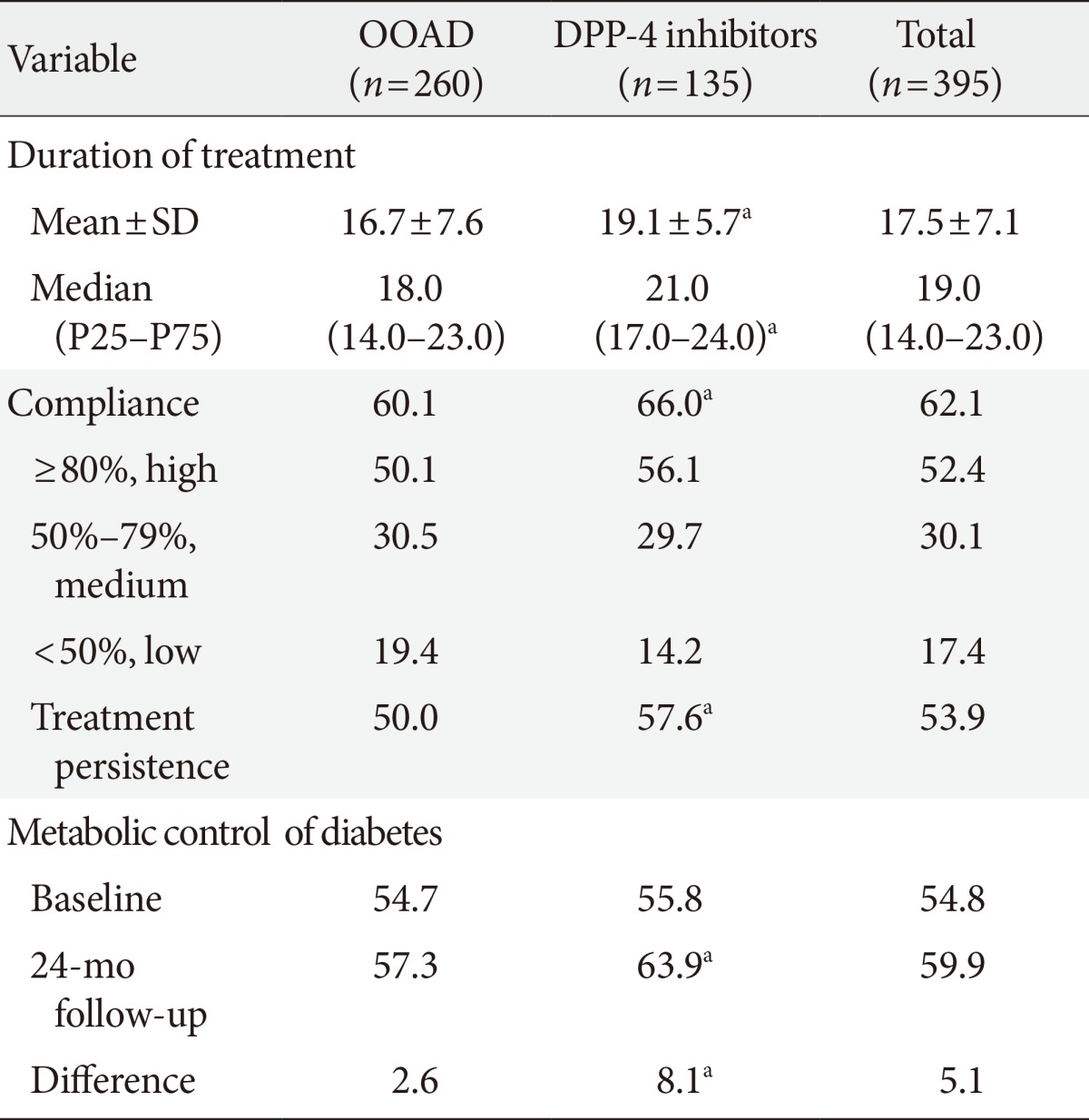
Values are presented as percentage.
OOAD, other oral antidiabetics (sulfonylureas and glitazones included); DPP-4, dipeptidyl peptidase-4.
aStatistically significant results: P<0.05. Adherence: ratio between the number of tablets dispensed between prescribed. Persistence: median time without abandoning the initial treatment or switching to another medication without at least 30 days after the initial prescription. Metabolic control: glycosylated hemoglobin <7%.
