- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 46(3); 2022 > Article
-
ReviewMetabolic Risk/Epidemiology Lifestyle Interventions for Non-Obese Patients Both with, and at Risk, of Non-Alcoholic Fatty Liver Disease
-
Xin-Lei Zhang1
 , Ting-Yao Wang2, Giovanni Targher3, Christopher D. Byrne4,5, Ming-Hua Zheng1,6,7
, Ting-Yao Wang2, Giovanni Targher3, Christopher D. Byrne4,5, Ming-Hua Zheng1,6,7
-
Diabetes & Metabolism Journal 2022;46(3):391-401.
DOI: https://doi.org/10.4093/dmj.2022.0048
Published online: May 25, 2022
1NAFLD Research Center, Department of Hepatology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
2Department of Nephrology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
3Section of Endocrinology, Diabetes and Metabolism, Department of Medicine, University of Verona, Verona, Italy
4Southampton National Institute for Health Research Biomedical Research Centre, University Hospital Southampton, Southampton General Hospital, Southampton, UK
5Nutrition and Metabolism, Faculty of Medicine, University of Southampton, Southampton, UK
6Institute of Hepatology, Wenzhou Medical University, Wenzhou, China
7Key Laboratory of Diagnosis and Treatment for the Development of Chronic Liver Disease in Zhejiang Province, Wenzhou, China
-
Corresponding author: Ming-Hua Zheng
 NAFLD Research Center, Department of Hepatology, The First Affiliated Hospital of Wenzhou Medical University, No. 2 Fuxue Lane, Wenzhou 325000, China E-mail: zhengmh@wmu.edu.cn
NAFLD Research Center, Department of Hepatology, The First Affiliated Hospital of Wenzhou Medical University, No. 2 Fuxue Lane, Wenzhou 325000, China E-mail: zhengmh@wmu.edu.cn
Copyright © 2022 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Non-alcoholic fatty liver disease occurring in non-obese subjects (the so-called non-obese NAFLD) is a highly prevalent but neglected liver condition, which is closely associated with metabolic disorders and suboptimal lifestyles. Landmark studies have shown that lifestyle interventions are potentially beneficial in decreasing the risk of developing non-obese NAFLD and in ameliorating NAFLD in non-obese individuals with pre-existing NAFLD. Lifestyle interventions usually refer to changes in eating habits and physical activity, both of which have a powerful effect on non-obese NAFLD and on risk factors for non-obese NAFLD. However, to date, patients and health-care professionals have a poor awareness and understanding of non-obese NAFLD and the beneficial effects of lifestyle interventions in this patient population. The aim of this narrative review is to briefly discuss the evidence for the effects of lifestyle changes and what changes are needed amongst medical personnel and other stakeholders in order to raise awareness of non-obese NAFLD.
- Non-obese non-alcoholic fatty liver disease (non-obese NAFLD), first discovered in Asia, has been increasingly recognized worldwide [1]. Currently, the number of individuals with non-obese NAFLD has reached epidemic proportions, with approximately 5% to 30% of the general population in different regions having this condition [2]. It has been reported that non-obese NAFLD prevalence is highest in Hispanic and Asian (35.1% and 35.6%, respectively) ethnic groups, followed by White (30.0%) and Black (11.6%; P<0.001) ethnic groups [3]. Non-obese NAFLD is influenced by an unhealthy diet and a sedentary lifestyle and these factors are also affected by host genetics, metabolism, endocrinology, and other factors such as gut microbiota [4-10]. The medical burden of non-obese NAFLD is aggravated by the fact that individuals with nonobese NAFLD may develop long-term liver-related complications and multiple extra-hepatic comorbidities, such as cardiovascular disease and chronic kidney disease [11-14]. For example, recent data has demonstrated that around 40% of the global NAFLD population are classified as non-obese, among which 39.0% (95% confidence interval [CI], 24.1 to 56.3) had non-alcoholic steatohepatitis (NASH), 29.2% (95% CI, 21.9 to 37.9) had significant fibrosis (stage ≥2), and 3.2% (95% CI, 1.5 to 5.7) had cirrhosis [11]. It has been estimated that the annual health care burden related to each patient with non-obese NAFLD is $1,613 in the USA and €1,163 in the Europe, respectively [15]. Consequently, it is now apposite that we should attach great importance to this long-term ignored and detrimental disease.
- The importance of lifestyle factors in the development of non-obese NAFLD creates an opportunity to utilize healthy lifestyle modifications as the first-line treatment to slow the increasing prevalence of non-obese NAFLD worldwide [16,17]. First, evidence from an Asian randomized controlled trial involving 154 NAFLD individuals showed that a 12-month lifestyle intervention aimed at improving physical activity and a healthy diet successfully induced remission of NAFLD (as assessed by proton-magnetic resonance spectroscopy) in 67% of non-obese subjects compared with 18% in the control group [18]. This trial also showed that non-obese subjects achieved remission of NAFLD with 3% to 5% weight reduction; the same could only be achieved in obese subjects with 7% to 10% weight reduction [18]. Second, the severity of metabolic abnormalities is found to be milder in patients with non-obese NAFLD compared to their obese counterparts with NAFLD (Tables 1 and 2) [3,11,18-24]. Finally, most of the drugs for non-obese NAFLD, targeting different molecules such as peroxisome proliferator-activated receptor (PPAR)-α/γ agonists, pan-PPAR agonists, sodium-glucose cotransporter 2 inhibitors, or glucagon-like peptide-1 receptor agonists, are still in clinical development or being tested in large randomized clinical trials [25]. Nonetheless, except for lifestyle interventions, strategies that promote population-level improvements in health are also needed to complement intensive-intervention approaches in high-risk populations [26].
INTRODUCTION
- The American Association for the Study of Liver Diseases (AASLD) has stated that lifestyle modifications, consisting of hypocaloric diet and exercise, should be advocated to treat most patients with NAFLD, regardless of the patient’s body weight [27]. Therefore, it is also clinically important for non-obese patients with NAFLD that they are encouraged to make lifestyle changes when appropriate.
- Healthy eating in the treatment of non-obese NAFLD
- It is difficult for individuals in society to maintain a healthy diet and healthy eating patterns with the advent of a “fast food” era. Not only has the consumption of refined sugar and fructose increased in recent times, but also unhealthy dietary habits, including frequent and irregular patterns of eating (≥6 times daily), have increased rapidly [4,28]. Preliminary studies have suggested that these behaviors are associated with a spectrum of liver injury in non-obese patients, including non-alcoholic fatty liver (NAFL), NASH, cirrhosis, and hepatocellular carcinoma [4]. The possible underlying mechanisms contributing to the development and progression of NAFLD in non-obese individuals include increased hepatic de novo lipogenesis (DNL), inhibition of fat oxidation, greater insulin resistance (IR), circadian rhythm disorders, and intestinal dysbiosis. Consequently, it is widely acknowledged that lifestyle changes targeting each of these processes should be advocated in non-obese patients both at risk of, and with, pre-existing NAFLD.
