
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
- Diabetes Promotes Myocardial Fibrosis via AMPK/EZH2/PPAR-γ Signaling Pathway
- Shan-Shan Li, Lu Pan, Zhen-Ye Zhang, Meng-Dan Zhou, Xu-Fei Chen, Ling-Ling Qian, Min Dai, Juan Lu, Zhi-Ming Yu, Shipeng Dang, Ru-Xing Wang
- Received February 3, 2023 Accepted November 13, 2023 Published online February 27, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0031 [Epub ahead of print]
- 921 View
- 54 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
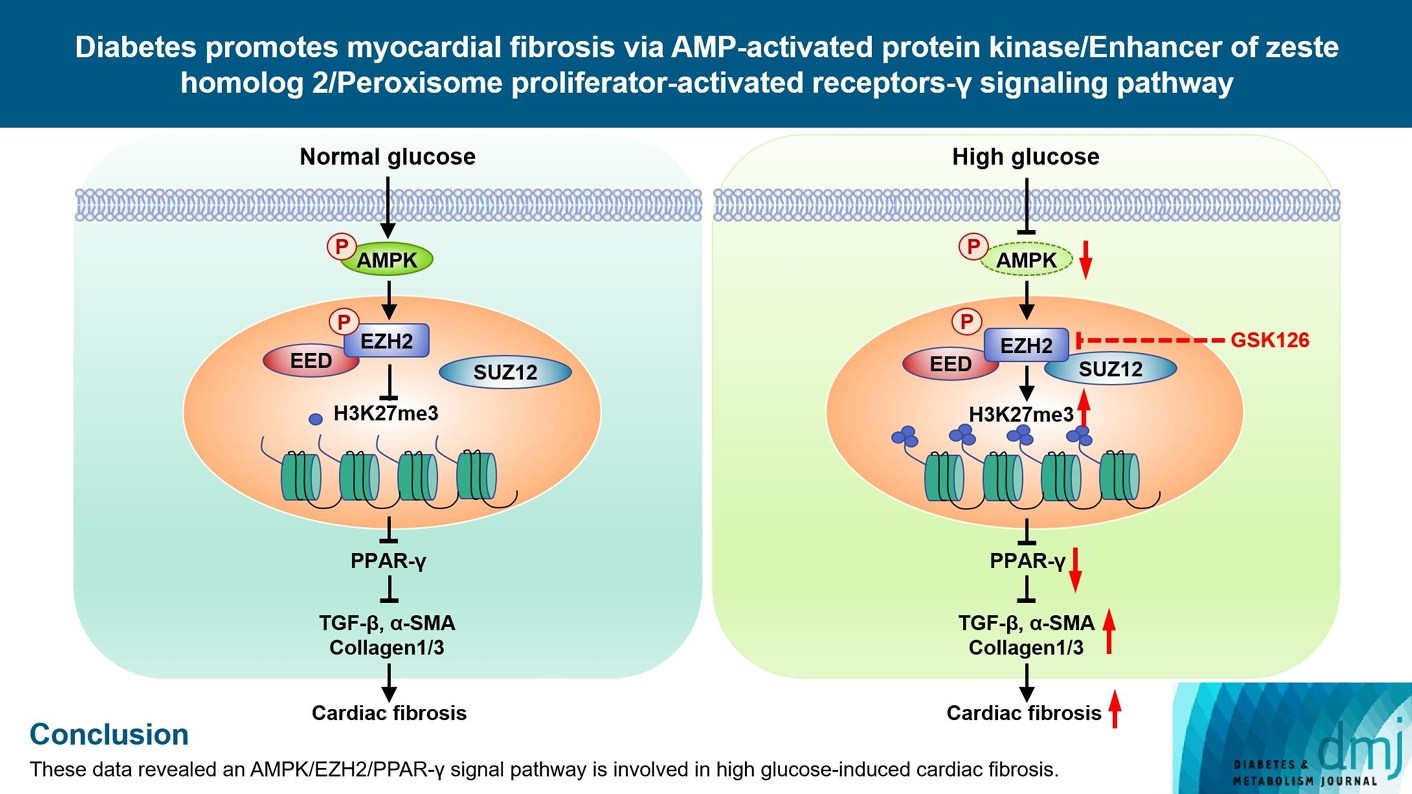
ePub - Background
Diabetes-induced cardiac fibrosis is one of the main mechanisms of diabetic cardiomyopathy. As a common histone methyltransferase, enhancer of zeste homolog 2 (EZH2) has been implicated in fibrosis progression in multiple organs. However, the mechanism of EZH2 in diabetic myocardial fibrosis has not been clarified.
Methods
In the current study, rat and mouse diabetic model were established, the left ventricular function of rat and mouse were evaluated by echocardiography and the fibrosis of rat ventricle was evaluated by Masson staining. Primary rat ventricular fibroblasts were cultured and stimulated with high glucose (HG) in vitro. The expression of histone H3 lysine 27 (H3K27) trimethylation, EZH2, and myocardial fibrosis proteins were assayed.
Results
In STZ-induced diabetic ventricular tissues and HG-induced primary ventricular fibroblasts in vitro, H3K27 trimethylation was increased and the phosphorylation of EZH2 was reduced. Inhibition of EZH2 with GSK126 suppressed the activation, differentiation, and migration of cardiac fibroblasts as well as the overexpression of the fibrotic proteins induced by HG. Mechanical study demonstrated that HG reduced phosphorylation of EZH2 on Thr311 by inactivating AMP-activated protein kinase (AMPK), which transcriptionally inhibited peroxisome proliferator-activated receptor γ (PPAR-γ) expression to promote the fibroblasts activation and differentiation.
Conclusion
Our data revealed an AMPK/EZH2/PPAR-γ signal pathway is involved in HG-induced cardiac fibrosis.
- Pathophysiology
- Primordial Drivers of Diabetes Heart Disease: Comprehensive Insights into Insulin Resistance
- Yajie Fan, Zhipeng Yan, Tingting Li, Aolin Li, Xinbiao Fan, Zhongwen Qi, Junping Zhang
- Diabetes Metab J. 2024;48(1):19-36. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0110
- 2,249 View
- 185 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
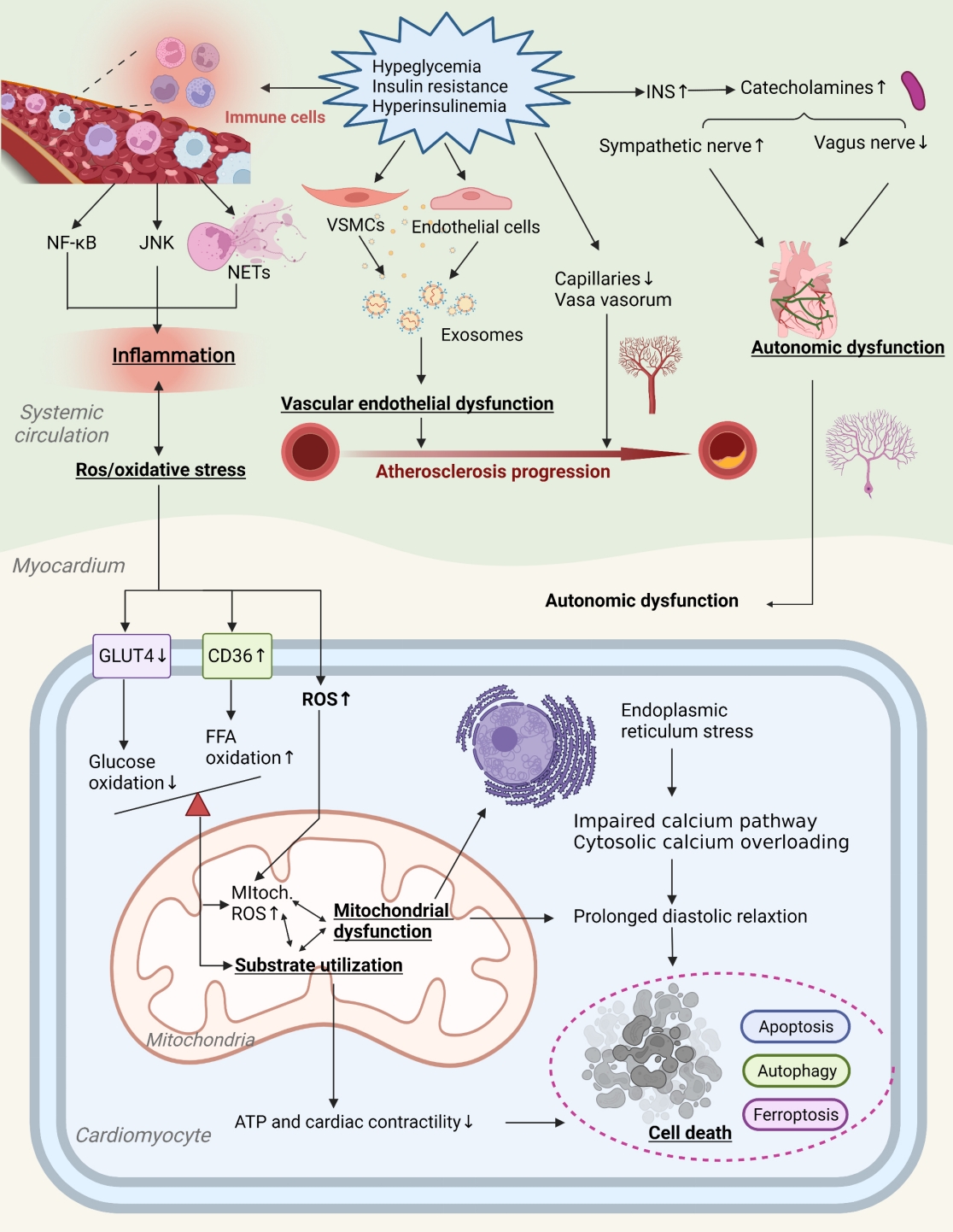
ePub - Insulin resistance has been regarded as a hallmark of diabetes heart disease (DHD). Numerous studies have shown that insulin resistance can affect blood circulation and myocardium, which indirectly cause cardiac hypertrophy and ventricular remodeling, participating in the pathogenesis of DHD. Meanwhile, hyperinsulinemia, hyperglycemia, and hyperlipidemia associated with insulin resistance can directly impair the metabolism and function of the heart. Targeting insulin resistance is a potential therapeutic strategy for the prevention of DHD. Currently, the role of insulin resistance in the pathogenic development of DHD is still under active research, as the pathological roles involved are complex and not yet fully understood, and the related therapeutic approaches are not well developed. In this review, we describe insulin resistance and add recent advances in the major pathological and physiological changes and underlying mechanisms by which insulin resistance leads to myocardial remodeling and dysfunction in the diabetic heart, including exosomal dysfunction, ferroptosis, and epigenetic factors. In addition, we discuss potential therapeutic approaches to improve insulin resistance and accelerate the development of cardiovascular protection drugs.
- Basic Research
- Pharmacologic Activation of Angiotensin-Converting Enzyme II Alleviates Diabetic Cardiomyopathy in db/db Mice by Reducing Reactive Oxidative Stress
- Donghyun Kim, Wooju Jeong, Yumin Kim, Jibeom Lee, Sung Woo Cho, Chang-Myung Oh, Raekil Park
- Diabetes Metab J. 2023;47(4):487-499. Published online April 25, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0125
- 2,327 View
- 150 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
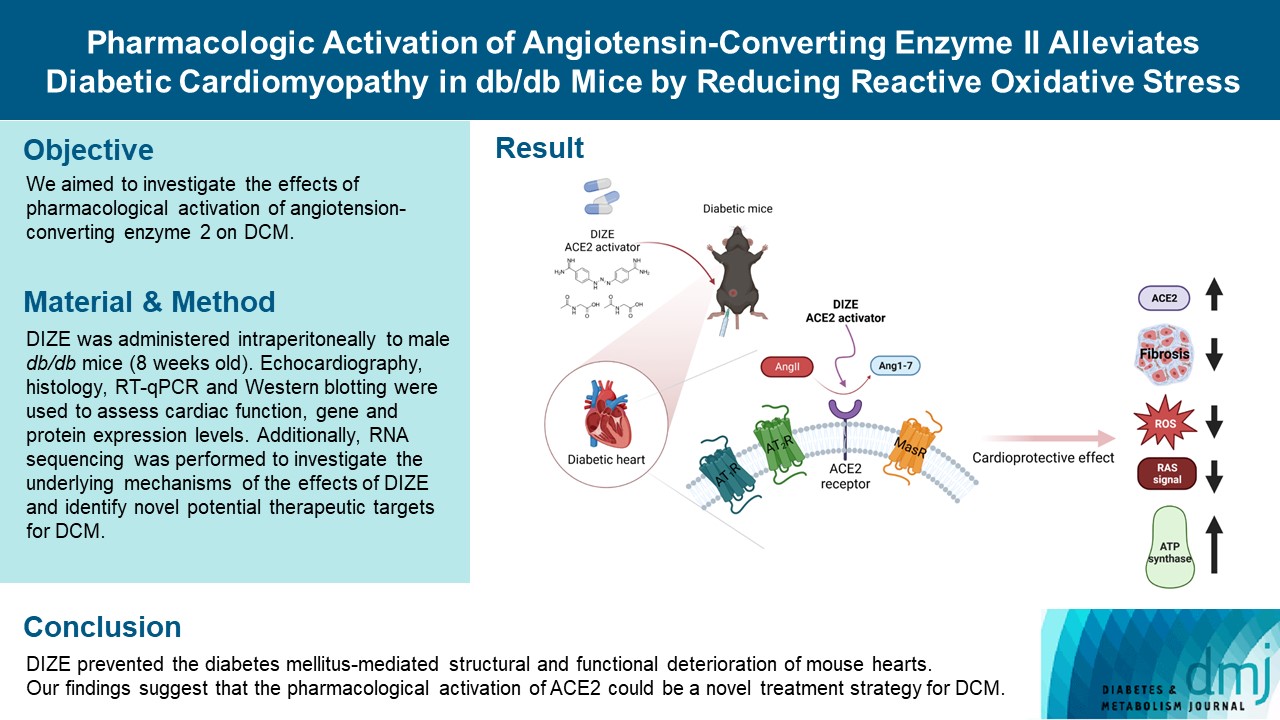
ePub - Background
Diabetes mellitus is one of the most common chronic diseases worldwide, and cardiovascular disease is the leading cause of morbidity and mortality in diabetic patients. Diabetic cardiomyopathy (DCM) is a phenomenon characterized by a deterioration in cardiac function and structure, independent of vascular complications. Among many possible causes, the renin-angiotensin-aldosterone system and angiotensin II have been proposed as major drivers of DCM development. In the current study, we aimed to investigate the effects of pharmacological activation of angiotensin-converting enzyme 2 (ACE2) on DCM.
