
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
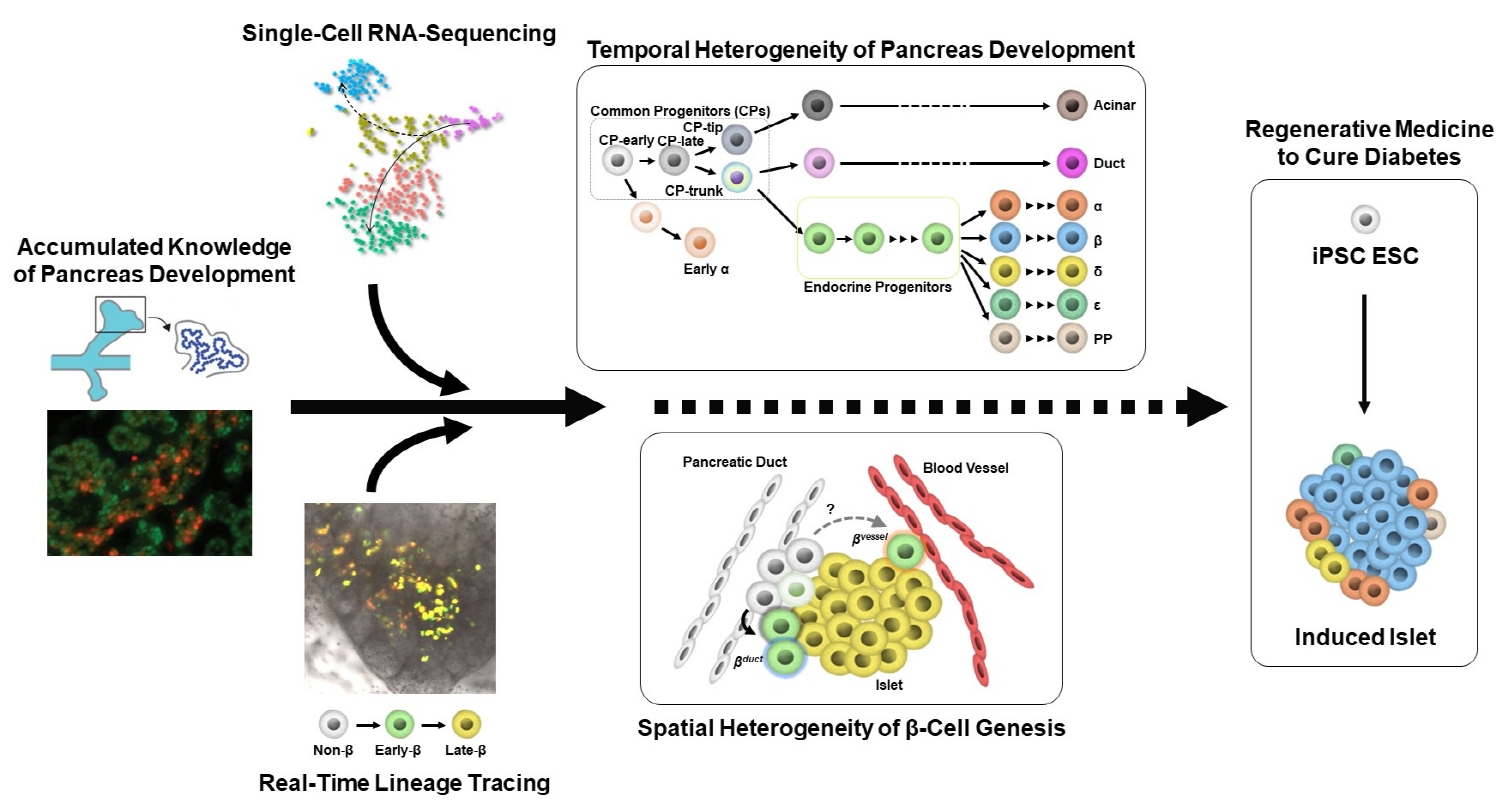
- Heterogeneity of Islet Cells during Embryogenesis and Differentiation
- Shugo Sasaki, Takeshi Miyatsuka
- Diabetes Metab J. 2023;47(2):173-184. Published online January 12, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0324
- 3,744 View
- 248 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Diabetes is caused by insufficient insulin secretion due to β-cell dysfunction and/or β-cell loss. Therefore, the restoration of functional β-cells by the induction of β-cell differentiation from embryonic stem (ES) and induced-pluripotent stem (iPS) cells, or from somatic non-β-cells, may be a promising curative therapy. To establish an efficient and feasible method for generating functional insulin-producing cells, comprehensive knowledge of pancreas development and β-cell differentiation, including the mechanisms driving cell fate decisions and endocrine cell maturation is crucial. Recent advances in single-cell RNA sequencing (scRNA-seq) technologies have opened a new era in pancreas development and diabetes research, leading to clarification of the detailed transcriptomes of individual insulin-producing cells. Such extensive high-resolution data enables the inference of developmental trajectories during cell transitions and gene regulatory networks. Additionally, advancements in stem cell research have not only enabled their immediate clinical application, but also has made it possible to observe the genetic dynamics of human cell development and maturation in a dish. In this review, we provide an overview of the heterogeneity of islet cells during embryogenesis and differentiation as demonstrated by scRNA-seq studies on the developing and adult pancreata, with implications for the future application of regenerative medicine for diabetes.
