
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
- Multiple Roles of Sirtuin 6 in Adipose Tissue Inflammation
- Eun Ju Bae, Byung-Hyun Park
- Diabetes Metab J. 2023;47(2):164-172. Published online January 12, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0270
- 3,792 View
- 230 Download
- 4 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
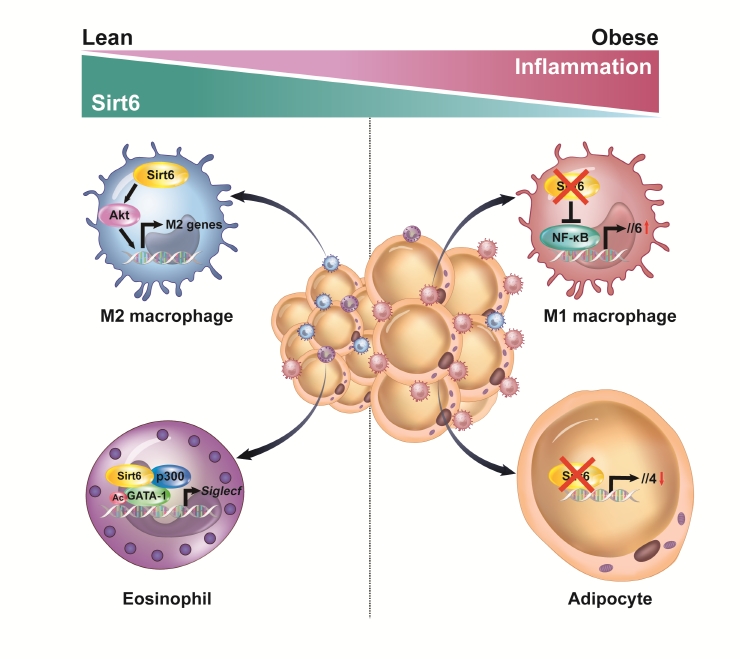
ePub - Adipose tissue (AT) inflammation is strongly associated with obesity-induced insulin resistance. When subjected to metabolic stress, adipocytes become inflamed and secrete a plethora of cytokines and chemokines, which recruit circulating immune cells to AT. Although sirtuin 6 (Sirt6) is known to control genomic stabilization, aging, and cellular metabolism, it is now understood to also play a pivotal role in the regulation of AT inflammation. Sirt6 protein levels are reduced in the AT of obese humans and animals and increased by weight loss. In this review, we summarize the potential mechanism of AT inflammation caused by impaired action of Sirt6 from the immune cells’ point of view. We first describe the properties and functions of immune cells in obese AT, with an emphasis on discrete macrophage subpopulations which are central to AT inflammation. We then highlight data that links Sirt6 to functional phenotypes of AT inflammation. Importantly, we discuss in detail the effects of Sirt6 deficiency in adipocytes, macrophages, and eosinophils on insulin resistance or AT browning. In our closing perspectives, we discuss emerging issues in this field that require further investigation.
-
Citations
Citations to this article as recorded by- The Role of Increased Expression of Sirtuin 6 in the Prevention of Premature Aging Pathomechanisms
Adrianna Dzidek, Olga Czerwińska-Ledwig, Małgorzata Żychowska, Wanda Pilch, Anna Piotrowska
International Journal of Molecular Sciences.2023; 24(11): 9655. CrossRef - Exploring the Influence of Age, Gender and Body Mass Index on Colorectal Cancer Location
Dorel Popovici, Cristian Stanisav, Sorin Saftescu, Serban Negru, Radu Dragomir, Daniel Ciurescu, Razvan Diaconescu
Medicina.2023; 59(8): 1399. CrossRef
- The Role of Increased Expression of Sirtuin 6 in the Prevention of Premature Aging Pathomechanisms
- Pathophysiology
- Low-Frequency Intermittent Hypoxia Suppresses Subcutaneous Adipogenesis and Induces Macrophage Polarization in Lean Mice
- Yan Wang, Mary Yuk Kwan Lee, Judith Choi Wo Mak, Mary Sau Man Ip
- Diabetes Metab J. 2019;43(5):659-674. Published online April 23, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0196
- 4,286 View
- 47 Download
- 4 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background The relationship between obstructive sleep apnoea (OSA) and metabolic disorders is complex and highly associated. The impairment of adipogenic capacity in pre-adipocytes may promote adipocyte hypertrophy and increase the risk of further metabolic dysfunction. We hypothesize that intermittent hypoxia (IH), as a pathophysiologic feature of OSA, may regulate adipogenesis by promoting macrophage polarization.
Methods Male C57BL/6N mice were exposed to either IH (240 seconds of 10% O2 followed by 120 seconds of 21% O2, i.e., 10 cycles/hour) or intermittent normoxia (IN) for 6 weeks. Stromal-vascular fractions derived from subcutaneous (SUB-SVF) and visceral (VIS-SVF) adipose tissues were cultured and differentiated. Conditioned media from cultured RAW 264.7 macrophages after air (Raw) or IH exposure (Raw-IH) were incubated with SUB-SVF during adipogenic differentiation.
Results Adipogenic differentiation of SUB-SVF but not VIS-SVF from IH-exposed mice was significantly downregulated in comparison with that derived from IN-exposed mice. IH-exposed mice compared to IN-exposed mice showed induction of hypertrophic adipocytes and increased preferential infiltration of M1 macrophages in subcutaneous adipose tissue (SAT) compared to visceral adipose tissue. Complementary
in vitro analysis demonstrated that Raw-IH media significantly enhanced inhibition of adipogenesis of SUB-SVF compared to Raw media, in agreement with corresponding gene expression levels of differentiation-associated markers and adipogenic transcription factors.Conclusion Low frequency IH exposure impaired adipogenesis of SAT in lean mice, and macrophage polarization may be a potential mechanism for the impaired adipogenesis.