Methods
The ACE2 activator diminazene aceturate (DIZE) was administered intraperitoneally to male db/db mice (8 weeks old) for 8 weeks. Transthoracic echocardiography was used to assess cardiac mass and function in mice. Cardiac structure and fibrotic changes were examined using histology and immunohistochemistry. Gene and protein expression levels were examined using quantitative reverse transcription polymerase chain reaction and Western blotting, respectively. Additionally, RNA sequencing was performed to investigate the underlying mechanisms of the effects of DIZE and identify novel potential therapeutic targets for DCM.
Results
Echocardiography revealed that in DCM, the administration of DIZE significantly improved cardiac function as well as reduced cardiac hypertrophy and fibrosis. Transcriptome analysis revealed that DIZE treatment suppresses oxidative stress and several pathways related to cardiac hypertrophy.
Conclusion
DIZE prevented the diabetes mellitus-mediated structural and functional deterioration of mouse hearts. Our findings suggest that the pharmacological activation of ACE2 could be a novel treatment strategy for DCM.
- Basic Research
- Application of Animal Models in Diabetic Cardiomyopathy
- Wang-Soo Lee, Jaetaek Kim
- Diabetes Metab J. 2021;45(2):129-145. Published online March 25, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0285
- 9,199 View
- 332 Download
- 9 Web of Science
- 14 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub 
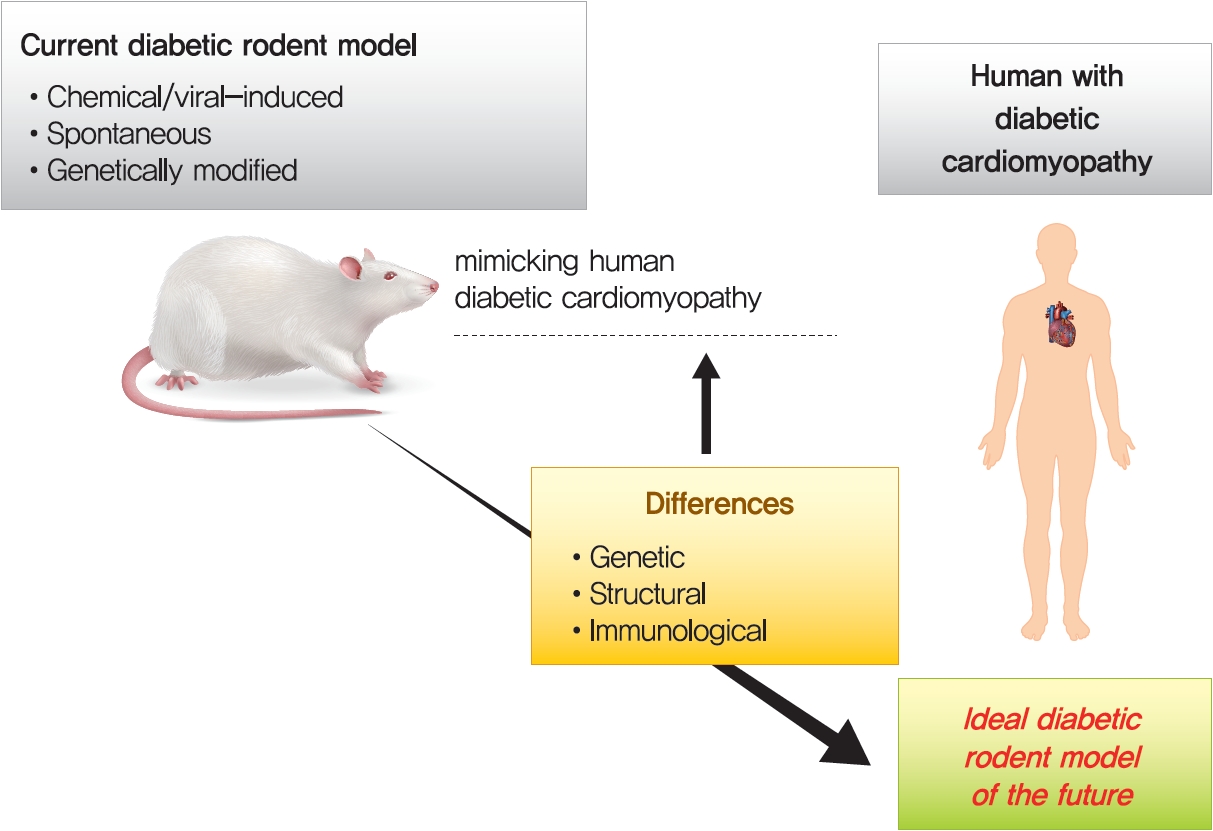
- Diabetic heart disease is a growing and important public health risk. Apart from the risk of coronary artery disease or hypertension, diabetes mellitus (DM) is a well-known risk factor for heart failure in the form of diabetic cardiomyopathy (DiaCM). Currently, DiaCM is defined as myocardial dysfunction in patients with DM in the absence of coronary artery disease and hypertension. The underlying pathomechanism of DiaCM is partially understood, but accumulating evidence suggests that metabolic derangements, oxidative stress, increased myocardial fibrosis and hypertrophy, inflammation, enhanced apoptosis, impaired intracellular calcium handling, activation of the renin-angiotensin-aldosterone system, mitochondrial dysfunction, and dysregulation of microRNAs, among other factors, are involved. Numerous animal models have been used to investigate the pathomechanisms of DiaCM. Despite some limitations, animal models for DiaCM have greatly advanced our understanding of pathomechanisms and have helped in the development of successful disease management strategies. In this review, we summarize the current pathomechanisms of DiaCM and provide animal models for DiaCM according to its pathomechanisms, which may contribute to broadening our understanding of the underlying mechanisms and facilitating the identification of possible new therapeutic targets.
-
Citations
Citations to this article as recorded by- Chitosan Versus Dapagliflozin in a Diabetic Cardiomyopathy Mouse Model
Georgică Târtea, Aurel Popa-Wagner, Veronica Sfredel, Smaranda Ioana Mitran, Alexandra Oltea Dan, Anca-Maria Țucă, Alexandra Nicoleta Preda, Victor Raicea, Eugen Țieranu, Dragoș Cozma, Radu Vătășescu
International Journal of Molecular Sciences.2024; 25(4): 2118. CrossRef - Mitochondrial energy metabolism in diabetic cardiomyopathy: Physiological adaption, pathogenesis, and therapeutic targets
Wanlin Ye, Kun Han, Maodi Xie, Sheyu Li, Guo Chen, Yanyan Wang, Tao Li
Chinese Medical Journal.2024; 137(8): 936. CrossRef - Liraglutide Attenuates Diabetic Cardiomyopathy via the ILK/PI3K/AKT/PTEN Signaling Pathway in Rats with Streptozotocin-Induced Type 2 Diabetes Mellitus
Shatha M. Alobaid, Rahaf M. Alshahrani, Asma S. Alonazi, Nawal M. Alrasheed, Maha A. Alamin, Tahani K. Alshammari, Anfal F. Bin Dayel, Doaa M. Elnagar, Rana R. Alotaibi, Lama A. Almuthnabi, Dalia H. Almasud, Shahad E. Al-Ammar, Shahad O. Almadhi, Reema A.
Pharmaceuticals.2024; 17(3): 374. CrossRef - An Overview of Diabetic Cardiomyopathy
Abdul Quaiyoom, Ranjeet Kumar
Current Diabetes Reviews.2024;[Epub] CrossRef - Evaluation and Management of Patients With Diabetes and Heart Failure: A Korean Diabetes Association and Korean Society of Heart Failure Consensus Statement
Kyu-Sun Lee, Junghyun Noh, Seong-Mi Park, Kyung Mook Choi, Seok-Min Kang, Kyu-Chang Won, Hyun-Jai Cho, Min Kyong Moon
International Journal of Heart Failure.2023; 5(1): 1. CrossRef - Evaluation and Management of Patients with Diabetes and Heart Failure: A Korean Diabetes Association and Korean Society of Heart Failure Consensus Statement
Kyu-Sun Lee, Junghyun Noh, Seong-Mi Park, Kyung Mook Choi, Seok-Min Kang, Kyu-Chang Won, Hyun-Jai Cho, Min Kyong Moon
Diabetes & Metabolism Journal.2023; 47(1): 10. CrossRef - Machine learning for spatial stratification of progressive cardiovascular dysfunction in a murine model of type 2 diabetes mellitus
Andrya J. Durr, Anna S. Korol, Quincy A. Hathaway, Amina Kunovac, Andrew D. Taylor, Saira Rizwan, Mark V. Pinti, John M. Hollander, Yoshihiro Fukumoto
PLOS ONE.2023; 18(5): e0285512. CrossRef - Hyperglycemic memory in diabetic cardiomyopathy
Jiabing Zhan, Chen Chen, Dao Wen Wang, Huaping Li
Frontiers of Medicine.2022; 16(1): 25. CrossRef - Murine Models of Obesity
Tânia Martins, Catarina Castro-Ribeiro, Sílvia Lemos, Tiago Ferreira, Elisabete Nascimento-Gonçalves, Eduardo Rosa, Paula Alexandra Oliveira, Luís Miguel Antunes
Obesities.2022; 2(2): 127. CrossRef - The Role of Mitochondria in Metabolic Syndrome–Associated Cardiomyopathy
Jiayu Li, Jingye Li, Yijun Chen, Wenyu Hu, Xuhe Gong, Hui Qiu, Hui Chen, Yanguo Xin, Hongwei Li, Tao Li
Oxidative Medicine and Cellular Longevity.2022; 2022: 1. CrossRef - Guidelines on models of diabetic heart disease
Lisa C. Heather, Anne D. Hafstad, Ganesh V. Halade, Romain Harmancey, Kimberley M. Mellor, Paras K. Mishra, Erin E. Mulvihill, Miranda Nabben, Michinari Nakamura, Oliver J. Rider, Matthieu Ruiz, Adam R. Wende, John R. Ussher
American Journal of Physiology-Heart and Circulatory Physiology.2022; 323(1): H176. CrossRef - Extracellular vesicle therapy for non-ischemic heart failure: A systematic review of preclinical studies
Ramana Vaka, Sophie Van Remortel, Valentina Ly, Darryl R. Davis
Extracellular Vesicle.2022; 1: 100009. CrossRef - Effect of a Six-week Endurance Exercise Program and Empagliflozin Consumption on Some Structural and Functional Indices of the Heart in Male Diabetic Rats
Eftekhar Mohammadi, Mohammad Fathi, Farzaneh Chehel Cheraghi, Afshin Nazari
journal of ilam university of medical sciences.2022; 30(3): 1. CrossRef - Cardiac Phosphodiesterases Are Differentially Increased in Diabetic Cardiomyopathy
Rita Hanna, Wared Nour-Eldine, Youakim Saliba, Carole Dagher-Hamalian, Pia Hachem, Pamela Abou-Khalil, Delphine Mika, Audrey Varin, Magali Samia El Hayek, Laëtitia Pereira, Nassim Farès, Grégoire Vandecasteele, Aniella Abi-Gerges
Life Sciences.2021; 283: 119857. CrossRef
- Chitosan Versus Dapagliflozin in a Diabetic Cardiomyopathy Mouse Model
- Cardiovascular Risk/Epidemiology
- Epidemiology, Pathophysiology, Diagnosis and Treatment of Heart Failure in Diabetes
- Jin Joo Park
- Diabetes Metab J. 2021;45(2):146-157. Published online March 25, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0282
- Correction in: Diabetes Metab J 2021;45(5):796

- 13,879 View
- 1,211 Download
- 51 Web of Science
- 56 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
- The cardiovascular disease continuum begins with risk factors such as diabetes mellitus (DM), progresses to vasculopathy and myocardial dysfunction, and finally ends with cardiovascular death. Diabetes is associated with a 2- to 4-fold increased risk for heart failure (HF). Moreover, HF patients with DM have a worse prognosis than those without DM. Diabetes can cause myocardial ischemia via micro- and macrovasculopathy and can directly exert deleterious effects on the myocardium. Hyperglycemia, hyperinsulinemia, and insulin resistance can cause alterations in vascular homeostasis. Then, reduced nitric oxide and increased reactive oxygen species levels favor inflammation leading to atherothrombotic progression and myocardial dysfunction. The classification, diagnosis, and treatment of HF for a patient with and without DM remain the same. Until now, drugs targeting neurohumoral and metabolic pathways improved mortality and morbidity in HF with reduced ejection fraction (HFrEF). Therefore, all HFrEF patients should receive guideline-directed medical therapy. By contrast, drugs modulating neurohumoral activity did not improve survival in HF with preserved ejection fraction (HFpEF) patients. Trials investigating whether sodium-glucose cotransporter-2 inhibitors are effective in HFpEF are on-going. This review will summarize the epidemiology, pathophysiology, and treatment of HF in diabetes.
