
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Clinical Diabetes & Therapeutics
- Glucagon-Like Peptide-1 Receptor Agonists for the Treatment of Type 2 Diabetes Mellitus: A Position Statement of the Korean Diabetes Association
- Hyun Jin Kim, Seok O Park, Seung-Hyun Ko, Sang Youl Rhee, Kyu-Yeon Hur, Nan-Hee Kim, Min Kyong Moon, Byung-Wan Lee, Jin Hwa Kim, Kyung Mook Choi
- Diabetes Metab J. 2017;41(6):423-429. Published online December 19, 2017
- DOI: https://doi.org/10.4093/dmj.2017.41.6.423
- 5,818 View
- 71 Download
- 5 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
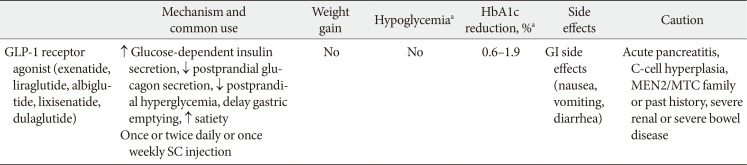
PubReader The glucagon-like peptide-1 receptor agonists (GLP-1RAs) were recommended as a monotherapy or combination therapy with oral hypoglycemic agents or basal insulin in the position statement of the Korean Diabetes Association 2017 for pharmacological therapy. Many randomized clinical trials and systematic reviews report that GLP-1RAs have considerable glucose-lowering effect and lead to weight reduction and low risk of hypoglycemia when used as a monotherapy or combination therapy. The cardiovascular safety of GLP-1RAs has been assessed in several randomized clinical trials and systematic reviews. The results of cardiovascular outcome trials of long-acting GLP-1RAs (liraglutide, semaglutide) demonstrated cardiovascular benefits in subjects with type 2 diabetes mellitus and a high risk of cardiovascular disease. The GLP-1RA may be a choice of therapy when weight control and avoidance of hypoglycemia are important, and patients with high risk of cardiovascular disease might also favor choosing GLP-1RA.
-
Citations
Citations to this article as recorded by- Anti-inflammatory effect of glucagon-like Peptide-1 receptor agonist on the neurosensory retina in an acute optic nerve injury rat model
Yeon Woong Chung, Ji Young Lee, Hyun Hee Ju, Jin A. Choi
European Journal of Pharmacology.2022; 933: 175269. CrossRef - Diabetes Risk Data Mining Method Based on Electronic Medical Record Analysis
Yang Liu, Zhaoxiang Yu, Yunlong Yang, Zhihan Lv
Journal of Healthcare Engineering.2021; 2021: 1. CrossRef - Paradigm Shift for the Treatment of Type 2 Diabetes Mellitus in Patients with Cardiovascular Disease: Cardiologist's Perspective
Doo Soo Jeon
Cardiovascular Prevention and Pharmacotherapy.2020; 2(1): 11. CrossRef - The Role of Glucagon-Like Peptide-1 Receptor Agonists in Type 2 Diabetes in Asia
Ju-Ming Lu
Advances in Therapy.2019; 36(4): 798. CrossRef - A Review of Practical Issues on the Use of Glucagon-Like Peptide-1 Receptor Agonists for the Management of Type 2 Diabetes
Irene Romera, Ana Cebrián-Cuenca, Fernando Álvarez-Guisasola, Fernando Gomez-Peralta, Jesús Reviriego
Diabetes Therapy.2019; 10(1): 5. CrossRef - Glucagon-Like Peptide-1 Receptor Agonists for the Treatment of Type 2 Diabetes Mellitus: A Position Statement of the Korean Diabetes Association
Hyun Jin Kim
The Journal of Korean Diabetes.2018; 19(1): 35. CrossRef
- Anti-inflammatory effect of glucagon-like Peptide-1 receptor agonist on the neurosensory retina in an acute optic nerve injury rat model
- Islet Studies and Transplantation
- An Update on the Effect of Incretin-Based Therapies on β-Cell Function and Mass
- Suk Chon, Jean-François Gautier
- Diabetes Metab J. 2016;40(2):99-114. Published online April 25, 2016
- DOI: https://doi.org/10.4093/dmj.2016.40.2.99
- 4,977 View
- 111 Download
- 43 Web of Science
- 41 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Type 2 diabetes mellitus (T2DM) is a multifactorial disease with a complex and progressive pathogenesis. The two primary mechanisms of T2DM pathogenesis are pancreatic β-cell dysfunction and insulin resistance. Pancreatic β-cell dysfunction is recognized to be a prerequisite for the development of T2DM. Therapeutic modalities that improve β-cell function are considered critical to T2DM management; however, blood glucose control remains a challenge for many patients due to suboptimal treatment efficacy and the progressive nature of T2DM. Incretin-based therapies are now the most frequently prescribed antidiabetic drugs in Korea. Incretin-based therapies are a favorable class of drugs due to their ability to reduce blood glucose by targeting the incretin hormone system and, most notably, their potential to improve pancreatic β-cell function. This review outlines the current understanding of the incretin hormone system in T2DM and summarizes recent updates on the effect of incretin-based therapies on β-cell function and β-cell mass in animals and humans.
