
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
Original Articles
- Basic Research
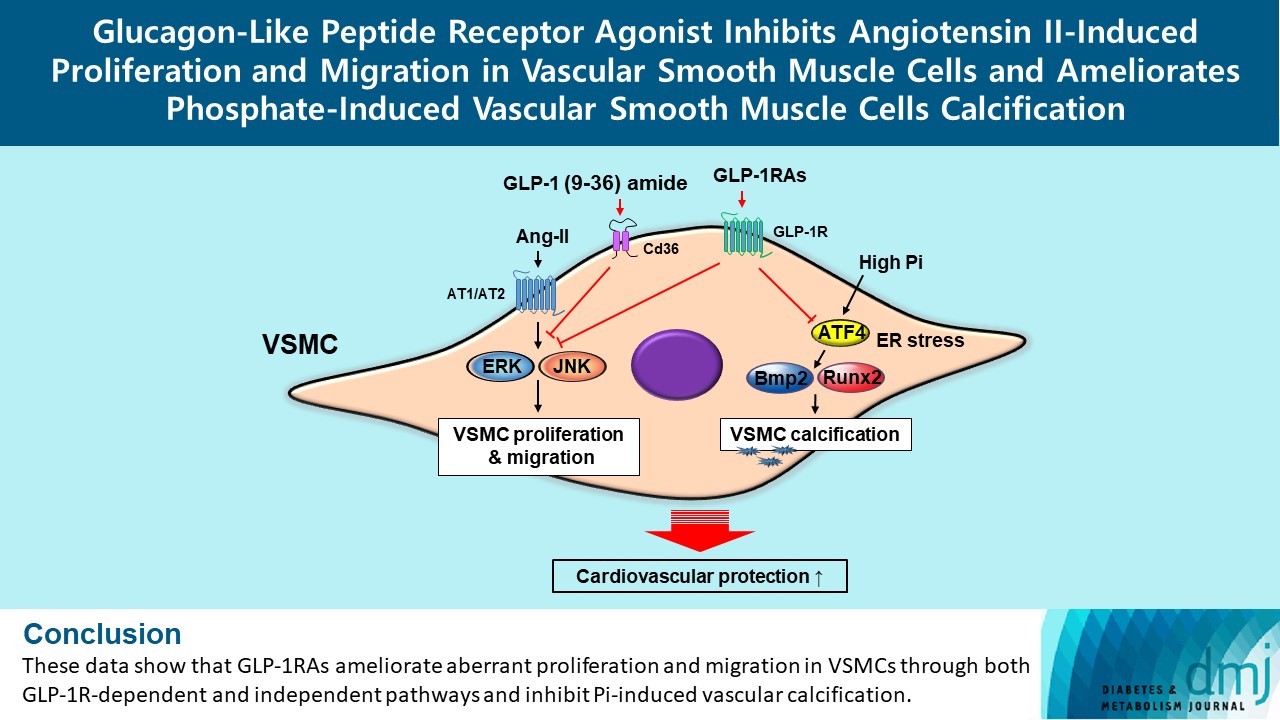
- Glucagon-Like Peptide Receptor Agonist Inhibits Angiotensin II-Induced Proliferation and Migration in Vascular Smooth Muscle Cells and Ameliorates Phosphate-Induced Vascular Smooth Muscle Cells Calcification
- Jinmi Lee, Seok-Woo Hong, Min-Jeong Kim, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
- Diabetes Metab J. 2024;48(1):83-96. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0363
- 1,932 View
- 174 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Glucagon-like peptide-1 receptor agonist (GLP-1RA), which is a therapeutic agent for the treatment of type 2 diabetes mellitus, has a beneficial effect on the cardiovascular system.
Methods
To examine the protective effects of GLP-1RAs on proliferation and migration of vascular smooth muscle cells (VSMCs), A-10 cells exposed to angiotensin II (Ang II) were treated with either exendin-4, liraglutide, or dulaglutide. To examine the effects of GLP-1RAs on vascular calcification, cells exposed to high concentration of inorganic phosphate (Pi) were treated with exendin-4, liraglutide, or dulaglutide.
