
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
- Umbilical Cord-Mesenchymal Stem Cell-Conditioned Medium Improves Insulin Resistance in C2C12 Cell
- Kyung-Soo Kim, Yeon Kyung Choi, Mi Jin Kim, Jung Wook Hwang, Kyunghoon Min, Sang Youn Jung, Soo-Kyung Kim, Yong-Soo Choi, Yong-Wook Cho
- Diabetes Metab J. 2021;45(2):260-269. Published online July 10, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0191
- 9,582 View
- 206 Download
- 8 Web of Science
- 8 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
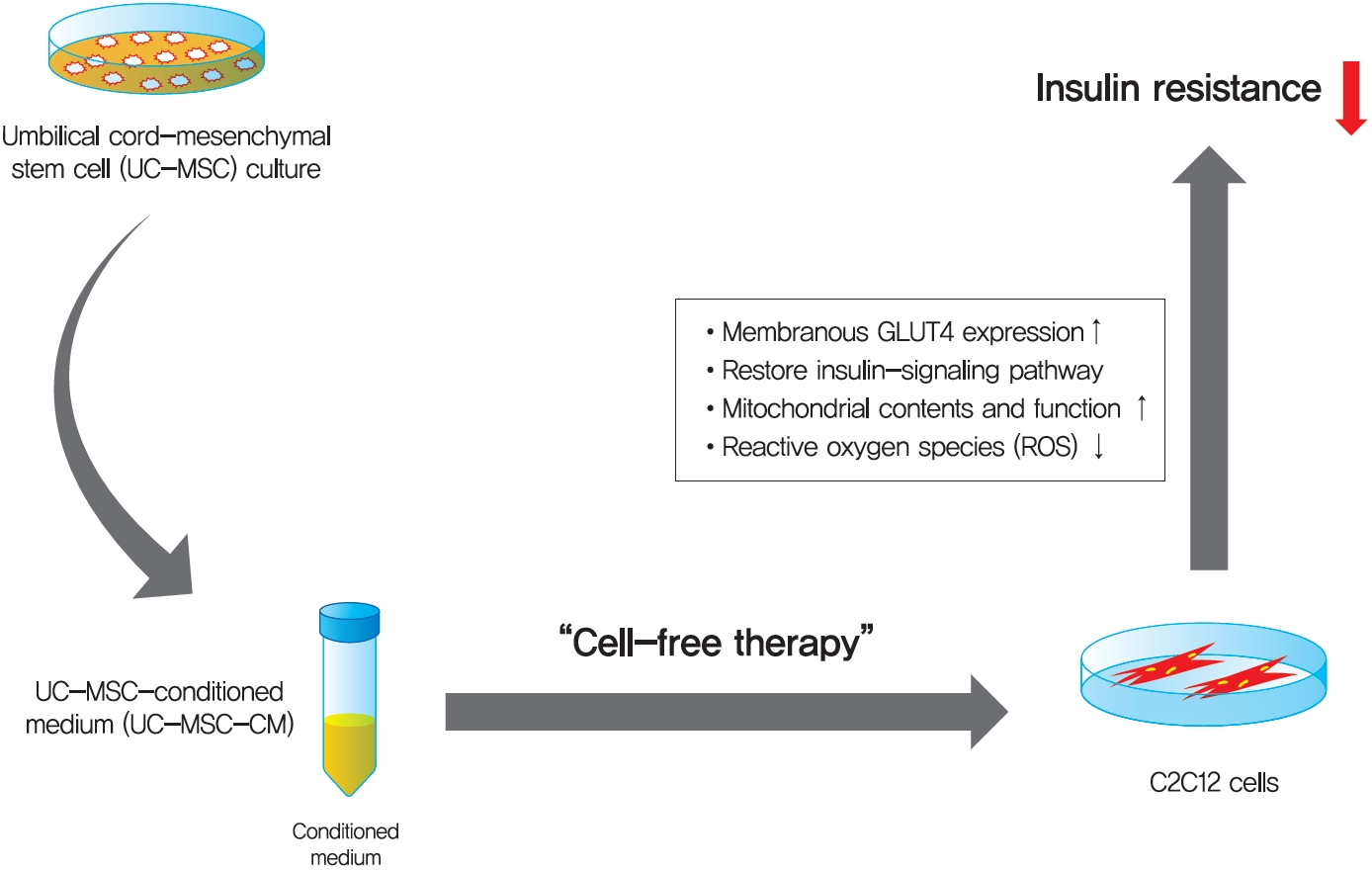
Background Umbilical cord-mesenchymal stem cell-conditioned medium (UC-MSC-CM) has emerged as a promising cell-free therapy. The aim of this study was to explore the therapeutic effects of UC-MSC-CM on insulin resistance in C2C12 cell.
