- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Ahead-of print > Article
-
Brief ReportGenetics Clinical Characteristics of Diabetes in People with Mitochondrial DNA 3243A>G Mutation in Korea
-
Eun Hoo Rho1
 , Sang Ik Baek2, Heerah Lee3, Moon-Woo Seong3, Jong-Hee Chae4, Kyong Soo Park1, Soo Heon Kwak1
, Sang Ik Baek2, Heerah Lee3, Moon-Woo Seong3, Jong-Hee Chae4, Kyong Soo Park1, Soo Heon Kwak1
-
DOI: https://doi.org/10.4093/dmj.2023.0078
Published online: February 1, 2024
- 506 Views
- 31 Download
1Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
2Department of Internal Medicine, Green Hospital, Seoul, Korea
3Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea
4Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea
- Corresponding author: Soo Heon Kwak Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea E-mail: shkwak@snu.ac.kr
Copyright © 2024 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
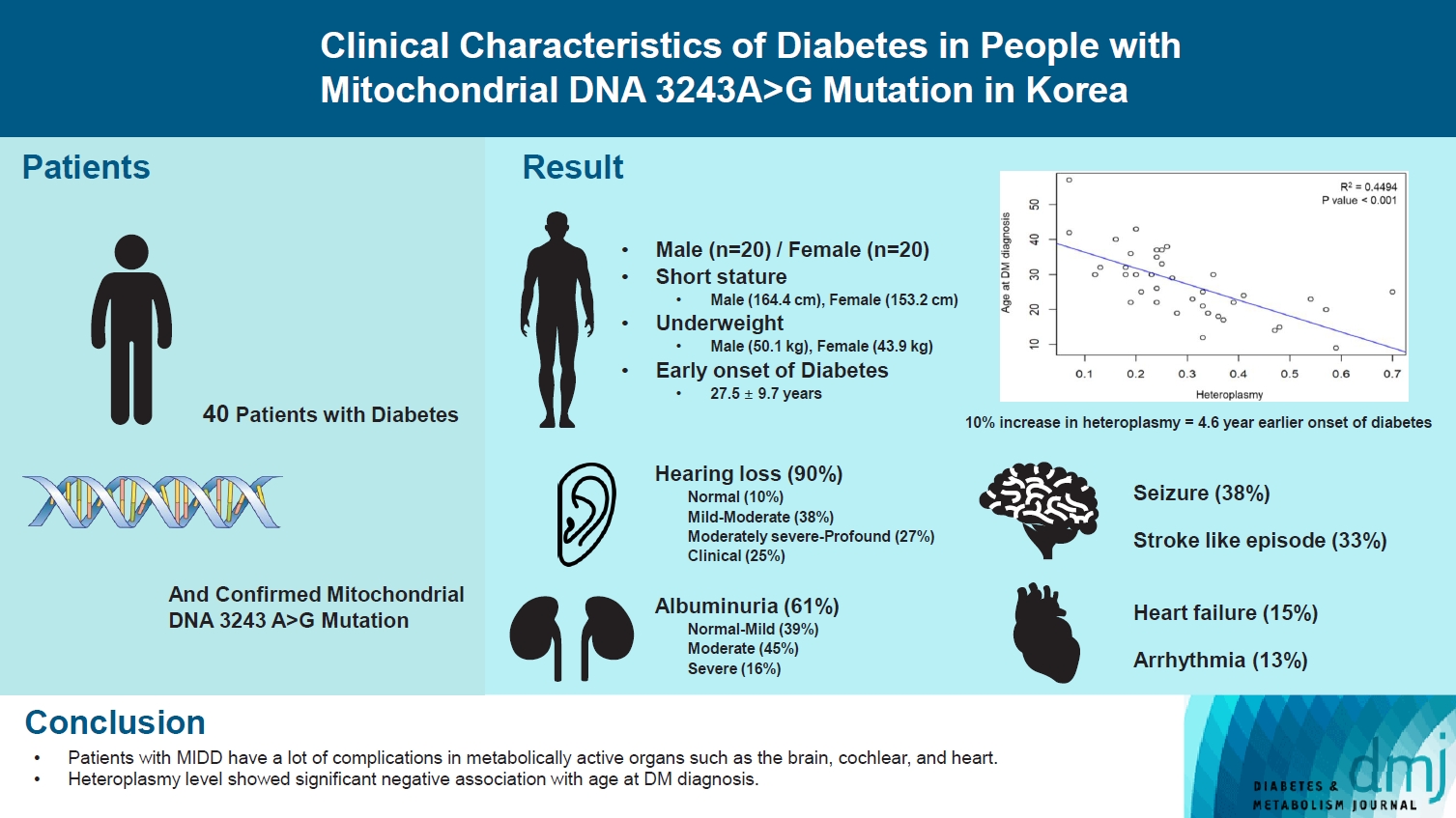
- Maternally inherited diabetes and deafness (MIDD) is a rare mitochondrial disorder primarily resulting from m.3243A>G mutation. The clinical characteristics of MIDD exhibit significant heterogeneity. Our study aims to delineate these characteristics and determine the potential correlation with m.3243A>G heteroplasmy levels. This retrospective, descriptive study encompassed patients with confirmed m.3243A>G mutation and diabetes mellitus at Seoul National University Hospital. Our cohort comprises 40 patients with MIDD, with a mean age at study enrollment of 33.3±12.9 years and an average % of heteroplasmy of 30.0%±14.6% in the peripheral blood. The most prevalent comorbidity was hearing loss (90%), followed by albuminuria (61%), seizure (38%), and stroke (33%). We observed a significant negative correlation between % of heteroplasmy and age at diabetes diagnosis. These clinical features can aid in the suspicion of MIDD and further consideration of genetic testing for m.3243A>G mutation.
- • Patients with MIDD often exhibited short stature, underweight, and early onset of DM.
- • Patients with MIDD commonly have comorbidities like hearing loss, albuminuria, seizure, and stroke.
- • Heteroplasmy level showed a significant negative association with age at DM diagnosis.
- • These features can suggest MIDD, prompting m.3243A>G mutation testing.
Highlights