-
Citations
Citations to this article as recorded by- Efficacy of Platelet-Rich Plasma in the Treatment of Diabetic Foot Ulcers: A Systematic Review and Meta-Analysis
Yundong Peng, JiePing Wang, Xinyao Liu, Yanqing Zhou, Shaohui Jia, Jinrong Xu, Cheng Zheng
Annals of Vascular Surgery.2024; 98: 365. CrossRef - Diabetic Cardiomyopathy: 2023 Update by the International Multidisciplinary Board of Experts
Ashot Avagimyan, Federica Fogacci, Nana Pogosova, Lev Kakrurskiy, Eugenia Kogan, Olga Urazova, Zhanna Kobalava, Liudmila Mikhaleva, Rositsa Vandysheva, Gioeva Zarina, Artem Trofimenko, Grizelda Navasardyan, Lusine Mkrtchyan, Mattia Galli, Zinaida Jndoyan,
Current Problems in Cardiology.2024; 49(1): 102052. CrossRef - Emerging Biomarkers in the Laboratory and in Practice: A Novel Approach to Diagnosing Heart Failure in Diabetes
Rachel E. Aaron, Tiffany Tian, G. Alexander Fleming, David B. Sacks, James L. Januzzi, MD FACC, Rodica Pop-Busui, Ibrahim A. Hashim, Alan H. B. Wu, Ambarish Pandey, David C. Klonoff
Journal of Diabetes Science and Technology.2024; 18(3): 733. CrossRef - Research Progress on the Relationship between HbA1c and Diabetes Complicated with Chronic Heart Failure
·依沙 迪达尔
Advances in Clinical Medicine.2024; 14(01): 1117. CrossRef - Association Between Use of Sodium-Glucose Cotransporter-2 Inhibitors or Angiotensin Receptor-Neprilysin Inhibitor and the Risk of Atherosclerotic Cardiovascular Disease With Coexisting Diabetes and Heart Failure
Ya-Wen Lin, Chun-Hsiang Lin, Cheng-Li Lin, Che-Huei Lin, Ming-Hung Lin
Journal of Cardiovascular Pharmacology and Therapeutics.2024;[Epub] CrossRef - The Role of Epicardial Adipose Tissue in Acute Coronary Syndromes, Post-Infarct Remodeling and Cardiac Regeneration
Kamil Krauz, Marcel Kempiński, Paweł Jańczak, Karol Momot, Maciej Zarębiński, Izabela Poprawa, Małgorzata Wojciechowska
International Journal of Molecular Sciences.2024; 25(7): 3583. CrossRef - Molecular mechanisms of metabolic dysregulation in diabetic cardiomyopathy
Yue Zeng, Yilang Li, Wenyue Jiang, Ning Hou
Frontiers in Cardiovascular Medicine.2024;[Epub] CrossRef - Association between nonalcoholic fatty liver disease and left ventricular diastolic dysfunction: A 7-year retrospective cohort study of 3,380 adults using serial echocardiography
Gyuri Kim, Tae Yang Yu, Jae Hwan Jee, Ji Cheol Bae, Mira Kang, Jae Hyeon Kim
Diabetes & Metabolism.2024; 50(3): 101534. CrossRef - SGLT2 Inhibitors in the Cardiovascular Disease
Jin Joo Park
The Journal of Korean Diabetes.2024; 25(1): 26. CrossRef - Metabolic Alteration Bridging the Prediabetic State and Colorectal Cancer
Antonino Colloca, Isabella Donisi, Camilla Anastasio, Maria Luisa Balestrieri, Nunzia D’Onofrio
Cells.2024; 13(8): 663. CrossRef - Inflammation in diabetes complications: molecular mechanisms and therapeutic interventions
Lu Zhao, Haoran Hu, Lin Zhang, Zheting Liu, Yunchao Huang, Qian Liu, Liang Jin, Meifei Zhu, Ling Zhang
MedComm.2024;[Epub] CrossRef - Methods to predict heart failure in diabetes patients
Alexander E. Berezin, Tetiana A Berezina, Uta C. Hoppe, Michael Lichtenauer, Alexander A. Berezin
Expert Review of Endocrinology & Metabolism.2024; : 1. CrossRef - Research advances on molecular mechanism and natural product therapy of iron metabolism in heart failure
Tianqing Zhang, Li Luo, Qi He, Sijie Xiao, Yuwei Li, Junpeng Chen, Tao Qin, Zhenni Xiao, Qingliang Ge
European Journal of Medical Research.2024;[Epub] CrossRef - The latest emerging drugs for the treatment of diabetic cardiomyopathy
Minghao Li, Lin Liu, Chunyu Zhang, Li Deng, Yi Zhong, Bin Liao, Xiuying Li, Ying Wan, Jian Feng
Expert Opinion on Pharmacotherapy.2024; : 1. CrossRef - Association between Dapagliflozin, Cardiac Biomarkers and Cardiac Remodeling in Patients with Diabetes Mellitus and Heart Failure
Andrew Xanthopoulos, Nikolaos Katsiadas, Spyridon Skoularigkis, Dimitrios E. Magouliotis, Niki Skopeliti, Sotirios Patsilinakos, Alexandros Briasoulis, Filippos Triposkiadis, John Skoularigis
Life.2023; 13(8): 1778. CrossRef - Empagliflozin for Patients with Heart Failure and Type 2 Diabetes Mellitus: Clinical Evidence in Comparison with Other Sodium-Glucose Co-transporter-2 Inhibitors and Potential Mechanism
Bo Liang, Rui Li, Peng Zhang, Ning Gu
Journal of Cardiovascular Translational Research.2023; 16(2): 327. CrossRef - Causes and Determinants of Heart Failure Readmissions Post Transcutaneous Aortic Valve Replacement: A Systematic Review and Meta-Analysis
Farah Yasmin, Muhammad Aamir, Abdul Moeed, Kinza Iqbal, Aymen Iqbal, Muhammad Sohaib Asghar, Waqas Ullah, Indranee Rajapreyar, Yevgeniy Brailovsky
Current Problems in Cardiology.2023; 48(1): 101428. CrossRef - Discussion of a study on the role of EMS in prognosis of elderly patients with AHF
Qingzhuo Yang, Hui Wu, Di Liu, Yunzhao Li, Gang Zhou, Dong Zhang, Yanfang Liu, Yi Li
International Journal of Cardiology.2023; 377: 91. CrossRef - The management correlation between metabolic index, cardiovascular health, and diabetes combined with cardiovascular disease
Yi Zhang, Chao Liu, Yijing Xu, Yanlei Wang, Fang Dai, Honglin Hu, Tian Jiang, Yunxia Lu, Qiu Zhang
Frontiers in Endocrinology.2023;[Epub] CrossRef - Evaluation and Management of Patients With Diabetes and Heart Failure: A Korean Diabetes Association and Korean Society of Heart Failure Consensus Statement
Kyu-Sun Lee, Junghyun Noh, Seong-Mi Park, Kyung Mook Choi, Seok-Min Kang, Kyu-Chang Won, Hyun-Jai Cho, Min Kyong Moon
International Journal of Heart Failure.2023; 5(1): 1. CrossRef - Evaluation and Management of Patients with Diabetes and Heart Failure: A Korean Diabetes Association and Korean Society of Heart Failure Consensus Statement
Kyu-Sun Lee, Junghyun Noh, Seong-Mi Park, Kyung Mook Choi, Seok-Min Kang, Kyu-Chang Won, Hyun-Jai Cho, Min Kyong Moon
Diabetes & Metabolism Journal.2023; 47(1): 10. CrossRef - Lower Circulating Cell-Free Mitochondrial DNA Is Associated with Heart Failure in Type 2 Diabetes Mellitus Patients
Tetiana A. Berezina, Mykola P. Kopytsya, Olga V. Petyunina, Alexander A. Berezin, Zeljko Obradovic, Lukas Schmidbauer, Michael Lichtenauer, Alexander E. Berezin
Cardiogenetics.2023; 13(1): 15. CrossRef - Novel model predicts diastolic cardiac dysfunction in type 2 diabetes
Mingyu Hao, Xiaohong Huang, Xueting Liu, Xiaokang Fang, Haiyan Li, Lingbo Lv, Liming Zhou, Tiecheng Guo, Dewen Yan
Annals of Medicine.2023; 55(1): 766. CrossRef - Cholinergic drugs reduce metabolic inflammation and diabetic myocardial injury by regulating the gut bacterial component lipopolysaccharide‐induced ERK/Egr‐1 pathway
Qing Wu, Ming Zhao, Dongling Li, Xi He, Weijin Zang
The FASEB Journal.2023;[Epub] CrossRef - Ferroptosis: roles and molecular mechanisms in diabetic cardiomyopathy
Yangting Zhao, Binjing Pan, Xiaoyu Lv, Chongyang Chen, Kai Li, Yawen Wang, Jingfang Liu
Frontiers in Endocrinology.2023;[Epub] CrossRef - Cardiorenal syndrome and diabetes: an evil pairing
Ana Belén Méndez Fernández, Ander Vergara Arana, Aleix Olivella San Emeterio, Maria Antonieta Azancot Rivero, Toni Soriano Colome, Maria Jose Soler Romeo
Frontiers in Cardiovascular Medicine.2023;[Epub] CrossRef - Therapeutic Potential of Hibiscus sabdariffa Linn. in Attenuating Cardiovascular Risk Factors
Syaifuzah Sapian, Asma Ali Ibrahim Mze, Fatin Farhana Jubaidi, Nor Anizah Mohd Nor, Izatus Shima Taib, Zariyantey Abd Hamid, Satirah Zainalabidin, Nur Najmi Mohamad Anuar, Haliza Katas, Jalifah Latip, Juriyati Jalil, Nur Faizah Abu Bakar, Siti Balkis Budi
Pharmaceuticals.2023; 16(6): 807. CrossRef - Exploring the prospect of intrinsic wave propagation in evaluating myocardial stiffness among patients with type 2 diabetes
Qiao Cheng, Xiao Huang, Xinying Fan, Jie Sun, Jun Zhang, Qiaoying Tang, Youbin Deng, Xiaojun Bi
Frontiers in Cardiovascular Medicine.2023;[Epub] CrossRef - The multidimensional prognostic index (MPI) predicts long-term mortality in old type 2 diabetes mellitus patients: a 13-year follow-up study
F. Salis, E. Cossu, A. Mandas
Journal of Endocrinological Investigation.2023; 47(1): 191. CrossRef - PRDM16 exerts critical role in myocardial metabolism and energetics in type 2 diabetes induced cardiomyopathy
Tongtong Hu, Qingqing Wu, Qi Yao, Jiabin Yu, Kebing Jiang, Ying Wan, Qizhu Tang
Metabolism.2023; 146: 155658. CrossRef - Empagliflozin Reduces Interleukin-6 Levels in Patients with Heart Failure
Michael Gotzmann, Pauline Henk, Ulrik Stervbo, Arturo Blázquez-Navarro, Andreas Mügge, Nina Babel, Timm H. Westhoff
Journal of Clinical Medicine.2023; 12(13): 4458. CrossRef - Features of the course and complications of acute myocardial infarction in type 2 diabetes mellitus
M. N. Zatsepina, F. O. Ushanova, T. L. Bogacheva
FOCUS. Endocrinology.2023; 4(2): 42. CrossRef - Admission triglyceride-glucose index is predictor of long-term mortality and appropriate implantable cardiac defibrillator therapy in patients with heart failure
Kazım S Özcan, Mert İ Hayıroğlu, Tufan Çınar
Biomarkers in Medicine.2023; 17(10): 487. CrossRef - Emerging Roles of Phospholipase C Beta Isozymes as Potential Biomarkers in Cardiac Disorders
Antonietta Fazio, Camilla Evangelisti, Alessandra Cappellini, Sara Mongiorgi, Foteini-Dionysia Koufi, Irene Neri, Maria Vittoria Marvi, Michele Russo, Alessandra Ghigo, Lucia Manzoli, Roberta Fiume, Stefano Ratti
International Journal of Molecular Sciences.2023; 24(17): 13096. CrossRef - Research progress of dihydromyricetin in the treatment of diabetes mellitus
Ziyuan Wang, Zhuoran Cao, Zhiying Yue, Zhengfeng Yang
Frontiers in Endocrinology.2023;[Epub] CrossRef - Assessment of subclinical left ventricular myocardial systolic dysfunction in type 2 diabetes mellitus patients with or without hypertension by global and segmental myocardial work
Guang-An Li, Jun Huang, Xiao Sheng, Li Fan
Diabetology & Metabolic Syndrome.2023;[Epub] CrossRef - Managing heart failure in diabetics with dual acting sotagliflozin—A review
Kushal Seni, Pooja A Chawla
Health Sciences Review.2023; 9: 100130. CrossRef - Association between triglyceride glucose-body mass index and heart failure in subjects with diabetes mellitus or prediabetes mellitus: a cross-sectional study
Shuping Yang, Xiangxiang Shi, Wanlu Liu, Zhaokai Wang, Ruoshui Li, Xianzhi Xu, Chaofan Wang, Lei Li, Ruili Wang, Tongda Xu
Frontiers in Endocrinology.2023;[Epub] CrossRef - Influence of Sociodemographic Variables and Healthy Habits on the Values of Insulin Resistance Indicators in 386,924 Spanish Workers
Miguel Mestre Font, Carla Busquets-Cortés, José Ignacio Ramírez-Manent, Pilar Tomás-Gil, Hernán Paublini, Ángel Arturo López-González
Nutrients.2023; 15(24): 5122. CrossRef - The effect of dapagliflozin on ventricular arrhythmias, cardiac arrest, or sudden death in people with heart failure: a tick in another box for sodium-glucose cotransporter 2 inhibitors
Theocharis Koufakis, George Giannakoulas, Pantelis Zebekakis, Kalliopi Kotsa
Expert Opinion on Pharmacotherapy.2022; 23(3): 321. CrossRef - The peculiar role of vitamin D in the pathophysiology of cardiovascular and neurodegenerative diseases
Milijana Janjusevic, Giulia Gagno, Alessandra Lucia Fluca, Laura Padoan, Antonio Paolo Beltrami, Gianfranco Sinagra, Rita Moretti, Aneta Aleksova
Life Sciences.2022; 289: 120193. CrossRef - Mechanisms of cardiac dysfunction in diabetic cardiomyopathy: molecular abnormalities and phenotypical variants
Francesca Romana Prandi, Isabella Evangelista, Domenico Sergi, Alberto Palazzuoli, Francesco Romeo
Heart Failure Reviews.2022; 28(3): 597. CrossRef - Modern Approaches to Treatment of Chronic Heart Failure in Patients with Type 2 Diabetes Mellitus
Yu. G. Gorb, S. A. Serik, O. V. Tkachenko, V. V. Ryabukha
Ukraïnsʹkij žurnal medicini, bìologìï ta sportu.2022; 7(1): 14. CrossRef - Metabonomic Characteristics of Myocardial Diastolic Dysfunction in Type 2 Diabetic Cardiomyopathy Patients
Mingyu Hao, Jianxin Deng, Xiaohong Huang, Haiyan Li, Huiting Ou, Xiangsheng Cai, Jiajie She, Xueting Liu, Ling Chen, Shujuan Chen, Wenlan Liu, Dewen Yan
Frontiers in Physiology.2022;[Epub] CrossRef - Serum Levels of Irisin Predict Cumulative Clinical Outcomes in Heart Failure Patients With Type 2 Diabetes Mellitus
Alexander A. Berezin, Michael Lichtenauer, Elke Boxhammer, Ivan M. Fushtey, Alexander E. Berezin
Frontiers in Physiology.2022;[Epub] CrossRef - Heart Failure: An Underappreciated Complication of Diabetes. A Consensus Report of the American Diabetes Association
Rodica Pop-Busui, James L. Januzzi, Dennis Bruemmer, Sonia Butalia, Jennifer B. Green, William B. Horton, Colette Knight, Moshe Levi, Neda Rasouli, Caroline R. Richardson
Diabetes Care.2022; 45(7): 1670. CrossRef - Von Willebrand factor (vWF) in patients with heart failure with preserved ejection fraction (HFpEF): A retrospective observational study
Mayila Abudoukelimu, Bayinsilema Ba, Yan Kai Guo, Jie Xu
Medicine.2022; 101(31): e29854. CrossRef - Why Does the Intravenous Iron Supplementation Not Work in Heart Failure Patients on Hemodialysis?