-
Citations
Citations to this article as recorded by- Melatonin attenuates chronic intermittent hypoxia-induced intestinal barrier dysfunction in mice
Xinyi Li, Fan Wang, Zhenfei Gao, Weijun Huang, Xiaoman Zhang, Feng Liu, Hongliang Yi, Jian Guan, Xiaolin Wu, Huajun Xu, Shankai Yin
Microbiological Research.2023; 276: 127480. CrossRef - Clinical outcomes and plaque characteristics in patients with coronary artery disease and concomitant sleep-disordered breathing treated by continuous positive airway pressure
Kazuhiro Fujiyoshi, Taiki Tojo, Yoshiyasu Minami, Kohki Ishida, Miwa Ishida, Ken-ichiro Wakabayashi, Takayuki Inomata, Junya Ako
Sleep Medicine.2023; 101: 543. CrossRef - Potential Pathophysiological Pathways in the Complex Relationships between OSA and Cancer
Manuel Sánchez-de-la-Torre, Carolina Cubillos, Olivia J. Veatch, Francisco Garcia-Rio, David Gozal, Miguel Angel Martinez-Garcia
Cancers.2023; 15(4): 1061. CrossRef - Effects of Chronic Intermittent Hypoxia and Chronic Sleep Fragmentation on Gut Microbiome, Serum Metabolome, Liver and Adipose Tissue Morphology
Fan Wang, Juanjuan Zou, Huajun Xu, Weijun Huang, Xiaoman Zhang, Zhicheng Wei, Xinyi Li, Yupu Liu, Jianyin Zou, Feng Liu, Huaming Zhu, Hongliang Yi, Jian Guan, Shankai Yin
Frontiers in Endocrinology.2022;[Epub] CrossRef - C‐X3‐C motif chemokine ligand 1/receptor 1 regulates the M1 polarization and chemotaxis of macrophages after hypoxia/reoxygenation injury
Shuiming Guo, Lei Dong, Junhua Li, Yuetao Chen, Ying Yao, Rui Zeng, Nelli Shushakova, Hermann Haller, Gang Xu, Song Rong
Chronic Diseases and Translational Medicine.2021; 7(4): 254. CrossRef
- Melatonin attenuates chronic intermittent hypoxia-induced intestinal barrier dysfunction in mice
- Pathophysiology
- Role of NO/VASP Signaling Pathway against Obesity-Related Inflammation and Insulin Resistance
- Yu Mi Kang, Francis Kim, Woo Je Lee
- Diabetes Metab J. 2017;41(2):89-95. Published online November 15, 2016
- DOI: https://doi.org/10.4093/dmj.2017.41.2.89
- 4,478 View
- 59 Download
- 20 Web of Science
- 19 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Obesity has quickly become a worldwide pandemic, causing major adverse health outcomes such as dyslipidemia, type 2 diabetes mellitus, cardiovascular disease and cancers. Obesity-induced insulin resistance is the key for developing these metabolic disorders, and investigation to understand the molecular mechanisms involved has been vibrant for the past few decades. Of these, low-grade chronic inflammation is suggested as a critical concept in the development of obesity-induced insulin resistance, and the anti-inflammatory effect of nitric oxide (NO) signaling has been reported to be linked to improvement of insulin resistance in multiple organs involved in glucose metabolism. Recently, a body of evidence suggested that vasodilatory-stimulated phosphoprotein (VASP), a downstream mediator of NO signaling plays a crucial role in the anti-inflammatory effect and improvement of peripheral insulin resistance. These preclinical studies suggest that NO/VASP signaling could be an ideal therapeutic target in the treatment of obesity-related metabolic dysfunction. In this review, we introduce studies that investigated the protective role of NO/VASP signaling against obesity-related inflammation and insulin resistance in various tissues.
-
Citations
Citations to this article as recorded by- Hippocampal proteomic changes in high-fat diet-induced obese mice associated with memory decline
Ping Lu, Cun-Xiu Gao, Fei-Jian Luo, Yu-Ting Huang, Mei-Mei Gao, Yue-Sheng Long
The Journal of Nutritional Biochemistry.2024; 125: 109554. CrossRef - Leptina, obesidad y enfermedades cardiovasculares
Jorly Mejia-Montilla , Nadia Reyna-Villasmil, Andreina Fernández-Ramírez, Eduardo Reyna-Villasmil
Revista Repertorio de Medicina y Cirugía.2023; 32(3): 218. CrossRef - Urinary and circulatory netrin-1 as biomarker in diabetes and its related complications: a systematic review and meta-analysis
Amir Hossein Behnoush, Amirmohammad Khalaji, Zahra Shokri Varniab, Afshin Rahbarghazi, Elahe Amini, Aleksandra Klisic
Endocrine.2023;[Epub] CrossRef - Impact of ultraviolet radiation on cardiovascular and metabolic disorders: The role of nitric oxide and vitamin D
Qing‐Ling Quan, Kyeong‐No Yoon, Ji Su Lee, Eun Ju Kim, Dong Hun Lee
Photodermatology, Photoimmunology & Photomedicine.2023; 39(6): 573. CrossRef - Beneficial Metabolic Effects of Praliciguat, a Soluble Guanylate Cyclase Stimulator, in a Mouse Diet-Induced Obesity Model
Chad D. Schwartzkopf, John R. Hadcock, Guang Liu, Peter Germano, Julien Roux, Courtney M. Shea, Emmanuel S. Buys, Juli E. Jones
Frontiers in Pharmacology.2022;[Epub] CrossRef - Type 2 Diabetes Complicated With Heart Failure: Research on Therapeutic Mechanism and Potential Drug Development Based on Insulin Signaling Pathway
Hui Ye, Yanan He, Chuan Zheng, Fang Wang, Ming Yang, Junzhi Lin, Runchun Xu, Dingkun Zhang
Frontiers in Pharmacology.2022;[Epub] CrossRef - Effect of low-dose tadalafil once daily on glycemic control in patients with type 2 diabetes and erectile dysfunction: a randomized, double-blind, placebo-controlled pilot study
Min-Kyung Lee, Jae-Hyuk Lee, Seo-Young Sohn, Seo Yeon Lee, Tae-Yoong Jeong, Sae Chul Kim
Diabetology & Metabolic Syndrome.2022;[Epub] CrossRef - In situ hydrogel capturing nitric oxide microbubbles accelerates the healing of diabetic foot
Yingzheng Zhao, Lanzi Luo, Lantian Huang, Yingying Zhang, Mengqi Tong, Hanxiao Pan, Jianxun Shangguan, Qing Yao, Shihao Xu, Helin Xu
Journal of Controlled Release.2022; 350: 93. CrossRef - Amelioration effect of black seed oil against high‐fat diet‐induced obesity in rats through Nrf2/HO‐1 pathway
Nada F. Abo El‐Magd, Mohamed El‐Mesery, Amro El‐Karef, Mamdouh M. El‐Shishtawy
Journal of Food Biochemistry.2021;[Epub] CrossRef - An exploratory, randomised, placebo-controlled, 14 day trial of the soluble guanylate cyclase stimulator praliciguat in participants with type 2 diabetes and hypertension