- Maternally inherited diabetes and deafness (MIDD) is a rare mitochondrial disorder predominantly caused by the m.3243A>G mutation, with a prevalence in the diabetes population ranging from 0.5% to 3.0% depending on region and ethnic background [1]. East Asians are more commonly affected than Europeans. Although diabetes mellitus and hearing loss are the hallmark features of MIDD, additional manifestations such as renal disease, cardiac disease, neurological disease, and myopathy are commonly observed [2-5]. However, the clinical manifestations of MIDD are highly heterogeneous [1], and the underlying reasons for this variability remain unclear. The reason why one unique gene mutation can exhibit such various clinical features is not clearly understood. Possible factors include both genetic and environmental influences [1], as well as the phenomenon of heteroplasmy. The relative amount of mutant mitochondrial DNA (mtDNA) compared to wild-type mtDNA is termed the heteroplasmy [6] and % of heteroplasmy can vary from patient to patient and even between organs and tissues in a given patient [2,7]. Some studies have suggested a correlation between higher % of heteroplasmy and greater disease severity [4,8]. Other studies have observed a tendency for % of heteroplasmy to decrease with age, leading to the concept of age-adjusted heteroplasmy [1,3,8].
- Despite the relatively high prevalence of MIDD in East Asia, there have been few studies on this disorder in Korea. Therefore, our study seeks to describe the clinical characteristics of MIDD patients in Korea and investigate the potential association between these features and the % of m.3243A>G heteroplasmy.
INTRODUCTION
- Patient selection
- This is a retrospective, descriptive study that includes patients diagnosed with MIDD. We identified patients with confirmed m.3243A>G mutation at Seoul National University Hospital between May 1, 2001 and May 27, 2022. We only included patients with glycosylated hemoglobin (HbA1c) levels above 6.5% or those receiving diabetes treatment.
- Data collection
- Through retrospective medical record analysis, we evaluated the clinical characteristics of patients, including % of heteroplasmy, age at diabetes diagnosis, diabetes treatment, and comorbidities. In cases of maternal inheritance, we considered the family history of diabetes. Of the various comorbidities associated with MIDD, we focused on those affecting the brain, ear, heart, and kidney. We only utilized data collected within 1 year from the time the genetic mutation was confirmed.