Methods Insulin resistance was induced by palmitate. Effects of UC-MSC-CM on insulin resistance were evaluated using glucose uptake, glucose transporter type 4 (GLUT4) translocation, the insulin-signaling pathway, and mitochondrial contents and functions in C2C12 cell.
Results Glucose uptake was improved by UC-MSC-CM. UC-MSC-CM treatment increased only in membranous GLUT4 expression, not in cytosolic GLUT4 expression. It restored the insulin-signaling pathway in insulin receptor substrate 1 and protein kinase B. Mitochondrial contents evaluated by mitochondrial transcription factor A, mitochondrial DNA copy number, and peroxisome proliferator-activated receptor gamma coactivator 1-alpha were increased by UC-MSC-CM. In addition, UC-MSC-CM significantly decreased mitochondrial reactive oxygen species and increased fatty acid oxidation and mitochondrial membrane potential. There was no improvement in adenosine triphosphate (ATP) contents, but ATP synthesis was improved by UC-MSC-CM. Cytokine and active factor analysis of UC-MSC-CM showed that it contained many regulators inhibiting insulin resistance.
Conclusion UC-MSC-CM improves insulin resistance with multiple mechanisms in C2C12 cell.
-
Citations
Citations to this article as recorded by- Neurotransmitters in Type 2 Diabetes and the Control of Systemic and Central Energy Balance
Amnah Al-Sayyar, Maha M. Hammad, Michayla R. Williams, Mohammed Al-Onaizi, Jehad Abubaker, Fawaz Alzaid
Metabolites.2023; 13(3): 384. CrossRef - Neuroprotective Effect of Wharton’s Jelly-Derived Mesenchymal Stem Cell-Conditioned Medium (WJMSC-CM) on Diabetes-Associated Cognitive Impairment by Improving Oxidative Stress, Neuroinflammation, and Apoptosis
Zohre Aghaei, Narges Karbalaei, Mohammad Reza Namavar, Masoud Haghani, Mahboobeh Razmkhah, Mahdi Khorsand Ghaffari, Marzieh Nemati, Andrea Ballini
Stem Cells International.2023; 2023: 1. CrossRef - Mesenchymal-Stem Cell-Derived Conditioned Media Versus Exosomes in the Treatment of Rat Model of Polycystic Ovary: An Attempt to Understand the Underlying Mechanisms (Biochemical and Histological Study)
Soha Abd-elkawy Abd-elwahab, Noura Hassan Khamis, Rehab Ahmed Rifaai, Nashwa Fathy Gamal El-Tahawy, Randa Ahmed Ibrahim
Microscopy and Microanalysis.2023; 29(3): 1244. CrossRef - Therapeutic Potential of Mesenchymal Stem Cell‐Derived Conditioned Medium for Diabetes Mellitus and Related Complications
Basak Isildar, Serbay Ozkan, Meral Koyuturk
Advanced Therapeutics.2023;[Epub] CrossRef - Treatment of type 2 diabetes mellitus with stem cells and antidiabetic drugs: a dualistic and future-focused approach
Priyamvada Amol Arte, Kanchanlata Tungare, Mustansir Bhori, Renitta Jobby, Jyotirmoi Aich
Human Cell.2023; 37(1): 54. CrossRef - Perinatal Stem Cell Therapy to Treat Type 1 Diabetes Mellitus: A Never-Say-Die Story of Differentiation and Immunomodulation
Francesca Paris, Valeria Pizzuti, Pasquale Marrazzo, Andrea Pession, Francesco Alviano, Laura Bonsi
International Journal of Molecular Sciences.2022; 23(23): 14597. CrossRef - Mesenchymal Stem Cell-Derived Apoptotic Bodies: Biological Functions and Therapeutic Potential
Huixue Tang, Huikun Luo, Zihan Zhang, Di Yang
Cells.2022; 11(23): 3879. CrossRef - Human umbilical cord mesenchymal stem cells in type 2 diabetes mellitus: the emerging therapeutic approach
Andreia Gomes, Pedro Coelho, Raquel Soares, Raquel Costa
Cell and Tissue Research.2021; 385(3): 497. CrossRef
- Neurotransmitters in Type 2 Diabetes and the Control of Systemic and Central Energy Balance
- Obesity and Metabolic Syndrome
- Adult Stem Cells: Beyond Regenerative Tool, More as a Bio-Marker in Obesity and Diabetes
- Sabyasachi Sen
- Diabetes Metab J. 2019;43(6):744-751. Published online December 26, 2019
- DOI: https://doi.org/10.4093/dmj.2019.0175
- 4,481 View
- 66 Download
- 5 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Obesity, diabetes, and cardiovascular diseases are increasing rapidly worldwide and it is therefore important to know the effect of exercise and medications for diabetes and obesity on adult stem cells. Adult stem cells play a major role in remodeling and tissue regeneration. In this review we will focus mainly on two adult stem/progenitor cells such as endothelial progenitor cells and mesenchymal stromal cells in relation to aerobic exercise and diabetes medications, both of which can alter the course of regeneration and tissue remodelling. These two adult precursor and stem cells are easily obtained from peripheral blood or adipose tissue depots, as the case may be and are precursors to endothelium and mesenchymal tissue (fat, bone, muscle, and cartilage). They both are key players in maintenance of cardiovascular and metabolic homeostasis and can act also as useful biomarkers.