- Hypercaloric, high fat, low insoluble dietary fiber and high glycemic index diets may be involved in the pathophysiology of non-obese NAFLD, among which high dietary sucrose and fructose consumption seems to be the major contributor [16,28]. In recent years, added sugar in soft drinks, which is a common source of fructose, has increased rapidly with the introduction of sweeteners by the drinks industry [28]. For example, soft drinks intake has increased markedly over the last century and by the year 2000 accounted for approximately 10% of overall energy intake in the average American [29,30]. Furthermore, preliminary studies have also confirmed that the higher intake of sugar-sweetened beverages happens among younger people (adolescents and adults in their twenties) and among ethnic minorities (African Americans, Hispanics, and Native Americans) [31,32].
- Until now, some evidence shows that fructose when consumed at levels typical of Western diets can lead to an increase in visceral adipose tissue, liver fat accumulation (NAFL) and other ectopic fat depots in non-obese individuals [33]. A minority of non-obese patients with NAFL may then develop the more advanced forms of NAFLD, and the evidence also suggests that increased fructose intake may promote progression to more advanced forms of NAFLD in both non-obese children and adults [34,35]. In non-obese NAFLD individuals who consume a substantial amount of fructose; both carbohydrate responsive-element binding protein and sterol regulatory element binding protein 1c are indirectly activated by the high circulating levels of insulin and glucose associated with hepatic and whole-body IR, as well as the fructose-derived metabolites [33,36]. Activation of these two proteins upregulates the full complement of enzymes required for DNL, thereby contributing to the hepatic storage of free fatty acids [37-39]. Furthermore, hepatic metabolism of fructose also inhibits mitochondrial β-oxidation of long-chain fatty acids and impairs triglyceride export in individuals with non-obese NAFLD [40]. Due to the molecular instability of its five-membered furanose ring, fructose promotes protein fructosylation and formation of reactive oxygen species, which eventually results in hepatic inflammation and fibrosis in non-obese individuals with NAFLD [40].
- It is therefore important to consider how best to manage individuals who are non-obese and who are diagnosed with NAFLD [1]. Reduction of fructose consumption may decrease liver fat, visceral adipose tissue and DNL, and improve IR [41]. It is also important for subjects to pay more attention to the mode of fructose intake, because this may also affect its biological impact. For example, in animal studies, fructose provided in liquid form is more deleterious than that incorporated into solid food [42,43]. Additionally, sugar ingested as a single large daily bolus is more detrimental than frequent ingestion of smaller amounts of fructose [43]. Thus, it is advisable to ingest fructose in food and avoid large quantities of fructose in drinks.
- A 21st century lifestyle (for example, involving travelling, commuting, jet lag, night, or rotating shift work) creates “circadian misalignment” and has emerged as a major contributor to metabolic liver diseases, including NAFLD and NASH, regardless of the presence or absence of obesity [44]. Changes in circadian gene expression in hepatocytes, endothelial cells, and Kupffer cells, which in turn influence metabolism in the liver [45,46], may adversely affect the development and progression of non-obese NAFLD.
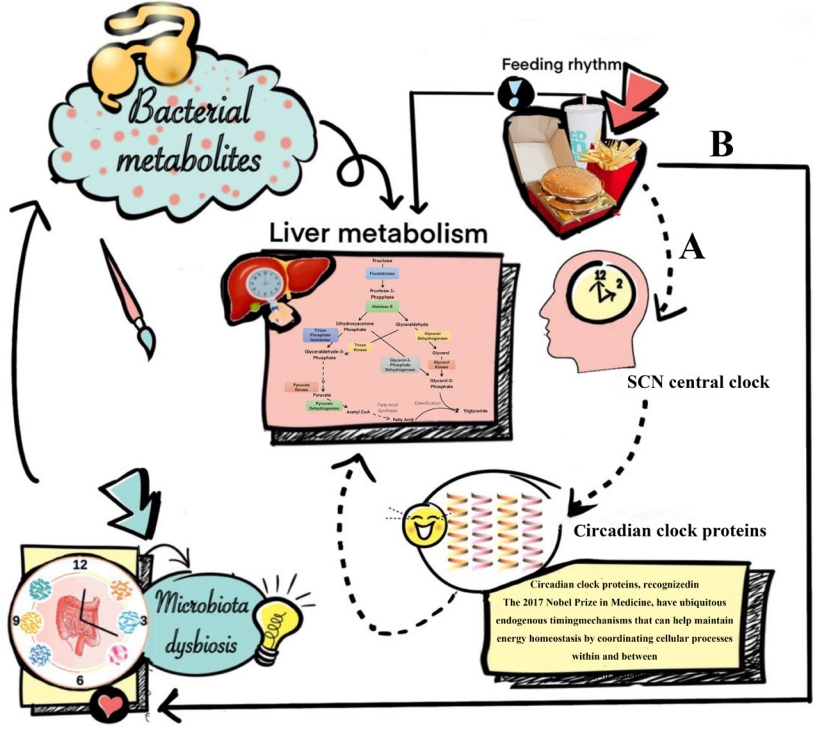
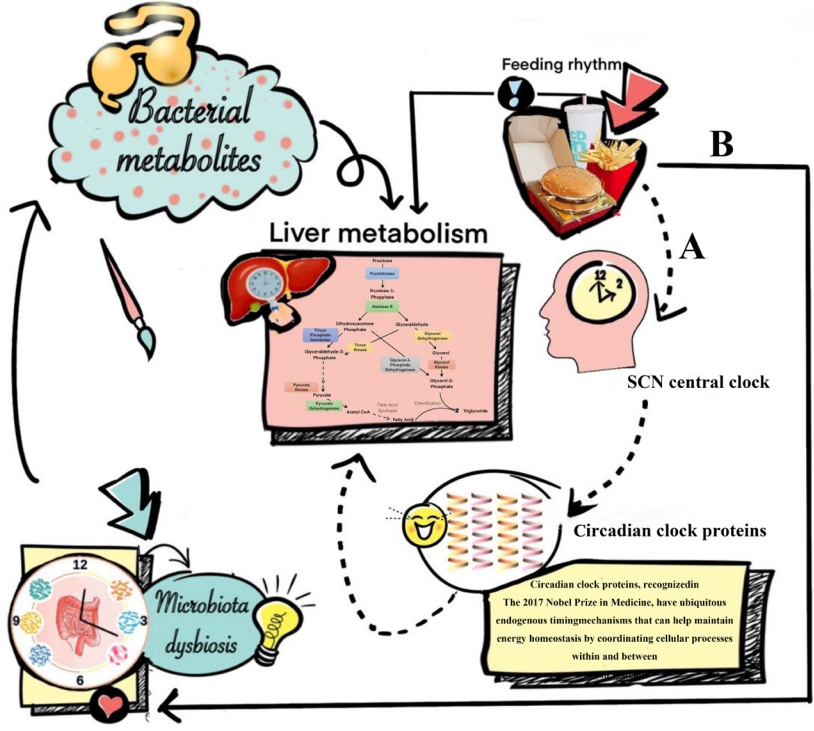
- In most cases, circadian rhythms in humans are mediated by the supra-chiasmatic nuclei (SCN), which is a master clock in the brain setting the time for all other peripheral body clocks in the liver and intestine [47]. Studies have shown that temporal signals such as the timing of food intake may reset peripheral tissue clocks without affecting the SCN central clock rhythms [48], and this may affect subsequent changes in non-obese NAFLD (Fig. 1). Except for abnormalities of systemic metabolism (including glucose, lipid, cholesterol, and bile acid metabolism), it has been suggested that disturbances of the circadian clock machinery may lead to autophagy, endoplasmic reticulum stress and increased oxidative stress in hepatocytes, all of which may promote the development of non-obese NAFLD and its progression to NASH [49]. Additionally, changes in feeding time may reshape the rhythmicity and composition of gut microbiota, which produces bacterial metabolites, such as secondary bile acids and short-chain fatty acids [50]. These gut microbiota-derived metabolites can affect liver metabolism, mainly through a variety of pathways, including contact-dependent mechanisms like pattern recognition receptors and contact-independent mechanisms, as well as changes in intestinal barrier integrity [50].
