
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
- Extracellular Vimentin Alters Energy Metabolism And Induces Adipocyte Hypertrophy
- Ji-Hae Park, Soyeon Kwon, Young Mi Park
- Diabetes Metab J. 2024;48(2):215-230. Published online September 26, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0332
- 2,422 View
- 207 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
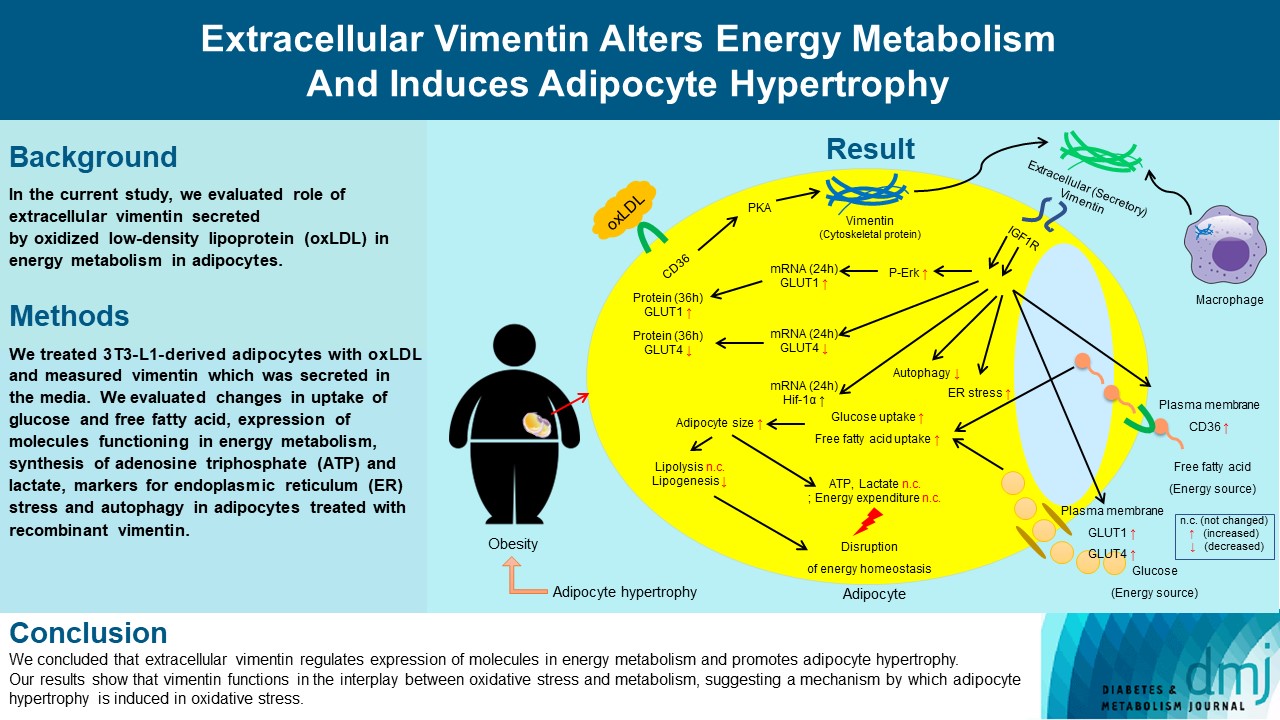
Previous studies have reported that oxidative stress contributes to obesity characterized by adipocyte hypertrophy. However, mechanism has not been studied extensively. In the current study, we evaluated role of extracellular vimentin secreted by oxidized low-density lipoprotein (oxLDL) in energy metabolism in adipocytes.
Methods
We treated 3T3-L1-derived adipocytes with oxLDL and measured vimentin which was secreted in the media. We evaluated changes in uptake of glucose and free fatty acid, expression of molecules functioning in energy metabolism, synthesis of adenosine triphosphate (ATP) and lactate, markers for endoplasmic reticulum (ER) stress and autophagy in adipocytes treated with recombinant vimentin.
Results
Adipocytes secreted vimentin in response to oxLDL. Microscopic evaluation revealed that vimentin treatment induced increase in adipocyte size and increase in sizes of intracellular lipid droplets with increased intracellular triglyceride. Adipocytes treated with vimentin showed increased uptake of glucose and free fatty acid with increased expression of plasma membrane glucose transporter type 1 (GLUT1), GLUT4, and CD36. Vimentin treatment increased transcription of GLUT1 and hypoxia-inducible factor 1α (Hif-1α) but decreased GLUT4 transcription. Adipose triglyceride lipase (ATGL), peroxisome proliferator-activated receptor γ (PPARγ), sterol regulatory element-binding protein 1 (SREBP1), diacylglycerol O-acyltransferase 1 (DGAT1) and 2 were decreased by vimentin treatment. Markers for ER stress were increased and autophagy was impaired in vimentin-treated adipocytes. No change was observed in synthesis of ATP and lactate in the adipocytes treated with vimentin.
Conclusion
We concluded that extracellular vimentin regulates expression of molecules in energy metabolism and promotes adipocyte hypertrophy. Our results show that vimentin functions in the interplay between oxidative stress and metabolism, suggesting a mechanism by which adipocyte hypertrophy is induced in oxidative stress.
- Complications
- Treatment of Diabetic Kidney Disease: Current and Future
- Tomotaka Yamazaki, Imari Mimura, Tetsuhiro Tanaka, Masaomi Nangaku
- Diabetes Metab J. 2021;45(1):11-26. Published online January 22, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0217
- 19,424 View
- 1,335 Download
- 92 Web of Science
- 92 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub 
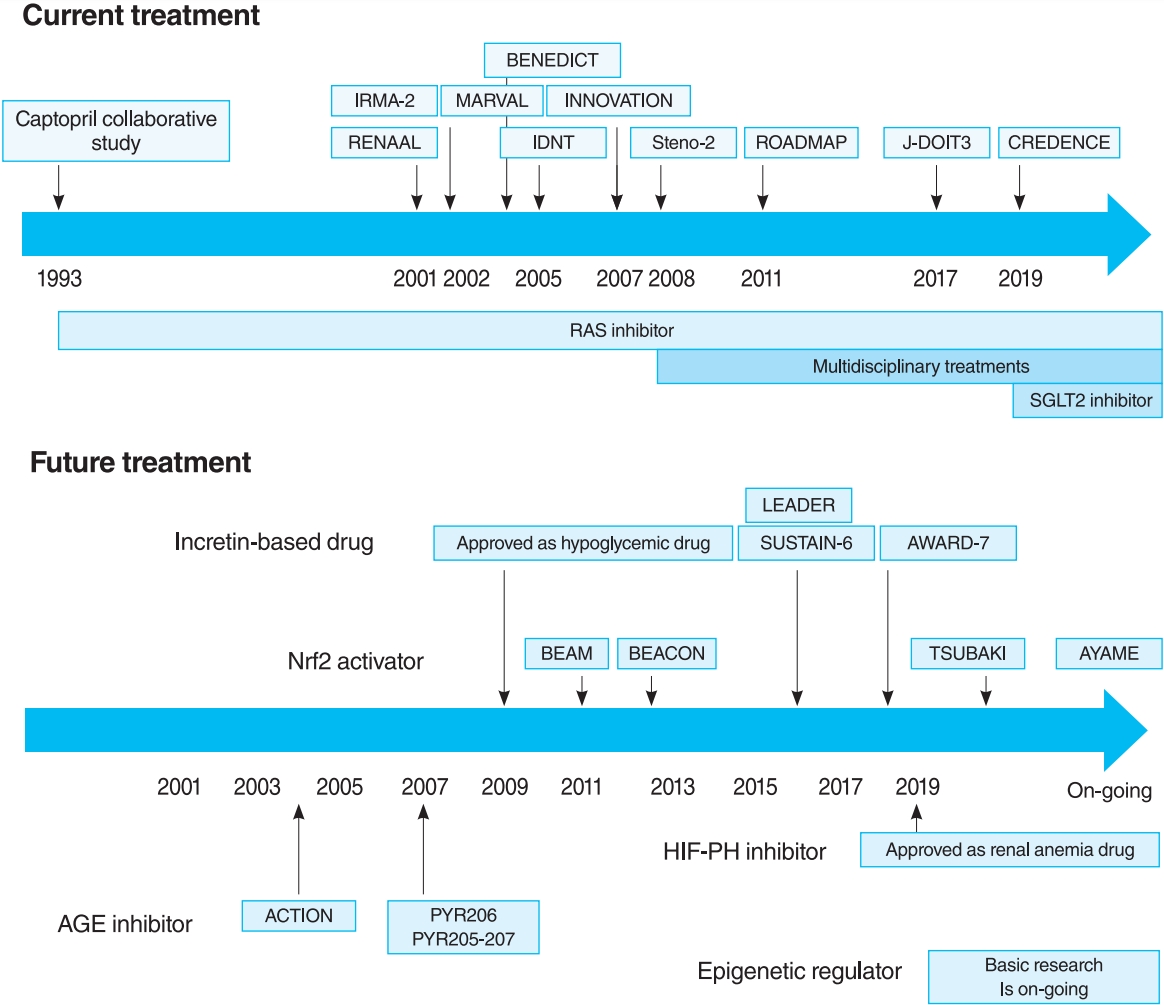
- Diabetic kidney disease (DKD) is the major cause of end-stage kidney disease. However, only renin-angiotensin system inhibitor with multidisciplinary treatments is effective for DKD. In 2019, sodium-glucose cotransporter 2 (SGLT2) inhibitor showed efficacy against DKD in Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial, adding a new treatment option. However, the progression of DKD has not been completely controlled. The patients with transient exposure to hyperglycemia develop diabetic complications, including DKD, even after normalization of their blood glucose. Temporary hyperglycemia causes advanced glycation end product (AGE) accumulations and epigenetic changes as metabolic memory. The drugs that improve metabolic memory are awaited, and AGE inhibitors and histone modification inhibitors are the focus of clinical and basic research. In addition, incretin-related drugs showed a renoprotective ability in many clinical trials, and these trials with renal outcome as their primary endpoint are currently ongoing. Hypoxia-inducible factor prolyl hydroxylase inhibitors recently approved for renal anemia may be renoprotective since they improve tubulointerstitial hypoxia. Furthermore, NF-E2–related factor 2 activators improved the glomerular filtration rate of DKD patients in Bardoxolone Methyl Treatment: Renal Function in chronic kidney disease/Type 2 Diabetes (BEAM) trial and Phase II Study of Bardoxolone Methyl in Patients with Chronic Kidney Disease and Type 2 Diabetes (TSUBAKI) trial. Thus, following SGLT2 inhibitor, numerous novel drugs could be utilized in treating DKD. Future studies are expected to provide new insights.