-
Citations
Citations to this article as recorded by- Newly discovered knowledge pertaining to glucagon and its clinical applications
Dan Kawamori, Shugo Sasaki
Journal of Diabetes Investigation.2023; 14(7): 829. CrossRef
- Newly discovered knowledge pertaining to glucagon and its clinical applications
- Altered Transendothelial Transport of Hormones as a Contributor to Diabetes
- Nanyoung Yoon, Thanh Q. Dang, Helen Chasiotis, Scott P. Kelly, Gary Sweeney
- Diabetes Metab J. 2014;38(2):92-99. Published online April 18, 2014
- DOI: https://doi.org/10.4093/dmj.2014.38.2.92
- 3,978 View
- 36 Download
- 8 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The vascular endothelium is a dynamic structure responsible for the separation and regulated movement of biological material between circulation and interstitial fluid. Hormones and nutrients can move across the endothelium either via a transcellular or paracellular route. Transcellular endothelial transport is well understood and broadly acknowledged to play an important role in the normal and abnormal physiology of endothelial function. However, less is known about the role of the paracellular route. Although the concept of endothelial dysfunction in diabetes is now widely accepted, we suggest that alterations in paracellular transport should be studied in greater detail and incorporated into this model. In this review we provide an overview of endothelial paracellular permeability and discuss its potential importance in contributing to the development of diabetes and associated complications. Accordingly, we also contend that if better understood, altered endothelial paracellular permeability could be considered as a potential therapeutic target for diabetes.
-
Citations
Citations to this article as recorded by- Use of 2-dimensional cell monolayers and 3-dimensional microvascular networks on microfluidic devices shows that iron increases transendothelial adiponectin flux via inducing ROS production
Nanyoung Yoon, Seunggyu Kim, Hye Kyoung Sung, Thanh Q. Dang, Jessie S. Jeon, Gary Sweeney
Biochimica et Biophysica Acta (BBA) - General Subjects.2021; 1865(2): 129796. CrossRef - Adiponectin Synthesis, Secretion and Extravasation from Circulation to Interstitial Space
Simone C. da Silva Rosa, Meilian Liu, Gary Sweeney
Physiology.2021; 36(3): 134. CrossRef - Tracking adiponectin biodistribution via fluorescence molecular tomography indicates increased vascular permeability after streptozotocin-induced diabetes
Nanyoung Yoon, Keith Dadson, Thanh Dang, Teresa Chu, Nina Noskovicova, Boris Hinz, Adeline Raignault, Eric Thorin, Seunggyu Kim, Jessie S. Jeon, James Jonkman, Trevor D. McKee, Justin Grant, Jeffrey D. Peterson, Scott P. Kelly, Gary Sweeney
American Journal of Physiology-Endocrinology and Metabolism.2019; 317(5): E760. CrossRef - Overview of the Components of Cardiac Metabolism
Elizabeth A. Hausner, Susan A. Elmore, Xi Yang
Drug Metabolism and Disposition.2019; 47(6): 673. CrossRef - Emerging Roles of Vascular Endothelium in Metabolic Homeostasis
Xinchun Pi, Liang Xie, Cam Patterson
Circulation Research.2018; 123(4): 477. CrossRef - Transendothelial movement of adiponectin is restricted by glucocorticoids
Thanh Q Dang, Nanyoung Yoon, Helen Chasiotis, Emily C Dunford, Qilong Feng, Pingnian He, Michael C Riddell, Scott P Kelly, Gary Sweeney
Journal of Endocrinology.2017; 234(2): 101. CrossRef - Insulin access to skeletal muscle is impaired during the early stages of diet‐induced obesity