- Molecular study
- The extraction of mtDNA from peripheral blood leukocytes was carried out using the QIAsymphony DSP DNA Midi Kit (QIAGEN, Hilden, Germany). Subsequently, polymerase chain reaction amplification of mtDNA was performed and Sanger sequencing was conducted on 31 patients using the BigDye Terminator v3.1 Cycle Sequencing Kit and ABI 3730 DNA analyzer (Thermo Scientific, Waltham, MA USA). For the remaining nine patients, targeted panel sequencing was performed as described above [9]. Among the 40 patients with m.3243 A>G mutation, % of heteroplasmy was available for 39 patients. The estimation of heteroplasmy was performed concurrently with the sequencing results. For the nine samples subjected to targeted sequencing, the DNA samples were deposited in the Seoul National University Hospital Human Biobank.
- Statistical analysis
- All statistical analyses were conducted using R 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria). We performed Pearson correlation analysis, Spearman correlation analysis, and logistic regression analysis to assess correlations between % of heteroplasmy and clinical features. We considered a P value of less than 0.05 to be statistically significant.
- Ethics statement
- This study was approved by the Institutional Review Board of Seoul National University Hospital, Seoul, Korea (IRB number 1606-023-768). Written informed consent by the patients was waived due to a retrospective nature of our study.
METHODS
- Baseline characteristics
- Forty patients were diagnosed with MIDD, with 20 of them being male. The mean age at study enrollment was 33.3±12.9 years. The mean age at the time of diabetes diagnosis was 27.5±9.7 years and 22 patients, which accounts for 63% of the total, had a confirmed maternal family history of diabetes. The mean % of heteroplasmy was 30%±14.6% and the mean age-adjusted % of heteroplasmy as suggested by Grady et al. [8] was 78%± 18.5%. Patient with MIDD exhibited characteristics of relatively short stature and low body mass index (BMI, 18.5±2.5 kg/m2 in male and 18.7±2.6 kg/m2 in female). The median HbA1c was 7.5% (interquartile range, 6.5% to 8.5%) and was measured while the patients were receiving treatment for diabetes (Table 1).
- Comorbidities
- Hearing loss was the most common comorbidity in MIDD patients, which was found in 36 (90%) patients (Table 1). The severity of hearing loss ranged from mild (15%) to profound (7%). Albuminuria was the next common comorbidity, found in 19 patients (61%), but only five patients (13%) had an estimated glomerular filtration rate of less than 60 mL/min/1.73 m2. Stroke-like episodes and seizures were identified in 13 (33%) and 15 (38%) patients, respectively (Table 1).
- Diabetes treatment
- Twenty-five patients (63%) out of the total 40 MIDD patients diagnosed with diabetes were receiving insulin treatment. The mean frequency of insulin injection was 2.3 times a day with a median dosage of 36 units a day. Twenty-four patients (60%) were treated with oral anti-diabetic drug, with metformin being the most used medication (Supplementary Table 1).