-
Citations
Citations to this article as recorded by- Clinical value of serum MMP-3 in chronic kidney disease
Yulin Fu, Cheng Song, Yuan Qin, Tianyu Zheng, Xiumei Zhou, Xueqin Zhao, Jian Zou, Biao Huang
Clinica Chimica Acta.2024; 553: 117725. CrossRef - β2-Adrenergic receptor agonists as a treatment for diabetic kidney disease
Ehtesham Arif, Danira Medunjanin, Ashish Solanki, Xiaofeng Zuo, Yanhui Su, Yujing Dang, Brennan Winkler, Kasey Lerner, Ahmed I. Kamal, Oleg Palygin, Marc-Andre Cornier, Bethany J. Wolf, Kelly J. Hunt, Joshua H. Lipschutz
American Journal of Physiology-Renal Physiology.2024; 326(1): F20. CrossRef - β2-Adrenergic receptor agonists: a new treatment for diabetic kidney disease?
Zhiwen Liu, Zheng Dong
American Journal of Physiology-Renal Physiology.2024; 326(1): F1. CrossRef - Urinary exosomal microRNA-145-5p and microRNA-27a-3p act as noninvasive diagnostic biomarkers for diabetic kidney disease
Lu-Lu Han, Sheng-Hai Wang, Ming-Yan Yao, Hong Zhou
World Journal of Diabetes.2024; 15(1): 92. CrossRef - Placenta-derived mesenchymal stem cells protect against diabetic kidney disease by upregulating autophagy-mediated SIRT1/FOXO1 pathway
Honghong Liu, Jiao Wang, Guanru Yue, Jixiong Xu
Renal Failure.2024;[Epub] CrossRef - Association of serum Nrf2 protein levels with disease activity and renal impairment in lupus nephritis
Jicui Li, Qiaoyan Guo, Xianping Wei, Yuexin Zhu, Manyu Luo, Ping Luo
Frontiers in Immunology.2024;[Epub] CrossRef - Effects of Qidan Tangshen Granule on diabetic kidney disease in patients with type 2 diabetes
Hua Yang, Shisi Xia, Yilei Cong, Xinyu Yang, Jie Min, Tengfei Wu
Diabetes Research and Clinical Practice.2024; 209: 111128. CrossRef - Comparison of conventional mathematical model and machine learning model based on recent advances in mathematical models for predicting diabetic kidney disease
Yingda Sheng, Caimei Zhang, Jing Huang, Dan Wang, Qian Xiao, Haocheng Zhang, Xiaoqin Ha
DIGITAL HEALTH.2024;[Epub] CrossRef - Network Pharmacology, Molecular Docking, and Experimental Verification to Reveal the Mitophagy-Associated Mechanism of Tangshen Formula in the Treatment of Diabetic Nephropathy
Yinfeng Chen, Xiaying Wang, Jie Min, Jie Zheng, Xuanli Tang, Xiaoling Zhu, Dongrong Yu, De Jin
Diabetes, Metabolic Syndrome and Obesity.2024; Volume 17: 739. CrossRef - Senolytic combination of dasatinib and quercetin protects against diabetic kidney disease by activating autophagy to alleviate podocyte dedifferentiation via the Notch pathway
Xinwang Zhu, Congxiao Zhang, Linlin Liu, Li Xu, Li Yao
International Journal of Molecular Medicine.2024;[Epub] CrossRef - Decreased risk of renal cell carcinoma in patients with type 2 diabetes treated with sodium glucose cotransporter‐2 inhibitors
Chun‐Huei Chiu, Wei‐Yao Wang, Hung‐Yi Chen, Pei‐Lun Liao, Gwo‐Ping Jong, Tsung‐Yuan Yang
Cancer Science.2024;[Epub] CrossRef - System Biology Approaches for Systemic Diseases: Emphasis on Type II Diabetes Mellitus and Allied Metabolism
Mohan Das, Moumita Chakraborty, Promi Das, Sayantan Santra, Abhishek Mukherjee, Sarobi Das, Krisztian Banyai, Souvik Roy, Lopamudra Choudhury, Rudrak Gupta, Tama Dey, Dibya Das, Anirbandeep Bose, Balasubramanian Ganesh, Rintu Banerjee
Biocatalysis and Agricultural Biotechnology.2024; : 103176. CrossRef - Beneficial effects of ginsenosides on diabetic nephropathy: A systematical review and meta-analysis of preclinical evidence
Xiao-Mei Chen, Gui-Xuan Lin, Xue Wang, Hong-Yan Ma, Ru-Shang Wang, Shu-Mei Wang, Dan Tang
Journal of Ethnopharmacology.2023; 302: 115860. CrossRef - Waist circumference and end‐stage renal disease based on glycaemic status: National Health Insurance Service data 2009–2018
Yun Kyung Cho, Ji Hye Huh, Shinje Moon, Yoon Jung Kim, Yang‐Hyun Kim, Kyung‐do Han, Jun Goo Kang, Seong Jin Lee, Sung‐Hee Ihm
Journal of Cachexia, Sarcopenia and Muscle.2023; 14(1): 585. CrossRef - A Narrative Review of New Treatment Options for Diabetic Nephropathy
Aadhira Pillai, Darshna Fulmali
Cureus.2023;[Epub] CrossRef - Shenkang recipe alleviates renal aging in diabetic kidney disease by interfering with the lysine-specific demethylase KDM6B to modulate the PPAR-γ signaling pathway
Anna Zuo, Jiarun Xie, Junqiao Shao, Shuyu Li, Haoyu Lin, Shaoting Wang, Wei Sun, Jinjin Xia, Weiqiang Jiang, Jia Sun, Ming Wang
Pharmacological Research - Modern Chinese Medicine.2023; 6: 100216. CrossRef - miR-223-3p mediates the diabetic kidney disease progression by targeting IL6ST/STAT3 pathway
Ping Tang, Yushan Xu, Jingrong Zhang, Juanli Nan, Ruxian Zhong, Jingmei Luo, Dazhi Xu, Shaoqing Shi, Lihua Zhang
Biochemical and Biophysical Research Communications.2023; 648: 50. CrossRef - miR‐124‐3p improves mitochondrial function of renal tubular epithelial cells in db/db mice
Luqun Liang, Chunxin Wo, Yao Yuan, Hongjuan Cao, Wanlin Tan, Xingcheng Zhou, Dan Wang, Rongyu Chen, Mingjun Shi, Fan Zhang, Ying Xiao, Lingling Liu, Yuxia Zhou, Tian Zhang, Yuanyuan Wang, Bing Guo
The FASEB Journal.2023;[Epub] CrossRef - Hypoxia-Inducible Factor-Prolyl-Hydroxylase and Sodium-Glucose Cotransporter 2 Inhibitors for Low-Risk Myelodysplastic Syndrome-Related Anemia in Patients with Chronic Kidney Disease: A Report of Three Cases
Satoshi Yamasaki, Takahiko Horiuchi
Hematology Reports.2023; 15(1): 180. CrossRef - Diagnostic significance of hsa_circ_0000146 and hsa_circ_0000072 biomarkers for Diabetic Kidney Disease in patients with type 2 diabetes mellitus
Amul Badr, Omayma Elkholy, Mona Said, Sally Fahim, Mohamed El-Khatib, Dina Sabry, Radwa Gaber
Journal of Medical Biochemistry.2023; 42(2): 239. CrossRef - The emerging insight into E3 ligases as the potential therapeutic target for diabetic kidney disease
Vivek Akhouri, Syamantak Majumder, Anil Bhanudas Gaikwad
Life Sciences.2023; 321: 121643. CrossRef - Klotho’s impact on diabetic nephropathy and its emerging connection to diabetic retinopathy
Anqi Tang, Yu Zhang, Ling Wu, Yong Lin, Lizeyu Lv, Liangbin Zhao, Bojun Xu, Youqun Huang, Mingquan Li
Frontiers in Endocrinology.2023;[Epub] CrossRef - Differences and Clinical Significance of Serum 25-Hydroxyvitamin D3 and Vasohibin-1 (VASH-1) Levels in Patients with Diabetic Nephropathy and Different Renal Injuries
Hui Liu, Dongyan Wang, Jingnan Tang, Linlin Yu, Shanshan Su
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 1085. CrossRef - Medial Arterial Calcification and the Risk of Amputation of Diabetic Foot Ulcer in Patients With Diabetic Kidney Disease
Joon Myeong So, Ji Ho Park, Jin Gyeong Kim, Il Rae Park, Eun Yeong Ha, Seung Min Chung, Jun Sung Moon, Chul Hyun Park, Woo-Sung Yun, Tae-Gon Kim, Woong Kim, Ji Sung Yoon, Kyu Chang Won, Hyoung Woo Lee
Journal of Korean Medical Science.2023;[Epub] CrossRef - Heparanase-2 protein and peptides have a protective effect on experimental glomerulonephritis and diabetic nephropathy
Baranca Buijsers, Marjolein Garsen, Mark de Graaf, Marinka Bakker-van Bebber, Chunming Guo, Xue Li, Johan van der Vlag
Frontiers in Pharmacology.2023;[Epub] CrossRef - Influence of non-alcoholic steatohepatitis on the renal functional status in patients with type 2 diabetes and diabetic kidney disease
Z.Ya. Кotsiubiichuk, O.S. Khukhlina, А.А. Аntoniv, O.Ye. Mandryk
INTERNATIONAL JOURNAL OF ENDOCRINOLOGY (Ukraine).2023; 19(2): 100. CrossRef - Roles of extracellular vesicles in ageing-related chronic kidney disease: Demon or angel
Siqi Yin, Zixuan Zhou, Peiwen Fu, Chaoying Jin, Peipei Wu, Cheng Ji, Yunjie Shan, Linru Shi, Min Xu, Hui Qian