Josiane L. Broussard, Ana V.B. Castro, Malini Iyer, Rebecca L. Paszkiewicz, Isaac Asare Bediako, Lidia S. Szczepaniak, Edward W. Szczepaniak, Richard N. Bergman, Cathryn M. Kolka
Obesity.2016; 24(9): 1922. CrossRef - Temporal and Molecular Analyses of Cardiac Extracellular Matrix Remodeling following Pressure Overload in Adiponectin Deficient Mice
Keith Dadson, Subat Turdi, Stellar Boo, Boris Hinz, Gary Sweeney, Nikolaos Frangogiannis
PLOS ONE.2015; 10(4): e0121049. CrossRef
- Use of 2-dimensional cell monolayers and 3-dimensional microvascular networks on microfluidic devices shows that iron increases transendothelial adiponectin flux via inducing ROS production
- The Hijacking of Cellular Signaling and the Diabetes Epidemic: Mechanisms of Environmental Disruption of Insulin Action and Glucose Homeostasis
- Robert M. Sargis
- Diabetes Metab J. 2014;38(1):13-24. Published online February 19, 2014
- DOI: https://doi.org/10.4093/dmj.2014.38.1.13
- 3,983 View
- 46 Download
- 40 Web of Science
- 39 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The burgeoning epidemic of metabolic disease causes significant societal and individual morbidity and threatens the stability of health care systems around the globe. Efforts to understand the factors that contribute to metabolic derangements are critical for reversing these troubling trends. While excess caloric consumption and physical inactivity superimposed on a susceptible genetic background are central drivers of this crisis, these factors alone fail to fully account for the magnitude and rapidity with which metabolic diseases have increased in prevalence worldwide. Recent epidemiological evidence implicates endocrine disrupting chemicals in the pathogenesis of metabolic diseases. These compounds represent a diverse array of chemicals to which humans are exposed via multiple routes in adulthood and during development. Furthermore, a growing ensemble of animal- and cell-based studies provides preclinical evidence supporting the hypothesis that environmental contaminants contribute to the development of metabolic diseases, including diabetes. Herein are reviewed studies linking specific endocrine disruptors to impairments in glucose homeostasis as well as tying these compounds to disturbances in insulin secretion and impairments in insulin signal transduction. While the data remains somewhat incomplete, the current body of evidence supports the hypothesis that our chemically polluted environment may play a contributing role in the current metabolic crisis.
-
Citations
Citations to this article as recorded by- Effects of Inorganic Arsenic on Type 2 Diabetes Mellitus In Vivo: the Roles and Mechanisms of miRNAs
Jackson Sira, Xiaodan Zhang, Lin Gao, Therese Martin Cheteu Wabo, Jinyu Li, Caselia Akiti, Wei Zhang, Dianjun Sun
Biological Trace Element Research.2024; 202(1): 111. CrossRef - Relationships Between Urinary Metals and Diabetes Traits Among Mexican Americans in Starr County, Texas, USA
Margaret C. Weiss, Yu-Hsuan Shih, Molly Scannell Bryan, Brian P. Jackson, David Aguilar, Craig L. Hanis, Maria Argos, Robert M. Sargis
Biological Trace Element Research.2023; 201(2): 529. CrossRef - Prenatal arsenic exposure induces immunometabolic alteration and renal injury in rats