-
Citations
Citations to this article as recorded by- Harnessing gut cells for functional insulin production: Strategies and challenges
Kelvin Baafi, John C. March
Biotechnology Notes.2023; 4: 7. CrossRef - Incretin and Pancreatic β-Cell Function in Patients with Type 2 Diabetes
Chang Ho Ahn, Tae Jung Oh, Se Hee Min, Young Min Cho
Endocrinology and Metabolism.2023; 38(1): 1. CrossRef - Weight loss maintenance with exercise and liraglutide improves glucose tolerance, glucagon response, and beta cell function
Simon B. K. Jensen, Christian R. Juhl, Charlotte Janus, Julie R. Lundgren, Christoffer Martinussen, Christoffer Wiingaard, Cecilie Knudsen, Ruth Frikke‐Schmidt, Bente M. Stallknecht, Jens J. Holst, Sten Madsbad, Signe S. Torekov
Obesity.2023; 31(4): 977. CrossRef - How do parasitic worms prevent diabetes? An exploration of their influence on macrophage and β-cell crosstalk
Inah Camaya, Bronwyn O’Brien, Sheila Donnelly
Frontiers in Endocrinology.2023;[Epub] CrossRef - An Imbalance of Pathophysiologic Factors in Late Postprandial Hypoglycemia Post Bariatric Surgery: A Narrative Review
Marah Alsayed Hasan, Stanley Schwartz, Victoria McKenna, Richard Ing
Obesity Surgery.2023; 33(9): 2927. CrossRef - Therapeutic Dilemma in Personalized Medicine
Ehab S. EL Desoky
Current Reviews in Clinical and Experimental Pharmacology.2022; 17(2): 94. CrossRef - Lessons from neonatal β-cell epigenomic for diabetes prevention and treatment
Amar Abderrahmani, Cécile Jacovetti, Romano Regazzi
Trends in Endocrinology & Metabolism.2022; 33(6): 378. CrossRef - Beneficial metabolic effects of recurrent periods of beta‐cell rest and stimulation using stable neuropeptide Y1 and glucagon‐like peptide‐1 receptor agonists
Neil Tanday, Ryan A. Lafferty, Peter R. Flatt, Nigel Irwin
Diabetes, Obesity and Metabolism.2022; 24(12): 2353. CrossRef - A Randomized Controlled Trial of R-Form Verapamil Added to Ongoing Metformin Therapy in Patients with Type 2 Diabetes
Chih-Yuan Wang, Kuo-Chin Huang, Chia-Wen Lu, Chih-Hsun Chu, Chien-Ning Huang, Harn-Shen Chen, I-Te Lee, Jung-Fu Chen, Ching-Chu Chen, Chung-Sen Chen, Chang-Hsun Hsieh, Kai-Jen Tien, Hung-Yu Chien, Yu-Yao Huang, Jui-Pao Hsu, Guang-Tzuu Shane, Ai-Ching Chan
The Journal of Clinical Endocrinology & Metabolism.2022; 107(10): e4063. CrossRef - Exenatide, Metformin, or Both for Prediabetes in PCOS: A Randomized, Open-label, Parallel-group Controlled Study
Tao Tao, Yi Zhang, Yu-Chen Zhu, Jia-Rong Fu, Yu-Ying Wang, Jie Cai, Jing-Yu Ma, Yu Xu, Yi-Ning Gao, Yun Sun, WuQiang Fan, Wei Liu
The Journal of Clinical Endocrinology & Metabolism.2021; 106(3): e1420. CrossRef - The utility of assessing C-peptide in patients with insulin-treated type 2 diabetes: a cross-sectional study
Tuccinardi Dario, Giorgino Riccardo, Pieralice Silvia, Watanabe Mikiko, Maggi Daria, Palermo Andrea, Defeudis Giuseppe, Fioriti Elvira, Pozzilli Paolo, Manfrini Silvia
Acta Diabetologica.2021; 58(4): 411. CrossRef - Type 2 diabetes: evidence-based medicine approach to glucose-lowering therapy
E. V. Biryukova, I. A. Morozova, S. V. Rodionova