- Recently, a dietary therapy called time restricted feeding (TRF) has attracted a lot of scientific interest and aims at aligning peripheral and central circadian rhythms, by restricting dietary intake to certain times of the day on every day. This style and pattern of eating has the potential for use as a treatment for NAFLD or NASH in non-obese individuals [49]. TRF does not necessarily reduce calorie intake and it has been shown that TRF increases hepatic fatty acid β-oxidation, decreases hepatic glucose production, as well as reduces macrophage infiltration and hepatic inflammation, all of which are potentially beneficial for the remission of NAFLD or NASH in non-obese individuals [51]. TRF may significantly change the gut microbiome diversity with a decrease of Escherichia and enrichment of Prevotellaceae and Bacteroideaceae [52]. Recently, it has been confirmed that Escherichia fergusonii may promote non-obese NAFLD by interfering with host hepatic lipid metabolism through its own msRNA 23487 [53]. Consequently, it may be important to advocate TRF especially restricting food intake to the middle of the day, as food intake at this time is associated with an abnormal metabolic and inflammatory response in the liver [54-58].
- Physical activity in the treatment of non-obese NAFLD
- Physical inactivity, driven by the high prevalence of sedentary behaviors within the population, is becoming increasingly common worldwide. For example, the most recent global estimates show that one in four (27%) adults [59] and more than three-quarters (81%) of adolescents [60] do not meet the recommendations for aerobic exercise, as outlined in the 2010 Global Recommendations on Physical Activity for Health [61]. In fact, sedentary behaviors, typically characterized by reduced muscle activity, are closely associated with sarcopenia, systemic IR and low-grade inflammation, all of which can contribute to the progression of non-obese NAFLD.
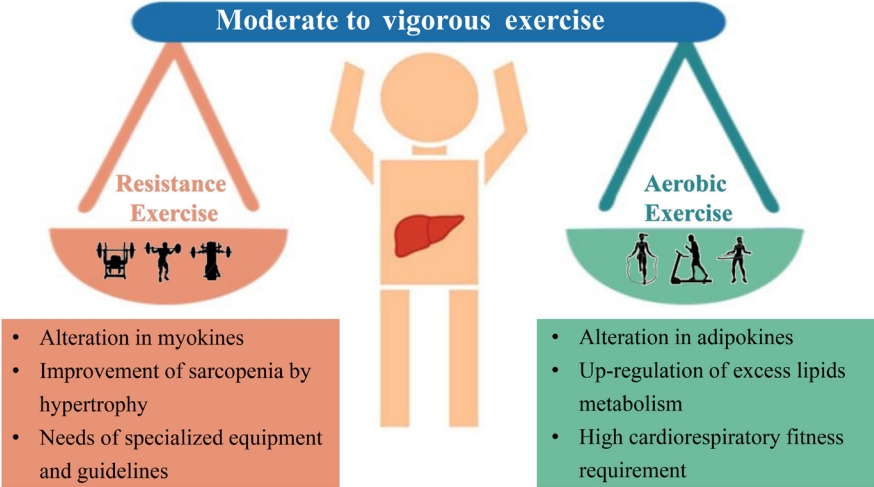
- The spectrum of evidence underpinning the link between physical activity and health is particularly compelling in relation to the development of non-obese NAFLD. Prospective observational research has reported that moderate to vigorous physical activity levels are consistent with current recommendations associated with a reduced risk of incident non-obese NAFLD and resolution of already present non-obese NAFLD [62]. Some mechanistic studies have described several pathways linking increased physical activity to improvement of non-obese NAFLD. First and foremost, there is a complex interaction between three major organs: adipose tissue, liver, and skeletal muscles in non-obese NAFLD, and physical exercise may improve IR in these three organs [63]. An improvement of IR in peripheral tissues may also reduce glucose transport to the liver from muscle tissue and decrease free fatty acid flux to the liver, thus reducing DNL and potentially decreasing liver fat content [63]. Second, exercise reduces the risk of sarcopenia, which is an important risk factor for non-obese NAFLD [64,65]. Furthermore, improved muscle function may further reduce systemic IR and improve hepatic inflammation [66]. Finally, exercise also increases cardiorespiratory fitness, which is particularly useful in decreasing cardiovascular risk in patients with non-obese NAFLD [64].
- There are two different groups of exercise for preventing or treating non-obese NAFLD, i.e., aerobic or resistance exercise (Fig. 2). Current evidence shows that both forms of exercise produce comparable therapeutic effects with similar frequency, duration, and periods of exercise (40–45 minute/session three times/week for 12 weeks) [67]. Aerobic exercise, such as running and cycling, is a low-cost, convenient, and extremely high energy-consuming intervention. Not only does aerobic exercise require good cardiorespiratory fitness because of its high oxygen consumption, but also it is sometimes not feasible in older patients due to arthritis, discomfort, and fatigue [68]. Therefore, resistance exercise, utilizing muscle strength mass and bone density, may be preferable for many patients with NAFLD and seems to produce metabolic benefits with less energy consumption [67]. Thus, subgroups such as the elderly who have sarcopenia, arthritis, or poor cardiorespiratory fitness, may prefer exercise with a low intensity exercise volume [4]. Until now, a beneficial exercise program has not been elucidated for patients with non-obese NAFLD. We propose that exercise targets should be similar for non-obese or obese patients, namely 4.5 metabolic equivalents (METs), 45 minutes/session, and three times/week for aerobic exercise or 3.5 METs, 45 minutes/session, and three times/week for resistance exercise [4].