Radha Dutt Singh, Ratnakar Tiwari, Vineeta Sharma, Hafizurrahman Khan, Siddhartha Gangopadhyay, Sukhveer Singh, Kavita Koshta, Shagun Shukla, Nidhi Arjaria, Kapil Mandrah, Pankaj Ramji Jagdale, Satyakam Patnaik, Somendu Kumar Roy, Dhirendra Singh, Ashok K
Frontiers in Medicine.2023;[Epub] CrossRef - Arsenic metabolism, diabetes prevalence, and insulin resistance among Mexican Americans: A mendelian randomization approach
Margaret C. Weiss, Yu-Hsuan Shih, Molly Scannell Bryan, Brian P. Jackson, David Aguilar, Eric L. Brown, Goo Jun, Craig L. Hanis, Maria Argos, Robert M. Sargis
Environmental Advances.2023; 12: 100361. CrossRef - Transportation-related Environmental Mixtures and Diabetes Prevalence and Control in Urban/Metropolitan Counties in the United States
Margaret C Weiss, Sneha Adusumilli, Jyotsna S Jagai, Robert M Sargis
Journal of the Endocrine Society.2023;[Epub] CrossRef - 24-Epibrassinolide alleviates diazinon oxidative damage by escalating activities of antioxidant defense systems in maize plants
Saeed Karami Mehrian, Nasser Karimi, Fatemeh Rahmani
Scientific Reports.2023;[Epub] CrossRef - Relationship Between Serum Levels of Arsenic, Cadmium, and Mercury and Body Mass Index and Fasting Plasma Glucose in a Mexican Adult Population
Héctor Hernández-Mendoza, Héctor Edmundo Álvarez-Loredo, Elizabeth Teresita Romero-Guzmán, Darío Gaytán-Hernández, Consuelo Chang-Rueda, Israel Martínez-Navarro, Bertha Irene Juárez-Flores, María Judith Rios-Lugo
Biological Trace Element Research.2022; 200(12): 4916. CrossRef - Health Behavior, Level of Hemoglobin A1c, and Quality of Life Among Agricultural Workers of Various Ethnicities in Thai Border Communities
Sorawit Boonyathee, Parichat Ong-Artborirak, Katekaew Seangpraw, Prakasit Tonchoy, Supakan Kantow, Sasivimol Bootsikeaw, Nisarat Auttama, Monchanok Choowanthanapakorn, Dech Dokpuang, Pitakpong Panta
Frontiers in Medicine.2022;[Epub] CrossRef - Perfluorooctanoic acid promotes pancreatic β cell dysfunction and apoptosis through ER stress and the ATF4/CHOP/TRIB3 pathway
Xiaowei He, Dan Wu, Yanan Xu, Yaqin Zhang, Yue Sun, Xiaoai Chang, Yunxia Zhu, Wei Tang
Environmental Science and Pollution Research.2022; 29(56): 84532. CrossRef - Increased gut serotonin production in response to bisphenol A structural analogs may contribute to their obesogenic effects
Nicole G. Barra, Yun Han Kwon, Katherine M. Morrison, Gregory R. Steinberg, Michael G. Wade, Waliul I Khan, Mathilakath M. Vijayan, Jonathan D. Schertzer, Alison C. Holloway
American Journal of Physiology-Endocrinology and Metabolism.2022; 323(1): E80. CrossRef - Combinatorial pathway disruption is a powerful approach to delineate metabolic impacts of endocrine disruptors
Kévin Bernal, Charbel Touma, Chedi Erradhouani, Talía Boronat‐Belda, Lucas Gaillard, Sara Al Kassir, Hélène Le Mentec, Corinne Martin‐Chouly, Normand Podechard, Dominique Lagadic‐Gossmann, Sophie Langouet, François Brion, Anja Knoll‐Gellida, Patrick J. Ba
FEBS Letters.2022; 596(24): 3107. CrossRef - Low-Dose Dioxin Reduced Glucose Uptake in C2C12 Myocytes: The Role of Mitochondrial Oxidative Stress and Insulin-Dependent Calcium Mobilization
Suyeol Im, Sora Kang, Ji Hwan Kim, Seung Jun Oh, Youngmi Kim Pak
Antioxidants.2022; 11(11): 2109. CrossRef - Environmental toxicants in the brain: A review of astrocytic metabolic dysfunction
Mondona S. McCann, Kathleen A. Maguire-Zeiss
Environmental Toxicology and Pharmacology.2021; 84: 103608. CrossRef - Environmental pollution and diabetes mellitus
Amany El-Sikaily, Mohamed Helal