-
Citations
Citations to this article as recorded by- Novel Therapeutics in Nonalcoholic Fatty Liver Disease: A Focus on Adult Stem Cells
Seshagiri Rao Nandula, Eric S. Nylen, Sabyasachi Sen
Metabolic Syndrome and Related Disorders.2023; 21(2): 71. CrossRef - Obesity and Wound Healing: Focus on Mesenchymal Stem Cells
Antonio Alma, Guya Diletta Marconi, Elena Rossi, Cristina Magnoni, Alessia Paganelli
Life.2023; 13(3): 717. CrossRef - Stem Cells in Tendon Regeneration and Factors governing Tenogenesis
Lingli Ding, BingYu Zhou, Yonghui Hou, Liangliang Xu
Current Stem Cell Research & Therapy.2022; 17(6): 503. CrossRef - Role of Canagliflozin on function of CD34+ve endothelial progenitor cells (EPC) in patients with type 2 diabetes
Seshagiri Rao Nandula, Nabanita Kundu, Hassan B. Awal, Beda Brichacek, Mona Fakhri, Nikhila Aimalla, Adrian Elzarki, Richard L. Amdur, Sabyasachi Sen
Cardiovascular Diabetology.2021;[Epub] CrossRef - Tailored generation of insulin producing cells from canine mesenchymal stem cells derived from bone marrow and adipose tissue
Watchareewan Rodprasert, Sirirat Nantavisai, Koranis Pathanachai, Prasit Pavasant, Thanaphum Osathanon, Chenphop Sawangmake
Scientific Reports.2021;[Epub] CrossRef
- Novel Therapeutics in Nonalcoholic Fatty Liver Disease: A Focus on Adult Stem Cells
- Obesity and Metabolic Syndrome
- Two Faces of White Adipose Tissue with Heterogeneous Adipogenic Progenitors
- Injae Hwang, Jae Bum Kim
- Diabetes Metab J. 2019;43(6):752-762. Published online December 26, 2019
- DOI: https://doi.org/10.4093/dmj.2019.0174
- 8,961 View
- 181 Download
- 36 Web of Science
- 38 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Chronic energy surplus increases body fat, leading to obesity. Since obesity is closely associated with most metabolic complications, pathophysiological roles of adipose tissue in obesity have been intensively studied. White adipose tissue is largely divided into subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT). These two white adipose tissues are similar in their appearance and lipid storage functions. Nonetheless, emerging evidence has suggested that SAT and VAT have different characteristics and functional roles in metabolic regulation. It is likely that there are intrinsic differences between VAT and SAT. In diet-induced obese animal models, it has been reported that adipogenic progenitors in VAT rapidly proliferate and differentiate into adipocytes. In obesity, VAT exhibits elevated inflammatory responses, which are less prevalent in SAT. On the other hand, SAT has metabolically beneficial effects. In this review, we introduce recent studies that focus on cellular and molecular components modulating adipogenesis and immune responses in SAT and VAT. Given that these two fat depots show different functions and characteristics depending on the nutritional status, it is feasible to postulate that SAT and VAT have different developmental origins with distinct adipogenic progenitors, which would be a key determining factor for the response and accommodation to metabolic input for energy homeostasis.