INTERVENTIONS AND TREATMENTS FOR NON-OBESE NAFLD
Diet: first started with quitting fructose
Clock: follow the “time restricted feeding”
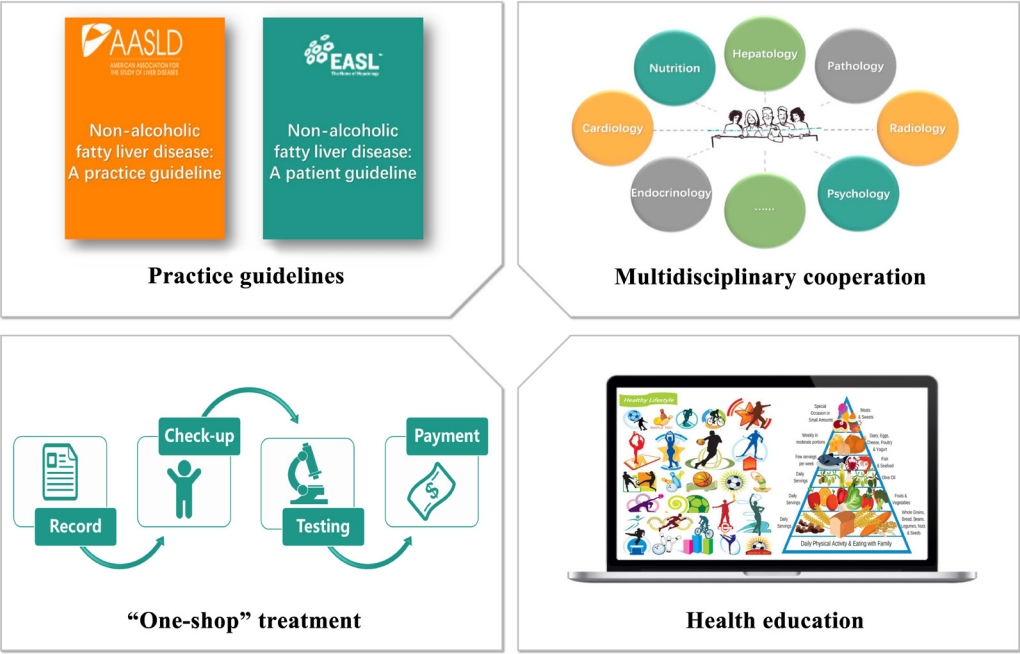
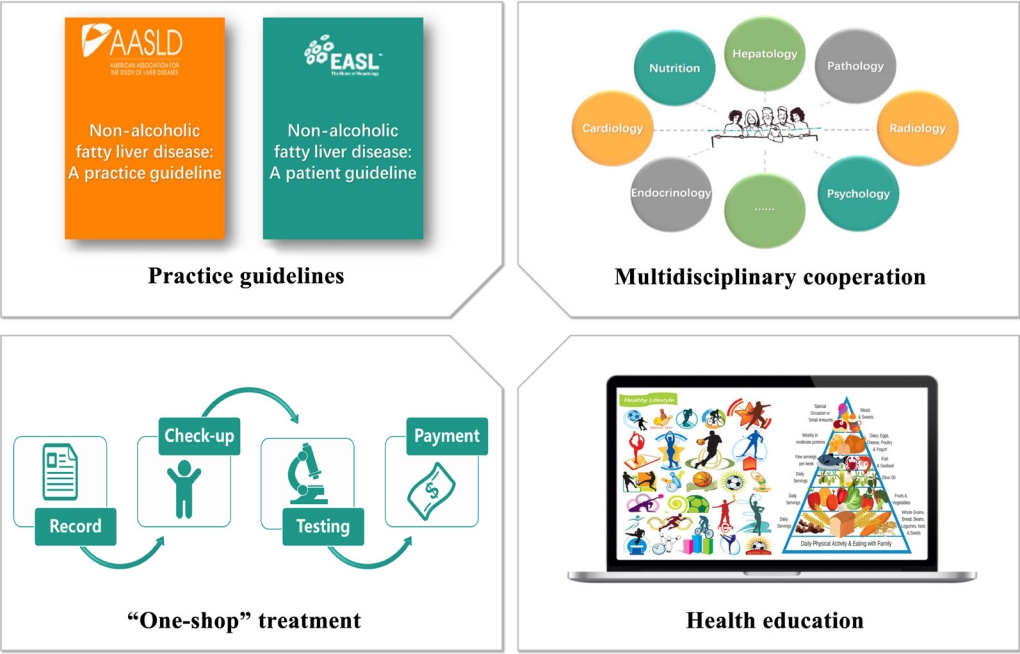
- Several compelling and practical guidelines suggest that treatment schemes for patients with NAFLD should be accompanied by population-level efforts to raise awareness. Such efforts should include “sustained government initiatives comprising advocacy, community support, private sector engagement, and continuous media communication” (Fig. 3).
- National government level strategies
- Despite the significant challenges that non-obese NAFLD present, guidelines and a strategy for non-obese NAFLD are largely absent from the current global health discourse, so they should be addressed in the future sustainable development goals [69]. Also, there are no unified methods for differentiating patients, which may be partially due to the outdated nomenclature and definition [70,71]. Consequently, it is crucial for organizations and governments to establish a framework which targets policy development and individual-level treatment making. The first step is to establish guidelines for non-obese NAFLD at the regional level, including health system structures and funding, as well as reimbursement systems in each country or region [26,72]. The emergence of guidelines can contribute to the unified management of non-obese NAFLD worldwide as well as being beneficial and educational for health-care professionals involved in the diagnosis and management of non-obese NAFLD [70]. In addition, based on the complexity of non-obese NAFLD and its close relationship with metabolic-dysfunction associated fatty liver disease, it is important to establish multidisciplinary care for this patient group [70,71,73-75]. Multidisciplinary clinics, which are emerging models of health care delivery, usually provide individuals with coordinated care services from different medical specialists to diagnose problems, evaluate disease severity, and develop treatment plans [74]. This approach may facilitate the cooperation of diverse services delivered at different levels of the health-care system (primary, secondary, and tertiary) [70], and also improve patient experience and quality of life in NAFLD care, while reducing health care costs [74]. Last, but not least, putting NAFLD on the global public health and development agendas, and promoting NAFLD as a global public health issue is central [76], because there is a significant knowledge gap between liver specialists and non-liver specialists [77].