Jin Joo Park
CardioMetabolic Syndrome Journal.2022; 2(2): 176. CrossRef - Severe hypoglycemia and risk of hospitalization for heart failure in adults with diabetes treated with oral medications with or without insulin: A population-based study
You-Bin Lee, Yoon-Jong Bae, Hoseob Kim, Jiyun Park, So Yoon Kwon, So Hee Park, Gyuri Kim, Kyu Yeon Hur, Jae Hyeon Kim, Sang-Man Jin
Diabetes Research and Clinical Practice.2022; 192: 110083. CrossRef - Blood Pressure Target in Type 2 Diabetes Mellitus
Hyun-Jin Kim, Kwang-il Kim
Diabetes & Metabolism Journal.2022; 46(5): 667. CrossRef - Application Value of Systemic Inflammatory Indexes in the Clinical Evaluation of Patients with Heart Failure with Preserved Ejection Fraction (HFpEF)
Ruxin Wang, Juan Wu, Haowen Ye, Xiaofang Zhang, Lihong Wang
Medicina.2022; 58(10): 1473. CrossRef - HFpEF and Atrial Fibrillation: The Enigmatic Interplay of Dysmetabolism, Biomarkers, and Vascular Endothelial Dysfunction
Jure Bosanac, Lara Straus, Marko Novaković, Daniel Košuta, Mojca Božič Mijovski, Jerneja Tasič, Borut Jug, Azizah Ugusman
Disease Markers.2022; 2022: 1. CrossRef - The importance of caveolin as a target in the prevention and treatment of diabetic cardiomyopathy
Weiyi Xia, Xia Li, Qingping Wu, Aimin Xu, Liangqing Zhang, Zhengyuan Xia
Frontiers in Immunology.2022;[Epub] CrossRef - Microvascular Burden and Incident Heart Failure Among Middle-Aged and Older Adults With Type 1 or Type 2 Diabetes
Fu-Rong Li, Daniel Nyarko Hukportie, Jing Yang, Huan-Huan Yang, Guo-Chong Chen, Xian-Bo Wu
Diabetes Care.2022; 45(12): 2999. CrossRef - C-reactive protein and statins in heart failure with reduced and preserved ejection fraction
Jin Joo Park, Minjae Yoon, Hyoung-Won Cho, Hyun-Jai Cho, Kye Hun Kim, Dong Heon Yang, Byung-Su Yoo, Seok-Min Kang, Sang Hong Baek, Eun-Seok Jeon, Jae-Joong Kim, Myeong-Chan Cho, Shung Chull Chae, Byung-Hee Oh, Dong-Ju Choi
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Pharmacological treatment of type 2 diabetes in elderly patients with heart failure: randomized trials and beyond
Angela Sciacqua, Elena Succurro, Giuseppe Armentaro, Sofia Miceli, Daniele Pastori, Giuseppe Rengo, Giorgio Sesti
Heart Failure Reviews.2021; 28(3): 667. CrossRef
- Efficacy of Platelet-Rich Plasma in the Treatment of Diabetic Foot Ulcers: A Systematic Review and Meta-Analysis
- Basic Research
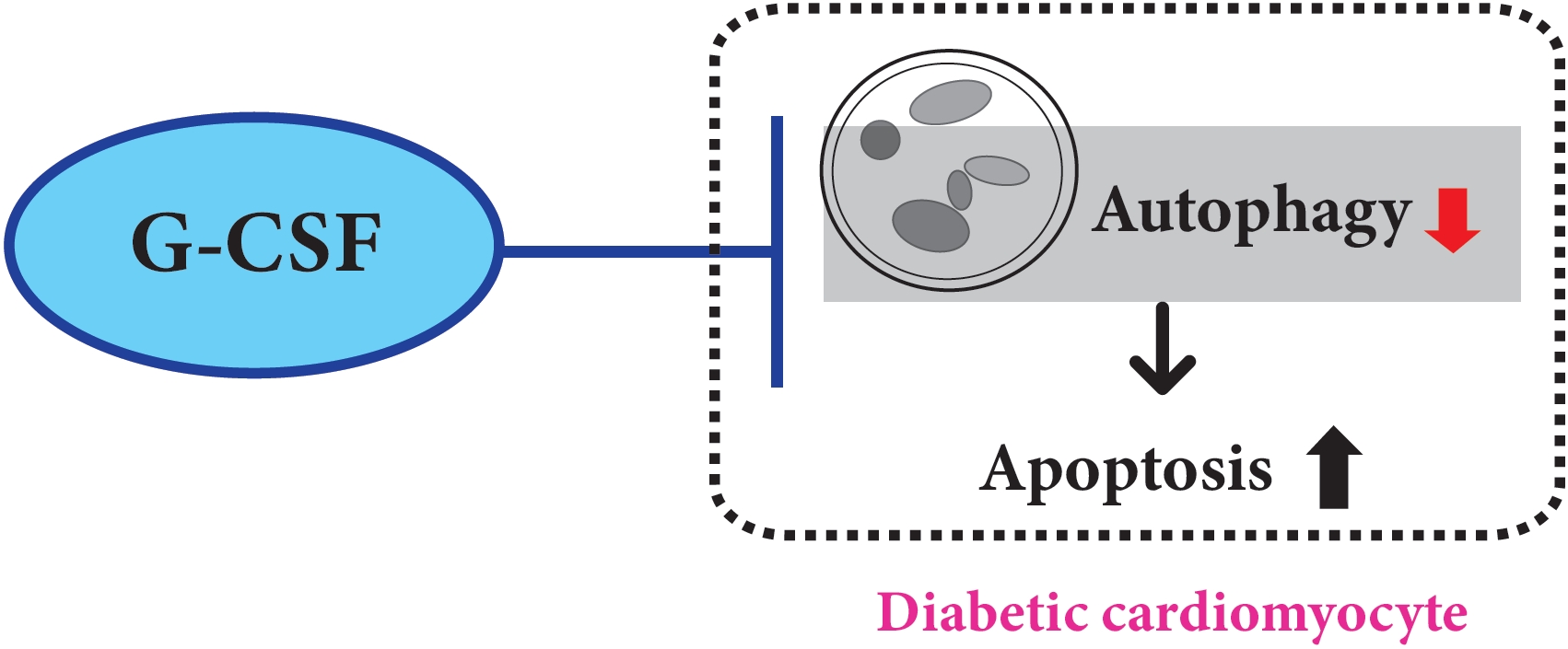
- Role of Autophagy in Granulocyte-Colony Stimulating Factor Induced Anti-Apoptotic Effects in Diabetic Cardiomyopathy
- Guang-Yin Shen, Jeong-Hun Shin, Yi-Sun Song, Hyun-Woo Joo, In-Hwa Park, Jin-Hee Seong, Na-Kyoung Shin, A-Hyeon Lee, Young Jong Cho, Yonggu Lee, Young-Hyo Lim, Hyuck Kim, Kyung-Soo Kim
- Diabetes Metab J. 2021;45(4):594-605. Published online February 26, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0049
- 7,254 View
- 147 Download
- 3 Web of Science
- 2 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
- Background
We previously, reported that granulocyte-colony stimulating factor (G-CSF) reduces cardiomyocyte apoptosis in diabetic cardiomyopathy. However, the underlying mechanisms are not yet fully understood. Therefore, we investigated whether the mechanisms underlying of the anti-apoptotic effects of G-CSF were associated with autophagy using a rat model of diabetic cardiomyopathy.
Methods
Diabetic cardiomyopathy was induced in rats through a high-fat diet combined with low-dose streptozotocin and the rats were then treated with G-CSF for 5 days. Rat H9c2 cardiac cells were cultured under high glucose conditions as an in vitro model of diabetic cardiomyopathy. The extent of apoptosis and protein levels related to autophagy (Beclin-1, microtubule-binding protein light chain 3 [LC3]-II/LC3-I ratio, and P62) were determined for both models. Autophagy determination was performed using an Autophagy Detection kit.
Results
G-CSF significantly reduced cardiomyocyte apoptosis in the diabetic myocardium in vivo and led to an increase in Beclin-1 level and the LC3-II/LC3-I ratio, and decreased P62 level. Similarly, G-CSF suppressed apoptosis, increased Beclin-1 level and LC3-II/LC3-I ratio, and decreased P62 level in high glucose-induced H9c2 cardiac cells in vitro. These effects of G-CSF were abrogated by 3-methyladenine, an autophagy inhibitor. In addition, G-CSF significantly increased autophagic flux in vitro.
Conclusion
Our results suggest that the anti-apoptotic effect of G-CSF might be significantly associated with the up-regulation of autophagy in diabetic cardiomyopathy. -
Citations
Citations to this article as recorded by- Ginkgo biloba extract protects against diabetic cardiomyopathy by restoring autophagy via adenosine monophosphate‐activated protein kinase/mammalian target of the rapamycin pathway modulation
Xueyao Yang, Xin Zhao, Yanfei Liu, Yue Liu, Libo Liu, Ziyu An, Haoran Xing, Jinfan Tian, Xiantao Song
Phytotherapy Research.2023; 37(4): 1377. CrossRef - Perspectives for Forkhead box transcription factors in diabetic cardiomyopathy: Their therapeutic potential and possible effects of salvianolic acids
Ronghui Han, Hemeng Huang, Weiyi Xia, Jingjin Liu, Hui Luo, Jing Tang, Zhengyuan Xia
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef
- Ginkgo biloba extract protects against diabetic cardiomyopathy by restoring autophagy via adenosine monophosphate‐activated protein kinase/mammalian target of the rapamycin pathway modulation
- Basic Research
- Mitochondrial Mechanisms in Diabetic Cardiomyopathy
- Johannes Gollmer, Andreas Zirlik, Heiko Bugger
- Diabetes Metab J. 2020;44(1):33-53. Published online February 21, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0185
- 10,262 View
- 214 Download
- 57 Web of Science
- 58 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Mitochondrial medicine is increasingly discussed as a promising therapeutic approach, given that mitochondrial defects are thought to contribute to many prevalent diseases and their complications. In individuals with diabetes mellitus (DM), defects in mitochondrial structure and function occur in many organs throughout the body, contributing both to the pathogenesis of DM and complications of DM. Diabetic cardiomyopathy (DbCM) is increasingly recognized as an underlying cause of increased heart failure in DM, and several mitochondrial mechanisms have been proposed to contribute to the development of DbCM. Well established mechanisms include myocardial energy depletion due to impaired adenosine triphosphate (ATP) synthesis and mitochondrial uncoupling, and increased mitochondrial oxidative stress. A variety of upstream mechanisms of impaired ATP regeneration and increased mitochondrial reactive oxygen species have been proposed, and recent studies now also suggest alterations in mitochondrial dynamics and autophagy, impaired mitochondrial Ca2+ uptake, decreased cardiac adiponectin action, increased O-GlcNAcylation, and impaired activity of sirtuins to contribute to mitochondrial defects in DbCM, among others. In the current review, we present and discuss the evidence that underlies both established and recently proposed mechanisms that are thought to contribute to mitochondrial dysfunction in DbCM.