Pharmacological Research.2023; 193: 106795. CrossRef - Role of Natural and Synthetic Compounds in Modulating NRF2/KEAP1 Signaling Pathway in Prostate Cancer
Giovanni Tossetta, Sonia Fantone, Daniela Marzioni, Roberta Mazzucchelli
Cancers.2023; 15(11): 3037. CrossRef - Risk factors for heart, cerebrovascular, and kidney diseases: evaluation of potential side effects of medications to control hypertension, hyperglycemia, and hypercholesterolemia
Kazumitsu Nawata
Frontiers in Cardiovascular Medicine.2023;[Epub] CrossRef - Rationale and design of a prospective, clinical study of kidney biopsies in people with type 2 diabetes and severely increased albuminuria (the PRIMETIME 2 study)
Marie Møller, Rikke Borg, Iain Bressendorff, Lisbeth N Fink, Eva Gravesen, Karina Haar Jensen, Torben Hansen, Dorrit Krustrup, Frederik Persson, Peter Rossing, Frederikke E Sembach, Anne C B Thuesen, Ditte Hansen
BMJ Open.2023; 13(6): e072216. CrossRef - Oral Chinese patent medicines for diabetic kidney disease: An overview of systematic reviews
Xue Xue, Ke-ying Li, Shang-zhi Liu, Jia-xuan Li, Xin-yan Jin, Xue-han Liu, La-mei Lin, Xin-rong Zou, Chun-li Lu, Fang-fang Zhao, Jian-ping Liu, Xiao-qin Wang
European Journal of Integrative Medicine.2023; 61: 102269. CrossRef - Recent Advances in Proteinuric Kidney Disease/Nephrotic Syndrome: Lessons from Knockout/Transgenic Mouse Models
Ryosuke Saiki, Kan Katayama, Kaoru Dohi
Biomedicines.2023; 11(7): 1803. CrossRef - Epigenetic regulation of angiogenesis and ischemic response by long noncoding RNA LEENE in diabetes
Imari Mimura, Masaomi Nangaku
Kidney International.2023; 104(6): 1048. CrossRef - Advances in the pharmacological study of Chinese herbal medicine to alleviate diabetic nephropathy by improving mitochondrial oxidative stress
Ming Chen, Yao Chen, Wenhui Zhu, Xiaoming Yan, Jing Xiao, Peiqing Zhang, Peng Liu, Ping Li
Biomedicine & Pharmacotherapy.2023; 165: 115088. CrossRef - A Systematic Review and Meta-Analysis on the Efficacy and Safety of Finerenone Therapy in Patients with Cardiovascular and Chronic Kidney Diseases in Type 2 Diabetes Mellitus
FNU Jyotsna, Kamran Mahfooz, Tirath Patel, FNU Parshant, Fnu Simran, Fnu Harsha, Fnu Neha, Dev Jyotishna, Dipesh Mishra, Sirjana Subedi, Mahima Khatri, Satesh Kumar, Giustino Varrassi
Cureus.2023;[Epub] CrossRef - Molecular implications of glycosaminoglycans in diabetes pharmacotherapy
Tanya Waseem, Madiha Ahmed, Tausif Ahmed Rajput, Mustafeez Mujtaba Babar
International Journal of Biological Macromolecules.2023; 247: 125821. CrossRef - SGLT2 Inhibitors in the Treatment of Diabetic Kidney Disease: More than Just Glucose Regulation
Jasna Klen, Vita Dolžan
Pharmaceutics.2023; 15(7): 1995. CrossRef - CUL3 induces mitochondrial dysfunction via MRPL12 ubiquitination in renal tubular epithelial cells
Xingzhao Ji, Xiaoli Yang, Xia Gu, Lingju Chu, Shengnan Sun, Jian Sun, Peng Song, Qian Mu, Ying Wang, Xiaoming Sun, Dun Su, Tong Su, Shaoshuai Hou, Yao Lu, Chen Ma, Mingqiang Liu, Tianyi Zhang, Weiying Zhang, Yi Liu, Qiang Wan
The FEBS Journal.2023; 290(22): 5340. CrossRef - HP1 induces ferroptosis of renal tubular epithelial cells through NRF2 pathway in diabetic nephropathy
Chuanqiang Zhou, Min Wu, Gaolun Liu, Li Zhou
Open Life Sciences.2023;[Epub] CrossRef - A Review of the Potential of Nuclear Factor [Erythroid-Derived 2]-like 2 Activation in Autoimmune Diseases
Ilker Ates, Ayşe Didem Yılmaz, Brigitta Buttari, Marzia Arese, Luciano Saso, Sibel Suzen
Brain Sciences.2023; 13(11): 1532. CrossRef - Astragalus membranaceus and Salvia miltiorrhiza ameliorate diabetic kidney disease via the “gut-kidney axis”
Zhen Shen, Tao Cui, Yao Liu, Shuai Wu, Cong Han, Jie Li
Phytomedicine.2023; 121: 155129. CrossRef - The relevance of the non-invasive biomarkers lncRNA GAS5/miR-21 ceRNA regulatory network in the early identification of diabetes and diabetic nephropathy
He Sun, Tong Chen, Xin Li, Yonghong Zhu, Shuang Zhang, Ping He, Yali Peng, Qiuling Fan
Diabetology & Metabolic Syndrome.2023;[Epub] CrossRef - Activation of acetyl-CoA synthetase 2 mediates kidney injury in diabetic nephropathy
Jian Lu, Xue Qi Li, Pei Pei Chen, Jia Xiu Zhang, Liang Liu, Gui Hua Wang, Xiao Qi Liu, Ting Ting Jiang, Meng Ying Wang, Wen Tao Liu, Xiong Zhong Ruan, Kun Ling Ma
JCI Insight.2023;[Epub] CrossRef - SET7, a lysine-specific methyl transferase: An intriguing epigenetic target to combat diabetic nephropathy
Samarth Dwivedi, Atharva Chavan, Atish T. Paul
Drug Discovery Today.2023; 28(10): 103754. CrossRef - Dznep, a histone modification inhibitor, inhibits HIF1α binding to TIMP2 gene and suppresses TIMP2 expression under hypoxia
Tomotaka Yamazaki, Imari Mimura, Yu Kurata, Tetsuhiro Tanaka, Masaomi Nangaku
Physiological Reports.2023;[Epub] CrossRef - GLP-1RAs inhibit the activation of the NLRP3 inflammasome signaling pathway to regulate mouse renal podocyte pyroptosis
Xiang Li, Xiao Jiang, Mei Jiang, Zhi-feng Wang, Tao Zhao, Si-ming Cao, Qiu-Mei Li
Acta Diabetologica.2023; 61(2): 225. CrossRef - Highly Sensitive, Portable Detection System for Multiplex Chemiluminescence Analysis
Yannan Yu, Wei Nie, Kaiqin Chu, Xi Wei, Zachary J. Smith
Analytical Chemistry.2023; 95(39): 14762. CrossRef - From normal population to prediabetes and diabetes: study of influencing factors and prediction models
Di Gong, Xiaohong Chen, Lin Yang, Yongjian Zhang, Qianqian Zhong, Jing Liu, Chen Yan, Yongjiang Cai, Weihua Yang, Jiantao Wang
Frontiers in Endocrinology.2023;[Epub] CrossRef - Diabetes Monitoring through Urine Analysis Using ATR-FTIR Spectroscopy and Machine Learning
Sajid Farooq, Denise Maria Zezell
Chemosensors.2023; 11(11): 565. CrossRef - Treatment and practical considerations of diabetic kidney disease
Yara Bilen, Allaa Almoushref, Kenda Alkwatli, Omar Osman, Ali Mehdi, Hanny Sawaf
Frontiers in Medicine.2023;[Epub] CrossRef - Application of Metabolomics and Traditional Chinese Medicine for Type 2 Diabetes Mellitus Treatment
Jing Li, Na Zhu, Yaqiong Wang, Yanlei Bao, Feng Xu, Fengjuan Liu, Xuefeng Zhou
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 4269. CrossRef - Cardiovascular autonomic neuropathy and incident diabetic kidney disease in patients with type 2 diabetes
Ji Eun Jun, Min Sun Choi, Jae Hyeon Kim
Diabetes Research and Clinical Practice.2022; 184: 109181. CrossRef - Lipidomic Analysis Reveals the Protection Mechanism of GLP-1 Analogue Dulaglutide on High-Fat Diet-Induced Chronic Kidney Disease in Mice
Martin Ho Yin Yeung, Ka Long Leung, Lai Yuen Choi, Jung Sun Yoo, Susan Yung, Pui-Kin So, Chi-Ming Wong
Frontiers in Pharmacology.2022;[Epub] CrossRef - GLP-1 receptor agonists in diabetic kidney disease: current evidence and future directions
Ji Hee Yu, So Young Park, Da Young Lee, Nan Hee Kim, Ji A Seo
Kidney Research and Clinical Practice.2022; 41(2): 136. CrossRef - Evolving Type 2 diabetes management focuses on clinical outcomes
Caroline Fenton, Connie Kang
Drugs & Therapy Perspectives.2022; 38(4): 165. CrossRef - Pathophysiologic Mechanisms and Potential Biomarkers in Diabetic Kidney Disease
Chan-Young Jung, Tae-Hyun Yoo
Diabetes & Metabolism Journal.2022; 46(2): 181. CrossRef - Critical shear stress of red blood cells as a novel integrated biomarker for screening chronic kidney diseases in cases of type 2 diabetes
Il Rae Park, Jimi Choi, Eun Young Ha, Seung Min Chung, Jun Sung Moon, Sehyun Shin, Sin Gon Kim, Kyu Chang Won
Clinical Hemorheology and Microcirculation.2022; 81(4): 293. CrossRef - Inhibition of ChREBP ubiquitination via the ROS/Akt-dependent downregulation of Smurf2 contributes to lysophosphatidic acid-induced fibrosis in renal mesangial cells