- Regional government level strategies
- The next level of public-health action refers to the community service, which also has a marked impact on disease management [78]. A primary public-health strategy should enable a “one-stop shop” approach, indicating that all check-ups, recordings and follow-up visits are completed on one site [79-81]. This approach ensures that care is well cooperated and integrated and enables patients’ demands to be assessed and resolved [70]. Additionally, education of managers is a pre-requisite for successful intervention programs, which include low glycemic index diet, TRF, and appropriate physical activity. Offering healthy lifestyle education in school can also be effective for disease prevention because it is useful for students to learn how to eat healthily and exercise [82]. Another effective public-health instrument is to construct community infrastructure including cycle pathways, leisure centers, public footpaths, and parks so that this may facilitate and encourage patients to engage in physical activity [83].
- Personal level strategies
- Non-obese NAFLD is often referred to as a self-inflicted disease, implying that personal behavioral choices are primary determinants of our chances of developing these conditions [73]. Thus, it is significant for individuals, especially those subjects who carry the patatin-like phospholipase domain-containing protein 3 (PNPLA3) rs738409 GG genotype, to develop an improved understanding of non-obese NAFLD, learn how to monitor their own condition, and assess the effectiveness of various therapeutic strategies [84-86]. First and foremost, individuals should be encouraged to participate in, and be offered health lectures, to facilitate them taking an active role in their own health-care (thereby learning directly from their doctors the importance of healthy diet and exercise as well as the direct impact that making these changes may have on their medical conditions). Second, individuals should be encouraged to monitor (where relevant) body weight, waist circumference, blood pressure, plasma glucose, and triglyceride concentrations, all of which are risk factors for non-obese NAFLD, as well as make a detailed diet and exercise plan.
SOCIAL PLANNING TOWARDS NON-OBESE NAFLD MANAGEMENT
- Non-obese NAFLD is now established as a major public-health challenge in both developing and developed countries. The potential to treat NAFLD in non-obese individuals by lifestyle interventions is now firmly established. A healthy diet, regular eating habits and increased physical activity are central to lifestyle modifications, and their promotion should form a key component of any therapeutic initiative for NAFLD, regardless of the patient’s body weight.
CONCLUSIONS
-
CONFLICTS OF INTEREST
Ming-Hua Zheng has been international editorial board members of the Diabetes & Metabolism Journal since 2022. He was not involved in the review process of this review. Otherwise, there was no conflict of interest.
-
FUNDING
This work was supported by grants from the National Natural Science Foundation of China (No. 82070588), High Level Creative Talents from the Department of Public Health in Zhejiang Province (No. S2032102600032), Project of New Century 551 Talent Nurturing in Wenzhou. Giovanni Targher is supported in part by grants from the University School of Medicine of Verona, Verona, Italy. Christopher D. Byrne is supported in part by the Southampton NIHR Biomedical Research Centre (IS-BRC-20004), UK. This work is a part of the PERSONS study.
NOTES
-
Acknowledgements
- None

| Study | Study type | Population | Non-obese vs. obese NAFLD |
|---|---|---|---|
| Wong et al. (2018) [18] | Randomized controlled trial | n=154, 1H-MRS-proven NAFLD | Lower WC/FPG |
| Feldman et al. (2017) [19] | Cross-sectional study | n=187, ultrasound-proven NAFLD | Lower WC/TG/FPG/HOMA-IR |
| Chen et al. (2020) [20] | Cross-sectional study | n=538, biopsy-proven NAFLD | Lower WHR/TG/FBG/HOMA-IR |
| Lower incidence of type 2 diabetes mellitus | |||
| Younes et al. (2022) [21] | Prospective longitudinal study | n=1,339, biopsy-proven NAFLD | Lower WC/TG/FPG |
| Lower incidence of type 2 diabetes mellitus | |||
| Tan et al. (2022) [22] | Cross-sectional study | n=1,812, biopsy-proven NAFLD | Lower WC |
| Lower prevalence of central obesity/pre- diabetes or diabetes/hypertension | |||
| Zou et al. (2020) [3] | Cross-sectional study | n=14,365, US-FLI-defined NAFLD | Lower WC/BP/FPG/HOMA-IR |
| Study | Study composition | Non-obese vs. obese NAFLD |
|---|---|---|
| Ye et al. (2020) [11] | n=10,576,383, from 93 studies (84 cross-sectional and 5 prospective) | Lower BP/HOMA-IR |
| Lu et al. (2020) [23] | n=205,307, from 33 studies (26 cross-sectional, 4 prospective, and 3 retrospective) | Lower WC/BP/HbA1c |
| Lower incidence of central obesity/dyslipidemia/diabetes/hypertension | ||
| Ito et al. (2021) [24] | n=258,531, from 73 studies (55 cross-sectional, 6 prospective, 9 outcome analyses, and 6 NAFLD characteristics data only) | Lower WC/FPG/HbA1c/HOMA-IR |
| Lower incidence of central obesity/dyslipidemia/diabetes/hypertension |
- 1. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11-20.ArticlePubMedPDF
- 2. Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol 2017;15:474-85.ArticlePubMed
- 3. Zou B, Yeo YH, Nguyen VH, Cheung R, Ingelsson E, Nguyen MH. Prevalence, characteristics and mortality outcomes of obese, nonobese and lean NAFLD in the United States, 1999-2016. J Intern Med 2020;288:139-51.ArticlePubMedPDF
- 4. Kamada Y, Takahashi H, Shimizu M, Kawaguchi T, Sumida Y, Fujii H, et al. Clinical practice advice on lifestyle modification in the management of nonalcoholic fatty liver disease in Japan: an expert review. J Gastroenterol 2021;56:1045-61.ArticlePubMedPDF