-
Citations
Citations to this article as recorded by- SIRT1: a promising therapeutic target in type 2 diabetes mellitus
Ainaz Mihanfar, Maryam Akbarzadeh, Saber Ghazizadeh Darband, Shirin Sadighparvar, Maryam Majidinia
Archives of Physiology and Biochemistry.2024; 130(1): 13. CrossRef - Effect of exercise on improving myocardial mitochondrial function in decreasing diabetic cardiomyopathy
Feng Zhang, Jian jian Lin, Hao nan Tian, Jun Wang
Experimental Physiology.2024; 109(2): 190. CrossRef - Cardioprotective effects of asiaticoside against diabetic cardiomyopathy: Activation of the AMPK/Nrf2 pathway
Chennian Xu, Lin Xia, Dengyue Xu, Yang Liu, Ping Jin, Mengen Zhai, Yu Mao, Yiwei Wang, Anguo Wen, Jian Yang, Lifang Yang
Journal of Cellular and Molecular Medicine.2024;[Epub] CrossRef - Ferroptosis: Mechanisms and role in diabetes mellitus and its complications
Pan Liu, Zhengdong Zhang, Yichen Cai, Zhaoying Li, Qian Zhou, Qiu Chen
Ageing Research Reviews.2024; 94: 102201. CrossRef - Salidroside Alleviates Myocardial Ischemia Reperfusion by Balancing Mitochondrial Homeostasis via Nrf2
Tingxu Yan, Xu Li, Xin Wang, Bosai He, Ying Jia, Wei Xiao, Rong He
Journal of Food Biochemistry.2024; 2024: 1. CrossRef - AGO2 Protects Against Diabetic Cardiomyopathy by Activating Mitochondrial Gene Translation
Jiabing Zhan, Kunying Jin, Rong Xie, Jiahui Fan, Yuyan Tang, Chen Chen, Huaping Li, Dao Wen Wang
Circulation.2024; 149(14): 1102. CrossRef - Cellular interplay between cardiomyocytes and non-myocytes in diabetic cardiomyopathy
Ren Jie Phang, Rebecca H Ritchie, Derek J Hausenloy, Jarmon G Lees, Shiang Y Lim
Cardiovascular Research.2023; 119(3): 668. CrossRef - Mitochondrial miRNA as epigenomic signatures: Visualizing aging-associated heart diseases through a new lens
Jasvinder Singh Bhatti, Naina Khullar, Rajesh Vijayvergiya, Umashanker Navik, Gurjit Kaur Bhatti, P. Hemachandra Reddy
Ageing Research Reviews.2023; 86: 101882. CrossRef - Eucommiae Folium and Active Compounds Protect Against Mitochondrial Dysfunction-Calcium Overload in Epileptic Hippocampal Neurons Through the Hypertrophic Cardiomyopathy Pathway
Shuai-nan Zhang, Hong-mei Li, Qi Liu, Xu-zhao Li, Wu-de Yang, Ying Zhou
Neurochemical Research.2023; 48(9): 2674. CrossRef - Speckle-tracking echocardiography provides sensitive measurements of subtle early alterations associated with cardiac dysfunction in T2DM rats
Yanchao Qi, Zhiyan Chen, Bingyan Guo, Zhe Liu, Lijie Wang, Suyun Liu, Lixiang Xue, Meifang Ma, Yajuan Yin, Yongjun Li, Gang Liu
BMC Cardiovascular Disorders.2023;[Epub] CrossRef - Novel insights into the role of mitochondria in diabetic cardiomyopathy: molecular mechanisms and potential treatments
Fumin Zhi, Qian Zhang, Li Liu, Xing Chang, Hongtao Xu
Cell Stress and Chaperones.2023; 28(6): 641. CrossRef - Transcription factor EB: A potential integrated network regulator in metabolic-associated cardiac injury
Weixing Wen, Haoxiao Zheng, Weiwen Li, Guolin Huang, Peng Chen, Xiaolin Zhu, Yue Cao, Jiahuan Li, Xiaohui Huang, Yuli Huang
Metabolism.2023; 147: 155662. CrossRef - Role of STIM1 in the Regulation of Cardiac Energy Substrate Preference
Panpan Liu, Zhuli Yang, Youjun Wang, Aomin Sun
International Journal of Molecular Sciences.2023; 24(17): 13188. CrossRef - Mitochondrial dysfunction at the crossroad of cardiovascular diseases and cancer
Carmine Rocca, Teresa Soda, Ernestina Marianna De Francesco, Marco Fiorillo, Francesco Moccia, Giuseppe Viglietto, Tommaso Angelone, Nicola Amodio
Journal of Translational Medicine.2023;[Epub] CrossRef - Triarylphosphonium compounds as effective vectors for mitochondria-targeted delivery systems: decoration strategies and prospects for clinical application
T. N. Pashirova, A. V. Nemtarev, E. B. Souto, V. F. Mironov
Russian Chemical Reviews.2023; 92(10): RCR5095. CrossRef - Moderate- and High-Intensity Endurance Training Alleviate Diabetes-Induced Cardiac Dysfunction in Rats
Sarah D’Haese, Maxim Verboven, Lize Evens, Dorien Deluyker, Ivo Lambrichts, BO Eijnde, Dominique Hansen, Virginie Bito
Nutrients.2023; 15(18): 3950. CrossRef - Alpha-lipoic acid enhances ischemic postconditioning-mediated improvement of myocardial infarction and apoptosis in diabetic rats with ischemia/reperfusion injury
Sanaz Gholami, Reza Badalzadeh, Alireza Alihemmati
Canadian Journal of Physiology and Pharmacology.2023; 101(12): 682. CrossRef - Concurrent diabetes and heart failure: interplay and novel therapeutic approaches
Qutuba G Karwi, Kim L Ho, Simran Pherwani, Ezra B Ketema, Qiuyu Sun, Gary D Lopaschuk
Cardiovascular Research.2022; 118(3): 686. CrossRef - Ca2+ mishandling and mitochondrial dysfunction: a converging road to prediabetic and diabetic cardiomyopathy
Carolina Jaquenod De Giusti, Julieta Palomeque, Alicia Mattiazzi
Pflügers Archiv - European Journal of Physiology.2022; 474(1): 33. CrossRef - Double-edge sword roles of iron in driving energy production versus instigating ferroptosis
Shuping Zhang, Wei Xin, Gregory J. Anderson, Ruibin Li, Ling Gao, Shuguang Chen, Jiajun Zhao, Sijin Liu
Cell Death & Disease.2022;[Epub] CrossRef - Differential remodelling of mitochondrial subpopulations and mitochondrial dysfunction are a feature of early stage diabetes
Bodour S. Rajab, Sarah Kassab, Connor D. Stonall, Hussam Daghistani, Stephen Gibbons, Mamas Mamas, David Smith, Aleksandr Mironov, Zainab AlBalawi, Yin Hua Zhang, Florence Baudoin, Min Zi, Sukhpal Prehar, Elizabeth J. Cartwright, Ashraf Kitmitto
Scientific Reports.2022;[Epub] CrossRef - GlyNAC (Glycine and N-Acetylcysteine) Supplementation Improves Impaired Mitochondrial Fuel Oxidation and Lowers Insulin Resistance in Patients with Type 2 Diabetes: Results of a Pilot Study
Rajagopal V. Sekhar
Antioxidants.2022; 11(1): 154. CrossRef - Glucose-derived posttranslational modification in cardiovascular disease
Michael Lehrke, Julia Moellmann, Florian Kahles, Nikolaus Marx
Molecular Aspects of Medicine.2022; 86: 101084. CrossRef - Mitochondrial Implications in Cardiovascular Aging and Diseases: The Specific Role of Mitochondrial Dynamics and Shifts
Anastasia V. Poznyak, Tatiana V. Kirichenko, Evgeny E. Borisov, Nikolay K. Shakhpazyan, Andrey G. Kartuesov, Alexander N. Orekhov
International Journal of Molecular Sciences.2022; 23(6): 2951. CrossRef - Editorial: Management of Diabetes and its Complications: A Focus on Endothelial Dysfunction
Shanhu Qiu, Jianhua Ma, Tongzhi Wu
Frontiers in Endocrinology.2022;[Epub] CrossRef - Alternative autophagy: mechanisms and roles in different diseases
Hong Feng, Nian Wang, Nan Zhang, Hai-han Liao
Cell Communication and Signaling.2022;[Epub] CrossRef - Mitochondria-Mediated Cardiovascular Benefits of Sodium-Glucose Co-Transporter 2 Inhibitors
Siarhei A. Dabravolski, Alexander D. Zhuravlev, Andrey G. Kartuesov, Evgeny E. Borisov, Vasily N. Sukhorukov, Alexander N. Orekhov
International Journal of Molecular Sciences.2022; 23(10): 5371. CrossRef - Animal Models of Dysregulated Cardiac Metabolism
Heiko Bugger, Nikole J. Byrne, E. Dale Abel
Circulation Research.2022; 130(12): 1965. CrossRef - Mitochondrial Dynamics and Mitophagy in Cardiometabolic Disease
Jianguo Lin, Jinlong Duan, Qingqing Wang, Siyu Xu, Simin Zhou, Kuiwu Yao
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Perspectives for Forkhead box transcription factors in diabetic cardiomyopathy: Their therapeutic potential and possible effects of salvianolic acids
Ronghui Han, Hemeng Huang, Weiyi Xia, Jingjin Liu, Hui Luo, Jing Tang, Zhengyuan Xia
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Molecular and cellular mechanisms in diabetic heart failure: Potential therapeutic targets
Misganaw Asmamaw Mengstie, Endeshaw Chekol Abebe, Awgichew Behaile Teklemariam, Anemut Tilahun Mulu, Assefa Agegnehu Teshome, Edgeit Abebe Zewde, Zelalem Tilahun Muche, Muluken Teshome Azezew
Frontiers in Endocrinology.2022;[Epub] CrossRef - Effect of Chronic Treatment with Uridine on Cardiac Mitochondrial Dysfunction in the C57BL/6 Mouse Model of High-Fat Diet–Streptozotocin-Induced Diabetes
Natalia V. Belosludtseva, Vlada S. Starinets, Irina B. Mikheeva, Maxim N. Belosludtsev, Mikhail V. Dubinin, Galina D. Mironova, Konstantin N. Belosludtsev
International Journal of Molecular Sciences.2022; 23(18): 10633. CrossRef - FGF21–Sirtuin 3 Axis Confers the Protective Effects of Exercise Against Diabetic Cardiomyopathy by Governing Mitochondrial Integrity
Leigang Jin, Leiluo Geng, Lei Ying, Lingling Shu, Kevin Ye, Ranyao Yang, Yan Liu, Yao Wang, Yin Cai, Xue Jiang, Qin Wang, Xingqun Yan, Boya Liao, Jie Liu, Fuyu Duan, Gary Sweeney, Connie Wai Hong Woo, Yu Wang, Zhengyuan Xia, Qizhou Lian, Aimin Xu
Circulation.2022; 146(20): 1537. CrossRef - Novel Insights Into Molecular Mechanism of Mitochondria in Diabetic Cardiomyopathy
Jing Bai, Chuanbin Liu, Pingjun Zhu, Yang Li
Frontiers in Physiology.2021;[Epub] CrossRef - Mitochondrial Dysfunction Increases Arrhythmic Triggers and Substrates; Potential Anti-arrhythmic Pharmacological Targets
Khalil Saadeh, Ibrahim Talal Fazmin
Frontiers in Cardiovascular Medicine.2021;[Epub] CrossRef - Prostaglandin E receptor subtype 4 protects against diabetic cardiomyopathy by modulating cardiac fatty acid metabolism via FOXO1/CD36 signalling
Fan Ying, Hao Liu, Eva Hoi Ching Tang, Ishan Lakhani, Ningning Liu, Zhengyuan Xia, Shiming Liu
Biochemical and Biophysical Research Communications.2021; 548: 196. CrossRef - Study of long non-coding RNA and mitochondrial dysfunction in diabetic rats
Haytham K. Sultan, Wael M. El-Ayat, Azza H. AbouGhalia, Noha N. Lasheen, Amr S. Moustafa
Tissue and Cell.2021; 71: 101516. CrossRef - A Role of Glucose Overload in Diabetic Cardiomyopathy in Nonhuman Primates
Xiu Wang, Shi Jin, Weina Hu, Gaetano Santulli
Journal of Diabetes Research.2021; 2021: 1. CrossRef - Dysregulation of circulating miRNAs promotes the pathogenesis of diabetes-induced cardiomyopathy
Uzair Ahmed, Usman Ali Ashfaq, Muhammad Qasim, Imtiaz Ahmad, Hafiz Usman Ahmad, Muhammad Tariq, Muhammad Shareef Masoud, Saba Khaliq, Muhammad Younas Khan Barozai
PLOS ONE.2021; 16(4): e0250773. CrossRef - MicroRNAs Regulating Mitochondrial Function in Cardiac Diseases
Guang-Qiong Zhang, Sheng-Quan Wang, Yan Chen, Ling-Yun Fu, Yi-Ni Xu, Ling Li, Ling Tao, Xiang-Chun Shen
Frontiers in Pharmacology.2021;[Epub] CrossRef - Effects of omega-3 fatty acids and metformin combination on diabetic cardiomyopathy in rats through autophagic pathway
Salma M. Eraky, Nehal M. Ramadan
The Journal of Nutritional Biochemistry.2021; 97: 108798. CrossRef - Adropin Alleviates Myocardial Fibrosis in Diabetic Cardiomyopathy Rats: A Preliminary Study
Mao Liu, Jiao Ai, Zhuang Shuai, Kai Tang, Zongyu Li, Yin Huang
Frontiers in Cardiovascular Medicine.2021;[Epub] CrossRef - Fibrosis of the diabetic heart: Clinical significance, molecular mechanisms, and therapeutic opportunities
Izabela Tuleta, Nikolaos G. Frangogiannis
Advanced Drug Delivery Reviews.2021; 176: 113904. CrossRef - Effect of the MPT Pore Inhibitor Alisporivir on the Development of Mitochondrial Dysfunction in the Heart Tissue of Diabetic Mice