Donghee Kim, Ga-Young Nam, Eunhui Seo, Hee-Sook Jun
Journal of Biomedical Science.2022;[Epub] CrossRef - The Pathophysiological Basis of Diabetic Kidney Protection by Inhibition of SGLT2 and SGLT1
Yuji Oe, Volker Vallon
Kidney and Dialysis.2022; 2(2): 349. CrossRef - Dapagliflozin for the treatment of chronic kidney disease
Yu Kurata, Masaomi Nangaku
Expert Review of Endocrinology & Metabolism.2022; 17(4): 275. CrossRef - Repurposing drugs for highly prevalent diseases: pentoxifylline, an old drug and a new opportunity for diabetic kidney disease
Javier Donate-Correa, María Dolores Sanchez-Niño, Ainhoa González-Luis, Carla Ferri, Alberto Martín-Olivera, Ernesto Martín-Núñez, Beatriz Fernandez-Fernandez, Víctor G Tagua, Carmen Mora-Fernández, Alberto Ortiz, Juan F Navarro-González
Clinical Kidney Journal.2022; 15(12): 2200. CrossRef - Cyproheptadine, a SET7/9 inhibitor, reduces hyperglycaemia-induced ER stress alleviating inflammation and fibrosis in renal tubular epithelial cells
Himanshu Sankrityayan, Ajinath Kale, Vishwadeep Shelke, Anil Bhanudas Gaikwad
Archives of Physiology and Biochemistry.2022; : 1. CrossRef - Pan-Src kinase inhibitor treatment attenuates diabetic kidney injury via inhibition of Fyn kinase-mediated endoplasmic reticulum stress
Debra Dorotea, Songling Jiang, Eun Seon Pak, Jung Beom Son, Hwan Geun Choi, Sung-Min Ahn, Hunjoo Ha
Experimental & Molecular Medicine.2022; 54(8): 1086. CrossRef - Renoprotective Mechanism of Sodium-Glucose Cotransporter 2 Inhibitors: Focusing on Renal Hemodynamics
Nam Hoon Kim, Nan Hee Kim
Diabetes & Metabolism Journal.2022; 46(4): 543. CrossRef - Effect of once-weekly dulaglutide on renal function in patients with chronic kidney disease
Sungmin Kim, Jung Nam An, Young Rim Song, Sung Gyun Kim, Hyung Seok Lee, AJin Cho, Jwa-Kyung Kim, Tomislav Bulum
PLOS ONE.2022; 17(8): e0273004. CrossRef - Oxidative Stress and NRF2/KEAP1/ARE Pathway in Diabetic Kidney Disease (DKD): New Perspectives
Daniela Maria Tanase, Evelina Maria Gosav, Madalina Ioana Anton, Mariana Floria, Petronela Nicoleta Seritean Isac, Loredana Liliana Hurjui, Claudia Cristina Tarniceriu, Claudia Florida Costea, Manuela Ciocoiu, Ciprian Rezus
Biomolecules.2022; 12(9): 1227. CrossRef - Preventive and healing effect of high dosing grape seed flour on CKD patients of various stages and aetiologies
Wiem Bejaoui, Mohamed Mahmoudi, Kamel Charradi, Monia Abbes-Belhadj, Habib Boukhalfa, Mossadok Ben-Attia, Ferid Limam, Ezzedine Aouani
Biomarkers.2022; 27(8): 795. CrossRef - Heart failure with preserved ejection fraction (HFpEF) in type 2 diabetes mellitus: from pathophysiology to therapeutics
Miyesaier Abudureyimu, Xuanming Luo, Xiang Wang, James R Sowers, Wenshuo Wang, Junbo Ge, Jun Ren, Yingmei Zhang, Wei-Ping Jia
Journal of Molecular Cell Biology.2022;[Epub] CrossRef - Recent Advances in the Emerging Therapeutic Strategies for Diabetic Kidney Diseases
Wei Huang, Yi-Yuan Chen, Zi-Qi Li, Fang-Fang He, Chun Zhang
International Journal of Molecular Sciences.2022; 23(18): 10882. CrossRef - Serum isthmin-1 levels are positively and independently correlated with albuminuria in patients with type 2 diabetes mellitus
Chuan Wang, Mingyue Xu, Ruiying Feng, Lei Zhang, Xiaofei Yin, Ruoqi Feng, Kai Liang, Jinbo Liu
BMJ Open Diabetes Research & Care.2022; 10(5): e002972. CrossRef - hucMSC-sEVs-Derived 14-3-3ζ Serves as a Bridge between YAP and Autophagy in Diabetic Kidney Disease
Siqi Yin, Wanzhu Liu, Cheng Ji, Yuan Zhu, Yunjie Shan, Zixuan Zhou, Wenya Chen, Leilei Zhang, Zixuan Sun, Wenqin Zhou, Hui Qian, Chaoliang Tang
Oxidative Medicine and Cellular Longevity.2022; 2022: 1. CrossRef - Adenosine receptors as emerging therapeutic targets for diabetic kidney disease
Eun Seon Pak, Jin Joo Cha, Dae Ryong Cha, Keizo Kanasaki, Hunjoo Ha
Kidney Research and Clinical Practice.2022; 41(Suppl 2): S74. CrossRef - REDD1 Ablation Attenuates the Development of Renal Complications in Diabetic Mice
Siddharth Sunilkumar, Esma I. Yerlikaya, Allyson L. Toro, William P. Miller, Han Chen, Kebin Hu, Scot R. Kimball, Michael D. Dennis
Diabetes.2022; 71(11): 2412. CrossRef - The Role of Hypoxia-Inducible Factor-1 Alpha in Renal Disease
Huixia Liu, Yujuan Li, Jing Xiong
Molecules.2022; 27(21): 7318. CrossRef - Resistant Starch as a Dietary Intervention to Limit the Progression of Diabetic Kidney Disease
Anna M. Drake, Melinda T. Coughlan, Claus T. Christophersen, Matthew Snelson
Nutrients.2022; 14(21): 4547. CrossRef - Aggravated renal fibrosis is positively associated with the activation of HMGB1-TLR2/4 signaling in STZ-induced diabetic mice
Yan Yuan, Yuanxia Liu, Mengyao Sun, Huijing Ye, Yuchen Feng, Zhenzhen Liu, Lingyu Pan, Hongbo Weng
Open Life Sciences.2022; 17(1): 1451. CrossRef - Single-cell multiomics reveals the complexity of TGFβ signalling to chromatin in iPSC-derived kidney organoids
Jessica L. Davis, Ciaran Kennedy, Shane Clerkin, Niall J. Treacy, Thomas Dodd, Catherine Moss, Alison Murphy, Derek P. Brazil, Gerard Cagney, Dermot F. Brougham, Rabi Murad, Darren Finlay, Kristiina Vuori, John Crean
Communications Biology.2022;[Epub] CrossRef - Oxidized Albumin: Evaluation of Oxidative Stress as a Marker for the Progression of Kidney Disease
Hiroshi Watanabe
Biological and Pharmaceutical Bulletin.2022; 45(12): 1728. CrossRef - Whether Renal Pathology Is an Independent Predictor for End-Stage Renal Disease in Diabetic Kidney Disease Patients with Nephrotic Range Proteinuria: A Biopsy-Based Study
Tingli Wang, Junlin Zhang, Yiting Wang, Lijun Zhao, Yucheng Wu, Honghong Ren, Yutong Zou, Rui Zhang, Huan Xu, Zhonglin Chai, Mark Cooper, Jie Zhang, Fang Liu
Journal of Clinical Medicine.2022; 12(1): 88. CrossRef - What’s New in the Molecular Mechanisms of Diabetic Kidney Disease: Recent Advances
Kimio Watanabe, Emiko Sato, Eikan Mishima, Mariko Miyazaki, Tetsuhiro Tanaka
International Journal of Molecular Sciences.2022; 24(1): 570. CrossRef - Clinical efficacy and safety of astragalus injection combined with ACEI/ARB in the treatment of diabetic kidney disease: Protocol for a systematic review and meta-analysis
Zhiyue Zhu, Qi Zhang, Le Liu, Pengjie Bao, Shilin Liu, Chaoqun Song, Wenbo Yang, Zheng Nan
Medicine.2022; 101(49): e31490. CrossRef - Cudrania tricuspidata Root Extract Prevents Methylglyoxal-Induced Inflammation and Oxidative Stress via Regulation of the PKC-NOX4 Pathway in Human Kidney Cells
Donghee Kim, Jayeon Cheon, Haelim Yoon, Hee-Sook Jun, Evangelia Dounousi
Oxidative Medicine and Cellular Longevity.2021; 2021: 1. CrossRef - Pleiotropic Effects of Sodium-Glucose Cotransporter-2 Inhibitors: Renoprotective Mechanisms beyond Glycemic Control
Tomoaki Takata, Hajime Isomoto
International Journal of Molecular Sciences.2021; 22(9): 4374. CrossRef - HIF-α Prolyl Hydroxylase Inhibitors and Their Implications for Biomedicine: A Comprehensive Review
Kiichi Hirota
Biomedicines.2021; 9(5): 468. CrossRef - Nephropathie bei Diabetes
Roland E. Schmieder
CardioVasc.2021; 21(3): 31. CrossRef - Clinical Predictors of Nondiabetic Kidney Disease in Patients with Diabetes: A Single-Center Study
Francesco Fontana, Rossella Perrone, Francesco Giaroni, Gaetano Alfano, Silvia Giovanella, Giulia Ligabue, Riccardo Magistroni, Gianni Cappelli, Udeme Ekrikpo
International Journal of Nephrology.2021; 2021: 1. CrossRef - Activated Histone Acetyltransferase p300/CBP-Related Signalling Pathways Mediate Up-Regulation of NADPH Oxidase, Inflammation, and Fibrosis in Diabetic Kidney