John P. Hanrahan, Jelena P. Seferovic, James D. Wakefield, Phebe J. Wilson, Jennifer G. Chickering, Joon Jung, Kenneth E. Carlson, Daniel P. Zimmer, Andrew L. Frelinger, Alan D. Michelson, Linda Morrow, Michael Hall, Mark G. Currie, G. Todd Milne, Albert
Diabetologia.2020; 63(4): 733. CrossRef - Advantages of Phosphodiesterase Type 5 Inhibitors in the Management of Glucose Metabolism Disorders: A Clinical and Translational Issue
Cristina Antinozzi, Paolo Sgrò, Luigi Di Luigi
International Journal of Endocrinology.2020; 2020: 1. CrossRef - Association of endothelial dysfunction with incident prediabetes, type 2 diabetes and related traits: the KORA F4/FF4 study
Marie-Theres Huemer, Cornelia Huth, Florian Schederecker, Stefanie J Klug, Christa Meisinger, Wolfgang Koenig, Wolfgang Rathmann, Annette Peters, Barbara Thorand
BMJ Open Diabetes Research & Care.2020; 8(1): e001321. CrossRef - Embelin from Embelia ribes ameliorates oxidative stress and inflammation in high-fat diet-fed obese C57BL/6 mice
Priyanka Bansal, Uma Bhandari, Sayeed Ahmad
Pharmacognosy Magazine.2020; 16(5): 443. CrossRef - Weight change is significantly associated with risk of thyroid cancer: A nationwide population-based cohort study
Hyemi Kwon, Kyung-Do Han, Cheol-Young Park
Scientific Reports.2019;[Epub] CrossRef - Diabetes and Cancer: Cancer Should Be Screened in Routine Diabetes Assessment
Sunghwan Suh, Kwang-Won Kim
Diabetes & Metabolism Journal.2019; 43(6): 733. CrossRef - Antioxidant, antihyperglycemic, and antidiabetic activity of Apis mellifera bee tea
Janielle da Silva Melo da Cunha, Tamaeh Monteiro Alfredo, Jéssica Maurino dos Santos, Valter Vieira Alves Junior, Luiza Antas Rabelo, Emerson Silva Lima, Ana Paula de Araújo Boleti, Carlos Alexandre Carollo, Edson Lucas dos Santos, Kely de Picoli Souza, M
PLOS ONE.2018; 13(6): e0197071. CrossRef - Relationship Between Circulating Netrin-1 Concentration, Impaired Fasting Glucose, and Newly Diagnosed Type 2 Diabetes
Jisook Yim, Gyuri Kim, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Jeong-Ho Kim, Jin Won Cho, Sang-Guk Lee, Yong-ho Lee
Frontiers in Endocrinology.2018;[Epub] CrossRef - Glycyrrhizin ameliorates high fat diet-induced obesity in rats by activating NrF2 pathway
Nada F. Abo El-Magd, Mohamed El-Mesery, Amro El-Karef, Mamdouh M. El-Shishtawy
Life Sciences.2018; 193: 159. CrossRef - cGMP-dependent protein kinase I (cGKI) modulates human hepatic stellate cell activation
Andras Franko, Marketa Kovarova, Susanne Feil, Robert Feil, Robert Wagner, Martin Heni, Alfred Königsrainer, Marc Ruoß, Andreas K. Nüssler, Cora Weigert, Hans-Ulrich Häring, Stefan Z. Lutz, Andreas Peter
Metabolism.2018; 88: 22. CrossRef
- Hippocampal proteomic changes in high-fat diet-induced obese mice associated with memory decline
- Resistin in Rodents and Humans
- Hyeong Kyu Park, Rexford S. Ahima
- Diabetes Metab J. 2013;37(6):404-414. Published online December 12, 2013
- DOI: https://doi.org/10.4093/dmj.2013.37.6.404
- 5,612 View
- 54 Download
- 122 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Obesity is characterized by excess accumulation of lipids in adipose tissue and other organs, and chronic inflammation associated with insulin resistance and an increased risk of type 2 diabetes. Obesity, type 2 diabetes, and cardiovascular diseases are major health concerns. Resistin was first discovered as an adipose-secreted hormone (adipokine) linked to obesity and insulin resistance in rodents. Adipocyte-derived resistin is increased in obese rodents and strongly related to insulin resistance. However, in contrast to rodents, resistin is expressed and secreted from macrophages in humans and is increased in inflammatory conditions. Some studies have also suggested an association between increased resistin levels and insulin resistance, diabetes and cardiovascular disease. Genetic studies have provided additional evidence for a role of resistin in insulin resistance and inflammation. Resistin appears to mediate the pathogenesis of atherosclerosis by promoting endothelial dysfunction, vascular smooth muscle cell proliferation, arterial inflammation, and formation of foam cells. Indeed, resistin is predictive of atherosclerosis and poor clinical outcomes in patients with coronary artery disease and ischemic stroke. There is also growing evidence that elevated resistin is associated with the development of heart failure. This review will focus on the biology of resistin in rodents and humans, and evidence linking resistin with type 2 diabetes, atherosclerosis, and cardiovascular disease.
-
Citations
Citations to this article as recorded by- Resistin – A Plausible Therapeutic Target in the Pathogenesis of Psoriasis
Manupati Srikanth, Mahaboobkhan Rasool
Immunological Investigations.2024; 53(2): 115. CrossRef - MHO or MUO? White adipose tissue remodeling
Jing Yi Zhao, Li Juan Zhou, Kai Le Ma, Rui Hao, Min Li
Obesity Reviews.2024;[Epub] CrossRef - Resistin in endocrine pancreas of sheep: Presence and expression related to different diets
Margherita Maranesi, Elisa Palmioli, Cecilia Dall'Aglio, Daniele Marini, Polina Anipchenko, Elena De Felice, Paola Scocco, Francesca Mercati
General and Comparative Endocrinology.2024; 348: 114452. CrossRef - Adipocytokines levels as potential biomarkers for discriminating patients with a diagnosis of depressive disorder from healthy controls
Elżbieta Małujło-Balcerska, Tadeusz Pietras
Journal of Psychiatric Research.2024; 171: 163. CrossRef - Adipokines in atopic dermatitis: the link between obesity and atopic dermatitis
Shiyun Zhang, Bingjie Zhang, Yuehua Liu, Li Li
Lipids in Health and Disease.2024;[Epub] CrossRef - The Role of Adipokines in the Control of Pituitary Functions
Barbara Kaminska, Beata Kurowicka, Marta Kiezun, Kamil Dobrzyn, Katarzyna Kisielewska, Marlena Gudelska, Grzegorz Kopij, Karolina Szymanska, Barbara Zarzecka, Oguzhan Koker, Ewa Zaobidna, Nina Smolinska, Tadeusz Kaminski
Animals.2024; 14(2): 353. CrossRef - Adipokine imbalance and its role in the pathogenesis of novel coronavirus infection