Natalia V. Belosludtseva, Vlada S. Starinets, Irina B. Mikheeva, Dmitriy A. Serov, Maxim E. Astashev, Maxim N. Belosludtsev, Mikhail V. Dubinin, Konstantin N. Belosludtsev
Biology.2021; 10(9): 839. CrossRef - Characterisation of the Myocardial Mitochondria Structural and Functional Phenotype in a Murine Model of Diabetic Cardiomyopathy
Alex M. Parker, Mitchel Tate, Darnel Prakoso, Minh Deo, Andrew M. Willis, David M. Nash, Daniel G. Donner, Simon Crawford, Helen Kiriazis, Cesare Granata, Melinda T. Coughlan, Miles J. De Blasio, Rebecca H. Ritchie
Frontiers in Physiology.2021;[Epub] CrossRef - Radiation‑induced dysfunction of energy metabolism in the heart results in the fibrosis of cardiac tissues
Peng Xu, Yali Yi, Yijing Luo, Zhicheng Liu, Yilin Xu, Jing Cai, Zhimin Zeng, Anwen Liu
Molecular Medicine Reports.2021;[Epub] CrossRef - Heart Failure and Diabetes: Perspective of a Dangerous Association
Liliana E. Favaloro, Roxana D. Ratto, Carla Musso
Current Hypertension Reviews.2021; 17(2): 85. CrossRef - CTRP9 Mediates Protective Effects in Cardiomyocytes via AMPK- and Adiponectin Receptor-Mediated Induction of Anti-Oxidant Response
Bernd Niemann, Ling Li, Dorothee Siegler, Benedikt H. Siegler, Fabienne Knapp, Jakob Hanna, Muhammad Aslam, Michael Kracht, Rainer Schulz, Susanne Rohrbach
Cells.2020; 9(5): 1229. CrossRef - The Hippo Pathway Orchestrates Mitochondrial Quality Control: A Novel Focus on Cardiovascular Diseases
Ying Tan, Cai Lei, Huifang Tang, Xiao Zhu, Guanghui Yi
DNA and Cell Biology.2020; 39(9): 1494. CrossRef - Adeno-Associated Viral Transfer of Glyoxalase-1 Blunts Carbonyl and Oxidative Stresses in Hearts of Type 1 Diabetic Rats
Fadhel A. Alomar, Abdullah Al-Rubaish, Fahad Al-Muhanna, Amein K. Al-Ali, JoEllyn McMillan, Jaipaul Singh, Keshore R. Bidasee
Antioxidants.2020; 9(7): 592. CrossRef - Loss of function of transcription factor EB remodels lipid metabolism and cell death pathways in the cardiomyocyte
Purvi C. Trivedi, Jordan J. Bartlett, Angella Mercer, Logan Slade, Marc Surette, Andrea Ballabio, Stephane Flibotte, Bahira Hussein, Brian Rodrigues, Petra C. Kienesberger, Thomas Pulinilkunnil
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2020; 1866(10): 165832. CrossRef - Rescue of myocardial energetic dysfunction in diabetes through the correction of mitochondrial hyperacetylation by honokiol
Matthew Kerr, Jack J. Miller, Dharendra Thapa, Sophie Stiewe, Kerstin N. Timm, Claudia N. Montes Aparicio, Iain Scott, Damian J. Tyler, Lisa C. Heather
JCI Insight.2020;[Epub] CrossRef - Diabetes Mellitus, Mitochondrial Dysfunction and Ca2+-Dependent Permeability Transition Pore
Konstantin N. Belosludtsev, Natalia V. Belosludtseva, Mikhail V. Dubinin
International Journal of Molecular Sciences.2020; 21(18): 6559. CrossRef - Effects of Standardized Green Tea Extract and Its Main Component, EGCG, on Mitochondrial Function and Contractile Performance of Healthy Rat Cardiomyocytes
Rocchina Vilella, Gianluca Sgarbi, Valeria Naponelli, Monia Savi, Leonardo Bocchi, Francesca Liuzzi, Riccardo Righetti, Federico Quaini, Caterina Frati, Saverio Bettuzzi, Giancarlo Solaini, Donatella Stilli, Federica Rizzi, Alessandra Baracca
Nutrients.2020; 12(10): 2949. CrossRef - Diabetic cardiomyopathy: definition, diagnosis criteria, treatment directions and prevention of heart failure
N. A. Koziolova, P. G. Karavaev, A. S. Veklich
South Russian Journal of Therapeutic Practice.2020; 1(2): 93. CrossRef - Adiponectin protects HL-1 cardiomyocytes against rotenone-induced cytotoxicity through AMPK activation
Biao Li, Baojian Zhang, Na Liu, Keke Wu, Yingxu Ma, Wanyun Zuo, Zuodong Ning, Yaozhong Liu, Chao Sun, Yichao Xiao, Tao Tu, Qiming Liu
Toxicology Letters.2020; 335: 82. CrossRef - Nutrient Sensor mTOR and OGT: Orchestrators of Organelle Homeostasis in Pancreatic β-Cells
Nicholas Esch, Seokwon Jo, Mackenzie Moore, Emilyn U. Alejandro, Yingke Xu
Journal of Diabetes Research.2020; 2020: 1. CrossRef - Where Does Metformin Stand in Modern Day Management of Type 2 Diabetes?
Ehtasham Ahmad, Jack Sargeant, Francesco Zaccardi, Kamlesh Khunti, David Webb, Melanie Davies
Pharmaceuticals.2020; 13(12): 427. CrossRef
- SIRT1: a promising therapeutic target in type 2 diabetes mellitus
- Technology/Device
- Role of MicroRNA-34a in Anti-Apoptotic Effects of Granulocyte-Colony Stimulating Factor in Diabetic Cardiomyopathy
- In-Hwa Park, Yi-Sun Song, Hyun-Woo Joo, Guang-Yin Shen, Jin-Hee Seong, Na-Kyoung Shin, Young Jong Cho, Yonggu Lee, Jeong Hun Shin, Young-Hyo Lim, Hyuck Kim, Kyung-Soo Kim
- Diabetes Metab J. 2020;44(1):173-185. Published online April 23, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0211
- 5,529 View
- 75 Download
- 11 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background Recent studies have shown that microRNAs (miRNAs) are involved in the process of cardiomyocyte apoptosis. We have previously reported that granulocyte-colony stimulating factor (G-CSF) ameliorated diastolic dysfunction and attenuated cardiomyocyte apoptosis in a rat model of diabetic cardiomyopathy. In this study, we hypothesized a regulatory role of cardiac miRNAs in the mechanism of the anti-apoptotic effect of G-CSF in a diabetic cardiomyopathy rat model.
Methods Rats were given a high-fat diet and low-dose streptozotocin injection and then randomly allocated to receive treatment with either G-CSF or saline. H9c2 rat cardiomyocytes were cultured under a high glucose (HG) condition to induce diabetic cardiomyopathy
in vitro . We examined the extent of apoptosis, miRNA expression, and miRNA target genes in the myocardium and H9c2 cells.Results G-CSF treatment significantly decreased apoptosis and reduced miR-34a expression in diabetic myocardium and H9c2 cells under the HG condition. G-CSF treatment also significantly increased B-cell lymphoma 2 (Bcl-2) protein expression as a target for miR-34a. In addition, transfection with an miR-34a mimic significantly increased apoptosis and decreased Bcl-2 luciferase activity in H9c2 cells.
Conclusion Our results indicate that G-CSF might have an anti-apoptotic effect through down-regulation of miR-34a in a diabetic cardiomyopathy rat model.
-
Citations
Citations to this article as recorded by- The study of the mechanism of non-coding RNA regulation of programmed cell death in diabetic cardiomyopathy
Bingrui Zhang, Hua Wu, Jingwen Zhang, Cong Cong, Lin Zhang
Molecular and Cellular Biochemistry.2024;[Epub] CrossRef - Non-coding RNAs in the pathophysiology of heart failure with preserved ejection fraction
Elisabeth A. Jalink, Amber W. Schonk, Reinier A. Boon, Rio P. Juni
Frontiers in Cardiovascular Medicine.2024;[Epub] CrossRef - Dynamic interplay of microRNA in diseases and therapeutic
Neha Kargutkar, Priya Hariharan, Anita Nadkarni
Clinical Genetics.2023; 103(3): 268. CrossRef - LGR5+ Intestinal Stem Cells Display Sex-Dependent Radiosensitivity
Ryan C. Zitter, Rishi Man Chugh, Payel Bhanja, Bruce F. Kimler, Subhrajit Saha
Cells.2023; 13(1): 46. CrossRef - Female Mice are More Resistant to the Mixed-Field (67% Neutron + 33% Gamma) Radiation-Induced Injury in Bone Marrow and Small Intestine than Male Mice due to Sustained Increases in G-CSF and the Bcl-2/Bax Ratio and Lower miR-34a and MAPK Activation
Juliann G. Kiang, Georgetta Cannon, Matthew G. Olson, Joan T. Smith, Marsha N. Anderson, Min Zhai, M. Victoria Umali, Kevin Ho, Connie Ho, Wanchang Cui, Mang Xiao
Radiation Research.2022;[Epub] CrossRef - Potential Role of Natural Plant Medicine Cyclocarya paliurus in the Treatment of Type 2 Diabetes Mellitus
Han Wang, Cheng Tang, Zezheng Gao, Yishan Huang, Boxun Zhang, Jiahua Wei, Linhua Zhao, Xiaolin Tong, Yong Xu
Journal of Diabetes Research.2021; 2021: 1. CrossRef - Ghrelin, a novel therapy, corrects cytokine and NF-κB-AKT-MAPK network and mitigates intestinal injury induced by combined radiation and skin-wound trauma
Juliann G. Kiang, Joan T. Smith, Georgetta Cannon, Marsha N. Anderson, Connie Ho, Min Zhai, Wanchang Cui, Mang Xiao
Cell & Bioscience.2020;[Epub] CrossRef - Evaluation of biomarkers in liver following Solanum melongena green calyx administration in diabetic rats
Shiva Roshankhah, Ahmad Shabanizadeh, Amir Abdolmaleki, Mohammad Reza Gholami, Mohammad Reza Salahshoor
Journal of Diabetes & Metabolic Disorders.2020; 19(2): 1115. CrossRef - Diabetic cardiomyopathy: definition, diagnosis criteria, treatment directions and prevention of heart failure
N. A. Koziolova, P. G. Karavaev, A. S. Veklich
South Russian Journal of Therapeutic Practice.2020; 1(2): 93. CrossRef - The Potential Role of MicroRNA in Diabetic Cardiomyopathy
Jin Hwa Kim
Diabetes & Metabolism Journal.2020; 44(1): 54. CrossRef
- The study of the mechanism of non-coding RNA regulation of programmed cell death in diabetic cardiomyopathy
- Metabolic Risk/Epidemiology
- Association between Non-Alcoholic Steatohepatitis and Left Ventricular Diastolic Dysfunction in Type 2 Diabetes Mellitus
- Hokyou Lee, Gyuri Kim, Young Ju Choi, Byung Wook Huh, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Eun Jig Lee, Yong-ho Lee, Kap Bum Huh
- Diabetes Metab J. 2020;44(2):267-276. Published online February 28, 2019
- DOI: https://doi.org/10.4093/dmj.2019.0001
- 6,921 View
- 152 Download
- 25 Web of Science
- 27 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Impaired diastolic heart function has been observed in persons with non-alcoholic fatty liver disease (NAFLD) and/or with type 2 diabetes mellitus (T2DM). However, it is unclear whether NAFLD fibrotic progression, i.e., non-alcoholic steatohepatitis, poses an independent risk for diastolic dysfunction in T2DM. We investigated the association between liver fibrosis and left ventricular (LV) diastolic dysfunction in T2DM.
Methods We analyzed 606 patients with T2DM, aged ≥50 years, who had undergone liver ultrasonography and pulsed-wave Doppler echocardiography. Insulin sensitivity was measured by short insulin tolerance test. Presence of NAFLD and/or advanced liver fibrosis was determined by abdominal ultrasonography and NAFLD fibrosis score (NFS). LV diastolic dysfunction was defined according to transmitral peak early to late ventricular filling (E/A) ratio and deceleration time, using echocardiography.
Results LV diastolic dysfunction was significantly more prevalent in the NAFLD versus non-NAFLD group (59.7% vs. 49.0%,
P =0.011). When NAFLD was stratified by NFS, subjects with advanced liver fibrosis exhibited a higher prevalence of diastolic dysfunction (49.0%, 50.7%, 61.8%; none, simple steatosis, advanced fibrosis, respectively;P for trend=0.003). In multivariable logistic regression, liver fibrosis was independently associated with diastolic dysfunction (odds ratio [OR], 1.58; 95% confidence interval [CI], 1.07 to 2.34;P =0.022) after adjusting for insulin resistance and cardiometabolic risk factors. This association remained significant in patients without insulin resistance (OR, 4.32; 95% CI, 1.73 to 11.51;P =0.002).Conclusions Liver fibrosis was associated with LV diastolic dysfunction in patients with T2DM and may be an independent risk factor for diastolic dysfunction, especially in patients without systemic insulin resistance.