- 5. Zhang X, Goh GB, Chan WK, Wong GL, Fan JG, Seto WK, et al. Unhealthy lifestyle habits and physical inactivity among Asian patients with non-alcoholic fatty liver disease. Liver Int 2020;40:2719-31.ArticlePubMedPDF
- 6. Jung Y, Lee MK, Puri P, Koo BK, Joo SK, Jang SY, et al. Circulating lipidomic alterations in obese and non-obese subjects with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2020;52:1603-14.ArticlePubMedPDF
- 7. Lee G, You HJ, Bajaj JS, Joo SK, Yu J, Park S, et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat Commun 2020;11:4982.ArticlePubMedPMCPDF
- 8. Lin H, Wong GL, Whatling C, Chan AW, Leung HH, Tse CH, et al. Association of genetic variations with NAFLD in lean individuals. Liver Int 2022;42:149-60.ArticlePubMedPDF
- 9. Jung Y, Koo BK, Jang SY, Kim D, Lee H, Lee DH, et al. Association between circulating bile acid alterations and nonalcoholic steatohepatitis independent of obesity and diabetes mellitus. Liver Int 2021;41:2892-902.ArticlePubMedPDF
- 10. Chen SD, Zhang H, Rios RS, Li YY, Zhu PW, Jin Y, et al. J-shaped relationship between serum zinc levels and the severity of hepatic necro-inflammation in patients with MAFLD. Nutr Metab Cardiovasc Dis 2022;32:1259-65.ArticlePubMed
- 11. Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020;5:739-52.ArticlePubMed
- 12. Rios RS, Zheng KI, Zheng MH. Non-alcoholic steatohepatitis and risk of hepatocellular carcinoma. Chin Med J (Engl) 2021;134:2911-21.ArticlePubMedPMC
- 13. Wang TY, Wang RF, Bu ZY, Targher G, Byrne CD, Sun DQ, et al. Association of metabolic dysfunction-associated fatty liver disease with kidney disease. Nat Rev Nephrol 2022;18:259-68.ArticlePubMedPDF
- 14. Zheng KI, Zheng MH. The uprising of metabolic dysfunction-associated fatty liver disease (MAFLD) in acute-on-chronic liver failure (ACLF). Hepatobiliary Surg Nutr 2021;10:857-9.ArticlePubMedPMC
- 15. Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016;64:1577-86.ArticlePubMedPDF
- 16. Ahadi M, Molooghi K, Masoudifar N, Namdar AB, Vossoughinia H, Farzanehfar M. A review of non-alcoholic fatty liver disease in non-obese and lean individuals. J Gastroenterol Hepatol 2021;36:1497-507.ArticlePubMedPDF
- 17. Zou TT, Zhang C, Zhou YF, Han YJ, Xiong JJ, Wu XX, et al. Lifestyle interventions for patients with nonalcoholic fatty liver disease: a network meta-analysis. Eur J Gastroenterol Hepatol 2018;30:747-55.ArticlePubMed
- 18. Wong VW, Wong GL, Chan RS, Shu SS, Cheung BH, Li LS, et al. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J Hepatol 2018;69:1349-56.ArticlePubMed
- 19. Feldman A, Eder SK, Felder TK, Kedenko L, Paulweber B, Stadlmayr A, et al. Clinical and metabolic characterization of lean Caucasian subjects with non-alcoholic fatty liver. Am J Gastroenterol 2017;112:102-10.ArticlePubMedPDF
- 20. Chen F, Esmaili S, Rogers GB, Bugianesi E, Petta S, Marchesini G, et al. Lean NAFLD: a distinct entity shaped by differential metabolic adaptation. Hepatology 2020;71:1213-27.ArticlePubMedPDF
- 21. Younes R, Govaere O, Petta S, Miele L, Tiniakos D, Burt A, et al. Caucasian lean subjects with non-alcoholic fatty liver disease share long-term prognosis of non-lean: time for reappraisal of BMI-driven approach? Gut 2022;71:382-90.ArticlePubMed
- 22. Tan EX, Lee JW, Jumat NH, Chan WK, Treeprasertsuk S, Goh GB, et al. Non-obese non-alcoholic fatty liver disease (NAFLD) in Asia: an international registry study. Metabolism 2022;126:154911.ArticlePubMed
- 23. Lu FB, Zheng KI, Rios RS, Targher G, Byrne CD, Zheng MH. Global epidemiology of lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Gastroenterol Hepatol 2020;35:2041-50.ArticlePubMedPDF
- 24. Ito T, Ishigami M, Zou B, Tanaka T, Takahashi H, Kurosaki M, et al. The epidemiology of NAFLD and lean NAFLD in Japan: a meta-analysis with individual and forecasting analysis, 1995-2040. Hepatol Int 2021;15:366-79.ArticlePubMedPDF
- 25. Kuchay MS, Martinez-Montoro JI, Choudhary NS, Fernandez-Garcia JC, Ramos-Molina B. Non-alcoholic fatty liver disease in lean and non-obese individuals: current and future challenges. Biomedicines 2021;9:1346.ArticlePubMedPMC
- 26. Lazarus JV, Mark HE, Villota-Rivas M, Palayew A, Carrieri P, Colombo M, et al. The global NAFLD policy review and preparedness index: are countries ready to address this silent public health challenge? J Hepatol 2022;76:771-80.PubMed
- 27. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328-57.ArticlePubMedPDF
- 28. Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, Roncal C, et al. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J Hepatol 2018;68:1063-75.ArticlePubMedPMC
- 29. Nielsen SJ, Popkin BM. Changes in beverage intake between 1977 and 2001. Am J Prev Med 2004;27:205-10.ArticlePubMed
- 30. Anderson TA. Recent trends in carbohydrate consumption. Annu Rev Nutr 1982;2:113-32.ArticlePubMed
- 31. Yracheta JM, Alfonso J, Lanaspa MA, Roncal-Jimenez C, Johnson SB, Sanchez-Lozada LG, et al. Hispanic Americans living in the United States and their risk for obesity, diabetes and kidney disease: genetic and environmental considerations. Postgrad Med 2015;127:503-10.ArticlePubMed
- 32. Yracheta JM, Lanaspa MA, Le MT, Abdelmalak MF, Alfonso J, Sanchez-Lozada LG, et al. Diabetes and kidney disease in American Indians: potential role of sugar-sweetened beverages. Mayo Clin Proc 2015;90:813-23.ArticlePubMed
- 33. Herman MA, Birnbaum MJ. Molecular aspects of fructose metabolism and metabolic disease. Cell Metab 2021;33:2329-54.ArticlePubMedPMC
- 34. Mosca A, Nobili V, De Vito R, Crudele A, Scorletti E, Villani A, et al. Serum uric acid concentrations and fructose consumption are independently associated with NASH in children and adolescents. J Hepatol 2017;66:1031-6.ArticlePubMed
- 35. Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 2010;51:1961-71.ArticlePubMedPMC