Alexandra-Gela Lazar, Mihaela-Loredana Vlad, Adrian Manea, Maya Simionescu, Simona-Adriana Manea
Antioxidants.2021; 10(9): 1356. CrossRef - Plasma and urine biomarkers in chronic kidney disease: closer to clinical application
Azadeh Zabetian, Steven G. Coca
Current Opinion in Nephrology & Hypertension.2021; 30(6): 531. CrossRef - Therapeutic effect and mechanism of combined use of FGF21 and insulin on diabetic nephropathy
Fanrui Meng, Yukai Cao, Mir Hassan Khoso, Kai Kang, Guiping Ren, Wei Xiao, Deshan Li
Archives of Biochemistry and Biophysics.2021; 713: 109063. CrossRef - Mineralocorticoid Receptor Antagonists in Diabetic Kidney Disease
Daiji Kawanami, Yuichi Takashi, Yoshimi Muta, Naoki Oda, Dai Nagata, Hiroyuki Takahashi, Makito Tanabe
Frontiers in Pharmacology.2021;[Epub] CrossRef - Transcription Factor ChREBP Mediates High Glucose-Evoked Increase in HIF-1α Content in Epithelial Cells of Renal Proximal Tubules
Aleksandra Owczarek, Katarzyna B. Gieczewska, Robert Jarzyna, Zuzanna Frydzinska, Katarzyna Winiarska
International Journal of Molecular Sciences.2021; 22(24): 13299. CrossRef - The effect of modern hypoglycemic therapy on the course of chronic kidney disease in patients with type 2 diabetes mellitus
V.I. Katerenchuk
INTERNATIONAL JOURNAL OF ENDOCRINOLOGY (Ukraine).2021; 17(8): 624. CrossRef
- Clinical value of serum MMP-3 in chronic kidney disease
- Basic Research
- Hypoxia Increases β-Cell Death by Activating Pancreatic Stellate Cells within the Islet
- Jong Jin Kim, Esder Lee, Gyeong Ryul Ryu, Seung-Hyun Ko, Yu-Bae Ahn, Ki-Ho Song
- Diabetes Metab J. 2020;44(6):919-927. Published online May 11, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0181
- 5,975 View
- 146 Download
- 15 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
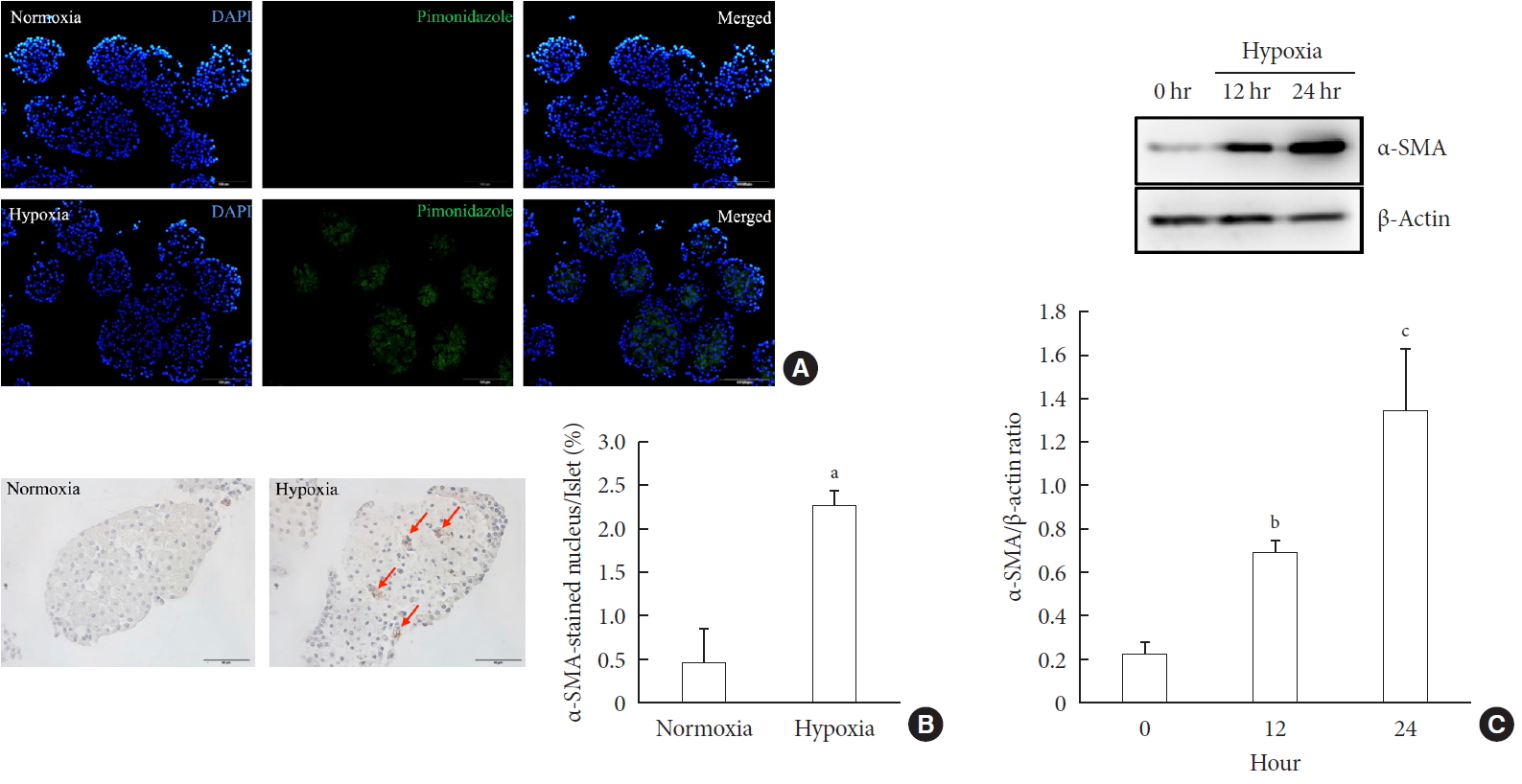
ePub Background Hypoxia can occur in pancreatic islets in type 2 diabetes mellitus. Pancreatic stellate cells (PSCs) are activated during hypoxia. Here we aimed to investigate whether PSCs within the islet are also activated in hypoxia, causing β-cell injury.
Methods Islet and primary PSCs were isolated from Sprague Dawley rats, and cultured in normoxia (21% O2) or hypoxia (1% O2). The expression of α-smooth muscle actin (α-SMA), as measured by immunostaining and Western blotting, was used as a marker of PSC activation. Conditioned media (hypoxia-CM) were obtained from PSCs cultured in hypoxia.
Results Islets and PSCs cultured in hypoxia exhibited higher expressions of α-SMA than did those cultured in normoxia. Hypoxia increased the production of reactive oxygen species. The addition of N-acetyl-L-cysteine, an antioxidant, attenuated the hypoxia-induced PSC activation in islets and PSCs. Islets cultured in hypoxia-CM showed a decrease in cell viability and an increase in apoptosis.
Conclusion PSCs within the islet are activated in hypoxia through oxidative stress and promote islet cell death, suggesting that hypoxia-induced PSC activation may contribute to β-cell loss in type 2 diabetes mellitus.
-
Citations
Citations to this article as recorded by- Effects of hypoxia in the diabetic corneal stroma microenvironment
Purnima Sharma, Jian-Xing Ma, Dimitrios Karamichos
Experimental Eye Research.2024; 240: 109790. CrossRef - Visualizing hypoxic modulation of beta cell secretions via a sensor augmented oxygen gradient
Kai Duan, Mengyang Zhou, Yong Wang, Jose Oberholzer, Joe F. Lo
Microsystems & Nanoengineering.2023;[Epub] CrossRef - Pancreatic stellate cells promote pancreatic β-cell death through exosomal microRNA transfer in hypoxia
Esder Lee, Gyeong Ryul Ryu, Seung-Hyun Ko, Yu-Bae Ahn, Ki-Ho Song
Molecular and Cellular Endocrinology.2023; 572: 111947. CrossRef - Pancreatic stellate cells in pancreatic cancer: as potential targets for future therapy
Zhengfeng Wang, Ru He, Shi Dong, Wence Zhou
Frontiers in Oncology.2023;[Epub] CrossRef - Recent advances in the development of bioartificial pancreas using 3D bioprinting for the treatment of type 1 diabetes: a review
Anushikha Ghosh, Arka Sanyal, Abhik Mallick
Exploration of Medicine.2023; : 886. CrossRef - Pancreas and islet morphology in cystic fibrosis: clues to the etiology of cystic fibrosis-related diabetes
Sarah S. Malik, Diksha Padmanabhan, Rebecca L. Hull-Meichle
Frontiers in Endocrinology.2023;[Epub] CrossRef - Diabetic mellitus, vascular calcification and hypoxia: A complex and neglected tripartite relationship
Xue-Jiao Sun, Nai-Feng Liu
Cellular Signalling.2022; 91: 110219. CrossRef - HIF-1 and NRF2; Key Molecules for Malignant Phenotypes of Pancreatic Cancer
Shin Hamada, Ryotaro Matsumoto, Atsushi Masamune
Cancers.2022; 14(2): 411. CrossRef - Pancreatic Stellate Cells and Metabolic Alteration: Physiology and Pathophysiology
Shin Hamada, Ryotaro Matsumoto, Atsushi Masamune
Frontiers in Physiology.2022;[Epub] CrossRef - Exosomal miR-140–3p and miR-143–3p from TGF-β1-treated pancreatic stellate cells target BCL2 mRNA to increase β-cell apoptosis
Xiangyun Zhu, Dechen Liu, Guoqing Li, Mengmeng Zhi, Ji Sun, Liang Qi, Jingbo Li, Stephen J. Pandol, Ling Li
Molecular and Cellular Endocrinology.2022; 551: 111653. CrossRef - Mitochondria oxidative stress mediated nicotine-promoted activation of pancreatic stellate cells by regulating mitochondrial dynamics
Yue Yuan, Zhiren Li, Miaomiao Li, Tong Jin, Xiaoyun Zhang, Xinjuan Liu, Jianyu Hao
Toxicology in Vitro.2022; 84: 105436. CrossRef - Antioxidant Mitoquinone Alleviates Chronic Pancreatitis via Anti-Fibrotic and Antioxidant Effects
Miaomiao Li, Yue Yuan, Xue Han, Xinjuan Liu, Weizhen Zhang, Jianyu Hao
Journal of Inflammation Research.2022; Volume 15: 4409. CrossRef - Diabetic Ferroptosis and Pancreatic Cancer: Foe or Friend?
Le Li, Xing-jia Yu, Lei Gao, Long Cheng, Bei Sun, Gang Wang
Antioxidants & Redox Signaling.2022; 37(16-18): 1206. CrossRef - Melatonin Induces Apoptosis and Modulates Cyclin Expression and MAPK Phosphorylation in Pancreatic Stellate Cells Subjected to Hypoxia
Matias Estaras, Manuel R. Gonzalez-Portillo, Miguel Fernandez-Bermejo, Jose M. Mateos, Daniel Vara, Gerardo Blanco-Fernandez, Diego Lopez-Guerra, Vicente Roncero, Gines M. Salido, Antonio González
International Journal of Molecular Sciences.2021; 22(11): 5555. CrossRef - Integrated pancreatic microcirculatory profiles of streptozotocin‐induced and insulin‐administrated type 1 diabetes mellitus
Yuan Li, Bingwei Li, Bing Wang, Mingming Liu, Xiaoyan Zhang, Ailing Li, Jian Zhang, Honggang Zhang, Ruijuan Xiu
Microcirculation.2021;[Epub] CrossRef - Pancreatic stellate cells - rising stars in pancreatic pathologies
P Hrabák, M Kalousová, T Krechler, T Zima
Physiological Research.2021; (S4): S597. CrossRef
- Effects of hypoxia in the diabetic corneal stroma microenvironment
- Pathophysiology
- Low-Frequency Intermittent Hypoxia Suppresses Subcutaneous Adipogenesis and Induces Macrophage Polarization in Lean Mice
- Yan Wang, Mary Yuk Kwan Lee, Judith Choi Wo Mak, Mary Sau Man Ip
- Diabetes Metab J. 2019;43(5):659-674. Published online April 23, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0196
- 4,292 View
- 47 Download
- 4 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background The relationship between obstructive sleep apnoea (OSA) and metabolic disorders is complex and highly associated. The impairment of adipogenic capacity in pre-adipocytes may promote adipocyte hypertrophy and increase the risk of further metabolic dysfunction. We hypothesize that intermittent hypoxia (IH), as a pathophysiologic feature of OSA, may regulate adipogenesis by promoting macrophage polarization.