World Journal of Meta-Analysis.2021; 9(3): 234. CrossRef -
Effects of PCB126 on Adipose-to-Muscle Communication in an
in Vitro
Model
Audrey Caron, Fozia Ahmed, Vian Peshdary, Léa Garneau, Ella Atlas, Céline Aguer
Environmental Health Perspectives.2020;[Epub] CrossRef - Interventions to Address Environmental Metabolism-Disrupting Chemicals: Changing the Narrative to Empower Action to Restore Metabolic Health
Robert M. Sargis, Jerrold J. Heindel, Vasantha Padmanabhan
Frontiers in Endocrinology.2019;[Epub] CrossRef - Retinol binding protein 4 mediates MEHP-induced glucometabolic abnormalities in HepG2 cells
Fei Wang, Chong Chang, Ruobi Li, Zhen Zhang, Hongmei Jiang, Ni Zeng, Daochuan Li, Liping Chen, Yongmei Xiao, Wen Chen, Qing Wang
Toxicology.2019; 424: 152236. CrossRef - Percentage fractions of urinary di(2-ethylhexyl) phthalate metabolites: Association with obesity and insulin resistance in Korean girls
Shin-Hye Kim, Ji-won On, Heesoo Pyo, Kyung Soo Ko, Jong Chul Won, Jiyeon Yang, Mi Jung Park, Elena Baixeras
PLOS ONE.2018; 13(11): e0208081. CrossRef - Association between diabetes and pesticides: a case-control study among Thai farmers
Chudchawal Juntarawijit, Yuwayong Juntarawijit
Environmental Health and Preventive Medicine.2018;[Epub] CrossRef - Environmental toxicant exposures and type 2 diabetes mellitus: Two interrelated public health problems on the rise
Marcelo G. Bonini, Robert M. Sargis
Current Opinion in Toxicology.2018; 7: 52. CrossRef - Unravelling the Effect of p,p′-Dichlorodiphenyldichloroethylene (DDE) in Hypertension of Wistar Rats
Carla Sá, Diogo Pestana, Conceição Calhau, Ana Faria
Journal of Agricultural and Food Chemistry.2018; 66(48): 12847. CrossRef - Exposure to bisphenol A is directly associated with inflammation in healthy Korean adults
Yong Jun Choi, Kyoung Hwa Ha, Dae Jung Kim
Environmental Science and Pollution Research.2017; 24(1): 284. CrossRef - Adipose tissue dysfunction as a central mechanism leading to dysmetabolic obesity triggered by chronic exposure to p,p’-DDE
Diogo Pestana, Diana Teixeira, Manuela Meireles, Cláudia Marques, Sónia Norberto, Carla Sá, Virgínia C. Fernandes, Luísa Correia-Sá, Ana Faria, Luísa Guardão, João T. Guimarães, Wendy N. Cooper, Ionel Sandovici, Valentina F. Domingues, Cristina Delerue-Ma
Scientific Reports.2017;[Epub] CrossRef - BDE-47 and BDE-85 stimulate insulin secretion in INS-1 832/13 pancreatic β-cells through the thyroid receptor and Akt
Shpetim Karandrea, Huquan Yin, Xiaomei Liang, Emma A. Heart
Environmental Toxicology and Pharmacology.2017; 56: 29. CrossRef - Association between Blood Mercury Level and Visceral Adiposity in Adults
Jong Suk Park, Kyoung Hwa Ha, Ka He, Dae Jung Kim
Diabetes & Metabolism Journal.2017; 41(2): 113. CrossRef - Polychlorinated biphenyls and links to cardiovascular disease
Jordan T. Perkins, Michael C. Petriello, Bradley J. Newsome, Bernhard Hennig
Environmental Science and Pollution Research.2016; 23(3): 2160. CrossRef - Polychlorinated biphenyl 126 exposure in L6 myotubes alters glucose metabolism: a pilot study
Jean-François Mauger, Lucien Nadeau, Audrey Caron, Natalie Ann Chapados, Céline Aguer
Environmental Science and Pollution Research.2016; 23(8): 8133. CrossRef - Direct effect of p,p'- DDT on mice liver
Bárbara Arroyo-Salgado, Jesús Olivero-Verbel, Angélica Guerrero-Castilla
Brazilian Journal of Pharmaceutical Sciences.2016; 52(2): 287. CrossRef - More β-cell researchers are wanted!