- Correlation between heteroplasmy and clinical feature
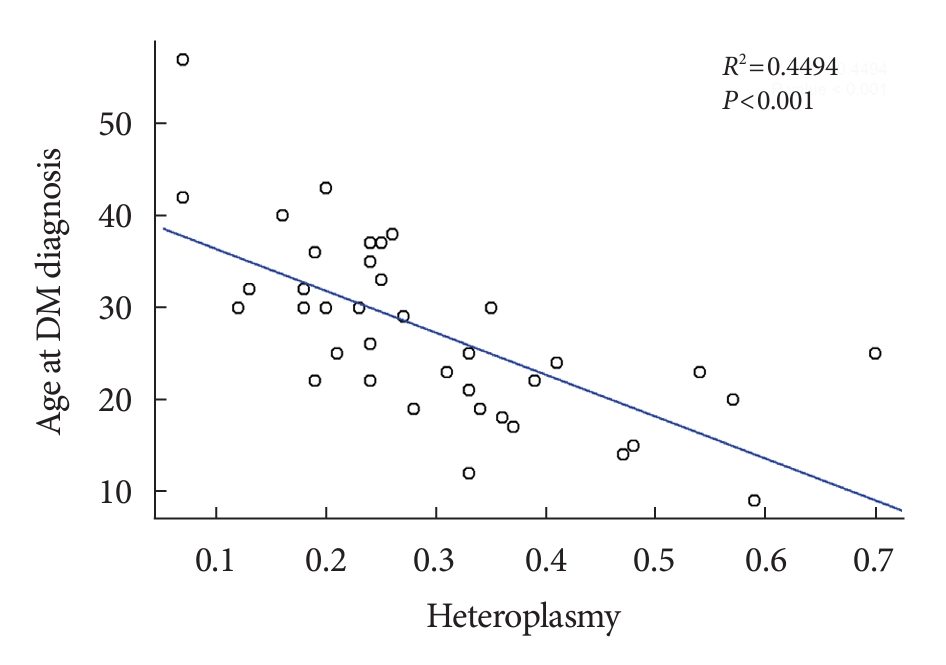
- We investigated whether there was a correlation between % of heteroplasmy and clinical features. The only significant correlation found was between % of heteroplasmy and age at the time of diabetes diagnosis. Age at diabetes diagnosis was negatively correlated with % of heteroplasmy as shown in Fig. 1 (R2=0.449, P<0.001), as well as with age-adjusted % of heteroplasmy (R2=0.309, P<0.001) (Supplementary Tables 2 and 3).
RESULTS
- MIDD is a rare mitochondrial genetic disease, predominantly caused by the m.3243A>G mutation, and characterized by a highly heterogeneous array of clinical features primarily affecting metabolically active organs [2]. This heterogeneity makes the diagnosis of MIDD a formidable challenge, even with a single point mutation. Given the limited research on MIDD in Korea, our study aimed to provide the most comprehensive investigation on this topic conducted in the country thus far.
- Our study enrolled a total of 40 patients diagnosed with MIDD. The average % of heteroplasmy was 30%±14.6%, which was consistent with previous research findings [5]. The patients had various comorbidities, with hearing loss being the most prevalent and linked to 90% of the cases, in accordance with prior studies [5,10]. The mean age at diabetes diagnosis was 27.5 years, earlier than that of diabetic patients with other forms. This finding aligns with reports indicating impaired insulin secretion in patients with MIDD [11]. As a result, 63% of our cohort received insulin treatment. Additionally, it is noteworthy that these patients displayed characteristics of short stature and low BMI.
- Numerous previous studies have consistently demonstrated a negative correlation between % of heteroplasmy and the age at diabetes diagnosis [4,5,8]. Our data corroborated this trend, revealing a negative correlation between % of heteroplasmy and the age at diabetes diagnosis, which indicated a 4.6-year earlier onset of diabetes for every 10% increase in heteroplasmy. Age-adjusted heteroplasmy also displayed a negative correlation with the age at diabetes diagnosis, albeit to a lesser degree.
- Our study possesses several strengths. First, despite being a rare genetic disease, a relatively sizeable sample of 40 patients was recruited for the study. Second, the correlation between clinical features and % of heteroplasmy was analyzed not only for the % of heteroplasmy itself but also for age-adjusted % of heteroplasmy. Conversely, our study also has some limitations. Although the sample size of 40 patients was notable, the statistical power required to test the association between the % of heteroplasmy and various other clinical characteristics was inadequate. Furthermore, our study was retrospective, and thus unable to evaluate a more diverse range of clinical features as well as incident events.
- MIDD showcases an extensive range of clinical features, with only 57% of patients displaying both hearing loss and maternal inheritance of diabetes. As such, diagnosing the disease is a formidable challenge. However, our study highlights that early-onset diabetes, hearing loss, albuminuria, seizure, and stroke may all be indicative of MIDD, and should prompt consideration of genetic testing for the m.3243A>G mutation. Moreover, our findings demonstrate a significant negative association between the % of heteroplasmy and the age at which diabetes was diagnosed.
DISCUSSION
SUPPLEMENTARY MATERIALS
Supplementary Table 2.
Supplementary Table 3.
-
CONFLICTS OF INTEREST
Kyong Soo Park has been honorary editors of the Diabetes & Metabolism Journal since 2020. He was not involved in the review process of this article. Otherwise, there was no conflict of interest.