-
Citations
Citations to this article as recorded by- Lipodystrophy as a target to delay premature aging
Daniela G. Costa, Marisa Ferreira-Marques, Cláudia Cavadas
Trends in Endocrinology & Metabolism.2024; 35(2): 97. CrossRef - Subcutaneous fat predicts bone metastasis in breast cancer: A novel multimodality-based deep learning model
Shidi Miao, Haobo Jia, Wenjuan Huang, Ke Cheng, Wenjin Zhou, Ruitao Wang
Cancer Biomarkers.2024; 39(3): 171. CrossRef - Association between abdominal adiposity and clinical outcomes in patients with acute ischemic stroke
Kayo Wakisaka, Ryu Matsuo, Fumi Irie, Yoshinobu Wakisaka, Tetsuro Ago, Masahiro Kamouchi, Takanari Kitazono, Masaki Mogi
PLOS ONE.2024; 19(1): e0296833. CrossRef - NOTCH1 as a Negative Regulator of Avian Adipocyte Differentiation: Implications for Fat Deposition
Zheng Wang, Yue Su, Mingyu Zhao, Zhenhua Ma, Jianhui Li, Zhuocheng Hou, Huifeng Li
Animals.2024; 14(4): 585. CrossRef - Green tea beneficial effects involve changes in the profile of immune cells in the adipose tissue of obese mice
Kaue Tognolli, Victoria Silva, Celso Pereira Batista Sousa-Filho, Claudia Andrea Lima Cardoso, Renata Gorjão, Rosemari Otton
European Journal of Nutrition.2023; 62(1): 321. CrossRef - Mechanic Insight into the Distinct and Common Roles of Ovariectomy Versus Adrenalectomy on Adipose Tissue Remodeling in Female Mice
Weihao Chen, Fengyan Meng, Xianyin Zeng, Xiaohan Cao, Guixian Bu, Xiaogang Du, Guozhi Yu, Fanli Kong, Yunkun Li, Tian Gan, Xingfa Han
International Journal of Molecular Sciences.2023; 24(3): 2308. CrossRef - High-fat diet consumption by male rat offspring of obese mothers exacerbates adipose tissue hypertrophy and metabolic alterations in adult life
Guadalupe L. Rodríguez-González, Sergio De Los Santos, Dayana Méndez-Sánchez, Luis A. Reyes-Castro, Carlos A. Ibáñez, Patricia Canto, Elena Zambrano
British Journal of Nutrition.2023; 130(5): 783. CrossRef - Obesity and the risk of cardiometabolic diseases
Pedro L. Valenzuela, Pedro Carrera-Bastos, Adrián Castillo-García, Daniel E. Lieberman, Alejandro Santos-Lozano, Alejandro Lucia
Nature Reviews Cardiology.2023; 20(7): 475. CrossRef - Abdominal fat and muscle distributions in different stages of colorectal cancer
Jun Han, Xinyang Liu, Min Tang, Fan Yang, Zuoyou Ding, Guohao Wu
BMC Cancer.2023;[Epub] CrossRef - Expression Analysis of hsa-miR-181a-5p, hsa-miR-143-3p, hsa-miR-132-3p and hsa-miR-23a-3p as Biomarkers in Colorectal Cancer—Relationship to the Body Mass Index
Sofía Elena Tesolato, Daniel González-Gamo, Ana Barabash, Paula Claver, Sofía Cristina de la Serna, Inmaculada Domínguez-Serrano, Jana Dziakova, Carmen de Juan, Antonio José Torres, Pilar Iniesta
Cancers.2023; 15(13): 3324. CrossRef - Lower subcutaneous fat index predicts bone metastasis in breast cancer
Wen Wang, Wen-Juan Huang, Ping-Ping Liu, Shuang Fu, Meng-Lin Zhang, Xin Zhang, Rui-Tao Wang, Yuan-Xi Huang
Cancer Biomarkers.2023; 38(1): 121. CrossRef - RabGAP AS160/TBC1D4 deficiency increases long-chain fatty acid transport but has little additional effect on obesity and metabolic syndrome in ADMSCs-derived adipocytes of morbidly obese women
Agnieszka Mikłosz, Bartłomiej Łukaszuk, Elżbieta Supruniuk, Kamil Grubczak, Magdalena Kusaczuk, Adrian Chabowski
Frontiers in Molecular Biosciences.2023;[Epub] CrossRef - White adipose tissue: Distribution, molecular insights of impaired expandability, and its implication in fatty liver disease
Griselda Rabadán-Chávez, Rocío I. Díaz de la Garza, Daniel A. Jacobo-Velázquez
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2023; 1869(8): 166853. CrossRef - Histological pattern and gene expression profiling of thyroid tissue in subjects with obesity
A. Basolo, A. M. Poma, R. Giannini, G. Ceccarini, C. Pelosini, P. Fierabracci, M. U. Castany, S. Bechi Genzano, C. E. Ambrosini, G. Materazzi, L. Chiovato, F. Basolo, F. Santini, L. Torregrossa
Journal of Endocrinological Investigation.2022; 45(2): 413. CrossRef - Predictors of non-alcoholic fatty liver disease in children
Menglong Li, Wen Shu, Jiawulan Zunong, Nubiya Amaerjiang, Huidi Xiao, Dan Li, Sten H. Vermund, Yifei Hu
Pediatric Research.2022; 92(1): 322. CrossRef - Distinct properties of adipose stem cell subpopulations determine fat depot-specific characteristics
Hahn Nahmgoong, Yong Geun Jeon, Eun Seo Park, Yoon Ha Choi, Sang Mun Han, Jeu Park, Yul Ji, Jee Hyung Sohn, Ji Seul Han, Ye Young Kim, Injae Hwang, Yun Kyung Lee, Jin Young Huh, Sung Sik Choe, Tae Jung Oh, Sung Hee Choi, Jong Kyoung Kim, Jae Bum Kim
Cell Metabolism.2022; 34(3): 458. CrossRef - WT1 in Adipose Tissue: From Development to Adult Physiology
Karin M. Kirschner, Holger Scholz
Frontiers in Cell and Developmental Biology.2022;[Epub] CrossRef - Shall We Begin the Voyage of Adipose Tissue Exploration?