I. D. Bespalova, U. M. Mitrichenko, V. V. Kalyuzhin, E. S. Koroleva, Yu. I. Koshchavtseva, D. S. Romanov, D. E. Pershina
Bulletin of Siberian Medicine.2024; 22(4): 164. CrossRef - Association of adipokine levels with obesity in periodontal health and disease: A systematic review with meta‐analysis and meta‐regression
Eswar Kandaswamy, Chun‐Teh Lee, Soumya Bardvalli Gururaj, Sachin Shivanaikar, Vinayak M. Joshi
Journal of Periodontal Research.2024;[Epub] CrossRef - Role of epicardial adipose tissue in the pathogenesis of chronic inflammation in heart failure with preserved ejection fraction
O. N. Dzhioeva, Yu. S. Timofeev, V. A. Metelskaya, A. A. Bogdanova, T. Yu. Vedenikin, O. M. Drapkina
Cardiovascular Therapy and Prevention.2024; 23(3): 3928. CrossRef - Association of maternal body composition and diet on breast milk hormones and neonatal growth during the first month of lactation
David Ramiro-Cortijo, Pratibha Singh, Gloria Herranz Carrillo, Andrea Gila-Díaz, María A. Martín-Cabrejas, Camilia R. Martin, Silvia M. Arribas
Frontiers in Endocrinology.2023;[Epub] CrossRef - Upregulation of peripheral blood mononuclear cells resistin gene expression in severe obstructive sleep apnea and obstructive sleep apnea with coexisting type 2 diabetes mellitus
Branislava Rajkov, Marija Zdravković, Ana Ninić, Milica Brajković, Slobodan Klašnja, Vera Gardijan, Lidija Memon, Jelena Munjas, Marija Mihajlović, Vesna Spasojević- Kalimanovska, Vojislav Radosavljević, Miron Sopić
Sleep and Breathing.2023; 27(5): 2031. CrossRef - Fat-to-heart crosstalk in health and disease
Fleur Lodewijks, Timothy A. McKinsey, Emma L. Robinson
Frontiers in Genetics.2023;[Epub] CrossRef - Adipokines as Diagnostic and Prognostic Markers for the Severity of COVID-19
Thomas Grewal, Christa Buechler
Biomedicines.2023; 11(5): 1302. CrossRef - Role of adipokines in sarcopenia
Wenhao Lu, Wenjie Feng, Jieyu Lai, Dongliang Yuan, Wenfeng Xiao, Yusheng Li
Chinese Medical Journal.2023; 136(15): 1794. CrossRef - Resistin, TNF-α, and microRNA 124-3p expressions in peripheral blood mononuclear cells are associated with diabetic nephropathy
Amin Monjezi, Azam Khedri, Mehrnoosh Zakerkish, Ghorban Mohammadzadeh
International Journal of Diabetes in Developing Countries.2022; 42(1): 62. CrossRef - Resistin in Urine and Breast Milk: Relation to Type of Feeding and Anthropometry at 1-Month
Irena Santosa, Hiromichi Shoji, Kentaro Awata, Yoshiteru Arai, Hiroki Suganuma, Toshiaki Shimizu
Pediatric Reports.2022; 14(1): 86. CrossRef - High Serum Levels of Resistin is Associated With Acute Cerebral Infarction
Kee Ook Lee, Kyung-Yul Lee, Cheol-Young Lee, Ji Hoon Kim, Jaeku Kang, Hoi Young Lee, Sang-Jun Na, Seung-Hun Oh, Ji Hoe Heo
The Neurologist.2022; 27(2): 41. CrossRef - Resistin production does not affect outcomes in a mouse model of acute surgical sepsis
Anthony S. Bonavia, Zissis C. Chroneos, Victor Ruiz-Velasco, Charles H. Lang, Partha Mukhopadhyay
PLOS ONE.2022; 17(3): e0265241. CrossRef - Single-nucleotide polymorphisms as important risk factors of diabetes among Middle East population
Iman Akhlaghipour, Amir Reza Bina, Mohammad Reza Mogharrabi, Ali Fanoodi, Amir Reza Ebrahimian, Soroush Khojasteh Kaffash, Atefeh Babazadeh Baghan, Mohammad Erfan Khorashadizadeh, Negin Taghehchian, Meysam Moghbeli
Human Genomics.2022;[Epub] CrossRef - Synergistic Effects of Weighted Genetic Risk Scores and Resistin and sST2 Levels on the Prognostication of Long-Term Outcomes in Patients with Coronary Artery Disease
Hsin-Hua Chou, Lung-An Hsu, Jyh-Ming Jimmy Juang, Fu-Tien Chiang, Ming-Sheng Teng, Semon Wu, Yu-Lin Ko
International Journal of Molecular Sciences.2022; 23(8): 4292. CrossRef - Hypoxia Increases the Potential for Neutrophil-mediated Endothelial Damage in Chronic Obstructive Pulmonary Disease
Katharine M. Lodge, Arlette Vassallo, Bin Liu, Merete Long, Zhen Tong, Paul R. Newby, Danya Agha-Jaffar, Koralia Paschalaki, Clara E. Green, Kylie B. R. Belchamber, Victoria C. Ridger, Robert A. Stockley, Elizabeth Sapey, Charlotte Summers, Andrew S. Cowb
American Journal of Respiratory and Critical Care Medicine.2022; 205(8): 903. CrossRef - The Role of the Adipokine Resistin in the Pathogenesis and Progression of Epithelial Ovarian Cancer
Klaudia Parafiniuk, Wiktoria Skiba, Anna Pawłowska, Dorota Suszczyk, Aleksandra Maciejczyk, Iwona Wertel
Biomedicines.2022; 10(4): 920. CrossRef - Resistin Modulates Low-Density Lipoprotein Cholesterol Uptake in Human Placental Explants via PCSK9
Sonia Nava-Salazar, Arturo Flores-Pliego, Giovanni Pérez-Martínez, Sandra Parra-Hernández, America Vanoye-Carlo, Francisco Ibarguengoitia-Ochoa, Otilia Perichart-Perera, Enrique Reyes-Muñoz, Juan Mario Solis-Paredes, Salvador Espino y Sosa, Guadalupe Estr
Reproductive Sciences.2022; 29(11): 3242. CrossRef - Differential Association of Selected Adipocytokines, Adiponectin, Leptin, Resistin, Visfatin and Chemerin, with the Pathogenesis and Progression of Type 2 Diabetes Mellitus (T2DM) in the Asir Region of Saudi Arabia: A Case Control Study
Mohammad Muzaffar Mir, Rashid Mir, Mushabab Ayed Abdullah Alghamdi, Javed Iqbal Wani, Zia Ul Sabah, Mohammed Jeelani, Vijaya Marakala, Shahzada Khalid Sohail, Mohamed O’haj, Muffarah Hamid Alharthi, Mohannad Mohammad S. Alamri
Journal of Personalized Medicine.2022; 12(5): 735. CrossRef - Immune system and sarcopenia: Presented relationship and future perspective
Xuzhi Zhang, Hengzhen Li, Miao He, Jingyu Wang, Yuxiang Wu, Yusheng Li
Experimental Gerontology.2022; 164: 111823. CrossRef - Adipose Tissue Secretion Pattern Influences β-Cell Wellness in the Transition from Obesity to Type 2 Diabetes
Giuseppina Biondi, Nicola Marrano, Anna Borrelli, Martina Rella, Giuseppe Palma, Isabella Calderoni, Edoardo Siciliano, Pasquale Lops, Francesco Giorgino, Annalisa Natalicchio
International Journal of Molecular Sciences.2022; 23(10): 5522. CrossRef - Supplemental hydroxychloroquine therapy regulates adipokines in patients with systemic lupus erythematosus with stable disease