Meditsinskiy sovet = Medical Council.2021; (21): 160. CrossRef - Emerging Role of Caveolin-1 in GLP-1 Action
Alessandra Puddu, Davide Maggi
Frontiers in Endocrinology.2021;[Epub] CrossRef - Improvements in HOMA indices and pancreatic endocrinal tissues in type 2-diabetic rats by DPP-4 inhibition and antioxidant potential of an ethanol fruit extract of Withania coagulans
Heera Ram, Pramod Kumar, Ashok Purohit, Priya Kashyap, Suresh Kumar, Shivani Kumar, Garima Singh, Abdulaziz A. Alqarawi, Abeer Hashem, Elsayed Fathi Abd-Allah, Al-Bandari Fahad Al-Arjani, Bhim Pratap Singh
Nutrition & Metabolism.2021;[Epub] CrossRef -
Chop
/
Ddit3
depletion in β cells alleviates ER stress and corrects hepatic steatosis in mice
Jing Yong, Vishal S. Parekh, Shannon M. Reilly, Jonamani Nayak, Zhouji Chen, Cynthia Lebeaupin, Insook Jang, Jiangwei Zhang, Thazha P. Prakash, Hong Sun, Sue Murray, Shuling Guo, Julio E. Ayala, Leslie S. Satin, Alan R. Saltiel, Randal J. Kaufman
Science Translational Medicine.2021;[Epub] CrossRef - Assessment of Insulin Secretion and Insulin Resistance in Human
So Young Park, Jean-François Gautier, Suk Chon
Diabetes & Metabolism Journal.2021; 45(5): 641. CrossRef - Targeted pharmacological therapy restores β-cell function for diabetes remission
Stephan Sachs, Aimée Bastidas-Ponce, Sophie Tritschler, Mostafa Bakhti, Anika Böttcher, Miguel A. Sánchez-Garrido, Marta Tarquis-Medina, Maximilian Kleinert, Katrin Fischer, Sigrid Jall, Alexandra Harger, Erik Bader, Sara Roscioni, Siegfried Ussar, Annett
Nature Metabolism.2020; 2(2): 192. CrossRef - The Relationship Between Timing of Initiation on a Glucagon-like Peptide-1 Receptor Agonist and Glycosylated Hemoglobin Values Among Patients With Type 2 Diabetes
Kristina S. Boye, Reema Mody, Maureen J. Lage, Raleigh E. Malik
Clinical Therapeutics.2020; 42(9): 1812. CrossRef - Short-term renal and metabolic effects of low dose vildagliptin treatment added-on insulin therapy in non-proteinuric patients with type 2 diabetes: open-label randomized prospective study
Valentina K. Bayrasheva, Ivan Y. Pchelin, Vladimir A. Dobronravov, Alina Yu. Babenko, Svetlana G. Chefu, Ivan S. Shatalov, Volha N. Vasilkova, Natalia V. Hudiakova, Alexandra N. Ivanova, Pavel A. Andoskin, Elena N. Grineva
Archives of Endocrinology and Metabolism.2020;[Epub] CrossRef - A Phenotypic Screen Identifies Calcium Overload as a Key Mechanism of β-Cell Glucolipotoxicity
Jennifer Vogel, Jianning Yin, Liansheng Su, Sharon X. Wang, Richard Zessis, Sena Fowler, Chun-Hao Chiu, Aaron C. Wilson, Amy Chen, Frederic Zecri, Gordon Turner, Thomas M. Smith, Brian DeChristopher, Heming Xing, Deborah M. Rothman, Xinming Cai, Alina Ber
Diabetes.2020; 69(5): 1032. CrossRef - Neuropeptide 26RFa (QRFP) is a key regulator of glucose homeostasis and its activity is markedly altered in obese/hyperglycemic mice
Gaëtan Prévost, Arnaud Arabo, Marie-Anne Le Solliec, Justine Bons, Marie Picot, Julie Maucotel, Hind Berrahmoune, Mouna El Mehdi, Saloua Cherifi, Alexandre Benani, Emmanuelle Nédélec, Moïse Coëffier, Jérôme Leprince, Anneli Nordqvist, Valéry Brunel, Pierr