Yong Geun Jeon
Molecules and Cells.2022; 45(6): 362. CrossRef - Mammalian adipogenesis regulator (Areg) cells use retinoic acid signalling to be non‐ and anti‐adipogenic in age‐dependent manner
Magda Zachara, Pernille Y Rainer, Horia Hashimi, Julie M Russeil, Daniel Alpern, Radiana Ferrero, Maria Litovchenko, Bart Deplancke
The EMBO Journal.2022;[Epub] CrossRef - The fates of different types of adipose tissue after transplantation in mice
Shenglu Jiang, Jiayan Lin, Qian Zhang, Yunjun Liao, Feng Lu, Junrong Cai
The FASEB Journal.2022;[Epub] CrossRef - Analysis of different adipose depot gene expression in cachectic patients with gastric cancer
Jun Han, Zuoyou Ding, Qiulin Zhuang, Lei Shen, Fan Yang, Szechun Sah, Guohao Wu
Nutrition & Metabolism.2022;[Epub] CrossRef - Adipose Stromal/Stem Cell-Derived Extracellular Vesicles: Potential Next-Generation Anti-Obesity Agents
Mariachiara Zuccarini, Patricia Giuliani, Valentina Di Liberto, Monica Frinchi, Francesco Caciagli, Vanni Caruso, Renata Ciccarelli, Giuseppa Mudò, Patrizia Di Iorio
International Journal of Molecular Sciences.2022; 23(3): 1543. CrossRef - Is There a Link between Obesity Indices and Skin Autofluorescence? A Response from the ILERVAS Project
Enric Sánchez, Marta Sánchez, Carolina López-Cano, Marcelino Bermúdez-López, José Manuel Valdivielso, Cristina Farràs-Sallés, Reinald Pamplona, Gerard Torres, Dídac Mauricio, Eva Castro, Elvira Fernández, Albert Lecube
Nutrients.2022; 15(1): 203. CrossRef - Insights behind the Relationship between Colorectal Cancer and Obesity: Is Visceral Adipose Tissue the Missing Link?
Alice Chaplin, Ramon Maria Rodriguez, Juan José Segura-Sampedro, Aina Ochogavía-Seguí, Dora Romaguera, Gwendolyn Barceló-Coblijn
International Journal of Molecular Sciences.2022; 23(21): 13128. CrossRef - Potential effects of nutrition-based weight loss therapies in reversing obesity-related breast cancer epigenetic marks
Paula M. Lorenzo, Ana B. Crujeiras
Food & Function.2021; 12(4): 1402. CrossRef - Metabolomic Profiles in Adipocytes Differentiated from Adipose-Derived Stem Cells Following Exercise Training or High-Fat Diet
Seita Osawa, Hisashi Kato, Yuki Maeda, Hisashi Takakura, Junetsu Ogasawara, Tetsuya Izawa
International Journal of Molecular Sciences.2021; 22(2): 966. CrossRef - Adipocytes Are the Control Tower That Manages Adipose Tissue Immunity by Regulating Lipid Metabolism
Jeu Park, Jee Hyung Sohn, Sang Mun Han, Yoon Jeong Park, Jin Young Huh, Sung Sik Choe, Jae Bum Kim
Frontiers in Immunology.2021;[Epub] CrossRef - Ceramides and Sphingosino-1-Phosphate in Obesity
Ilona Juchnicka, Mariusz Kuźmicki, Jacek Szamatowicz
Frontiers in Endocrinology.2021;[Epub] CrossRef - Contribution of Adipose Tissue to the Chronic Immune Activation and Inflammation Associated With HIV Infection and Its Treatment
Christine Bourgeois, Jennifer Gorwood, Anaelle Olivo, Laura Le Pelletier, Jacqueline Capeau, Olivier Lambotte, Véronique Béréziat, Claire Lagathu
Frontiers in Immunology.2021;[Epub] CrossRef - Subcutaneous, but not visceral, adipose tissue as a marker for prognosis in gastric cancer patients with cachexia
Jun Han, Min Tang, Chaocheng Lu, Lei Shen, Jiaqi She, Guohao Wu