Risa Wakiya, Kiyo Ueeda, Hiromi Shimada, Shusaku Nakashima, Tomohiro Kameda, Nobuyuki Miyatake, Mikiya Kato, Taichi Miyagi, Koichi Sugihara, Mao Mizusaki, Rina Mino, Norimitsu Kadowaki, Hiroaki Dobashi
Clinical Rheumatology.2022; 41(11): 3345. CrossRef - Can soy isoflavones in combination with soy protein change serum concentration of adiponectin and resistin? A systematic review and meta‐analysis on randomized clinical trials
Mitra Hariri, Bahareh Amirkalali, Ensiyeh Mollanoroozy, Ali Gholami
Food Science & Nutrition.2022; 10(12): 4126. CrossRef - Adipokines: Deciphering the cardiovascular signature of adipose tissue
Joseph C. Galley, Shubhnita Singh, Wanessa M.C. Awata, Juliano V. Alves, Thiago Bruder-Nascimento
Biochemical Pharmacology.2022; 206: 115324. CrossRef - Evaluation of the Anti-Obesity Effect of Zeaxanthin and Exercise in HFD-Induced Obese Rats
Mona Al-thepyani, Salha Algarni, Hana Gashlan, Mohamed Elzubier, Lina Baz
Nutrients.2022; 14(23): 4944. CrossRef - Single High-Dose Vitamin D Supplementation as an Approach for Reducing Ultramarathon-Induced Inflammation: A Double-Blind Randomized Controlled Trial
Jan Mieszkowski, Andżelika Borkowska, Błażej Stankiewicz, Andrzej Kochanowicz, Bartłomiej Niespodziński, Marcin Surmiak, Tomasz Waldziński, Rafał Rola, Miroslav Petr, Jędrzej Antosiewicz
Nutrients.2021; 13(4): 1280. CrossRef - Resistin mitigates stemness and metabolic profile of human adipose-derived mesenchymal stem cells via insulin resistance
Komal Rawal, Kishan M. Purohit, Tushar P. Patel, Neeta Karont, Sarita Gupta
Cytokine.2021; 138: 155374. CrossRef - Resistin is co-secreted with adiponectin in white mouse adipocytes
Saliha Musovic, Man Mohan Shrestha, Ali M. Komai, Charlotta S. Olofsson
Biochemical and Biophysical Research Communications.2021; 534: 707. CrossRef - Resistin: Potential biomarker and therapeutic target in atherosclerosis
Li Zhou, Jun-Yi Li, Ping-Ping He, Xiao-Hua Yu, Chao-Ke Tang
Clinica Chimica Acta.2021; 512: 84. CrossRef - The circulating levels of CTRP1 and CTRP5 are associated with obesity indices and carotid intima-media thickness (cIMT) value in patients with type 2 diabetes: a preliminary study
Ziba Majidi, Solaleh Emamgholipour, Abolfazl Omidifar, Soheil Rahmani Fard, Hossein Poustchi, Mehrnoosh Shanaki
Diabetology & Metabolic Syndrome.2021;[Epub] CrossRef - Corylin reduces obesity and insulin resistance and promotes adipose tissue browning through SIRT-1 and β3-AR activation
Chin-Chuan Chen, Chen-Hsin Kuo, Yann-Lii Leu, Shu-Huei Wang
Pharmacological Research.2021; 164: 105291. CrossRef - A Focused Review of the Metabolic Side-Effects of Clozapine
Jessica W. Y. Yuen, David D. Kim, Ric M. Procyshyn, William J. Panenka, William G. Honer, Alasdair M. Barr
Frontiers in Endocrinology.2021;[Epub] CrossRef - A Negative Energy Balance Is Associated with Metabolic Dysfunctions in the Hypothalamus of a Humanized Preclinical Model of Alzheimer’s Disease, the 5XFAD Mouse
Antonio J. López-Gambero, Cristina Rosell-Valle, Dina Medina-Vera, Juan Antonio Navarro, Antonio Vargas, Patricia Rivera, Carlos Sanjuan, Fernando Rodríguez de Fonseca, Juan Suárez
International Journal of Molecular Sciences.2021; 22(10): 5365. CrossRef - Resistin in pregnancy: Analysis of determinants in pairs of umbilical cord blood and maternal serum
Anne Floeck, Nina Ferrari, Christine Joisten, Maria T. Puth, Brigitte Strizek, Ramona Dolscheid-Pommerich, Ulrich Gembruch, Waltraut M. Merz
Cytokine: X.2021; 3(2): 100052. CrossRef - Is resistin the master link between inflammation and inflammation-related chronic diseases?
Mohammed Taouis, Yacir Benomar
Molecular and Cellular Endocrinology.2021; 533: 111341. CrossRef - The dynamics of human bone marrow adipose tissue in response to feeding and fasting
Pouneh K. Fazeli, Miriam A. Bredella, Gisela Pachon-Peña, Wenxiu Zhao, Xun Zhang, Alexander T. Faje, Megi Resulaj, Sai P. Polineni, Tara M. Holmes, Hang Lee, Elizabeth K. O’Donnell, Ormond A. MacDougald, Mark C. Horowitz, Clifford J. Rosen, Anne Klibanski
JCI Insight.2021;[Epub] CrossRef - Resistin: A journey from metabolism to cancer
Ankita Deb, Bhavana Deshmukh, Pranay Ramteke, Firoz Khan Bhati, Manoj Kumar Bhat
Translational Oncology.2021; 14(10): 101178. CrossRef - Obesity is the basis of metabolic syndrome
A. F. Verbovoy, N. I. Verbovaya, Yu. A. Dolgikh
Obesity and metabolism.2021; 18(2): 142. CrossRef - Human Milk Metabolic Hormones: Analytical Methods and Current Understanding
Majed A. Suwaydi, Zoya Gridneva, Sharon L. Perrella, Mary E. Wlodek, Ching Tat Lai, Donna T. Geddes
International Journal of Molecular Sciences.2021; 22(16): 8708. CrossRef - Adipokines as Immune Cell Modulators in Multiple Sclerosis
Merel Rijnsburger, Niek Djuric, Inge A. Mulder, Helga E. de Vries
International Journal of Molecular Sciences.2021; 22(19): 10845. CrossRef - The Role of Adipokines in Cardiovascular Pathology
Valery Podzolkov , Anna Pokrovskaya, Ulyana Bazhanova , Tatyana Vargina , Svetlana Anatolievna Knyazeva , Daria Vanina
Open Access Macedonian Journal of Medical Sciences.2021; 9(F): 794. CrossRef - Measurement of Plasma Resistin Concentrations in Horses with Metabolic and Inflammatory Disorders
Beatriz Fuentes-Romero, Alberto Muñoz-Prieto, José J. Cerón, María Martín-Cuervo, Manuel Iglesias-García, Escolástico Aguilera-Tejero, Elisa Díez-Castro
Animals.2021; 12(1): 77. CrossRef - EFFECT OF DIET AND EXERCISE-INDUCE WEIGHT LOSS ON LEVEL OF RESISTIN IN PATIENT WITH OBESITY
О. I. Tokarenko, I. O. Andreieva, O. O. Tokarenko, M. M. Surmilo
Modern medical technology.2021; (4): 11. CrossRef - Alteration of gut microbiota affects expression of adiponectin and resistin through modifying DNA methylation in high-fat diet-induced obese mice
Hongyang Yao, Chaonan Fan, Yuanyuan Lu, Xiuqin Fan, Lulu Xia, Ping Li, Rui Wang, Tiantian Tang, Yuanyuan Wang, Kemin Qi
Genes & Nutrition.2020;[Epub] CrossRef - Resistin hormone in diabetic kidney disease and its relation to iron status and hepcidin
Zhian Sherzad Hayder, Zrar Saleem Kareem