American Journal of Physiology-Endocrinology and Metabolism.2019; 317(1): E147. CrossRef - Gastrin analogue administration adds no significant glycaemic benefit to a glucagon‐like peptide‐1 receptor agonist acutely or after washout of both analogues
Krister Bokvist, Ying Ding, William H. Landschulz, Vikram Sinha, Aleksandra Pastrak, Ruth M. Belin
Diabetes, Obesity and Metabolism.2019; 21(7): 1606. CrossRef - Effects of boschnaloside from Boschniakia rossica on dysglycemia and islet dysfunction in severely diabetic mice through modulating the action of glucagon-like peptide-1
Lie-Chwen Lin, Lin-Chien Lee, Cheng Huang, Chiung-Tong Chen, Jen-Shin Song, Young-Ji Shiao, Hui-Kang Liu
Phytomedicine.2019; 62: 152946. CrossRef - The future of new drugs for diabetes management
Clifford J. Bailey, Caroline Day
Diabetes Research and Clinical Practice.2019; 155: 107785. CrossRef - Compact fluidic system for functional assessment of pancreatic islets
Takeshi Hori, Kei Yamane, Takayuki Anazawa, Osamu Kurosawa, Hiroo Iwata
Biomedical Microdevices.2019;[Epub] CrossRef - Pharmacokinetics of Exenatide in nonhuman primates following its administration in the form of sustained-release PT320 and Bydureon
Yazhou Li, Kelli L. Vaughan, David Tweedie, Jin Jung, Hee Kyung Kim, Ho-Il Choi, Dong Seok Kim, Julie A. Mattison, Nigel H. Greig
Scientific Reports.2019;[Epub] CrossRef - Pharmacokinetics and efficacy of PT302, a sustained-release Exenatide formulation, in a murine model of mild traumatic brain injury
Miaad Bader, Yazhou Li, Daniela Lecca, Vardit Rubovitch, David Tweedie, Elliot Glotfelty, Lital Rachmany, Hee Kyung Kim, Ho-Il Choi, Barry J. Hoffer, Chaim G. Pick, Nigel H. Greig, Dong Seok Kim
Neurobiology of Disease.2019; 124: 439. CrossRef - Novel dual incretin agonist peptide with antidiabetic and neuroprotective potential
N.M. Pathak, V. Pathak, V.A. Gault, S. McClean, N. Irwin, P.R. Flatt
Biochemical Pharmacology.2018; 155: 264. CrossRef - Human EndoC-βH1 β-cells form pseudoislets with improved glucose sensitivity and enhanced GLP-1 signaling in the presence of islet-derived endothelial cells
Michael G. Spelios, Lauren A. Afinowicz, Regine C. Tipon, Eitan M. Akirav
American Journal of Physiology-Endocrinology and Metabolism.2018; 314(5): E512. CrossRef - Vildagliptin: ten years in the service for type 2 diabetes mellitus patients. The journey of discovery, innovation and success in clinical practice
Tatiana Yu. Demidova
Problems of Endocrinology.2018; 64(5): 336. CrossRef - Thromboxane-Dependent Platelet Activation in Obese Subjects with Prediabetes or Early Type 2 Diabetes: Effects of Liraglutide- or Lifestyle Changes-Induced Weight Loss
Paola Simeone, Rossella Liani, Romina Tripaldi, Augusto Di Castelnuovo, Maria Guagnano, Armando Tartaro, Riccardo Bonadonna, Virginia Federico, Francesco Cipollone, Agostino Consoli, Francesca Santilli
Nutrients.2018; 10(12): 1872. CrossRef - Nutrient regulation of β-cell function: what do islet cell/animal studies tell us?