Clinical Nutrition.2021; 40(9): 5156. CrossRef - miR-410-3P inhibits adipocyte differentiation by targeting IRS-1 in cancer-associated cachexia patients
Diya Sun, Zuoyou Ding, Lei Shen, Fan Yang, Jun Han, Guohao Wu
Lipids in Health and Disease.2021;[Epub] CrossRef - Multipotent Stromal Cells from Subcutaneous Adipose Tissue of Normal Weight and Obese Subjects: Modulation of Their Adipogenic Differentiation by Adenosine A1 Receptor Ligands
Mariachiara Zuccarini, Catia Lambertucci, Marzia Carluccio, Patricia Giuliani, Maurizio Ronci, Andrea Spinaci, Rosaria Volpini, Renata Ciccarelli, Patrizia Di Iorio
Cells.2021; 10(12): 3560. CrossRef - Obesity: The Crossroads of Opinion, Knowledge, and Opportunity
L. A. Ruyatkina, D. S. Ruyatkin
Meditsinskiy sovet = Medical Council.2020; (7): 108. CrossRef - Sex Differences in Long-term Metabolic Effects of Maternal Resveratrol Intake in Adult Rat Offspring
Purificación Ros, Francisca Díaz, Alejandra Freire-Regatillo, Pilar Argente-Arizón, Vicente Barrios, Jesús Argente, Julie A Chowen
Endocrinology.2020;[Epub] CrossRef - Adipose stem cells in obesity: challenges and opportunities
Sunhye Shin, Asma S. El-Sabbagh, Brandon E. Lukas, Skylar J. Tanneberger, Yuwei Jiang
Bioscience Reports.2020;[Epub] CrossRef - Clinical and pathogenetic rationale for the prevention and treatment of obesity
O.M. Korzh
Shidnoevropejskij zurnal vnutrisnoi ta simejnoi medicini.2020; 2020(2): 146. CrossRef - OBESITY: CLINICAL AND PATHOGENETIC JUSTIFICATION OF PREVENTION AND TREATMENT
O. M. Korzh
International Medical Journal.2020; (2): 5. CrossRef - The Effect and Mechanism of Subcutaneous and Visceral Adipose Tissue Loss on Gastric Cancer Patients With Cachexia
Jun Han, Min Tang, Guyue Zhang, Chaocheng Lu, Jiaqi She, Guohao Wu
SSRN Electronic Journal .2020;[Epub] CrossRef
- Lipodystrophy as a target to delay premature aging
- Cell Therapy for Diabetic Neuropathy Using Adult Stem or Progenitor Cells
- Ji Woong Han, Min Young Sin, Young-sup Yoon
- Diabetes Metab J. 2013;37(2):91-105. Published online April 16, 2013
- DOI: https://doi.org/10.4093/dmj.2013.37.2.91
- 4,664 View
- 54 Download
- 26 Web of Science
- 23 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Diabetic neuropathy (DN) is the most common and disabling complication of diabetes that may lead to foot ulcers and limb amputations. Despite widespread awareness of DN, the only effective treatments are glucose control and pain management. A growing body of evidence suggests that DN is characterized by reduction of vascularity in peripheral nerves and deficiency in neurotrophic and angiogenic factors. Previous studies have tried to introduce neurotrophic or angiogenic factors in the form of protein or gene for therapy, but the effect was not significant. Recent studies have shown that bone marrow (BM)-derived stem or progenitor cells have favorable effects on the repair of cardiovascular diseases. Since these BM-derived stem or progenitor cells contain various angiogenic and neurotrophic factors, these cells have been attempted for treating experimental DN, and turned out to be effective for reversing various manifestations of experimental DN. These evidences suggest that cell therapy, affecting both vascular and neural components, can represent a novel therapeutic option for treatment of clinical DN.