-
Citations
Citations to this article as recorded by- Anti-hepatopathy and anti-nephropathy activities of Taraxacum officinale in a rat model of Streptozotocin diabetes-induced hepatorenal toxicity and dyslipidemia via attenuation of oxidative stress, inflammation, apoptosis, electrolyte imbalances, and mito
Sunday Aderemi Adelakun, Aniah Julius Akomaye, Olusegun Dare Omotoso, Olukayode Abimbola Arowosegbe
Aspects of Molecular Medicine.2024; 3: 100034. CrossRef - Epidemiology of heart failure in diabetes: a disease in disguise
Anna G. Hoek, Elisa Dal Canto, Eva Wenker, Navin Bindraban, M. Louis Handoko, Petra J. M. Elders, Joline W. J. Beulens
Diabetologia.2024; 67(4): 574. CrossRef - NASH triggers cardiometabolic HFpEF in aging mice
Dániel Kucsera, Mihály Ruppert, Nabil V. Sayour, Viktória E. Tóth, Tamás Kovács, Zsombor I. Hegedűs, Zsófia Onódi, Alexandra Fábián, Attila Kovács, Tamás Radovits, Béla Merkely, Pál Pacher, Péter Ferdinandy, Zoltán V. Varga
GeroScience.2024;[Epub] CrossRef - Inhibition of visceral adipose tissue-derived pathogenic signals by activation of adenosine A2AR improves hepatic and cardiac dysfunction of NASH mice
Chia-Chang Huang, Hsiao-Yun Yeh, Roger Lin, Tsai-Ling Liao, Hsiao-Chin Shen, Ying-Ying Yang, Han-Chieh Lin
American Journal of Physiology-Gastrointestinal and Liver Physiology.2024; 326(4): G385. CrossRef - Is non-alcoholic fatty liver disease a sign of left ventricular diastolic dysfunction in patients with type 2 diabetes mellitus? A systematic review and meta-analysis
Sicheng Wang, Xiangyuan Zhang, Qiqi Zhang, Boxun Zhang, Linhua Zhao
BMJ Open Diabetes Research & Care.2023; 11(1): e003198. CrossRef - The effect of metabolic dysfunction-associated fatty liver disease and diabetic kidney disease on the risk of hospitalization of heart failure in type 2 diabetes: a retrospective cohort study
Seung Eun Lee, Juhwan Yoo, Bong-Seong Kim, Han Seok Choi, Kyungdo Han, Kyoung-Ah Kim
Diabetology & Metabolic Syndrome.2023;[Epub] CrossRef - Metabolic Dysfunction-Associated Fatty Liver Disease and Mortality: A Population-Based Cohort Study
Kyung-Soo Kim, Sangmo Hong, Hong-Yup Ahn, Cheol-Young Park
Diabetes & Metabolism Journal.2023; 47(2): 220. CrossRef - Therapies for patients with coexisting heart failure with reduced ejection fraction and non-alcoholic fatty liver disease
Jose Arriola-Montenegro, Renato Beas, Renato Cerna-Viacava, Andres Chaponan-Lavalle, Karla Hernandez Randich, Diego Chambergo-Michilot, Herson Flores Sanga, Pornthira Mutirangura
World Journal of Cardiology.2023; 15(7): 328. CrossRef - Non-alcoholic Fatty Liver Disease and Its Association With Left Ventricular Diastolic Dysfunction: A Systematic Review
Namra V Gohil, Nida Tanveer, Vijaya Krishna Makkena, Arturo P Jaramillo, Babatope L Awosusi, Javaria Ayyub, Karan Nareshbhai Dabhi, Tuheen Sankar Nath
Cureus.2023;[Epub] CrossRef - Associations of advanced liver fibrosis with heart failure with preserved ejection fraction in type 2 diabetic patients according to obesity and metabolic goal achievement status
Wangyan Jiang, Zhelong Liu, Shaohua Liu, Tingting Du
Frontiers in Endocrinology.2023;[Epub] CrossRef - Non-Alcoholic Fatty Liver Disease and Echocardiographic Parameters of Left Ventricular Diastolic Function: A Systematic Review and Meta-Analysis
Athina Goliopoulou, Panagiotis Theofilis, Evangelos Oikonomou, Artemis Anastasiou, Panteleimon Pantelidis, Maria Ioanna Gounaridi, Georgios E. Zakynthinos, Ourania Katsarou, Eva Kassi, Vaia Lambadiari, Dimitris Tousoulis, Manolis Vavuranakis, Gerasimos Si
International Journal of Molecular Sciences.2023; 24(18): 14292. CrossRef - Non-alcoholic fatty liver disease and risk of cardiovascular diseases: clinical association, pathophysiological mechanisms, and management
Rong Yang, Jian-Gao Fan
Cardiology Plus.2023; 8(4): 217. CrossRef - Association of cardiovascular factors in diabetic patients with non-alcoholic fatty liver disease
Evangelos Cholongitas, Dimitrios Tsilingiris, Panagiota Diamantopoulou, Elpida Mastrogianni, Anastasios Tentolouris, Dimitrios Karagiannakis, Ioannis Moyssakis, George V. Papatheodoridis, Nikolaos Tentolouris
Hormones.2022; 21(1): 133. CrossRef - Non-alcoholic fatty liver disease association with structural heart, systolic and diastolic dysfunction: a meta-analysis
Jie Ning Yong, Cheng Han Ng, Chloe Wen-Min Lee, Yu Yi Chan, Ansel Shao Pin Tang, Margaret Teng, Darren Jun Hao Tan, Wen Hui Lim, Jingxuan Quek, Jieling Xiao, Yip Han Chin, Roger Foo, Mark Chan, Weiqin Lin, Mazen Noureddin, Mohammad Shadab Siddiqui, Mark D
Hepatology International.2022; 16(2): 269. CrossRef - Triglyceride and glucose index is a simple and easy‐to‐calculate marker associated with nonalcoholic fatty liver disease
Kyung‐Soo Kim, Sangmo Hong, Hong‐Yup Ahn, Cheol‐Young Park
Obesity.2022; 30(6): 1279. CrossRef - Association of Metabolic Dysfunction-Associated Fatty Liver Disease With Left Ventricular Diastolic Function and Cardiac Morphology
Dandan Peng, Zhenqiu Yu, Mingwei Wang, Junping Shi, Lei Sun, Yuanyuan Zhang, Wenbin Zhao, Chen Chen, Jiake Tang, Chunyi Wang, Jie Ni, Wen Wen, Jingjie Jiang
Frontiers in Endocrinology.2022;[Epub] CrossRef - NAFLD in Cardiovascular Diseases: A Contributor or Comorbidity?
Bing Chen, W.H. Wilson Tang, Mario Rodriguez, Kathleen E. Corey, Arun J. Sanyal, Patrick S. Kamath, Biykem Bozkurt, Hafeez Ul Hassan Virk, Gregg S. Pressman, Jeffrey V. Lazarus, Hashem B. El-Serag, Chayakrit Krittanawong
Seminars in Liver Disease.2022; 42(04): 465. CrossRef - Nonalcoholic fatty liver disease is associated with early left ventricular diastolic dysfunction in patients with type 2 diabeteS
Walaa Sheba, Eman Morsy, Salah Altahan, Mona Ayaad, Sameh A. Lashen
Alexandria Journal of Medicine.2022; 58(1): 117. CrossRef - Cardiac abnormalities in patients with nonalcoholic fatty liver disease
Yu Dong, Guangsen Li
Herz.2021; 46(2): 158. CrossRef - Elafibranor improves diet-induced nonalcoholic steatohepatitis associated with heart failure with preserved ejection fraction in Golden Syrian hamsters
François Briand, Julie Maupoint, Emmanuel Brousseau, Natalia Breyner, Mélanie Bouchet, Clément Costard, Thierry Leste-Lasserre, Mathieu Petitjean, Li Chen, Audrey Chabrat, Virgile Richard, Rémy Burcelin, Caroline Dubroca, Thierry Sulpice
Metabolism.2021; 117: 154707. CrossRef - Association of the Non‐Alcoholic Fatty Liver Disease Fibrosis Score with subclinical myocardial remodeling in patients with type 2 diabetes: A cross‐sectional study in China
Nengguang Fan, Xiaoying Ding, Qin Zhen, Liping Gu, Aifang Zhang, Tingting Shen, Yufan Wang, Yongde Peng
Journal of Diabetes Investigation.2021; 12(6): 1035. CrossRef - Nonalcoholic fatty liver disease, diastolic dysfunction, and impaired myocardial glucose uptake in patients with type 2 diabetes
Minyoung Lee, Kwang Joon Kim, Tae‐Ha Chung, Jaehyun Bae, Yong‐ho Lee, Byung‐Wan Lee, Bong‐Soo Cha, Mijin Yun, Eun Seok Kang
Diabetes, Obesity and Metabolism.2021; 23(4): 1041. CrossRef - Interplay between Heart Disease and Metabolic Steatosis: A Contemporary Perspective
Mohammad Said Ramadan, Vincenzo Russo, Gerardo Nigro, Emanuele Durante-Mangoni, Rosa Zampino
Journal of Clinical Medicine.2021; 10(8): 1569. CrossRef - Correlation Between 25-Hydroxyvitamin D Level and Cardiac Diastolic Dysfunction in Chinese Adults with Early-Onset Type 2 Diabetes Mellitus: A Cross-Sectional Study
Lei Xiu, Xiao-ai Yao, Tao Jiang
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2021; Volume 14: 1823. CrossRef - Bi-directional and temporal relationship between elevated alanine aminotransferase and hypertension in a longitudinal study of Chinese adults
Guoxin Huang, Hui Zhou, Chao Shen, Yihui Sheng, Ruyu Xue, Chen Dong, Shaoyan Zhang
Clinical and Experimental Hypertension.2021; 43(8): 750. CrossRef - Response: Association between Non-Alcoholic Steatohepatitis and Left Ventricular Diastolic Dysfunction in Type 2 Diabetes Mellitus (Diabetes Metab J 2020;44:267–76)
Hokyou Lee, Gyuri Kim, Yong-ho Lee
Diabetes & Metabolism Journal.2020; 44(3): 486. CrossRef - Letter: Association between Non-Alcoholic Steatohepatitis and Left Ventricular Diastolic Dysfunction in Type 2 Diabetes Mellitus (Diabetes Metab J2020;44:267–76)
Sung Hoon Yu
Diabetes & Metabolism Journal.2020; 44(3): 482. CrossRef
- Anti-hepatopathy and anti-nephropathy activities of Taraxacum officinale in a rat model of Streptozotocin diabetes-induced hepatorenal toxicity and dyslipidemia via attenuation of oxidative stress, inflammation, apoptosis, electrolyte imbalances, and mito
- Complications
- My Sweetheart Is Broken: Role of Glucose in Diabetic Cardiomyopathy
- Manoja K. Brahma, Mark E. Pepin, Adam R. Wende
- Diabetes Metab J. 2017;41(1):1-9. Published online November 15, 2016
- DOI: https://doi.org/10.4093/dmj.2017.41.1.1
- 4,599 View
- 79 Download
- 41 Web of Science
- 39 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Despite overall reductions in heart disease prevalence, the risk of developing heart failure has remained 2-fold greater among people with diabetes. Growing evidence has supported that fluctuations in glucose level and uptake contribute to cardiovascular disease (CVD) by modifying proteins, DNA, and gene expression. In the case of glucose, clinical studies have shown that increased dietary sugars for healthy individuals or poor glycemic control in diabetic patients further increased CVD risk. Furthermore, even after decades of maintaining tight glycemic control, susceptibility to disease progression can persist following a period of poor glycemic control through a process termed "glycemic memory." In response to chronically elevated glucose levels, a number of studies have identified molecular targets of the glucose-mediated protein posttranslational modification by the addition of an
O -linked N-acetylglucosamine to impair contractility, calcium sensitivity, and mitochondrial protein function. Additionally, elevated glucose contributes to dysfunction in coupling glycolysis to glucose oxidation, pentose phosphate pathway, and polyol pathway. Therefore, in the "sweetened" environment associated with hyperglycemia, there are a number of pathways contributing to increased susceptibly to "breaking" the heart of diabetics. In this review we will discuss the unique contribution of glucose to heart disease and recent advances in defining mechanisms of action.-
Citations
Citations to this article as recorded by- Compound SJ-12 attenuates streptozocin-induced diabetic cardiomyopathy by stabilizing SERCA2a
Shuaijie Lou, Weiwei Zhu, Tianxiang Yu, Qianhui Zhang, Minxiu Wang, Leiming Jin, Yongqiang Xiong, Jiachen Xu, Qinyan Wang, Gaozhi Chen, Guang Liang, Xiang Hu, Wu Luo
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2024; 1870(5): 167140. CrossRef - O304 ameliorates hyperglycemia in mice by dually promoting muscle glucose effectiveness and preserving β-cell function
Stefan Norlin, Jan Axelsson, Madelene Ericsson, Helena Edlund
Communications Biology.2023;[Epub] CrossRef - The role of glycolytic metabolic pathways in cardiovascular disease and potential therapeutic approaches
Shuxian Chen, Yuanming Zou, Chunyu Song, Kexin Cao, Kexin Cai, Yanjiao Wu, Zhaobo Zhang, Danxi Geng, Wei Sun, Nanxiang Ouyang, Naijin Zhang, Zhao Li, Guozhe Sun, Yixiao Zhang, Yingxian Sun, Ying Zhang