R Carlessi, K N Keane, C Mamotte, P Newsholme
European Journal of Clinical Nutrition.2017; 71(7): 890. CrossRef - Effectiveness and safety of exenatide in Korean patients with type 2 diabetes inadequately controlled with oral hypoglycemic agents: an observational study in a real clinical practice
You-Cheol Hwang, Ari Kim, Euna Jo, Yeoree Yang, Jae-Hyoung Cho, Byung-Wan Lee
BMC Endocrine Disorders.2017;[Epub] CrossRef - Efficacy and safety of adding evogliptin versus sitagliptin for metformin‐treated patients with type 2 diabetes: A 24‐week randomized, controlled trial with open label extension
Sang‐Mo Hong, Cheol‐Young Park, Dong‐Min Hwang, Kyung Ah Han, Chang Beom Lee, Choon Hee Chung, Kun‐Ho Yoon, Ji‐Oh Mok, Kyong Soo Park, Sung‐Woo Park
Diabetes, Obesity and Metabolism.2017; 19(5): 654. CrossRef - The effects of vildagliptin compared with metformin on vascular endothelial function and metabolic parameters: a randomized, controlled trial (Sapporo Athero-Incretin Study 3)
Naoyuki Kitao, Hideaki Miyoshi, Tomoo Furumoto, Kota Ono, Hiroshi Nomoto, Aika Miya, Chiho Yamamoto, Atsushi Inoue, Kenichi Tsuchida, Naoki Manda, Yoshio Kurihara, Shin Aoki, Akinobu Nakamura, Tatsuya Atsumi
Cardiovascular Diabetology.2017;[Epub] CrossRef - Recent Advances in Effect‐directed Enzyme Assays based on Thin‐layer Chromatography
Sarah Bräm, Evelyn Wolfram
Phytochemical Analysis.2017; 28(2): 74. CrossRef - Efficacy and safety of gemigliptin, a dipeptidyl peptidase‐4 inhibitor, in patients with type 2 diabetes mellitus inadequately controlled with combination treatment of metformin and sulphonylurea: a 24‐week, multicentre, randomized, double‐blind, placebo‐
Chang Ho Ahn, Kyung Ah Han, Jae Myung Yu, Joo Young Nam, Kyu Jeung Ahn, Tae Keun Oh, Hyoung Woo Lee, Dae Ho Lee, Jaetaek Kim, Choon Hee Chung, Tae Sun Park, Byung Joon Kim, Seok Won Park, Hyeong Kyu Park, Kwang Jae Lee, Sang‐Wook Kim, Jeong Hyun Park, Kwa
Diabetes, Obesity and Metabolism.2017; 19(5): 635. CrossRef - Antihyperglycemic agent therapy for adult patients with type 2 diabetes mellitus 2017: a position statement of the Korean Diabetes Association
Seung-Hyun Ko, Kyu-Yeon Hur, Sang Youl Rhee, Nan-Hee Kim, Min Kyong Moon, Seok-O Park, Byung-Wan Lee, Hyun Jin Kim, Kyung Mook Choi, Jin Hwa Kim
The Korean Journal of Internal Medicine.2017; 32(6): 947. CrossRef - Antihyperglycemic Agent Therapy for Adult Patients with Type 2 Diabetes Mellitus 2017: A Position Statement of the Korean Diabetes Association
Seung-Hyun Ko, Kyu-Yeon Hur, Sang Youl Rhee, Nan-Hee Kim, Min Kyong Moon, Seok-O Park, Byung-Wan Lee, Hyun Jin Kim, Kyung Mook Choi, Jin Hwa Kim
Diabetes & Metabolism Journal.2017; 41(5): 337. CrossRef - DPP-4 inhibitors in diabetic complications: role of DPP-4 beyond glucose control
Eun Ju Bae
Archives of Pharmacal Research.2016; 39(8): 1114. CrossRef - Liraglutide Enhances the Efficacy of Human Mesenchymal Stem Cells in Preserving Islet ß-cell Function in Severe Non-obese Diabetic Mice
Li-rong Li, Jing Lu, Xiao-lei Jia, Hui Hui, Jie Zhang, Ying Liu, Wei-juan Cui, Qian-yue Xu, Da-long Zhu
Molecular Medicine.2016; 22(1): 800. CrossRef