-
Citations
Citations to this article as recorded by- Efficacy of adipose‐derived mesenchymal stem cell therapy in the treatment of chronic micro‐ and macrovascular complications of diabetes
Agnieszka Mikłosz, Adrian Chabowski
Diabetes, Obesity and Metabolism.2024; 26(3): 793. CrossRef - Natural molecules as promising players against diabetic peripheral neuropathy: an emerging nutraceutical approach
Rabia Akram, Haseeb Anwar, Muhammad Shahid Javed, Ali Imran, Azhar Rasul, Shoaib Ahmad Malik, Mobina Manzoor, Fakhar Islam, Ikram Ullah Khan, Faiqa Sajid, Tehreem Iman, Muhammad Ajmal Shah, Tao Sun, Ghulam Hussain, Mohd Asif Shah
International Journal of Food Properties.2023; 26(1): 894. CrossRef - A Receptor Story: Insulin Resistance Pathophysiology and Physiologic Insulin Resensitization’s Role as a Treatment Modality
Stanley T. Lewis, Frank Greenway, Tori R. Tucker, Michael Alexander, Levonika K. Jackson, Scott A. Hepford, Brian Loveridge, Jonathan R. T. Lakey
International Journal of Molecular Sciences.2023; 24(13): 10927. CrossRef - Nanochannel‐Based Poration Drives Benign and Effective Nonviral Gene Delivery to Peripheral Nerve Tissue
Jordan T. Moore, Christopher G. Wier, Luke R. Lemmerman, Lilibeth Ortega‐Pineda, Daniel J. Dodd, William R. Lawrence, Silvia Duarte‐Sanmiguel, Kavya Dathathreya, Ludmila Diaz‐Starokozheva, Hallie N. Harris, Chandan K. Sen, Ian L. Valerio, Natalia Higuita‐
Advanced Biosystems.2020;[Epub] CrossRef - Endothelial progenitor cells and peripheral neuropathy in subjects with type 2 diabetes mellitus
Ioanna Eleftheriadou, Natalia Dimitrakopoulou, Nikolitsa Kafasi, Anastasios Tentolouris, Aglaia Dimitrakopoulou, Ioanna A. Anastasiou, Iordanis Mourouzis, Edward Jude, Nikolaos Tentolouris
Journal of Diabetes and its Complications.2020; 34(4): 107517. CrossRef - Autologous Bone Marrow Mononuclear Cell Transplantation Therapy Improved Symptoms in Patients with Refractory Diabetic Sensorimotor Polyneuropathy via the Mechanisms of Paracrine and Immunomodulation: A Controlled Study
Wei Wei, Li Li, Lin Deng, Zhong-Jing Wang, Jing-Jian Dong, Xiao-Yu Lyu, Ting Jia, Li Wang, Hong-Xiang Wang, Hong Mao, Shi Zhao
Cell Transplantation.2020; 29: 096368972094925. CrossRef - Sodium nitrate preconditioning prevents progression of the neuropathic pain in streptozotocin-induced diabetes Wistar rats
Hajar Oghbaei, Gisou Mohaddes, GholamReza Hamidian, Rana Keyhanmanesh
Journal of Diabetes & Metabolic Disorders.2020; 19(1): 105. CrossRef - The Influence of Diabetic Foot Exercise in Sensory Peripheral Neuropathy with Monofilament Test on Diabetes Mellitus Clients
Tintin Sukartini, Candra Panji Asmoro, Nandani Alifah
Jurnal Ners.2020; 14(3): 340. CrossRef - Effects of mesenchymal stromal cells on motor function and collagen in the skeletal muscles of rats with type I diabetes
Genoveva L. F. Luna, Thiago L. Russo, Maria A. Sabadine, Yisel C. Estrada‐Bonilla, Ana L. M. Andrade, Patricia Brassolatti, Fernanda F. Anibal, Ângela M. O. Leal
International Journal of Experimental Pathology.2019; 100(5-6): 359. CrossRef - Efficacy of autologous bone marrow mononuclear cell transplantation therapy in patients with refractory diabetic peripheral neuropathy
Hong Mao, Wei Wei, Xiu-Li Fu, Jing-Jian Dong, Xiao-Yu Lyu, Ting Jia, Yang Tang, Shi Zhao
Chinese Medical Journal.2019; 132(1): 11. CrossRef - Effects of adipose derived stromal vascular fraction on diabetic neuropathy: an experimental study
Berrak Karatan, Ersin Akşam, Esra Erden, Mustafa Erol Demirseren
Journal of Plastic Surgery and Hand Surgery.2019; 53(6): 335. CrossRef - Addressing Stem Cell Therapeutic Approaches in Pathobiology of Diabetes and Its Complications
Bou-Yue Peng, Navneet Kumar Dubey, Viraj Krishna Mishra, Feng-Chou Tsai, Rajni Dubey, Win-Ping Deng, Hong-Jian Wei
Journal of Diabetes Research.2018; 2018: 1. CrossRef - Transplantation of human mobilized mononuclear cells improved diabetic neuropathy
Se Hee Min, Jung Hee Kim, Yu Mi Kang, Seung Hak Lee, Byung-Mo Oh, Kyou-Sup Han, Meihua Zhang, Hoe Suk Kim, Woo Kyung Moon, Hakmo Lee, Kyong Soo Park, Hye Seung Jung
Journal of Endocrinology.2018; 239(3): 277. CrossRef - Fluoxetine pretreatment enhances neurogenic, angiogenic and immunomodulatory effects of MSCs on experimentally induced diabetic neuropathy
Shaimaa A. Abdelrahman, Mai A. Samak, Sally M. Shalaby
Cell and Tissue Research.2018; 374(1): 83. CrossRef - Autologous Bone Marrow-Derived Stem Cells for Treating Diabetic Neuropathy in Metabolic Syndrome