Model of gross and adjusted costs according to the study groups (mean unit costs in euros) during the 2-year follow-up
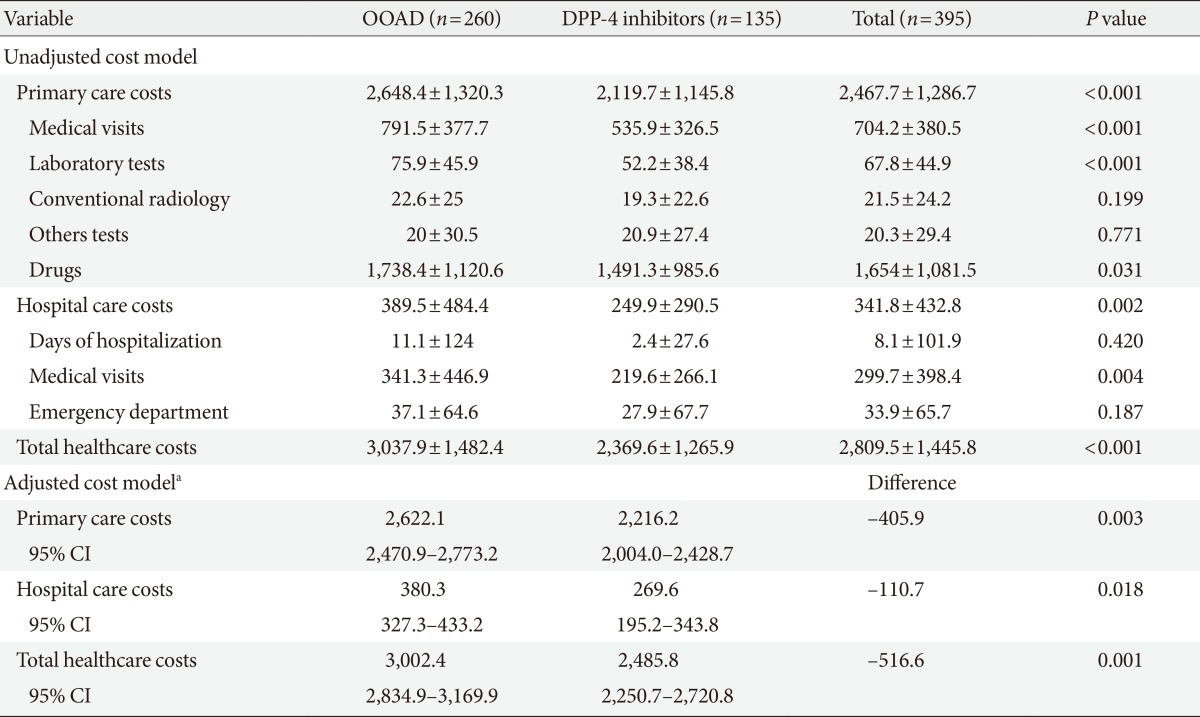
Values are presented as mean±standard deviation.
OOAD, other oral antidiabetics (includes sulfonylureas and glitazones); DPP-4, dipeptidyl peptidase-4; CI, confidence interval.
aCovariates: sex, age, co-mobility (Charlson index), and time of evolution since diagnosis of type 2 diabetes mellitus (Bonferroni correction): analysis of covariance model: the contrasts are based on pair wise comparisons, linearly independent, among the estimated marginal means.
Figure & Data
References
Citations

- Characteristics of Hypoglycemia Pateints Visiting the Emergency Department of a University Hospital
Sang-Hyeon Choi, Deok-Ki Youn, Moon-Gi Choi, Ohk-Hyun Ryu
The Journal of Korean Diabetes.2016; 17(3): 202. CrossRef - Response: Economic Impact of Combining Metformin with Dipeptidyl Peptidase-4 Inhibitors in Diabetic Patients with Renal Impairment in Spanish Patients (Diabetes Metab J2015;39:74-81)
Antoni Sicras-Mainar, Ruth Navarro-Artieda
Diabetes & Metabolism Journal.2015; 39(2): 173. CrossRef - Dipeptidyl Peptidase 4: A New Link between Diabetes Mellitus and Atherosclerosis?
Wellington Santana da Silva Júnior, Amélio Fernando de Godoy-Matos, Luiz Guilherme Kraemer-Aguiar
BioMed Research International.2015; 2015: 1. CrossRef - Letter: Economic Impact of Combining Metformin with Dipeptidyl Peptidase-4 Inhibitors in Diabetic Patients with Renal Impairment in Spanish Patients (Diabetes Metab J2015;39:74-81)
Hannah Seok
Diabetes & Metabolism Journal.2015; 39(2): 171. CrossRef

 KDA
KDA
 PubReader
PubReader Cite
Cite