- Harnessing gut cells for functional insulin production: Strategies and challenges
- The Role of Glucagon-Like Peptide-1 Receptor Agonists in Type 2 Diabetes: Understanding How Data Can Inform Clinical Practice in Korea
- Seungjoon Oh, Suk Chon, Kyu Jeong Ahn, In-Kyung Jeong, Byung-Joon Kim, Jun Goo Kang
- Diabetes Metab J. 2015;39(3):177-187. Published online June 15, 2015
- DOI: https://doi.org/10.4093/dmj.2015.39.3.177
- 4,212 View
- 47 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Glucagon-like peptide-1 receptor agonists (GLP-1RAs) reduce glycosylated hemoglobin (HbA1c, 0.5% to 1.0%), and are associated with moderate weight loss and a relatively low risk of hypoglycemia. There are differences between Asian and non-Asian populations. We reviewed available data on GLP-1RAs, focusing on Korean patients, to better understand their risk/benefit profile and help inform local clinical practice. Control of postprandial hyperglycemia is important in Asians in whom the prevalence of post-challenge hyperglycemia is higher (vs. non-Asians). The weight lowering effects of GLP-1RAs are becoming more salient as the prevalence of overweight and obesity among Korean patients increases. The higher rate of gastrointestinal adverse events amongst Asian patients in clinical trials may be caused by higher drug exposure due to the lower body mass index of the participants (vs. non-Asian studies). Data on the durability of weight loss, clinically important health outcomes, safety and optimal dosing in Korean patients are lacking. Use of GLP-1RAs is appropriate in several patient groups, including patients whose HbA1c is uncontrolled, especially if this is due to postprandial glucose excursions and patients who are overweight or obese due to dietary problems (e.g., appetite control). The potential for gastrointestinal adverse events should be explained to patients at treatment initiation to facilitate the promotion of better compliance.
-
Citations
Citations to this article as recorded by- Tolerability and Effectiveness of Switching to Dulaglutide in Patients With Type 2 Diabetes Inadequately Controlled With Insulin Therapy
Youngsook Kim, Ji Hye Huh, Minyoung Lee, Eun Seok Kang, Bong-Soo Cha, Byung-Wan Lee
Frontiers in Endocrinology.2022;[Epub] CrossRef - Antihyperglycemic Agent Therapy for Adult Patients with Type 2 Diabetes Mellitus 2017: A Position Statement of the Korean Diabetes Association
Seung-Hyun Ko, Kyu-Yeon Hur, Sang Youl Rhee, Nan-Hee Kim, Min Kyong Moon, Seok-O Park, Byung-Wan Lee, Hyun Jin Kim, Kyung Mook Choi, Jin Hwa Kim
Diabetes & Metabolism Journal.2017; 41(5): 337. CrossRef - Antihyperglycemic agent therapy for adult patients with type 2 diabetes mellitus 2017: a position statement of the Korean Diabetes Association
Seung-Hyun Ko, Kyu-Yeon Hur, Sang Youl Rhee, Nan-Hee Kim, Min Kyong Moon, Seok-O Park, Byung-Wan Lee, Hyun Jin Kim, Kyung Mook Choi, Jin Hwa Kim
The Korean Journal of Internal Medicine.2017; 32(6): 947. CrossRef
- Tolerability and Effectiveness of Switching to Dulaglutide in Patients With Type 2 Diabetes Inadequately Controlled With Insulin Therapy

 KDA
KDA
 First
First Prev
Prev