Wei Liu, Fengchun Yu, Zhenghong Zhou, Yi-Chen Li, Dongsheng Fan, Kai Zhu
BioMed Research International.2017; 2017: 1. CrossRef - Impaired olfactory function is related to the presence of neuropathy in adults with type 1 diabetes
Anna Duda-Sobczak, Aleksandra Araszkiewicz, Magdalena Urbas, Lukasz Borucki, Katarzyna Kulas, Maciej Chudzinski, Aleksandra Suwalska, Dorota Zozulinska-Ziolkiewicz
Diabetes and Vascular Disease Research.2017; 14(2): 139. CrossRef - New insights into the ameliorative effects of ferulic acid in pathophysiological conditions
Sumit Ghosh, Priyanka Basak, Sayanta Dutta, Sayantani Chowdhury, Parames C. Sil
Food and Chemical Toxicology.2017; 103: 41. CrossRef - Ferulic acid pretreatment could improve prognosis of autologous mesenchymal stromal cell transplantation for diabetic neuropathy
Solmaz Mirzamohammadi, Mohammad Hadi Nematollahi, Mehrzad Mehrbani, Mehrnaz Mehrabani
Cytotherapy.2016; 18(7): 925. CrossRef - Mesenchymal stem cells to treat diabetic neuropathy: a long and strenuous way from bench to the clinic
J Y Zhou, Z Zhang, G S Qian
Cell Death Discovery.2016;[Epub] CrossRef - Diabetes mellitus related bone metabolism and periodontal disease
Ying-Ying Wu, E Xiao, Dana T Graves
International Journal of Oral Science.2015; 7(2): 63. CrossRef - Microvessel permeability correlates with diabetic peripheral neuropathy in early stage of streptozotocin-induced diabetes rats
Liyuan Peng, Wei Liu, Fanglong Zhai, Li He, Hailan Wang
Journal of Diabetes and its Complications.2015; 29(7): 865. CrossRef - Mesenchymal Stem Cell-Based Treatment for Microvascular and Secondary Complications of Diabetes Mellitus
Grace C. Davey, Swapnil B. Patil, Aonghus O’Loughlin, Timothy O’Brien
Frontiers in Endocrinology.2014;[Epub] CrossRef - Emerging roles of hematopoietic cells in the pathobiology of diabetic complications
Hideto Kojima, Jongoh Kim, Lawrence Chan
Trends in Endocrinology & Metabolism.2014; 25(4): 178. CrossRef
- Efficacy of adipose‐derived mesenchymal stem cell therapy in the treatment of chronic micro‐ and macrovascular complications of diabetes
- Cell Replacement and Regeneration Therapy for Diabetes
- Hee-Sook Jun
- Korean Diabetes J. 2010;34(2):77-83. Published online April 30, 2010
- DOI: https://doi.org/10.4093/kdj.2010.34.2.77
- 2,285 View
- 26 Download
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Reduction of beta cell function and a beta cell mass is observed in both type 1 and type 2 diabetes. Therefore, restoration of this deficiency might be a therapeutic option for treatment of diabetes. Islet transplantation has benefits, such as reduced incidence of hypoglycemia and achievement of insulin independence. However, the major drawback is an insufficient supply of islet donors. Transplantation of cells differentiated
in vitro orin vivo regeneration of insulin-producing cells are possible approaches for beta cell/islet regenerative therapy. Embryonic and adult stem cells, pancreatic ductal progenitor cells, acinar cells, and other endocrine cells have been shown to differentiate into pancreatic beta cells. Formation of fully functional beta cells and the safety of these cells are critical issues for successful clinical application.-
Citations
Citations to this article as recorded by- Direct Reprogramming of Mice Skin Fibroblasts into Insulin-Producing CellsIn Vitro
Israa S. Salman, Ahmed Majeed Al-Shammari, Mukhtar Khamis Haba
Cellular Reprogramming.2022; 24(5): 271. CrossRef - Effects of β-like cell autotransplantation through hepatic arterial intervention on diabetic dogs
Yongxu Mu, Zhiming Hao, Junfeng He, Ruiqiang Yan, Haiyan Liu, Lei Zhang, Heming Liu, Xiaoyan Hu, Qiming Li
Artificial Cells, Nanomedicine, and Biotechnology.2016; 44(5): 1333. CrossRef - Meeting the Need for Regenerative Therapies I: Target-Based Incidence and Its Relationship to U.S. Spending, Productivity, and Innovation
Nancy Parenteau, Janet Hardin-Young, William Shannon, Patrick Cantini, Alan Russell
Tissue Engineering Part B: Reviews.2012; 18(2): 139. CrossRef - Glucose-stimulated insulin secretion of various mesenchymal stem cells after insulin-producing cell differentiation
Su-Jung Kim, Yong-Soo Choi, Eun-Sun Ko, Sang-Min Lim, Chang-Woo Lee, Dong-Il Kim
Journal of Bioscience and Bioengineering.2012; 113(6): 771. CrossRef
- Direct Reprogramming of Mice Skin Fibroblasts into Insulin-Producing CellsIn Vitro

 KDA
KDA
 First
First Prev
Prev