Methods Male C57BL/6N mice were exposed to either IH (240 seconds of 10% O2 followed by 120 seconds of 21% O2, i.e., 10 cycles/hour) or intermittent normoxia (IN) for 6 weeks. Stromal-vascular fractions derived from subcutaneous (SUB-SVF) and visceral (VIS-SVF) adipose tissues were cultured and differentiated. Conditioned media from cultured RAW 264.7 macrophages after air (Raw) or IH exposure (Raw-IH) were incubated with SUB-SVF during adipogenic differentiation.
Results Adipogenic differentiation of SUB-SVF but not VIS-SVF from IH-exposed mice was significantly downregulated in comparison with that derived from IN-exposed mice. IH-exposed mice compared to IN-exposed mice showed induction of hypertrophic adipocytes and increased preferential infiltration of M1 macrophages in subcutaneous adipose tissue (SAT) compared to visceral adipose tissue. Complementary
in vitro analysis demonstrated that Raw-IH media significantly enhanced inhibition of adipogenesis of SUB-SVF compared to Raw media, in agreement with corresponding gene expression levels of differentiation-associated markers and adipogenic transcription factors.Conclusion Low frequency IH exposure impaired adipogenesis of SAT in lean mice, and macrophage polarization may be a potential mechanism for the impaired adipogenesis.
-
Citations
Citations to this article as recorded by- Melatonin attenuates chronic intermittent hypoxia-induced intestinal barrier dysfunction in mice
Xinyi Li, Fan Wang, Zhenfei Gao, Weijun Huang, Xiaoman Zhang, Feng Liu, Hongliang Yi, Jian Guan, Xiaolin Wu, Huajun Xu, Shankai Yin
Microbiological Research.2023; 276: 127480. CrossRef - Clinical outcomes and plaque characteristics in patients with coronary artery disease and concomitant sleep-disordered breathing treated by continuous positive airway pressure
Kazuhiro Fujiyoshi, Taiki Tojo, Yoshiyasu Minami, Kohki Ishida, Miwa Ishida, Ken-ichiro Wakabayashi, Takayuki Inomata, Junya Ako
Sleep Medicine.2023; 101: 543. CrossRef - Potential Pathophysiological Pathways in the Complex Relationships between OSA and Cancer
Manuel Sánchez-de-la-Torre, Carolina Cubillos, Olivia J. Veatch, Francisco Garcia-Rio, David Gozal, Miguel Angel Martinez-Garcia
Cancers.2023; 15(4): 1061. CrossRef - Effects of Chronic Intermittent Hypoxia and Chronic Sleep Fragmentation on Gut Microbiome, Serum Metabolome, Liver and Adipose Tissue Morphology
Fan Wang, Juanjuan Zou, Huajun Xu, Weijun Huang, Xiaoman Zhang, Zhicheng Wei, Xinyi Li, Yupu Liu, Jianyin Zou, Feng Liu, Huaming Zhu, Hongliang Yi, Jian Guan, Shankai Yin
Frontiers in Endocrinology.2022;[Epub] CrossRef - C‐X3‐C motif chemokine ligand 1/receptor 1 regulates the M1 polarization and chemotaxis of macrophages after hypoxia/reoxygenation injury
Shuiming Guo, Lei Dong, Junhua Li, Yuetao Chen, Ying Yao, Rui Zeng, Nelli Shushakova, Hermann Haller, Gang Xu, Song Rong
Chronic Diseases and Translational Medicine.2021; 7(4): 254. CrossRef
- Melatonin attenuates chronic intermittent hypoxia-induced intestinal barrier dysfunction in mice
- Pathophysiology
- Mitochondrial Dysfunction in Adipocytes as a Primary Cause of Adipose Tissue Inflammation
- Chang-Yun Woo, Jung Eun Jang, Seung Eun Lee, Eun Hee Koh, Ki-Up Lee
- Diabetes Metab J. 2019;43(3):247-256. Published online March 27, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0221
- 8,594 View
- 257 Download
- 70 Web of Science
- 71 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Adipose tissue inflammation is considered a major contributing factor in the development of obesity-associated insulin resistance and cardiovascular diseases. However, the cause of adipose tissue inflammation is presently unclear. The role of mitochondria in white adipocytes has long been neglected because of their low abundance. However, recent evidence suggests that mitochondria are essential for maintaining metabolic homeostasis in white adipocytes. In a series of recent studies, we found that mitochondrial function in white adipocytes is essential to the synthesis of adiponectin, which is the most abundant adipokine synthesized from adipocytes, with many favorable effects on metabolism, including improvement of insulin sensitivity and reduction of atherosclerotic processes and systemic inflammation. From these results, we propose a new hypothesis that mitochondrial dysfunction in adipocytes is a primary cause of adipose tissue inflammation and compared this hypothesis with a prevailing concept that “adipose tissue hypoxia” may underlie adipose tissue dysfunction in obesity. Recent studies have emphasized the role of the mitochondrial quality control mechanism in maintaining mitochondrial function. Future studies are warranted to test whether an inadequate mitochondrial quality control mechanism is responsible for mitochondrial dysfunction in adipocytes and adipose tissue inflammation.
-
Citations
Citations to this article as recorded by- Prolonged Endurance Exercise Increases Macrophage Content and Mitochondrial Respiration in Adipose Tissue in Trained Men
Ronni Eg Sahl, Ioanna Patsi, Mikkel Thunestvedt Hansen, Tue Rømer, Jacob Frandsen, Hanne Kruuse Rasmusen, Arthur Ingersen, Steen Seier Poulsen, Flemming Dela, Steen Larsen, Jørn Wulff Helge
The Journal of Clinical Endocrinology & Metabolism.2024; 109(2): e799. CrossRef - Diabetes Mellitus, Energy Metabolism, and COVID-19
Caterina Conte, Elisa Cipponeri, Michael Roden
Endocrine Reviews.2024; 45(2): 281. CrossRef - The Role of Ion-Transporting Proteins in Human Disease
Yoshinori Marunaka
International Journal of Molecular Sciences.2024; 25(3): 1726. CrossRef - The Role of Obesity in Type 2 Diabetes Mellitus—An Overview
Preethi Chandrasekaran, Ralf Weiskirchen
International Journal of Molecular Sciences.2024; 25(3): 1882. CrossRef - The Metabolic Syndrome, a Human Disease
Marià Alemany
International Journal of Molecular Sciences.2024; 25(4): 2251. CrossRef - Inflammation‐mediated metabolic regulation in adipose tissue
Shujie Xu, Feng Lu, Jianhua Gao, Yi Yuan
Obesity Reviews.2024;[Epub] CrossRef - Sleeve Gastrectomy Reduces Oxidative Stress and Reverses Mitochondrial Dysfunction Associated with Metabolic Syndrome
Micaela M. Rossi, Franco J. Signorini, Tomas A. Castillo, María P. Scribano Parada, Federico Moser, Maria dC Baez
Obesity Surgery.2024;[Epub] CrossRef - The relationships between high-fat diet and metabolic syndrome: Potential mechanisms
Chao Tang, Yuxin Wang, Zeyu Xu, Dan Chen, Jingguo Xu, Duo Yang, Li Zhang, Jun Liu, Juan Kan
Food Bioscience.2024; 59: 104261. CrossRef - Could very low-calorie ketogenic diets turn off low grade inflammation in obesity? Emerging evidence
Luigi Barrea, Massimiliano Caprio, Mikiko Watanabe, Giuseppe Cammarata, Alessandra Feraco, Giovanna Muscogiuri, Ludovica Verde, Annamaria Colao, Silvia Savastano
Critical Reviews in Food Science and Nutrition.2023; 63(26): 8320. CrossRef - The emergent role of mitochondrial RNA modifications in metabolic alterations
Hatim Boughanem, Yvonne Böttcher, João Tomé‐Carneiro, María‐Carmen López de las Hazas, Alberto Dávalos, Akin Cayir, Manuel Macias‐González
WIREs RNA.2023;[Epub] CrossRef - Age‐associated adipose tissue inflammation promotes monocyte chemotaxis and enhances atherosclerosis
Jianrui Song, Diana Farris, Paola Ariza, Smriti Moorjani, Mita Varghese, Muriel Blin, Judy Chen, Daniel Tyrrell, Min Zhang, Kanakadurga Singer, Morgan Salmon, Daniel R. Goldstein
Aging Cell.2023;[Epub] CrossRef - Obesity, diabetes mellitus, and cardiometabolic risk: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2023
Harold Edward Bays, Shagun Bindlish, Tiffany Lowe Clayton
Obesity Pillars.2023; 5: 100056. CrossRef - A role of STING signaling in obesity-induced lung inflammation
Yong Qi, Zhuhua Wu, Dan Chen, Li Zhu, Yunlei Yang
International Journal of Obesity.2023; 47(4): 325. CrossRef - Estrogens in Adipose Tissue Physiology and Obesity-Related Dysfunction
Alina Kuryłowicz
Biomedicines.2023; 11(3): 690. CrossRef - White Adipose Tissue Dysfunction: Pathophysiology and Emergent Measurements
Natalia Santillana, Camila Astudillo-Guerrero, Amanda D’Espessailles, Gonzalo Cruz
Nutrients.2023; 15(7): 1722. CrossRef - Pleiotropic and multi-systemic actions of physical exercise on PGC-1α signaling during the aging process
Ivo Vieira de Sousa Neto, Ana Paula Pinto, Vitor Rosetto Muñoz, Rita de Cássia Marqueti, José Rodrigo Pauli, Eduardo Rochete Ropelle, Adelino Sanchez Ramos da Silva