- 36. Smith GI, Shankaran M, Yoshino M, Schweitzer GG, Chondronikola M, Beals JW, et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J Clin Invest 2020;130:1453-60.ArticlePubMedPMC
- 37. Sanders FW, Griffin JL. De novo lipogenesis in the liver in health and disease: more than just a shunting yard for glucose. Biol Rev Camb Philos Soc 2016;91:452-68.ArticlePubMedPMCPDF
- 38. Kim MS, Krawczyk SA, Doridot L, Fowler AJ, Wang JX, Trauger SA, et al. ChREBP regulates fructose-induced glucose production independently of insulin signaling. J Clin Invest 2016;126:4372-86.ArticlePubMedPMC
- 39. Katz LS, Baumel-Alterzon S, Scott DK, Herman MA. Adaptive and maladaptive roles for ChREBP in the liver and pancreatic islets. J Biol Chem 2021;296:100623.ArticlePubMedPMC
- 40. Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol 2010;7:251-64.ArticlePubMedPDF
- 41. Schwarz JM, Noworolski SM, Erkin-Cakmak A, Korn NJ, Wen MJ, Tai VW, et al. Effects of dietary fructose restriction on liver fat, de novo lipogenesis, and insulin kinetics in children with obesity. Gastroenterology 2017;153:743-52.ArticlePubMedPMC
- 42. Togo J, Hu S, Li M, Niu C, Speakman JR. Impact of dietary sucrose on adiposity and glucose homeostasis in C57BL/6J mice depends on mode of ingestion: liquid or solid. Mol Metab 2019;27:22-32.ArticlePubMedPMC
- 43. Jang C, Wada S, Yang S, Gosis B, Zeng X, Zhang Z, et al. The small intestine shields the liver from fructose-induced steatosis. Nat Metab 2020;2:586-93.ArticlePubMedPMCPDF
- 44. Mukherji A, Bailey SM, Staels B, Baumert TF. The circadian clock and liver function in health and disease. J Hepatol 2019;71:200-11.ArticlePubMedPMC
- 45. Guan D, Xiong Y, Borck PC, Jang C, Doulias PT, Papazyan R, et al. Diet-induced circadian enhancer remodeling synchronizes opposing hepatic lipid metabolic processes. Cell 2018;174:831-42.ArticlePubMedPMC
- 46. Guan D, Xiong Y, Trinh TM, Xiao Y, Hu W, Jiang C, et al. The hepatocyte clock and feeding control chronophysiology of multiple liver cell types. Science 2020;369:1388-94.ArticlePubMedPMC
- 47. Reinke H, Asher G. Circadian clock control of liver metabolic functions. Gastroenterology 2016;150:574-80.ArticlePubMed
- 48. Guan D, Lazar MA. Interconnections between circadian clocks and metabolism. J Clin Invest 2021;131:e148278.ArticlePubMedPMC
- 49. Saran AR, Dave S, Zarrinpar A. Circadian rhythms in the pathogenesis and treatment of fatty liver disease. Gastroenterology 2020;158:1948-66.ArticlePubMedPMC
- 50. Bishehsari F, Voigt RM, Keshavarzian A. Circadian rhythms and the gut microbiota: from the metabolic syndrome to cancer. Nat Rev Endocrinol 2020;16:731-9.ArticlePubMedPMCPDF
- 51. Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab 2016;23:1048-59.ArticlePubMedPMC
- 52. Zeb F, Wu X, Chen L, Fatima S, Haq IU, Chen A, et al. Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br J Nutr 2020;123:1216-26.ArticlePubMed
- 53. Xin FZ, Zhao ZH, Liu XL, Pan Q, Wang ZX, Zeng L, et al. Escherichia fergusonii promotes nonobese nonalcoholic fatty liver disease by interfering with host hepatic lipid metabolism through its own msRNA 23487. Cell Mol Gastroenterol Hepatol 2022;13:827-41.ArticlePubMedPMC
- 54. Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, et al. Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur J Sport Sci 2017;17:200-7.ArticlePubMed
- 55. Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab 2015;22:789-98.ArticlePubMedPMC
- 56. Carlson O, Martin B, Stote KS, Golden E, Maudsley S, Najjar SS, et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism 2007;56:1729-34.ArticlePubMedPMC
- 57. Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med 2016;14:290.ArticlePubMedPMCPDF
- 58. Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr 2007;85:981-8.ArticlePubMedPMC
- 59. Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob Health 2018;6:e1077-86.ArticlePubMed
- 60. Guthold R, Stevens GA, Riley LM, Bull FC. Global trends in insufficient physical activity among adolescents: a pooled analysis of 298 population-based surveys with 1·6 million participants. Lancet Child Adolesc Health 2020;4:23-35.ArticlePubMedPMC
- 61. World Health Organization Guidelines Review Committee. Global recommendations on physical activity for health. Geneva: World Health Organization; 2010.
- 62. Keating SE, Adams LA. Exercise in NAFLD: just do it. J Hepatol 2016;65:671-3.ArticlePubMed
- 63. Berzigotti A, Saran U, Dufour JF. Physical activity and liver diseases. Hepatology 2016;63:1026-40.ArticlePubMedPDF
- 64. Cigrovski Berkovic M, Bilic-Curcic I, Mrzljak A, Cigrovski V. NAFLD and physical exercise: ready, steady, go! Front Nutr 2021;8:734859.ArticlePubMedPMC
- 65. Pan X, Han Y, Zou T, Zhu G, Xu K, Zheng J, et al. Sarcopenia contributes to the progression of nonalcoholic fatty liver disease-related fibrosis: a meta-analysis. Dig Dis 2018;36:427-36.ArticlePubMedPDF
- 66. Li G, Rios RS, Wang XX, Yu Y, Zheng KI, Huang OY, et al. Sex influences the association between appendicular skeletal muscle mass to visceral fat area ratio and non-alcoholic steatohepatitis in patients with biopsy-proven non-alcoholic fatty liver disease. Br J Nutr 2021 Jun 28 [Epub]. https://doi.org/10.1017/S0007114521002415.Article
- 67. Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol 2017;66:142-52.ArticlePubMed
- 68. Hallsworth K, Fattakhova G, Hollingsworth KG, Thoma C, Moore S, Taylor R, et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 2011;60:1278-83.ArticlePubMedPMC
- 69. Lazarus JV, Cortez-Pinto H, Anstee QM. Reply to: “Caveats for the implementation of global strategies against non-alcoholic fatty liver disease”. J Hepatol 2020;73:221-2.ArticlePubMed
- 70. Lazarus JV, Anstee QM, Hagstrom H, Cusi K, Cortez-Pinto H, Mark HE, et al. Defining comprehensive models of care for NAFLD. Nat Rev Gastroenterol Hepatol 2021;18:717-29.ArticlePubMedPDF