Results
Ang II increased proliferation and migration of VSMCs, gene expression levels of Ang II receptors AT1 and AT2, proliferation marker of proliferation Ki-67 (Mki-67), proliferating cell nuclear antigen (Pcna), and cyclin D1 (Ccnd1), and the protein expression levels of phospho-extracellular signal-regulated kinase (p-Erk), phospho-c-JUN N-terminal kinase (p-JNK), and phospho-phosphatidylinositol 3-kinase (p-Pi3k). Exendin-4, liraglutide, and dulaglutide significantly decreased the proliferation and migration of VSMCs, the gene expression levels of Pcna, and the protein expression levels of p-Erk and p-JNK in the Ang II-treated VSMCs. Erk inhibitor PD98059 and JNK inhibitor SP600125 decreased the protein expression levels of Pcna and Ccnd1 and proliferation of VSMCs. Inhibition of GLP-1R by siRNA reversed the reduction of the protein expression levels of p-Erk and p-JNK by exendin-4, liraglutide, and dulaglutide in the Ang II-treated VSMCs. Moreover, GLP-1 (9-36) amide also decreased the proliferation and migration of the Ang II-treated VSMCs. In addition, these GLP-1RAs decreased calcium deposition by inhibiting activating transcription factor 4 (Atf4) in Pi-treated VSMCs.
Conclusion
These data show that GLP-1RAs ameliorate aberrant proliferation and migration in VSMCs through both GLP-1Rdependent and independent pathways and inhibit Pi-induced vascular calcification. -
Citations
Citations to this article as recorded by- Incretin Hormone Secretion in Women with Polycystic Ovary Syndrome: Roles of Obesity, Insulin Sensitivity and Treatment with Metformin and GLP-1s
Andrea Etrusco, Mislav Mikuš, Antonio D’Amato, Fabio Barra, Petar Planinić, Trpimir Goluža, Giovanni Buzzaccarini, Jelena Marušić, Mara Tešanović, Antonio Simone Laganà
Biomedicines.2024; 12(3): 653. CrossRef
- Incretin Hormone Secretion in Women with Polycystic Ovary Syndrome: Roles of Obesity, Insulin Sensitivity and Treatment with Metformin and GLP-1s
- Mitogenic Effects and Signaling Pathway of Insulin-Like Growth Factor-I (IGF-I) in the Rat Beta Cell Line (INS-1).
- In Kyung Jeong, Ja Young Kim, Hyung Joon Yoo, Myung Shik Lee, Moon Kyu Lee, Kwang Won Kim
- Korean Diabetes J. 2004;28(6):478-489. Published online December 1, 2004
- 996 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Nutrients and growth factors are known to stimulate pancreatic beta cell mitogenesis. IGF-I acts as a survival factor by limiting apoptosis and stimulating proliferation in many cell types. However, the appropriate mitogenic signaling pathways have not been defined. The aim of this study is to elucidate the mitogenic effect and signaling pathways of IGF-I in the rat beta cell line (INS-I). METHODS: The studies were performed using the rat pancreatic beta cell line, INS-1. INS-1 cells were cultured in RPMI 1640 containing serum-free, 0.2% BSA and 11.1 mmol/L glucose media for 24 hours, and the cells were then treated with IGF-I and different concentrations of glucose or tyrosine phosphorylation inhibitors, or insulin. The cell proliferation was measured by the [3H]thymidine uptake and MTT assay. The cell cycle was analyzed by a flow cytometer by using propidium iodide staining. Western blot analyses were performed using antibodies against PY20 and phospho-MAPK. RESULTS: 1) MTT assay and the [3H]thymidine uptake showed that IGF-I stimulated the INS-1 cell proliferation in a dose dependent manner. Glucose was noted to independently increase the INS-1 cell proliferation. A combination of IGF-I and glucose has a synergistic effect on the proliferation of INS-I cells. Insulin did not influence on the mitogenic effect of IGF-I. 2) The S fraction of INS-1 cells treated with IGF-I was increased in a dose dependent manner. IGF-I stimulated the exit from G1 into the S phase of the cell cycle. 3) Investigation of the role of the PI3K and MAPK, by using of the inhibitors LY294002, wortmannin, and PD98059, demonstrated that the activation of MAPK, but not PI3K, required to stimulate the proliferation of INS-1 cells. 4) IGF-I stimulated the phosphorylation activation of pp60 and phospho-MAPK in the INS-1 cells. IGF-I induced the beta cell proliferation, and this was mediated via a signaling mechanism that was facilitated by MAPK. CONCLUSION: The proliferative effect of IGF-I on pancreatic beta cell seems to be mediated through MAPK signaling pathway.