Ageing Research Reviews.2023; 87: 101935. CrossRef - The impact of metabolic endotoxaemia on the browning process in human adipocytes
Farah Omran, Alice M. Murphy, Awais Z. Younis, Ioannis Kyrou, Jana Vrbikova, Vojtech Hainer, Petra Sramkova, Martin Fried, Graham Ball, Gyanendra Tripathi, Sudhesh Kumar, Philip G. McTernan, Mark Christian
BMC Medicine.2023;[Epub] CrossRef - Molecular Mechanisms of Obesity-Induced Development of Insulin Resistance and Promotion of Amyloid-β Accumulation: Dietary Therapy Using Weak Organic Acids via Improvement of Lowered Interstitial Fluid pH
Yoshinori Marunaka
Biomolecules.2023; 13(5): 779. CrossRef - From Obesity-Induced Low-Grade Inflammation to Lipotoxicity and Mitochondrial Dysfunction: Altered Multi-Crosstalk between Adipose Tissue and Metabolically Active Organs
Gina Cavaliere, Fabiano Cimmino, Giovanna Trinchese, Angela Catapano, Lidia Petrella, Margherita D’Angelo, Lucio Lucchin, Maria Pina Mollica
Antioxidants.2023; 12(6): 1172. CrossRef - Receptor for the Advanced Glycation End Products (RAGE) Pathway in Adipose Tissue Metabolism
Klaudia Gutowska, Krzysztof Czajkowski, Alina Kuryłowicz
International Journal of Molecular Sciences.2023; 24(13): 10982. CrossRef - Aerobic and Resistance Training Attenuate Differently Knee Joint Damage Caused by a High-Fat–High-Sucrose Diet in a Rat Model
Nada Abughazaleh, Kevin Boldt, Jaqueline Lourdes Rios, Stela Marcia Mattiello, Kelsey H. Collins, Ruth-Anne Seerattan, Walter Herzog
CARTILAGE.2023;[Epub] CrossRef - Exercise mitigates age-related metabolic diseases by improving mitochondrial dysfunction
Dandan Jia, Zhenjun Tian, Ru Wang
Ageing Research Reviews.2023; 91: 102087. CrossRef - Mitochondrial dynamics and metabolism across skin cells: implications for skin homeostasis and aging
Ines Martic, Federica Papaccio, Barbara Bellei, Maria Cavinato
Frontiers in Physiology.2023;[Epub] CrossRef - Influence of Breastfeeding on the State of Meta-Inflammation in Obesity—A Narrative Review
Dominika Mazur, Małgorzata Satora, Anna K. Rekowska, Zuzanna Kabała, Aleksandra Łomża, Żaneta Kimber-Trojnar, Bożena Leszczyńska-Gorzelak
Current Issues in Molecular Biology.2023; 45(11): 9003. CrossRef - AGER-1 Long Non-Coding RNA Levels Correlate with the Expression of the Advanced Glycosylation End-Product Receptor, a Regulator of the Inflammatory Response in Visceral Adipose Tissue of Women with Obesity and Type 2 Diabetes Mellitus
Klaudia Gutowska, Krzysztof Koźniewski, Michał Wąsowski, Marta Izabela Jonas, Zbigniew Bartoszewicz, Wojciech Lisik, Maurycy Jonas, Artur Binda, Paweł Jaworski, Wiesław Tarnowski, Bartłomiej Noszczyk, Monika Puzianowska-Kuźnicka, Krzysztof Czajkowski, Ali
International Journal of Molecular Sciences.2023; 24(24): 17447. CrossRef - Pharmacological treatment with FGF21 strongly improves plasma cholesterol metabolism to reduce atherosclerosis
Cong Liu, Milena Schönke, Enchen Zhou, Zhuang Li, Sander Kooijman, Mariëtte R Boon, Mikael Larsson, Kristina Wallenius, Niek Dekker, Louise Barlind, Xiao-Rong Peng, Yanan Wang, Patrick C N Rensen
Cardiovascular Research.2022; 118(2): 489. CrossRef - Obesity-Related Adipose Tissue Remodeling in the Light of Extracellular Mitochondria Transfer
Simon Lecoutre, Karine Clément, Isabelle Dugail
International Journal of Molecular Sciences.2022; 23(2): 632. CrossRef - IL-4 polarized human macrophage exosomes control cardiometabolic inflammation and diabetes in obesity
Tuan Anh Phu, Martin Ng, Ngan K. Vu, Laura Bouchareychas, Robert L. Raffai
Molecular Therapy.2022; 30(6): 2274. CrossRef - Insulin-inducible THRSP maintains mitochondrial function and regulates sphingolipid metabolism in human adipocytes
Maria A. Ahonen, Marcus Höring, Van Dien Nguyen, Sami Qadri, Juuso H. Taskinen, Meghana Nagaraj, Martin Wabitsch, Pamela Fischer-Posovszky, You Zhou, Gerhard Liebisch, P. A. Nidhina Haridas, Hannele Yki-Järvinen, Vesa M. Olkkonen
Molecular Medicine.2022;[Epub] CrossRef - Modulation of adipose inflammation by cellular retinoic acid-binding protein 1
Chin-Wen Wei, Jennifer Nhieu, Yu-Lung Lin, Li-Na Wei
International Journal of Obesity.2022; 46(10): 1759. CrossRef - The Role of Adipokines in Pancreatic Cancer
Qi Wang, Huizhi Wang, Yuntao Ding, Mengtian Wan, Min Xu
Frontiers in Oncology.2022;[Epub] CrossRef - Epigenetic Reprogramming of the Inflammatory Response in Obesity and Type 2 Diabetes
Federica Zatterale, Gregory Alexander Raciti, Immacolata Prevenzano, Alessia Leone, Michele Campitelli, Veronica De Rosa, Francesco Beguinot, Luca Parrillo
Biomolecules.2022; 12(7): 982. CrossRef - Cellular Metabolism and Bioenergetic Function in Human Fibroblasts and Preadipocytes of Type 2 Familial Partial Lipodystrophy
Cristina Algieri, Chiara Bernardini, Fabiana Trombetti, Elisa Schena, Augusta Zannoni, Monica Forni, Salvatore Nesci
International Journal of Molecular Sciences.2022; 23(15): 8659. CrossRef - Shared pathobiology identifies AMPK as a therapeutic target for obesity and autosomal dominant polycystic kidney disease
Ioan-Andrei Iliuta, Xuewen Song, Lauren Pickel, Amirreza Haghighi, Ravi Retnakaran, James Scholey, Hoon-Ki Sung, Gregory R. Steinberg, York Pei
Frontiers in Molecular Biosciences.2022;[Epub] CrossRef - Hypoxia as a Double-Edged Sword to Combat Obesity and Comorbidities
Ruwen Wang, Qin Sun, Xianmin Wu, Yiyin Zhang, Xiaorui Xing, Kaiqing Lin, Yue Feng, Mingqi Wang, Yibing Wang, Ru Wang
Cells.2022; 11(23): 3735. CrossRef - Macrophage and Adipocyte Mitochondrial Dysfunction in Obesity-Induced Metabolic Diseases
Liwen Wang, Jie Hu, Haiyan Zhou
The World Journal of Men's Health.2021; 39(4): 606. CrossRef - ESRRA (estrogen related receptor alpha) is a critical regulator of intestinal homeostasis through activation of autophagic flux via gut microbiota
Sup Kim, June-Young Lee, Seul Gi Shin, Jin Kyung Kim, Prashanta Silwal, Young Jae Kim, Na-Ri Shin, Pil Soo Kim, Minho Won, Sang-Hee Lee, Soo Yeon Kim, Miwa Sasai, Masahiro Yamamoto, Jin-Man Kim, Jin-Woo Bae, Eun-Kyeong Jo
Autophagy.2021; 17(10): 2856. CrossRef - GDF15 as a central mediator for integrated stress response and a promising therapeutic molecule for metabolic disorders and NASH
Kook Hwan Kim, Myung-Shik Lee
Biochimica et Biophysica Acta (BBA) - General Subjects.2021; 1865(3): 129834. CrossRef - The Influence of Obesity and Associated Fatty Acids on Placental Inflammation
Alison J. Eastman, Rebecca E. Moore, Steven D. Townsend, Jennifer A. Gaddy, David M. Aronoff
Clinical Therapeutics.2021; 43(2): 265. CrossRef - Targeting the G protein-coupled estrogen receptor (GPER) in obesity and diabetes
Geetanjali Sharma, Eric R. Prossnitz
Endocrine and Metabolic Science.2021; 2: 100080. CrossRef - Changes in Body Composition Are Associated with Metabolic Changes and the Risk of Metabolic Syndrome
Yun Hwan Oh, Seulggie Choi, Gyeongsil Lee, Joung Sik Son, Kyae Hyung Kim, Sang Min Park
Journal of Clinical Medicine.2021; 10(4): 745. CrossRef - N6-Adenosine Methylation (m6A) RNA Modification: an Emerging Role in Cardiovascular Diseases
Ye-shi Chen, Xin-ping Ouyang, Xiao-hua Yu, Petr Novák, Le Zhou, Ping-ping He, Kai Yin
Journal of Cardiovascular Translational Research.2021; 14(5): 857. CrossRef - From Metabolic Syndrome to Neurological Diseases: Role of Autophagy
Jessica Maiuolo, Micaela Gliozzi, Vincenzo Musolino, Cristina Carresi, Federica Scarano, Saverio Nucera, Miriam Scicchitano, Francesca Bosco, Stefano Ruga, Maria Caterina Zito, Roberta Macri, Rosamaria Bulotta, Carolina Muscoli, Vincenzo Mollace
Frontiers in Cell and Developmental Biology.2021;[Epub] CrossRef - Absent Exercise-Induced Improvements in Fat Oxidation in Women With Polycystic Ovary Syndrome After High-Intensity Interval Training
Sofie Lionett, Ida Almenning Kiel, Ragnhild Røsbjørgen, Stian Lydersen, Steen Larsen, Trine Moholdt
Frontiers in Physiology.2021;[Epub] CrossRef - Roles of interstitial fluid pH and weak organic acids in development and amelioration of insulin resistance
Yoshinori Marunaka
Biochemical Society Transactions.2021; 49(2): 715. CrossRef - The Role of Mitochondrial Adaptation and Metabolic Flexibility in the Pathophysiology of Obesity and Insulin Resistance: an Updated Overview
Dimitrios Tsilingiris, Evangelia Tzeravini, Chrysi Koliaki, Maria Dalamaga, Alexander Kokkinos
Current Obesity Reports.2021; 10(3): 191. CrossRef - Obesity-Related Inflammation and Endothelial Dysfunction in COVID-19: Impact on Disease Severity