Susumu Seino
Diabetology International.2016; 7(2): 101. CrossRef - New risk factors for obesity and diabetes: Environmental chemicals
Ja Young Jeon, Kyoung Hwa Ha, Dae Jung Kim
Journal of Diabetes Investigation.2015; 6(2): 109. CrossRef - Dietary Exposure to the Endocrine Disruptor Tolylfluanid Promotes Global Metabolic Dysfunction in Male Mice
Shane M. Regnier, Andrew G. Kirkley, Honggang Ye, Essam El-Hashani, Xiaojie Zhang, Brian A. Neel, Wakanene Kamau, Celeste C. Thomas, Ayanna K. Williams, Emily T. Hayes, Nicole L. Massad, Daniel N. Johnson, Lei Huang, Chunling Zhang, Robert M. Sargis
Endocrinology.2015; 156(3): 896. CrossRef - Impact of Cadmium Exposure on the Association between Lipopolysaccharide and Metabolic Syndrome
Seung Han, Kyoung Ha, Ja Jeon, Hae Kim, Kwan Lee, Dae Kim
International Journal of Environmental Research and Public Health.2015; 12(9): 11396. CrossRef - A Northern contaminant mixture impairs pancreas function in obese and lean JCR rats and inhibits insulin secretion in MIN6 cells
Ryan Mailloux, Accalia Fu, Maria Florian, Ivan Petrov, Qixuan Chen, Melanie C. Coughlan, Mahemuti Laziyan, Jin Yan, Don Caldwell, Dominique Patry, Michelle Lalande, Gen-Sheng Wang, William Willmore, Xiaolei Jin
Toxicology.2015; 334: 81. CrossRef - Metabolic disruption in context: Clinical avenues for synergistic perturbations in energy homeostasis by endocrine disrupting chemicals
Robert M Sargis
Endocrine Disruptors.2015; 3(1): e1080788. CrossRef - Mitochondrial Redox Dysfunction and Environmental Exposures
Samuel W. Caito, Michael Aschner
Antioxidants & Redox Signaling.2015; 23(6): 578. CrossRef - Transgenerational inheritance of heart disorders caused by paternal bisphenol A exposure
Marta Lombó, Cristina Fernández-Díez, Silvia González-Rojo, Claudia Navarro, Vanesa Robles, María Paz Herráez
Environmental Pollution.2015; 206: 667. CrossRef - Qizhi Jiangtang Jiaonang Improves Insulin Signaling and Reduces Inflammatory Cytokine Secretion and Reactive Oxygen Species Formation in Insulin Resistant HepG2 Cells
Xiao-Tian Zhang, Chun-Jiang Yu, Jian-Wei Liu, Yan-Ping Zhang, Chao Zhang, Chen-Xue Song, Jing-Shu Xie, Jing-Ying Sai, Jing-Tong Zheng, Fang Wang
Evidence-Based Complementary and Alternative Medicine.2015; 2015: 1. CrossRef - Thyroid hormone regulation of hepatic lipid and carbohydrate metabolism
Rohit A. Sinha, Brijesh K. Singh, Paul M. Yen
Trends in Endocrinology & Metabolism.2014; 25(10): 538. CrossRef - Environmental Endocrine Disruption of Energy Metabolism and Cardiovascular Risk
Andrew G. Kirkley, Robert M. Sargis
Current Diabetes Reports.2014;[Epub] CrossRef
- Effects of Inorganic Arsenic on Type 2 Diabetes Mellitus In Vivo: the Roles and Mechanisms of miRNAs
- Transdifferentiation of Enteroendocrine K-cells into Insulin-expressing Cells.