- 71. Zheng KI, Fan JG, Shi JP, Wong VW, Eslam M, George J, et al. From NAFLD to MAFLD: a “redefining” moment for fatty liver disease. Chin Med J (Engl) 2020;133:2271-73.ArticlePubMedPMC
- 72. Lazarus JV, Palayew A, Carrieri P, Ekstedt M, Marchesini G, Novak K, et al. European ‘NAFLD Preparedness Index’: is Europe ready to meet the challenge of fatty liver disease? JHEP Rep 2021;3:100234.ArticlePubMedPMC
- 73. Lazarus JV, Colombo M, Cortez-Pinto H, Huang TT, Miller V, Ninburg M, et al. NAFLD: sounding the alarm on a silent epidemic. Nat Rev Gastroenterol Hepatol 2020;17:377-9.ArticlePubMedPDF
- 74. Kumar S, Wong R, Newberry C, Yeung M, Pena JM, Sharaiha RZ. Multidisciplinary clinic models: a paradigm of care for management of NAFLD. Hepatology 2021;74:3472-8.ArticlePubMedPDF
- 75. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 2020;73:202-9.ArticlePubMed
- 76. Lazarus JV, Mark HE, Anstee QM, Arab JP, Batterham RL, Castera L, et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat Rev Gastroenterol Hepatol 2022;19:60-78.PubMed
- 77. Younossi ZM, Ong JP, Takahashi H, Yilmaz Y, Eguc Hi Y, El Kassas M, et al. A global survey of physicians knowledge about nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2022;20:e1456-68.ArticlePubMed
- 78. Zheng KI, Eslam M, George J, Zheng MH. When a new definition overhauls perceptions of MAFLD related cirrhosis care. Hepatobiliary Surg Nutr 2020;9:801-4.ArticlePubMedPMC
- 79. Moolla A, Motohashi K, Marjot T, Shard A, Ainsworth M, Gray A, et al. A multidisciplinary approach to the management of NAFLD is associated with improvement in markers of liver and cardio-metabolic health. Frontline Gastroenterol 2019;10:337-46.ArticlePubMedPMC
- 80. Armstrong MJ, Hazlehurst JM, Parker R, Koushiappi E, Mann J, Khan S, et al. Severe asymptomatic non-alcoholic fatty liver disease in routine diabetes care; a multi-disciplinary team approach to diagnosis and management. QJM 2014;107:33-41.ArticlePubMedPMC
- 81. DeVore S, Kohli R, Lake K, Nicholas L, Dietrich K, Balistreri WF, et al. A multidisciplinary clinical program is effective in stabilizing BMI and reducing transaminase levels in pediatric patients with NAFLD. J Pediatr Gastroenterol Nutr 2013;57:119-23.ArticlePubMedPMC
- 82. Wolfenden L, Nathan NK, Sutherland R, Yoong SL, Hodder RK, Wyse RJ, et al. Strategies for enhancing the implementation of school-based policies or practices targeting risk factors for chronic disease. Cochrane Database Syst Rev 2017;11:CD011677.ArticlePubMedPMC
- 83. Smith M, Hosking J, Woodward A, Witten K, MacMillan A, Field A, et al. Systematic literature review of built environment effects on physical activity and active transport: an update and new findings on health equity. Int J Behav Nutr Phys Act 2017;14:158.ArticlePubMedPMCPDF
- 84. Francque SM, Marchesini G, Kautz A, Walmsley M, Dorner R, Lazarus JV, et al. Non-alcoholic fatty liver disease: a patient guideline. JHEP Rep 2021;3:100322.ArticlePubMedPMC
- 85. Shen J, Wong GL, Chan HL, Chan RS, Chan HY, Chu WC, et al. PNPLA3 gene polymorphism and response to lifestyle modification in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol 2015;30:139-46.ArticlePubMedPDF
- 86. Sevastianova K, Kotronen A, Gastaldelli A, Perttila J, Hakkarainen A, Lundbom J, et al. Genetic variation in PNPLA3 (adiponutrin) confers sensitivity to weight loss-induced decrease in liver fat in humans. Am J Clin Nutr 2011;94:104-11.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Triglycerides Mediate the Influence of Body Mass Index on Non-Alcoholic Fatty Liver Disease in a Non-Obese Chinese Population with Normal Low-Density Lipoprotein Cholesterol Levels
Xixi Han, Jingwen Kong, Hemin Zhang, Yuan Zhao, Yafeng Zheng, Chao Wei
Obesity Facts.2024; 17(2): 191. CrossRef - Patients with NAFLD exhibit more advanced fibrosis in liver biopsy than patients with other chronic liver diseases
Lydia Rohr, Peter Lemmer, Marie Henning, Andrea Tannapfel, Theodor Baars, Paul Manka, Ali Canbay, Jan-Peter Sowa
Zeitschrift für Gastroenterologie.2023; 61(01): 29. CrossRef - Performance of Simple Fibrosis Score in Non-Alcoholic Fatty Liver Disease with and without Type 2 Diabetes
Seung Min Chung, Min Kyu Kang, Jun Sung Moon, Jung Gil Park
Endocrinology and Metabolism.2023; 38(2): 277. CrossRef - An international multidisciplinary consensus statement on MAFLD and the risk of CVD
Xiao-Dong Zhou, Giovanni Targher, Christopher D. Byrne, Virend Somers, Seung Up Kim, C. Anwar A. Chahal, Vincent Wai-Sun Wong, Jingjing Cai, Michael D. Shapiro, Mohammed Eslam, Philippe Gabriel Steg, Ki-Chul Sung, Anoop Misra, Jian-Jun Li, Carlos Brotons,
Hepatology International.2023; 17(4): 773. CrossRef - Lean or Non-obese Nonalcoholic Fatty Liver Disease Patients: Are They Really Lean?
Eugene Han, Yong-ho Lee
Clinical and Molecular Hepatology.2023; 29(4): 980. CrossRef - Sex-Based Differences and Risk Factors for Comorbid Nonalcoholic Fatty Liver Disease in Patients with Bipolar Disorder: A Cross-Sectional Retrospective Study
Ying Wang, Yiyi Liu, Xun Zhang, Qing Wu
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 3533. CrossRef - Benefits of Physical Exercise as Approach to Prevention and Reversion of Non-Alcoholic Fatty Liver Disease in Children and Adolescents with Obesity
Valeria Calcaterra, Vittoria Magenes, Matteo Vandoni, Clarissa Berardo, Luca Marin, Alice Bianchi, Erika Cordaro, Giustino Silvestro, Dario Silvestri, Vittoria Carnevale Pellino, Cristina Cereda, Gianvincenzo Zuccotti
Children.2022; 9(8): 1174. CrossRef - The effects of supplementation of probiotics, prebiotics, or synbiotics on patients with non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials
Wenmin Xing, Wenyan Gao, Xiaoling Lv, Zhenlei Zhao, Genxiang Mao, Xiaoyan Dong, Zuyong Zhang
Frontiers in Nutrition.2022;[Epub] CrossRef

 KDA
KDA PubReader
PubReader ePub Link
ePub Link Cite
Cite