International Urology and Nephrology.2020; 52(4): 749. CrossRef - Proteoglycans in Obesity-Associated Metabolic Dysfunction and Meta-Inflammation
Ariane R. Pessentheiner, G. Michelle Ducasa, Philip L. S. M. Gordts
Frontiers in Immunology.2020;[Epub] CrossRef - Resistin Is Increased in Periodontal Cells and Tissues: In Vitro and In Vivo Studies
Andressa V. B. Nogueira, Marjan Nokhbehsaim, Sema Tekin, Rafael S. de Molon, Luis C. Spolidorio, Svenja Memmert, Anna Damanaki, Andreas Jäger, Sigrun Eick, James Deschner, Joni A. Cirelli
Mediators of Inflammation.2020; 2020: 1. CrossRef - Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease
Alan Chait, Laura J. den Hartigh
Frontiers in Cardiovascular Medicine.2020;[Epub] CrossRef - The possible role of endocrine dysfunction of adipose tissue in gestational diabetes mellitus
Patrik Šimják, Kateřina Anderlová, Anna Cinkajzlová, Antonín Pařízek, Michal Kršek, Martin Haluzík
Minerva Endocrinologica.2020;[Epub] CrossRef - High Plasma Resistin Levels Portend the Insulin Resistance-Associated Susceptibility to Early Cognitive Decline in Patients with Type 2 Diabetes Mellitus
Chenchen Wang, Xi Huang, Sai Tian, Rong Huang, Dan Guo, Hongyan Lin, Jiaqi Wang, Shaohua Wang
Journal of Alzheimer's Disease.2020; 75(3): 807. CrossRef - Resistin in metabolism, inflammation, and disease
Deeksha Tripathi, Sashi Kant, Saurabh Pandey, Nasreen Z. Ehtesham
The FEBS Journal.2020; 287(15): 3141. CrossRef - Adipokines: New Potential Therapeutic Target for Obesity and Metabolic, Rheumatic, and Cardiovascular Diseases
Lucia Recinella, Giustino Orlando, Claudio Ferrante, Annalisa Chiavaroli, Luigi Brunetti, Sheila Leone
Frontiers in Physiology.2020;[Epub] CrossRef - Correlation of CCL18 with Levels of Adi-pokines in the Sera of Patients with Myocardial Infarction in a 6-Month Period: Case Series
Atefeh GamarTalepoor, Ehsan Dowlatshahi, Mehrnoush Doroudchi
Iranian South Medical Journal.2020; 23(3): 222. CrossRef - The Mesentery, Systemic Inflammation, and Crohn’s Disease
Edgardo D Rivera, John Calvin Coffey, Dara Walsh, Eli D Ehrenpreis
Inflammatory Bowel Diseases.2019; 25(2): 226. CrossRef - Resistin and adenylyl cyclase-associated protein 1 (CAP1) regulate the expression of genes related to insulin resistance in BNL CL.2 mouse liver cells
Dimiter Avtanski, Karin Chen, Leonid Poretsky
Data in Brief.2019; 25: 104112. CrossRef - Proteomic profile of patients with atrial fibrillation undergoing cardiac surgery†
Ilias P Doulamis, George Samanidis, Aspasia Tzani, Asier Antoranz, Anastasios Gkogkos, Panagiotis Konstantopoulos, Vaia Pliaka, Angeliki Minia, Leonidas G Alexopoulos, Despina N Perrea, Konstantinos Perreas
Interactive CardioVascular and Thoracic Surgery.2019; 28(1): 94. CrossRef - Angiotensin-(1-7), Adipokines and Inflammation
Deborah de Farias Lelis, Daniela Fernanda de Freitas, Amanda Souto Machado, Thaísa Soares Crespo, Sérgio Henrique Sousa Santos
Metabolism.2019; 95: 36. CrossRef - New Insights into Adipokines as Potential Biomarkers for Type-2 Diabetes Mellitus
Marta Olivera-Santa Catalina, Pedro C. Redondo, Maria P. Granados, Carlos Cantonero, Jose Sanchez-Collado, Letizia Albarran, Jose J. Lopez
Current Medicinal Chemistry.2019; 26(22): 4119. CrossRef - Myokine–adipokine cross-talk: potential mechanisms for the association between plasma irisin and adipokines and cardiometabolic risk factors in Mexican children with obesity and the metabolic syndrome
Adrian M. Gonzalez-Gil, Mariana Peschard-Franco, Elena C. Castillo, Gustavo Gutierrez-DelBosque, Victor Treviño, Christian Silva-Platas, Luisa Perez-Villarreal, Gerardo Garcia-Rivas, Leticia Elizondo-Montemayor
Diabetology & Metabolic Syndrome.2019;[Epub] CrossRef - Early Life Exposures to Perfluoroalkyl Substances in Relation to Adipokine Hormone Levels at Birth and During Childhood
Colleen Shelly, Philippe Grandjean, Youssef Oulhote, Peter Plomgaard, Ruth Frikke-Schmidt, Flemming Nielsen, Denis Zmirou-Navier, Pal Weihe, Damaskini Valvi
The Journal of Clinical Endocrinology & Metabolism.2019; 104(11): 5338. CrossRef - Overweight and obesity in childhood: Dietary, biochemical, inflammatory and lifestyle risk factors
Samah R. Albataineh, Eman F. Badran, Reema F. Tayyem
Obesity Medicine.2019; 15: 100112. CrossRef - Effects of major adipokines and the −420 C > G resistin gene polymorphism on the long-term outcome of patients with acute ischemic stroke
Stella Bouziana, Konstantinos Tziomalos, Antonis Goulas, Timoleon-Achilleas Vyzantiadis, Maria Papadopoulou, Athanasia Panderi, Apostolos Ι. Ηatzitolios
International Journal of Neuroscience.2019; 129(10): 978. CrossRef - The Complex Interactions Between Obesity, Metabolism and the Brain
Romina María Uranga, Jeffrey Neil Keller
Frontiers in Neuroscience.2019;[Epub] CrossRef - Resistin: A reappraisal
E. Acquarone, F. Monacelli, R. Borghi, A. Nencioni, P. Odetti
Mechanisms of Ageing and Development.2019; 178: 46. CrossRef - Implications of resistin in type 2 diabetes mellitus and coronary artery disease: Impairing insulin function and inducing pro‐inflammatory cytokines
Melissa Emamalipour, Khaled Seidi, Ali Jahanban‐Esfahlan, Rana Jahanban‐Esfahlan
Journal of Cellular Physiology.2019; 234(12): 21758. CrossRef - Serum-based soluble markers differentiate psoriatic arthritis from osteoarthritis
Vinod Chandran, Fatima Abji, Anthony V Perruccio, Rajiv Gandhi, Suzanne Li, Richard J Cook, Dafna D Gladman
Annals of the Rheumatic Diseases.2019; 78(6): 796. CrossRef - Telmisartan prevents diet-induced obesity and preserves leptin transport across the blood-brain barrier in high-fat diet-fed mice
Franziska Schuster, Gianna Huber, Ines Stölting, Emily E. Wing, Kathrin Saar, Norbert Hübner, William A. Banks, Walter Raasch
Pflügers Archiv - European Journal of Physiology.2018; 470(11): 1673. CrossRef - Adipokines in human breast milk
Juergen Kratzsch, Yoon Ju Bae, Wieland Kiess
Best Practice & Research Clinical Endocrinology & Metabolism.2018; 32(1): 27. CrossRef - Addressing the Perfect Storm: Biomarkers in Obesity and Pathophysiology of Cardiometabolic Risk
Krasimira Aleksandrova, Dariush Mozaffarian, Tobias Pischon