- Effects of Peroxisome Proliferator-activated Receptor-gamma(PPARgamma) on the Pancreatic beta Cell Proliferation.
- Jung Hyun Noh, Tae Young Yang, In Kyung Jeong, Jae Hun Chung, Yong Ki Min, Myung Shik Lee, Kwang Won Kim, Moon Kyu Lee
- Korean Diabetes J. 2003;27(3):241-252. Published online June 1, 2003
- 1,127 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The effects and mechanisms of PPARgamma ligands on the cell proliferation in pancreatic beta cells were examined. METHODS: PPARgamma 1 cDNA was overexpressed in INS-1 cells using an adenoviral vector. The cell proliferations were measured by the MTT assay method, following the treatments with troglitazone (TGZ), rosiglitazone (RGZ), 15d-prostaglandin J2 (15d-PGJ2) or retinoic acid (RA), at increasing doses, in INS-1 and PPARgamma overexpressed INS-1 cells. The apoptosis, telomere length and cell cycles were determined after the PPARgamma ligand treatment. RESULTS: The long-term incubation, with PPARgamma ligands over 24 hr, inhibited the INS-1 cell proliferation rate. Apoptosis was not observed with the PPARgamma ligand treatment. G1 cell cycle arrest was observed with the troglitazone treatment. The telomere length remained unchanged following the TGZ treatment. The basal cell proliferation rate was unaffected by the overexpression of PPARgamma . After 48 h of TGZ treatment, the proliferation of the INS-1 cells was inhibited, in a dose- dependent manner, both with and without the overexpression. Moreover, the degree of inhibition was exaggerated in the PPARgamma overexpressed cells compared to beta gal overexpressed cells. CONCLUSION: PPARgamma ligands have direct inhibitory effects on the proliferation of INS-1 cells. Although the basal cell proliferation rate was not affected by PPARgamma overexpression, the PPARgamma overexpression and PPARgamma ligands have a synergistic inhibitory effect on the cell proliferation rate in pancreatic beta cells. G1 cell cycle arrest may be involved in the reduction of cell proliferation due to PPARgamma ligands.
- The Effects of Troglitazone on Vascular Smooth Muscle Cell Proliferation.
- Yun Jae Chung, Kyeong Min Min, Eun Young Oh, Jae Hoon Chung, Yong Ki Min, Myung Shik Lee, Moon Kyu Lee, Kwang Won Kim
- Korean Diabetes J. 2000;24(3):348-355. Published online January 1, 2001
- 1,186 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Elevated fasting and postprandial insulin levels are frequently observed in patients with obesity and hypertension as well as type 2 diabetes mellitus. This phenomenon has been suggested as an independent risk factors for atherosclerotic cardiovascular diseases. Troglitazone, an insulin-sensitizing antidiabetic agent, has been shown to inhibit atherosclerotic process, but its mechanism of action is not yet elucidated. This study was undertaken to examine the effects of troglitazone, a peroxisome proliferator- activated receptor- (PPAR ) ligand, on vascular smooth muscle cell proliferation. METHODS: Aortic smooth muscle cells were isolated from Sprague-Dawley rats and the effects of several different agonists (insulin, ET-I, IGF-I) on cellular DNA synthesis were measured and compared with the effects of troglitazone. In addition, the mRNA of PPARgamma gene in rat aortic smooth muscle cells(RASMCs) was detected by RT-PCR methods. RESULTS:1. Insulin, endothelin-I and IGF-I significantly stimulated DNA synthesis in RASMCs (p<0.05). 2. Insulin-induced DNA synthesis was not significantly inhibited by coincubation with wortmannin or LY294002 but inhibited by PD98059. 3. Troglitazone significantly inhibited insulin, endothelin-I and IGF-I-induced DNA synthesis in RASMCs (p<0.05, respectively). 4. PPAR mRNA was detected in RASMCs by RT-PCR and its expression did not significantly increase by troglitazone treatment. CONCLUSION: Troglitazone could inhibit agonist-induced proliferation of vascular smooth muscle cells and might be a useful agent for treatment as well as prevention of atherosclerosis.

 KDA
KDA
 First
First Prev
Prev





