
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
- Extracellular Vimentin Alters Energy Metabolism And Induces Adipocyte Hypertrophy
- Ji-Hae Park, Soyeon Kwon, Young Mi Park
- Diabetes Metab J. 2024;48(2):215-230. Published online September 26, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0332
- 2,527 View
- 211 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
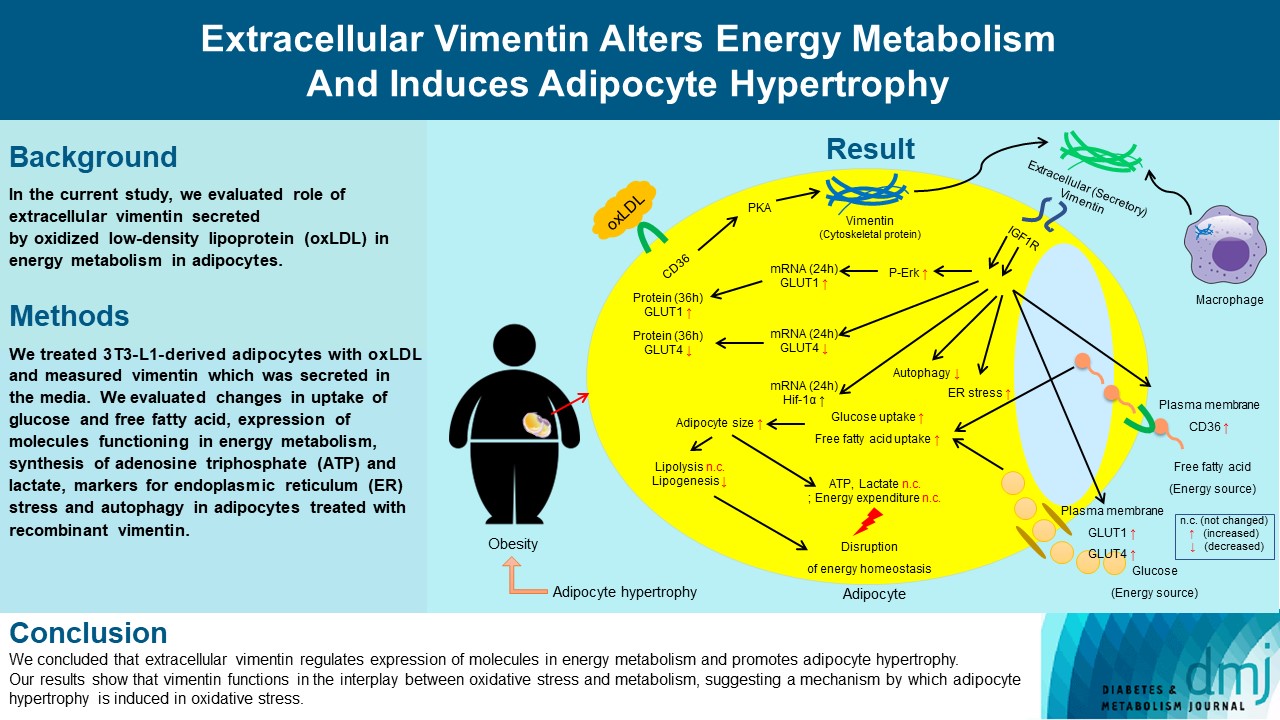
Previous studies have reported that oxidative stress contributes to obesity characterized by adipocyte hypertrophy. However, mechanism has not been studied extensively. In the current study, we evaluated role of extracellular vimentin secreted by oxidized low-density lipoprotein (oxLDL) in energy metabolism in adipocytes.
Methods
We treated 3T3-L1-derived adipocytes with oxLDL and measured vimentin which was secreted in the media. We evaluated changes in uptake of glucose and free fatty acid, expression of molecules functioning in energy metabolism, synthesis of adenosine triphosphate (ATP) and lactate, markers for endoplasmic reticulum (ER) stress and autophagy in adipocytes treated with recombinant vimentin.
Results
Adipocytes secreted vimentin in response to oxLDL. Microscopic evaluation revealed that vimentin treatment induced increase in adipocyte size and increase in sizes of intracellular lipid droplets with increased intracellular triglyceride. Adipocytes treated with vimentin showed increased uptake of glucose and free fatty acid with increased expression of plasma membrane glucose transporter type 1 (GLUT1), GLUT4, and CD36. Vimentin treatment increased transcription of GLUT1 and hypoxia-inducible factor 1α (Hif-1α) but decreased GLUT4 transcription. Adipose triglyceride lipase (ATGL), peroxisome proliferator-activated receptor γ (PPARγ), sterol regulatory element-binding protein 1 (SREBP1), diacylglycerol O-acyltransferase 1 (DGAT1) and 2 were decreased by vimentin treatment. Markers for ER stress were increased and autophagy was impaired in vimentin-treated adipocytes. No change was observed in synthesis of ATP and lactate in the adipocytes treated with vimentin.