Clinical Chemistry.2018; 64(1): 142. CrossRef - Adipocytokine Involvement in Innate Immune Mechanisms
Paulina Żelechowska, Elżbieta Kozłowska, Joanna Pastwińska, Justyna Agier, Ewa Brzezińska-Błaszczyk
Journal of Interferon & Cytokine Research.2018; 38(12): 527. CrossRef - The effect of a garlic supplement on the pro-inflammatory adipocytokines, resistin and tumor necrosis factor-alpha, and on pain severity, in overweight or obese women with knee osteoarthritis
Sahar Dehghani, Elham Alipoor, Ahmad Salimzadeh, Mehdi Yaseri, Mostafa Hosseini, Christine Feinle-Bisset, Mohammad Javad Hosseinzadeh-Attar
Phytomedicine.2018; 48: 70. CrossRef - Perivascular adipose tissue (PVAT) in atherosclerosis: a double-edged sword
Xiao-Yan Qi, Shun-Lin Qu, Wen-Hao Xiong, Oren Rom, Lin Chang, Zhi-Sheng Jiang
Cardiovascular Diabetology.2018;[Epub] CrossRef - Usefulness of the Adipokines as Biomarkers of Ischemic Cardiac Dysfunction
Larisa-Diana Mocan Hognogi, Cerasela-Mihaela Goidescu, Anca-Daniela Farcaş
Disease Markers.2018; 2018: 1. CrossRef - Circulating fibroblast growth factor 21 in patients with liver cirrhosis
Sabrina Krautbauer, Lisa Rein-Fischboeck, Elisabeth M Haberl, Rebekka Pohl, Reiner Wiest, Christa Buechler
Clinical and Experimental Medicine.2018; 18(1): 63. CrossRef - Association of Cord Blood Resistin with Neonatal Birth Weight and Gestational Age
Shahnaz Pourarian, Saeed Fotouhikia, Forough Saki
Journal of Comprehensive Pediatrics.2018;[Epub] CrossRef - Major Adipokines and the −420C>G Resistin Gene Polymorphism as Predictors of Acute Ischemic Stroke Severity and In-Hospital Outcome
Styliani D. Bouziana, Konstantinos Tziomalos, Antonios Goulas, Timoleon-Achilleas Vyzantiadis, Athanasia Panderi, Apostolos Ι. Ηatzitolios
Journal of Stroke and Cerebrovascular Diseases.2018; 27(4): 963. CrossRef - Resistin and NGAL are associated with inflammatory response, endothelial activation and clinical outcomes in sepsis
Stephen P. J. Macdonald, Erika Bosio, Claire Neil, Glenn Arendts, Sally Burrows, Lisa Smart, Simon G. A. Brown, Daniel M. Fatovich
Inflammation Research.2017; 66(7): 611. CrossRef - Reference values for fasting serum resistin in healthy children and adolescents
Ulrik Lausten-Thomsen, Michael Christiansen, Paula Louise Hedley, Tenna Ruest Haarmark Nielsen, Cilius Esmann Fonvig, Oluf Pedersen, Torben Hansen, Jens-Christian Holm
Clinica Chimica Acta.2017; 469: 161. CrossRef - Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis
Alexander Kalinkovich, Gregory Livshits
Ageing Research Reviews.2017; 35: 200. CrossRef - Is There Any Relationship between Plasma 25-Hydroxyvitamin D3, Adipokine Profiles and Excessive Body Weight in Type 2 Diabetic Patients?
Joanna Kocot, Piotr Dziemidok, Małgorzata Kiełczykowska, Jacek Kurzepa, Grzegorz Szcześniak, Irena Musik
International Journal of Environmental Research and Public Health.2017; 15(1): 19. CrossRef - Exogenous Adipokine Peptide Resistin Protects Against Focal Cerebral Ischemia/Reperfusion Injury in Mice
Jiangtao Zhu, Di Wu, Chenyu Zhao, Man Luo, Ronald C. Hamdy, Balvin H. L. Chua, Xingshun Xu, Zhigang Miao
Neurochemical Research.2017; 42(10): 2949. CrossRef - Adipokines in Liver Cirrhosis
Christa Buechler, Elisabeth Haberl, Lisa Rein-Fischboeck, Charalampos Aslanidis
International Journal of Molecular Sciences.2017; 18(7): 1392. CrossRef - The role of sex steroids in white adipose tissue adipocyte function
A E Newell-Fugate
Reproduction.2017; 153(4): R133. CrossRef - Odanacatib Inhibits Resistin-induced Cardiomyocyte Hypertrophy Through the Inactivation of ERK Signaling Pathway
Xian Zheng, Guanchang Cheng, Jianwei Luo, Qunhui Ye, Yongzhi Deng, Lin Wu
International Journal of Pharmacology.2017; 13(2): 212. CrossRef - Linking resistin, inflammation, and cardiometabolic diseases
Hyeong Kyu Park, Mi Kyung Kwak, Hye Jeong Kim, Rexford S. Ahima
The Korean Journal of Internal Medicine.2017; 32(2): 239. CrossRef - Translating the biology of adipokines in atherosclerosis and cardiovascular diseases: Gaps and open questions
M. Ruscica, A. Baragetti, A.L. Catapano, G.D. Norata
Nutrition, Metabolism and Cardiovascular Diseases.2017; 27(5): 379. CrossRef - Differences in Mean Levels of Maternal Resistin Serum between Early Onset Preeclampsia (EOPE) and Late Onset Preeclampsia (LOPE)
Yusrawati ., P. Alfajra, R. Machmud
Research Journal of Obstetrics and Gynecology.2016; 10(1): 1. CrossRef - Secret talk between adipose tissue and central nervous system via secreted factors—an emerging frontier in the neurodegenerative research
Avinash Parimisetty, Anne-Claire Dorsemans, Rana Awada, Palaniyandi Ravanan, Nicolas Diotel, Christian Lefebvre d’Hellencourt
Journal of Neuroinflammation.2016;[Epub] CrossRef - The role of adipokines in ischemic stroke risk stratification
Styliani Bouziana, Konstantinos Tziomalos, Antonios Goulas, Apostolos Ι Ηatzitolios
International Journal of Stroke.2016; 11(4): 389. CrossRef - The endocrine function of human placenta: an overview
Mariana A. Costa
Reproductive BioMedicine Online.2016; 32(1): 14. CrossRef - Ursolic acid plays a protective role in obesity-induced cardiovascular diseases
Yu-Ting Lin, Ya-Mei Yu, Weng-Cheng Chang, Su-Yin Chiang, Hsu-Chin Chan, Ming-Fen Lee
Canadian Journal of Physiology and Pharmacology.2016; 94(6): 627. CrossRef - Determinants of body weight regulation in humans
Milene Moehlecke, Luis Henrique Canani, Lucas Oliveira Junqueira e Silva, Manoel Roberto Maciel Trindade, Rogerio Friedman, Cristiane Bauermann Leitão
Archives of Endocrinology and Metabolism.2016; 60(2): 152. CrossRef - Sitagliptin decreases ventricular arrhythmias by attenuated glucose-dependent insulinotropic polypeptide (GIP)-dependent resistin signalling in infarcted rats
Tsung-Ming Lee, Wei-Ting Chen, Nen-Chung Chang
Bioscience Reports.2016;[Epub] CrossRef - Les adipokines : état des lieux et nouveautés
J.-P. Bastard, C. Bastard, S. Fellahi, C. Vatier, J. Capeau, B. Fève
Obésité.2016; 11(3): 181. CrossRef - Factors that promote macrophage homing to adipose tissue in metabolic syndrome