- Esder Lee, Jun Mo Yu, Min Kyung Lee, Gyeong Ryul Ryu, Seung Hyun Ko, Yu Bae Ahn, Sung Dae Moon, Ki Ho Song
- Korean Diabetes J. 2009;33(6):475-484. Published online December 1, 2009
- DOI: https://doi.org/10.4093/kdj.2009.33.6.475
- 2,219 View
- 19 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Despite a recent breakthough in human islet transplantation for treating type 1 diabetes mellitus, the limited availability of donor pancreases remains a major obstacle. Endocrine cells within the gut epithelium (enteroendocrine cells) and pancreatic beta cells share similar pathways of differentiation during embryonic development. In particular, K-cells that secrete glucose-dependent insulinotropic polypeptide (GIP) have been shown to express many of the key proteins found in beta cells. Therefore, we hypothesize that K-cells can be transdifferentiated into beta cells because both cells have remarkable similarities in their embryonic development and cellular phenotypes. METHODS: K-cells were purified from heterogeneous STC-1 cells originating from an endocrine tumor of a mouse intestine. In addition, a K-cell subclone expressing stable Nkx6.1, called "Kn4-cells," was successfully obtained. In vitro differentiation of K-cells or Kn4-cells into beta cells was completed after exendin-4 treatment and serum deprivation. The expressions of insulin mRNA and protein were examined by RT-PCR and immunocytochemistry. The interacellular insulin content was also measured. RESULTS: K-cells were found to express glucokinase and GIP as assessed by RT-PCR and Western blot analysis. RT-PCR showed that K-cells also expressed Pdx-1, NeuroD1/Beta2, and MafA, but not Nkx6.1. After exendin-4 treatment and serum deprivation, insulin mRNA and insulin or C-peptide were clearly detected in Kn4-cells. The intracellular insulin content was also increased significantly in these cells. CONCLUSION: K-cells are an attractive potential source of insulin-producing cells for treatment of type 1 diabetes mellitus. However, more experiments are necessary to optimize a strategy for converting K-cells into beta cells. -
Citations
Citations to this article as recorded by- Reprogramming of enteroendocrine K cells to pancreatic β-cells through the combined expression of Nkx6.1 and Neurogenin3, and reaggregation in suspension culture
Esder Lee, Gyeong Ryul Ryu, Sung-Dae Moon, Seung-Hyun Ko, Yu-Bae Ahn, Ki-Ho Song
Biochemical and Biophysical Research Communications.2014; 443(3): 1021. CrossRef
- Reprogramming of enteroendocrine K cells to pancreatic β-cells through the combined expression of Nkx6.1 and Neurogenin3, and reaggregation in suspension culture
- Stimulation of Glucagon Like Peptide-1 Secretion in Enteroendocrine L cells.
- Byung Joon Kim
- Korean Diabetes J. 2009;33(6):458-463. Published online December 1, 2009
- DOI: https://doi.org/10.4093/kdj.2009.33.6.458
- 1,892 View
- 20 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - GLP-1 (glucagon like peptide-1) is new anti-diabetic drug with a number of beneficial effects. It stimulates glucose dependant insulin secretion and restoration of beta cell mass through enhancement of islet mass. However, it is easily inactivated after being secreted from enteroendocrine L cells. Recent trial to increased GLP-1 is to directly stimulate L cells through its receptor located in the surface of L cell. Taste receptor in the apical surface of L cell is activated by various tastants contained in the food. Tongue perceives taste sense through the heterotrimeric G-protein (alpha-gustducin) and its downstream signaling cascades. Same taste receptors are also expressed in enteroendocrine cells. In duodenal L cell, alpha-gustducin was detected by immunofluorescence stainig at the luminal projections of enteroendocrine cells. And several other taste signaling elements were also found in L cells. Ingestion of sweet or bitter compounds revealed stimulation of GLP-1 secretion and the regulation of plasma insulin and glucose. In this review, I will briefly introduce the possibilities to stimulate GLP-1 secretion though the membrane receptor in enteroendocrine cell. And it will be the good candidate to develop the treatment modality for obesity, diabetes and abnormal gut motility.
-
Citations
Citations to this article as recorded by- Repression of sterol regulatory element-binding protein 1-c is involved in the protective effects of exendin-4 in pancreatic β-cell line
Seok-Woo Hong, Jinmi Lee, Se Eun Park, Eun-Jung Rhee, Cheol-Young Park, Ki-Won Oh, Sung-Woo Park, Won-Young Lee
Molecular and Cellular Endocrinology.2012; 362(1-2): 242. CrossRef - Exendin-4 Protects Oxidative Stress-Induced β-Cell Apoptosis through Reduced JNK and GSK3β Activity
Ju-Young Kim, Dong-Mee Lim, Chan Il Moon, Kyung-Jin Jo, Seong-Kyu Lee, Haing-Woon Baik, Ki-Ho Lee, Kang-Woo Lee, Keun-Young Park, Byung-Joon Kim
Journal of Korean Medical Science.2010; 25(11): 1626. CrossRef
- Repression of sterol regulatory element-binding protein 1-c is involved in the protective effects of exendin-4 in pancreatic β-cell line

 KDA
KDA
 First
First Prev
Prev