Andrea De Lorenzo, Vanessa Estato, Hugo C Castro-Faria-Neto, Eduardo Tibirica
Journal of Inflammation Research.2021; Volume 14: 2267. CrossRef - Thermogenic Fat: Development, Physiological Function, and Therapeutic Potential
Bruna B. Brandão, Ankita Poojari, Atefeh Rabiee
International Journal of Molecular Sciences.2021; 22(11): 5906. CrossRef - Metabolic Syndrome in an Aging Society – Role of Oxidant-Antioxidant Imbalance and Inflammation Markers in Disentangling Atherosclerosis
Sylwia Dziegielewska-Gesiak
Clinical Interventions in Aging.2021; Volume 16: 1057. CrossRef - Recruitment and remodeling of peridroplet mitochondria in human adipose tissue
Rebeca Acín-Perez, Anton Petcherski, Michaela Veliova, Ilan Y. Benador, Essam A. Assali, Georgia Colleluori, Saverio Cinti, Alexandra J. Brownstein, Siyouneh Baghdasarian, Masha J. Livhits, Michael W. Yeh, Karthickeyan Chella Krishnan, Laurent Vergnes, Na
Redox Biology.2021; 46: 102087. CrossRef - New Insights Into Mitochondrial Dysfunction at Disease Susceptibility Loci in the Development of Type 2 Diabetes
Hannah Maude, Winston Lau, Nikolas Maniatis, Toby Andrew
Frontiers in Endocrinology.2021;[Epub] CrossRef - Effects of sleeve gastrectomy on bone mass, microstructure of femurs and bone metabolism associated serum factors in obese rats
Ying Xue, Ran Li, Yong Zhao, Ling Li, Yun Zhou
BMC Endocrine Disorders.2021;[Epub] CrossRef - The cyclin dependent kinase inhibitor Roscovitine prevents diet-induced metabolic disruption in obese mice
Nabil Rabhi, Kathleen Desevin, Briana Noel Cortez, Ryan Hekman, Jean Z. Lin, Andrew Emili, Stephen R. Farmer
Scientific Reports.2021;[Epub] CrossRef - Reliability and variation in mitochondrial respiration in human adipose tissue
Ronni Eg Sahl, Eva Frederikke Høy Helms, Malte Schmücker, Mathias Flensted-Jensen, Arthur Ingersen, Thomas Morville, Flemming Dela, Jørn Wulff Helge, Steen Larsen
Adipocyte.2021; 10(1): 605. CrossRef - Inhibition of protein tyrosine phosphatase improves mitochondrial bioenergetics and dynamics, reduces oxidative stress, and enhances adipogenic differentiation potential in metabolically impaired progenitor stem cells
Katarzyna Kornicka-Garbowska, Lynda Bourebaba, Michael Röcken, Krzysztof Marycz
Cell Communication and Signaling.2021;[Epub] CrossRef - microRNAs in Human Adipose Tissue Physiology and Dysfunction
Alina Kurylowicz
Cells.2021; 10(12): 3342. CrossRef - Aging, obese-insulin resistance, and bone remodeling
Napatsorn Imerb, Chanisa Thonusin, Nipon Chattipakorn, Siriporn C. Chattipakorn
Mechanisms of Ageing and Development.2020; 191: 111335. CrossRef - Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes
Federica Zatterale, Michele Longo, Jamal Naderi, Gregory Alexander Raciti, Antonella Desiderio, Claudia Miele, Francesco Beguinot
Frontiers in Physiology.2020;[Epub] CrossRef - Is Mitochondrial Dysfunction a Common Root of Noncommunicable Chronic Diseases?
Alexis Diaz-Vegas, Pablo Sanchez-Aguilera, James R Krycer, Pablo E Morales, Matías Monsalves-Alvarez, Mariana Cifuentes, Beverly A Rothermel, Sergio Lavandero
Endocrine Reviews.2020;[Epub] CrossRef - Inflammatory Signaling and Brown Fat Activity
Farah Omran, Mark Christian
Frontiers in Endocrinology.2020;[Epub] CrossRef - Omega-3 fatty acids as regulators of brown/beige adipose tissue: from mechanisms to therapeutic potential
Marta Fernández-Galilea, Elisa Félix-Soriano, Ignacio Colón-Mesa, Xavier Escoté, Maria J. Moreno-Aliaga
Journal of Physiology and Biochemistry.2020; 76(2): 251. CrossRef - Anti-Inflammatory Strategies Targeting Metaflammation in Type 2 Diabetes
Alina Kuryłowicz, Krzysztof Koźniewski
Molecules.2020; 25(9): 2224. CrossRef - Obese Adipose Tissue Secretion Induces Inflammation in Preadipocytes: Role of Toll-Like Receptor-4
Mariana Renovato-Martins, Catharina Moreira-Nunes, Georgia C. Atella, Christina Barja-Fidalgo, João Alfredo de Moraes
Nutrients.2020; 12(9): 2828. CrossRef -
Diabetes and Metabolism Journal in 2020: Good to Great
In-Kyung Jeong
Diabetes & Metabolism Journal.2020; 44(1): 1. CrossRef - The Effect of Silibinin on Protein Expression Profile in White Adipose Tissue of Obese Mice
Fei Wang, Shuchun Chen, Luping Ren, Yichao Wang, Zelin Li, Tiantian Song, He Zhang, Qiwen Yang
Frontiers in Pharmacology.2020;[Epub] CrossRef - Beneficial Effects of Bariatric Surgery-Induced by Weight Loss on the Proteome of Abdominal Subcutaneous Adipose Tissue
Bárbara María Varela-Rodríguez, Paula Juiz-Valiña, Luis Varela, Elena Outeiriño-Blanco, Susana Belén Bravo, María Jesús García-Brao, Enrique Mena, José Francisco Noguera, Javier Valero-Gasalla, Fernando Cordido, Susana Sangiao-Alvarellos
Journal of Clinical Medicine.2020; 9(1): 213. CrossRef - Impact of Skeletal Muscle Mass on Metabolic Health
Gyuri Kim, Jae Hyeon Kim
Endocrinology and Metabolism.2020; 35(1): 1. CrossRef - Sea buckthorn (Hippophae rhamnoides L.) oil enhances proliferation, adipocytes differentiation and insulin sensitivity in 3T3-L1 cells
Ting Zhang, Xuze Qin, Yuxin Cao, Jianxin Zhang, Junxing Zhao
Food Science and Biotechnology.2020; 29(11): 1511. CrossRef - Adipose tissue secretory profile and cardiometabolic risk in obesity
Pengcheng Zhang, Daniels Konja, Yu Wang
Endocrine and Metabolic Science.2020; 1(3-4): 100061. CrossRef - Mitochondrial Dynamics in the Brain Are Associated With Feeding, Glucose Homeostasis, and Whole-Body Metabolism
Jessica L. Haigh, Lauryn E. New, Beatrice M. Filippi
Frontiers in Endocrinology.2020;[Epub] CrossRef - Adipogenesis: A Necessary but Harmful Strategy
Mohammed El Hafidi, Mabel Buelna-Chontal, Fausto Sánchez-Muñoz, Roxana Carbó
International Journal of Molecular Sciences.2019; 20(15): 3657. CrossRef
- Prolonged Endurance Exercise Increases Macrophage Content and Mitochondrial Respiration in Adipose Tissue in Trained Men
- Inducible Nitric Oxide Synthase (iNOS) Expression in the Hypoxic Injury to Pancreatic Beta (MIN6) Cells.
- Seung Hyun Ko, Seung Bum Kim, Kyung Ryul Ryu, Ji Won Kim, Yu Bai Ahn, Sung Dae Moon, Sung Rae Kim, Jung Min Lee, Hyuk Snag Kwon, Kun Ho Yoon, Ki Ho Song
- Korean Diabetes J. 2006;30(5):336-346. Published online September 1, 2006
- DOI: https://doi.org/10.4093/jkda.2006.30.5.336
- 2,184 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Islet transplantation is an alternative potential strategy to cure type 1 diabetes mellitus. However, two or more donors are usually needed for one recipient because a substantial part of the graft becomes nonfunctional due to several factors including hypoxia. Though hypoxic exposure of pancreatic beta cells has been reported to induce apoptotic cell death, the molecular processes involved in hypoxia-induced cell death are poorly understood. In type I diabetes, Nitric Oxide (NO) is known as an important cytokine, involved in the pathogenesis of beta cell dysfunction. Pancreatic beta cells are sensitive to the induction of inducible nitric oxide synthase (iNOS) when stimulated by TNF-a or IL-1beta. But contribution of iNOS in response to hypoxia is not yet fully understood. METHODS: Mouse insulinoma cells (MIN6) were incubated in an anaerobic chamber (75% N2/15% CO2/5% H2) for up to 12 hours. Cell viability was measured after AO/PI staining. Caspase-3 activation was also determined using Western blot analysis. Nitric Oxide (NO) release into culture medium was measured using a Griess reagent. The expression of iNOS and PDX-1 mRNA and iNOS protein was examined using real time PCR and Western blot analysis. RESULTS: Marked cell death was observed within 6 hours after hypoxic exposure of MIN6 cells (control, < 5%; 2 hr, 11.0+/-7.6%; 6 hr, 46.2+/-12.8%, P < 0.05). Immunoreactivity to activated caspase-3 was observed at 2, 4 and 6 hrs. NO production was increased in a time dependent manner. Expression of iNOS mRNA and protein was significantly increased at 4 and 6 hour after hypoxia. iNOS expression was confirmed by immunostaining. Of note, Pdx-1 mRNA expression was markedly attenuated by hypoxic treatment. Pretreatment with a selective iNOS inhibitor, 1400 W, significantly prevented beta cell death induced by hypoxic injury. CONCLUSION: Our data suggest that iNOS-NO play an important role in hypoxic injury to MIN6 cells. Therefore, iNOS-NO might be a potential therapeutic target for improving engraftment of the transplanted islets and suppression of iNOS would be helpful for prevention of beta cells damage to hypoxic injury.

 KDA
KDA
 First
First Prev
Prev