-
AUTHOR CONTRIBUTIONS
Conception or design: K.S.P., S.H.K.
Acquisition, analysis, or interpretation of data: E.H.R., S.I.B., H.L., M.W.S., J.H.C.
Drafting the work or revising: K.S.P., S.H.K.
Final approval of the manuscript: all authors.
-
FUNDING
This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (grant No. HI15C3131); and Seoul National University Hospital (0320220080). This work was supported by a grant (Soo Heon Kwak, 2021F-6) from the Korean Diabetes Association. Soo Heon Kwak is supported by National Human Genome Research Institute (NHGRI), grant FAIN# U01HG011723.
NOTES
-
Acknowledgements
- None
Values are presented as mean±standard deviation, percentage (number/total number), or median (interquartile range).
DM, diabetes mellitus; BMI, body mass index; HbA1c, glycosylated hemoglobin; FPG, fasting plasma glucose; BUN, blood urea nitrogen; Cr, creatinine; eGFR, estimated glomerular filtration rate.
- 1. Laloi-Michelin M, Meas T, Ambonville C, Bellanne-Chantelot C, Beaufils S, Massin P, et al. The clinical variability of maternally inherited diabetes and deafness is associated with the degree of heteroplasmy in blood leukocytes. J Clin Endocrinol Metab 2009;94:3025-30.ArticlePubMedPDF
- 2. Murphy R, Turnbull DM, Walker M, Hattersley AT. Clinical features, diagnosis and management of maternally inherited diabetes and deafness (MIDD) associated with the 3243A>G mitochondrial point mutation. Diabet Med 2008;25:383-99.ArticlePubMed
- 3. de Laat P, Koene S, van den Heuvel LP, Rodenburg RJ, Janssen MC, Smeitink JA. Clinical features and heteroplasmy in blood, urine and saliva in 34 Dutch families carrying the m.3243A > G mutation. J Inherit Metab Dis 2012;35:1059-69.ArticlePubMedPMC
- 4. Chae HW, Na JH, Kim HS, Lee YM. Mitochondrial diabetes and mitochondrial DNA mutation load in MELAS syndrome. Eur J Endocrinol 2020;183:505-12.ArticlePubMed
- 5. Yang M, Xu L, Xu C, Cui Y, Jiang S, Dong J, et al. The mutations and clinical variability in maternally inherited diabetes and deafness: an analysis of 161 patients. Front Endocrinol (Lausanne) 2021;12:728043.ArticlePubMedPMC
- 6. Cree LM, Samuels DC, Chinnery PF. The inheritance of pathogenic mitochondrial DNA mutations. Biochim Biophys Acta 2009;1792:1097-102.ArticlePubMedPMC
- 7. Frederiksen AL, Andersen PH, Kyvik KO, Jeppesen TD, Vissing J, Schwartz M. Tissue specific distribution of the 3243A->G mtDNA mutation. J Med Genet 2006;43:671-7.ArticlePubMedPMC
- 8. Grady JP, Pickett SJ, Ng YS, Alston CL, Blakely EL, Hardy SA, et al. mtDNA heteroplasmy level and copy number indicate disease burden in m.3243A>G mitochondrial disease. EMBO Mol Med 2018;10:e8262.PubMedPMC
- 9. Park SS, Jang SS, Ahn CH, Kim JH, Jung HS, Cho YM, et al. Identifying pathogenic variants of monogenic diabetes using targeted panel sequencing in an East Asian population. J Clin Endocrinol Metab 2019;104:4188-98.ArticlePubMedPDF
- 10. Suzuki S. Diabetes mellitus with mitochondrial gene mutations in Japan. Ann N Y Acad Sci 2004;1011:185-92.ArticlePubMed
- 11. Katagiri H, Asano T, Ishihara H, Inukai K, Anai M, Yamanouchi T, et al. Mitochondrial diabetes mellitus: prevalence and clinical characterization of diabetes due to mitochondrial tRNA(Leu (UUR)) gene mutation in Japanese patients. Diabetologia 1994;37:504-10.ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations


 KDA
KDA
 PubReader
PubReader ePub Link
ePub Link Cite
Cite