Ishwarlal Jialal, Beverley Adams-Huet, Sridevi Devaraj
Journal of Diabetes and its Complications.2016; 30(8): 1434. CrossRef - Uncovering Factors Related to Pancreatic Beta-Cell Function
Aoife M. Curran, Miriam F. Ryan, Elaine Drummond, Eileen R. Gibney, Michael J. Gibney, Helen M. Roche, Lorraine Brennan, Nigel Irwin
PLOS ONE.2016; 11(8): e0161350. CrossRef - Resistin’s, obesity and insulin resistance: the continuing disconnect between rodents and humans
X. Huang, Z. Yang
Journal of Endocrinological Investigation.2016; 39(6): 607. CrossRef - Adipocytokines in renal transplant recipients
Kristof Nagy, Shankar Prasad Nagaraju, Connie M. Rhee, Zoltan Mathe, Miklos Z. Molnar
Clinical Kidney Journal.2016; 9(3): 359. CrossRef - Endocrine alterations from concentric vs. eccentric muscle actions: A brief review
Robert R. Kraemer, V. Daniel Castracane
Metabolism.2015; 64(2): 190. CrossRef - Non-traditional cytokines: How catecholamines and adipokines influence macrophages in immunity, metabolism and the central nervous system
Mark A. Barnes, Monica J. Carson, Meera G. Nair
Cytokine.2015; 72(2): 210. CrossRef - Local and serum levels of adipokines in patients with obesity after periodontal therapy: one‐year follow‐up
Tiago Eduardo Dias Gonçalves, Glaucia Santos Zimmermann, Luciene Cristina Figueiredo, Monique de Carvalho Souza, Daniele Ferreira da Cruz, Marta Ferreira Bastos, Hélio Doyle Pereira da Silva, Poliana Mendes Duarte
Journal of Clinical Periodontology.2015; 42(5): 431. CrossRef - Newborn Adipokines and Birth Outcomes
Edwina H. Yeung, Alexander C. McLain, Nancy Anderson, David Lawrence, Nansi S. Boghossian, Charlotte Druschel, Erin Bell
Paediatric and Perinatal Epidemiology.2015; 29(4): 317. CrossRef - The effect of a preparation of minerals, vitamins and trace elements on the cardiac gene expression pattern in male diabetic rats
Márta Sárközy, Gergő Szűcs, Márton Pipicz, Ágnes Zvara, Katalin Éder, Veronika Fekete, Csilla Szűcs, Judit Bárkányi, Csaba Csonka, László G. Puskás, Csaba Kónya, Péter Ferdinandy, Tamás Csont
Cardiovascular Diabetology.2015;[Epub] CrossRef - Diet-induced variability of the resistin gene (Retn) transcript level and methylation profile in rats
Joanna Nowacka-Woszuk, Ewa Pruszynska-Oszmalek, Maciej Szydlowski, Slawomir Sadkowski, Izabela Szczerbal
BMC Genetics.2015;[Epub] CrossRef - INFLUENCE OF RESISTIN ON THE COURSE OF ISCHEMIC HEART DISEASE IN PATIENTS WITH TYPE 2 DIABETES MELLITUS
A. T. Teplyakov, Sh. D. Akhmedov, T. Ye. Suslova, А. V. Andriyanova, A. V. Kuznetsova, N. V. Protopopova, V. V. Kalyuzhin, O. N. Nasanova
Bulletin of Siberian Medicine.2015; 14(5): 73. CrossRef - The Effects of a Single Developmentally Entrained Pulse of Testosterone in Female Neonatal Mice on Reproductive and Metabolic Functions in Adult Life
Hyeran Jang, Shalender Bhasin, Tyler Guarneri, Carlo Serra, Mary Schneider, Mi-Jeong Lee, Wen Guo, Susan K. Fried, Karol Pencina, Ravi Jasuja
Endocrinology.2015; 156(10): 3737. CrossRef - The Resin fromProtium heptaphyllumPrevents High-Fat Diet-Induced Obesity in Mice: Scientific Evidence and Potential Mechanisms
Karine Maria Martins Bezerra Carvalho, José Delano Barreto Marinho Filho, Tiago Sousa de Melo, Ana Jérsia Araújo, Josiane da Silva Quetz, Maria do Perpétuo Socorro Saldanha da Cunha, Karina Moura de Melo, Armenio Andre de Carvalho Almeida da Silva, Adrian
Evidence-Based Complementary and Alternative Medicine.2015; 2015: 1. CrossRef - Evolution of the Vertebrate Resistin Gene Family
Qingda Hu, Huanran Tan, David M. Irwin, Marc Robinson-Rechavi
PLOS ONE.2015; 10(6): e0130188. CrossRef - Obesity, adipokines and neuroinflammation
Argel Aguilar-Valles, Wataru Inoue, Christoph Rummel, Giamal N. Luheshi
Neuropharmacology.2015; 96: 124. CrossRef - Adipokines at the crossroad between obesity and cardiovascular disease
Filippo Molica, Sandrine Morel, Brenda Kwak, Françoise Rohner-Jeanrenaud, Sabine Steffens
Thrombosis and Haemostasis.2015; 113(03): 553. CrossRef - Resistin – 420 C/G polymorphism and serum resistin level in Iranian patients with gestational diabetes mellitus
Mohammad Ali Takhshid, Zinab Zare
Journal of Diabetes & Metabolic Disorders.2015;[Epub] CrossRef - Ictal adipokines are associated with pain severity and treatment response in episodic migraine
Nu Cindy Chai, Bizu Gelaye, Gretchen E. Tietjen, Paul D. Dash, Barbara A. Gower, Linda W. White, Thomas N. Ward, Ann I. Scher, B. Lee Peterlin
Neurology.2015; 84(14): 1409. CrossRef - Inflammation and insulin/IGF-1 resistance as the possible link between obesity and neurodegeneration
Lindsay J. Spielman, Jonathan P. Little, Andis Klegeris
Journal of Neuroimmunology.2014; 273(1-2): 8. CrossRef - Wild Blueberries (Vaccinium myrtillus) Alleviate Inflammation and Hypertension Associated with Developing Obesity in Mice Fed with a High-Fat Diet
Otto T. Mykkänen, Anne Huotari, Karl-Heinz Herzig, Thomas W. Dunlop, Hannu Mykkänen, Pirkka V. Kirjavainen, Michael Müller
PLoS ONE.2014; 9(12): e114790. CrossRef - Role of fat and adipokines in intestinal inflammation
LeaI Kredel, Arvind Batra, Britta Siegmund
Current Opinion in Gastroenterology.2014; 30(6): 559. CrossRef -
13C metabolic flux analysis shows that resistin impairs the metabolic response to insulin in L6E9 myotubes
Shirley Guzmán, Silvia Marin, Anibal Miranda, Vitaly A Selivanov, Josep J Centelles, Romain Harmancey, Fatima Smih, Annie Turkieh, Yves Durocher, Antonio Zorzano, Philippe Rouet, Marta Cascante
BMC Systems Biology.2014;[Epub] CrossRef - Bee Pollen Improves Muscle Protein and Energy Metabolism in Malnourished Old Rats through Interfering with the Mtor Signaling Pathway and Mitochondrial Activity
Jérôme Salles, Nicolas Cardinault, Véronique Patrac, Alexandre Berry, Christophe Giraudet, Marie-Laure Collin, Audrey Chanet, Camille Tagliaferri, Philippe Denis, Corinne Pouyet, Yves Boirie, Stéphane Walrand
Nutrients.2014; 6(12): 5500. CrossRef
- Resistin – A Plausible Therapeutic Target in the Pathogenesis of Psoriasis

 KDA
KDA
 First
First Prev
Prev