Conclusion
We concluded that extracellular vimentin regulates expression of molecules in energy metabolism and promotes adipocyte hypertrophy. Our results show that vimentin functions in the interplay between oxidative stress and metabolism, suggesting a mechanism by which adipocyte hypertrophy is induced in oxidative stress.
- Clinical Diabetes & Therapeutics
- Comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus
- You-Cheol Hwang, Ji Eun Jun, In-Kyung Jeong, Kyu Jeung Ahn, Ho Yeon Chung
- Diabetes Metab J. 2019;43(5):582-589. Published online January 16, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0124
- 6,649 View
- 186 Download
- 14 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The apolipoprotein B/A1 (apoB/A1) ratio is a stronger predictor of future cardiovascular disease than is the level of conventional lipids. Statin and ezetimibe combination therapy have shown additional cardioprotective effects over statin monotherapy.
Methods This was a single-center, randomized, open-label, active-controlled study in Korea. A total of 36 patients with type 2 diabetes mellitus were randomized to either rosuvastatin monotherapy (20 mg/day,
n =20) or rosuvastatin/ezetimibe (5 mg/10 mg/day,n =16) combination therapy for 6 weeks.Results After the 6-week treatment, low density lipoprotein cholesterol (LDL-C) and apoB reduction were comparable between the two groups (−94.3±15.4 and −62.0±20.9 mg/dL in the rosuvastatin group, −89.9±22.7 and −66.8±21.6 mg/dL in the rosuvastatin/ezetimibe group,
P =0.54 andP =0.86, respectively). In addition, change in apoB/A1 ratio (−0.44±0.16 in the rosuvastatin group and −0.47±0.25 in the rosuvastatin/ezetimibe group,P =0.58) did not differ between the two groups. On the other hand, triglyceride and free fatty acid (FFA) reductions were greater in the rosuvastatin/ezetimibe group than in the rosuvastatin group (−10.5 mg/dL [interquartile range (IQR), −37.5 to 29.5] and 0.0 µEq/L [IQR, −136.8 to 146.0] in the rosuvastatin group, −49.5 mg/dL [IQR, −108.5 to −27.5] and −170.5 µEq/L [IQR, −353.0 to 0.8] in the rosuvastatin/ezetimibe group,P =0.010 andP =0.049, respectively). Both treatments were generally well tolerated, and there were no differences in muscle or liver enzyme elevation.Conclusion A 6-week combination therapy of low-dose rosuvastatin and ezetimibe showed LDL-C, apoB, and apoB/A1 ratio reduction comparable to that of high-dose rosuvastatin monotherapy in patients with type 2 diabetes mellitus. Triglyceride and FFA reductions were greater with the combination therapy than with rosuvastatin monotherapy.
-
Citations
Citations to this article as recorded by- Moderate-Intensity Rosuvastatin/Ezetimibe Combination versus Quadruple-Dose Rosuvastatin Monotherapy: A Meta-Analysis and Systemic Review
Yura Kang, Jung Mi Park, Sang-Hak Lee
Yonsei Medical Journal.2024; 65(1): 19. CrossRef - Combination Therapy of Ezetimibe and Rosuvastatin for Dyslipidemia: Current Insights
Maya R Chilbert, Dylan VanDuyn, Sara Salah, Collin M Clark, Qing Ma
Drug Design, Development and Therapy.2022; Volume 16: 2177. CrossRef - Ezetimibe and diabetes mellitus:a new strategy for lowering cholesterol
V.A. Serhiyenko, A.A. Serhiyenko
INTERNATIONAL JOURNAL OF ENDOCRINOLOGY (Ukraine).2022; 18(5): 302. CrossRef - The Effect of Rosuvastatin on Plasma/Serum Levels of High-Sensitivity C-Reactive Protein, Interleukin-6, and D-Dimer in People Living with Human Immunodeficiency Virus: A Systematic Review and Meta-Analysis
Akililu Alemu Ashuro, Yin-Guang Fan, Yuan-Sheng Fu, Dong-Sheng Di, Napoleon Bellua Sam, Hai-Feng Pan, Dong-Qing Ye
AIDS Research and Human Retroviruses.2021; 37(11): 821. CrossRef - Comparison of the Efficacy and Safety of Rosuvastatin/Ezetimibe Combination Therapy and Rosuvastatin Monotherapy on Lipoprotein in Patients With Type 2 Diabetes: Multicenter Randomized Controlled Study
Jiwoo Lee, You-Cheol Hwang, Woo Je Lee, Jong Chul Won, Kee-Ho Song, Cheol-Young Park, Kyu Jeung Ahn, Joong-Yeol Park
Diabetes Therapy.2020; 11(4): 859. CrossRef - Comparison of Renal Effects of Ezetimibe–Statin Combination versus Statin Monotherapy: A Propensity-Score-Matched Analysis
Jaehyun Bae, Namki Hong, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Yong-ho Lee
Journal of Clinical Medicine.2020; 9(3): 798. CrossRef - Combined use of rosuvastatin and ezetimibe improves hepatic steatosis in patients with dyslipidemia
Won Dong Lee, Beom Kyung Kim, Jun Yong Park, Do Young Kim, Sang Hoon Ahn, Kwang-Hyub Han, Seung Up Kim
European Journal of Gastroenterology & Hepatology.2020; 32(12): 1538. CrossRef - Influence of rosuvastatin dose on total fatty acids and free fatty acids in plasma
Cristian I. Ciucanu, Sonia Olariu, Daliborca C. Vlad, Victor Dumitraşcu
Medicine.2020; 99(48): e23356. CrossRef - The effect of switching from statin-monotherapy to statin/ezetimibe combination therapy on lipid profiles in patients with type 2 diabetes and dyslipidemia: a multicenter open-label study (EUCLID)
Mitsuhide Takeshita, Atsushi Tanaka, Atsushi Kawaguchi, Keiko Sato, Shigeru Toyoda, Teruo Inoue, Koichi Node
Vascular Failure.2020; 4(1): 22. CrossRef - Response: Comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus (Diabetes Metab J 2019;43:582–9)
You-Cheol Hwang
Diabetes & Metabolism Journal.2019; 43(6): 915. CrossRef - Letter: Comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus (Diabetes Metab J2019;43:582–9)
Tae Seo Sohn
Diabetes & Metabolism Journal.2019; 43(6): 909. CrossRef - Changes in Plasma Free Fatty Acids Associated with Type-2 Diabetes
Amélie I. S. Sobczak, Claudia A. Blindauer, Alan J. Stewart
Nutrients.2019; 11(9): 2022. CrossRef
- Moderate-Intensity Rosuvastatin/Ezetimibe Combination versus Quadruple-Dose Rosuvastatin Monotherapy: A Meta-Analysis and Systemic Review
- Obesity and Metabolic Syndrome
In Vitro Effect of Fatty Acids Identified in the Plasma of Obese Adolescents on the Function of Pancreatic β-Cells- Claudia Velasquez, Juan Sebastian Vasquez, Norman Balcazar
- Diabetes Metab J. 2017;41(4):303-315. Published online May 24, 2017
- DOI: https://doi.org/10.4093/dmj.2017.41.4.303
- 3,814 View
- 38 Download
- 8 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The increase in circulating free fatty acid (FFA) levels is a major factor that induces malfunction in pancreatic β-cells. We evaluated the effect of FFAs reconstituted according to the profile of circulating fatty acids found in obese adolescents on the viability and function of the murine insulinoma cell line (mouse insulinoma [MIN6]).
Methods From fatty acids obtained commercially, plasma-FFA profiles of three different youth populations were reconstituted: obese with metabolic syndrome; obese without metabolic syndrome; and normal weight without metabolic syndrome. MIN6 cells were treated for 24 or 48 hours with the three FFA profiles, and glucose-stimulated insulin secretion, cell viability, mitochondrial function and antioxidant activity were evaluated.
Results The high FFA content and high polyunsaturated ω6/ω3 ratio, present in plasma of obese adolescents with metabolic syndrome had a toxic effect on MIN6 cell viability and function, increasing oxidative stress and decreasing glucose-dependent insulin secretion.
Conclusion These results could help to guide nutritional management of obese young individuals, encouraging the increase of ω-3-rich food consumption in order to reduce the likelihood of deterioration of β-cells and the possible development of type 2 diabetes mellitus.
-
Citations
Citations to this article as recorded by- The reversible effects of free fatty acids on sulfonylurea-stimulated insulin secretion are related to the expression and dynamin-mediated endocytosis of KATP channels in pancreatic β cells
Chenmin Wei, Zichen Zhang, Qi Fu, Yunqiang He, Tao Yang, Min Sun
Endocrine Connections.2023;[Epub] CrossRef - Preparation of fatty acid solutions exerts significant impact on experimental outcomes in cell culture models of lipotoxicity
Axel Römer, Divya Rawat, Thomas Linn, Sebastian F Petry
Biology Methods and Protocols.2022;[Epub] CrossRef - Lipotoxic Impairment of Mitochondrial Function in β-Cells: A Review
Axel Römer, Thomas Linn, Sebastian F. Petry
Antioxidants.2021; 10(2): 293. CrossRef - Contribution of Oxidative Stress and Impaired Biogenesis of Pancreatic β-Cells to Type 2 Diabetes
Petr Ježek, Martin Jabůrek, Lydie Plecitá-Hlavatá
Antioxidants & Redox Signaling.2019; 31(10): 722. CrossRef - The role of polyunsaturated fatty acids (n-3 PUFAs) on the pancreatic β-cells and insulin action
Habtamu Wondifraw Baynes, Seifu Mideksa, Sintayehu Ambachew
Adipocyte.2018; : 1. CrossRef - Fatty Acid-Stimulated Insulin Secretion vs. Lipotoxicity
Petr Ježek, Martin Jabůrek, Blanka Holendová, Lydie Plecitá-Hlavatá
Molecules.2018; 23(6): 1483. CrossRef
- The reversible effects of free fatty acids on sulfonylurea-stimulated insulin secretion are related to the expression and dynamin-mediated endocytosis of KATP channels in pancreatic β cells

 KDA
KDA
 First
First Prev
Prev