Basic Research in Cardiology.2023;[Epub] CrossRef - Silibinin ameliorates the cardiovascular oxidative and inflammatory effects of type-2-diabetic rats exposed to air particulate matter
Olamide O. Awolaja, Akeem O. Lawal, Ibukun M. Folorunso, Olusola O. Elekofehinti, Haruna I. Umar
Polycyclic Aromatic Compounds.2023; 43(7): 6570. CrossRef - The Protective Effect of 11-Keto-β-Boswellic Acid against Diabetic Cardiomyopathy in Rats Entails Activation of AMPK
Jozaa Z. AlTamimi, Nora A. AlFaris, Ghedeir M. Alshammari, Reham I. Alagal, Dalal H. Aljabryn, Mohammed Abdo Yahya
Nutrients.2023; 15(7): 1660. CrossRef - The role of CD36 in cardiovascular disease
Hongyang Shu, Yizhong Peng, Weijian Hang, Jiali Nie, Ning Zhou, Dao Wen Wang
Cardiovascular Research.2022; 118(1): 115. CrossRef - The world congress on insulin resistance, diabetes, and cardiovascular disease (WCIRDC)
Zachary Bloomgarden
Journal of Diabetes.2022; 14(3): 163. CrossRef - Pharmacological modulation of prostaglandin E2 (PGE2) EP receptors improves cardiomyocyte function under hyperglycemic conditions
Karin J. Bosma, Monica Ghosh, Spencer R. Andrei, Lin Zhong, Jennifer C. Dunn, Valerie F. Ricciardi, Juliann B. Burkett, Antonis K. Hatzopoulos, Derek S. Damron, Maureen Gannon
Physiological Reports.2022;[Epub] CrossRef - The efficacy and safety of traditional Chinese medicine treating diabetic cardiomyopathy: A protocol for systematic review and meta-analysis
Shuo Han, Yuan Hou, Huaman Liu, Quanlin Zhao
Medicine.2022; 101(47): e31269. CrossRef - High Throughput Screen Identifies Small Molecule Effectors That Modulate Thin Filament Activation in Cardiac Muscle
Priyanka Parijat, Laszlo Kondacs, Alexander Alexandrovich, Mathias Gautel, Alexander J. A. Cobb, Thomas Kampourakis
ACS Chemical Biology.2021; 16(1): 225. CrossRef - Prostaglandin E receptor subtype 4 protects against diabetic cardiomyopathy by modulating cardiac fatty acid metabolism via FOXO1/CD36 signalling
Fan Ying, Hao Liu, Eva Hoi Ching Tang, Ishan Lakhani, Ningning Liu, Zhengyuan Xia, Shiming Liu
Biochemical and Biophysical Research Communications.2021; 548: 196. CrossRef - Intracellular Toxic AGEs (TAGE) Triggers Numerous Types of Cell Damage
Masayoshi Takeuchi, Akiko Sakasai-Sakai, Takanobu Takata, Jun-ichi Takino, Yoshiki Koriyama, Chigusa Kikuchi, Ayako Furukawa, Kentaro Nagamine, Takamitsu Hori, Tamihide Matsunaga
Biomolecules.2021; 11(3): 387. CrossRef - Phosphorylated and O-GlcNAc Modified IRS-1 (Ser1101) and -2 (Ser1149) Contribute to Human Diabetes Type II
Afshan Kaleem, Sabahat Javed, Nayab Rehman, Roheena Abdullah, Mehwish Iqtedar, Mohammad Nauman Aftab, Daniel C. Hoessli, Ikram-Ul Haq
Protein & Peptide Letters.2021; 28(3): 333. CrossRef - Diabetes and Heart Failure: Multi-Omics Approaches
Akram Tayanloo-Beik, Peyvand Parhizkar Roudsari, Mostafa Rezaei-Tavirani, Mahmood Biglar, Ozra Tabatabaei-Malazy, Babak Arjmand, Bagher Larijani
Frontiers in Physiology.2021;[Epub] CrossRef - Asymptomatic Diabetic Cardiomyopathy: an Underrecognized Entity in Type 2 Diabetes
Ana Maria Stanton, Muthiah Vaduganathan, Lee-Shing Chang, Alexander Turchin, James L. Januzzi, Vanita R. Aroda
Current Diabetes Reports.2021;[Epub] CrossRef - Increased Glucose Availability Attenuates Myocardial Ketone Body Utilization
Manoja K. Brahma, Chae‐Myeong Ha, Mark E. Pepin, Sobuj Mia, Zhihuan Sun, John C. Chatham, Kirk M. Habegger, Evan Dale Abel, Andrew J. Paterson, Martin E. Young, Adam R. Wende
Journal of the American Heart Association.2020;[Epub] CrossRef - Gene therapy targeting cardiac phosphoinositide 3-kinase (p110α) attenuates cardiac remodeling in type 2 diabetes
Darnel Prakoso, Miles J. De Blasio, Mitchel Tate, Helen Kiriazis, Daniel G. Donner, Hongwei Qian, David Nash, Minh Deo, Kate L. Weeks, Laura J. Parry, Paul Gregorevic, Julie R. McMullen, Rebecca Helen Ritchie
American Journal of Physiology-Heart and Circulatory Physiology.2020; 318(4): H840. CrossRef - The Potential Role of MicroRNA in Diabetic Cardiomyopathy
Jin Hwa Kim
Diabetes & Metabolism Journal.2020; 44(1): 54. CrossRef - Re-balancing cellular energy substrate metabolism to mend the failing heart
Jan F.C. Glatz, Miranda Nabben, Martin E. Young, P. Christian Schulze, Heinrich Taegtmeyer, Joost J.F.P. Luiken
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2020; 1866(5): 165579. CrossRef - The Peroxisome Proliferator-Activated Receptor-Gamma Coactivator-1α–Heme Oxygenase 1 Axis, a Powerful Antioxidative Pathway with Potential to Attenuate Diabetic Cardiomyopathy
Maayan Waldman, Michael Arad, Nader G. Abraham, Edith Hochhauser
Antioxidants & Redox Signaling.2020; 32(17): 1273. CrossRef - miR‑155 modulates high glucose‑induced cardiac fibrosis via the Nrf2/HO‑1 signaling pathway
Yu Li, Jing‑Zhu Duan, Qian He, Chong‑Quan Wang
Molecular Medicine Reports.2020;[Epub] CrossRef - Disruption of energy utilization in diabetic cardiomyopathy; a mini review
Shinsuke Nirengi, Carmem Peres Valgas da Silva, Kristin I Stanford
Current Opinion in Pharmacology.2020; 54: 82. CrossRef - Reduced fatty acid uptake aggravates cardiac contractile dysfunction in streptozotocin-induced diabetic cardiomyopathy
Yogi Umbarawan, Ryo Kawakami, Mas Rizky A. A. Syamsunarno, Norimichi Koitabashi, Hideru Obinata, Aiko Yamaguchi, Hirofumi Hanaoka, Takako Hishiki, Noriyo Hayakawa, Hiroaki Sunaga, Hiroki Matsui, Masahiko Kurabayashi, Tatsuya Iso
Scientific Reports.2020;[Epub] CrossRef - Metabolomics biomarkers and the risk of overall mortality and ESRD in CKD: Results from the Progredir Cohort
Silvia M. Titan, Gabriela Venturini, Kallyandra Padilha, Alessandra C. Goulart, Paulo A. Lotufo, Isabela J. Bensenor, Jose E. Krieger, Ravi I. Thadhani, Eugene P. Rhee, Alexandre C. Pereira, Vivekanand Jha
PLOS ONE.2019; 14(3): e0213764. CrossRef - Chronic hyperglycemia as a risk factor in implant therapy
Fawad Javed, Georgios E. Romanos
Periodontology 2000.2019; 81(1): 57. CrossRef - Recent advances in the pathogenesis of microvascular complications in diabetes
Sungmi Park, Hyeon-Ji Kang, Jae-Han Jeon, Min-Ji Kim, In-Kyu Lee
Archives of Pharmacal Research.2019; 42(3): 252. CrossRef - Nutrient regulation of signaling and transcription
Gerald W. Hart
Journal of Biological Chemistry.2019; 294(7): 2211. CrossRef - Intracellular toxic advanced glycation end-products in cardiomyocytes may cause cardiovascular disease
Takanobu Takata, Akiko Sakasai-Sakai, Tadashi Ueda, Masayoshi Takeuchi
Scientific Reports.2019;[Epub] CrossRef - Effects of Exercise to Improve Cardiovascular Health
Kelsey Pinckard, Kedryn K. Baskin, Kristin I. Stanford
Frontiers in Cardiovascular Medicine.2019;[Epub] CrossRef - A new hyperpolarized 13C ketone body probe reveals an increase in acetoacetate utilization in the diabetic rat heart
Desiree Abdurrachim, Chern Chiuh Woo, Xing Qi Teo, Wei Xin Chan, George K. Radda, Philip Teck Hock Lee
Scientific Reports.2019;[Epub] CrossRef - Chronic O-GlcNAcylation and Diabetic Cardiomyopathy: The Bitterness of Glucose
Simon Ducheix, Jocelyne Magré, Bertrand Cariou, Xavier Prieur
Frontiers in Endocrinology.2018;[Epub] CrossRef - Proteomics of the Rat Myocardium during Development of Type 2 Diabetes Mellitus Reveals Progressive Alterations in Major Metabolic Pathways
Anders Valdemar Edhager, Jonas Agerlund Povlsen, Bo Løfgren, Hans Erik Bøtker, Johan Palmfeldt
Journal of Proteome Research.2018; 17(7): 2521. CrossRef - Protective effects of astaxanthin on diabetic cardiomyopathy in rats
Bingshan Zhang, Di Xu
CyTA - Journal of Food.2018; 16(1): 909. CrossRef - Au cœur de la cardiomyopathie diabétique
Alexandre Lugat, Michael Joubert, Bertrand Cariou, Xavier Prieur
médecine/sciences.2018; 34(6-7): 563. CrossRef - Redox imbalance stress in diabetes mellitus: Role of the polyol pathway
Liang‐jun Yan
Animal Models and Experimental Medicine.2018; 1(1): 7. CrossRef - Heart rate dynamics during cardio-pulmonary exercise testing are associated with glycemic control in individuals with type 1 diabetes
Othmar Moser, Max L. Eckstein, Olivia McCarthy, Rachel Deere, Stephen C. Bain, Hanne L. Haahr, Eric Zijlstra, Tim Heise, Richard M. Bracken, Petter Bjornstad
PLOS ONE.2018; 13(4): e0194750. CrossRef - Resistance exercise improves cardiac function and mitochondrial efficiency in diabetic rat hearts
Tae Hee Ko, Jubert C. Marquez, Hyoung Kyu Kim, Seung Hun Jeong, SungRyul Lee, Jae Boum Youm, In Sung Song, Dae Yun Seo, Hye Jin Kim, Du Nam Won, Kyoung Im Cho, Mun Gi Choi, Byoung Doo Rhee, Kyung Soo Ko, Nari Kim, Jong Chul Won, Jin Han
Pflügers Archiv - European Journal of Physiology.2018; 470(2): 263. CrossRef - Adriamycin-induced cardiomyopathy can serve as a model for diabetic cardiomyopathy – a hypothesis
Kaviyarasi Renu, V.G. Abilash, P.B. Tirupathi Pichiah, Thabassum Akthar Syeda, Sankarganesh Arunachalam
Asian Pacific Journal of Tropical Biomedicine.2017; 7(11): 1041. CrossRef - Proteome profiling in the aorta and kidney of type 1 diabetic rats
Moustafa Al Hariri, Mohamad Elmedawar, Rui Zhu, Miran A. Jaffa, Jingfu Zhao, Parvin Mirzaei, Adnan Ahmed, Firas Kobeissy, Fuad N. Ziyadeh, Yehia Mechref, Ayad A. Jaffa, Michael Bader
PLOS ONE.2017; 12(11): e0187752. CrossRef
- Compound SJ-12 attenuates streptozocin-induced diabetic cardiomyopathy by stabilizing SERCA2a
- Diabetic Cardiomyopathy and Its Prevention by Nrf2: Current Status
- Jing Chen, Zhiguo Zhang, Lu Cai
- Diabetes Metab J. 2014;38(5):337-345. Published online October 17, 2014
- DOI: https://doi.org/10.4093/dmj.2014.38.5.337
- 5,117 View
- 55 Download
- 76 Web of Science
- 75 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Diabetic cardiomyopathy (DCM), as one of the major cardiac complications in diabetic patients, is known to related with oxidative stress that is due to a severe imbalance between reactive oxygen species (ROS) and/or reactive nitrogen species (RNS) generation and their clearance by antioxidant defense systems. Transcription factor nuclear factor NF-E2-related factor 2 (Nrf2) plays an important role in maintaining the oxidative homeostasis by regulating multiple downstream antioxidants. Diabetes may up-regulate several antioxidants in the heart as a compensative mechanism at early stage, but at late stage, diabetes not only generates extra ROS and/or RNS but also impairs antioxidant capacity in the heart, including Nrf2. In an early study, we have established that Nrf2 protect the cardiac cells and heart from high level of glucose
in vitro and hyperglycemiain vivo , and in the following study demonstrated the significant down-regulation of cardiac Nrf2 expression in diabetic animals and patients. Using Nrf2-KO mice or Nrf2 inducers, blooming evidence has indicated the important protection by Nrf2 from cardiac pathogenesis in the diabetes. Therefore, this brief review summarizes the status of studies on Nrf2's role in preventing DCM and even other complications, the need for new and safe Nrf2 inducer screening and the precaution for the undesirable side of Nrf2 under certain conditions.-
Citations
Citations to this article as recorded by- Natural Products and Health
Joanna Bartkowiak-Wieczorek, Edyta Mądry
Nutrients.2024; 16(3): 415. CrossRef - Nrf2 prevents diabetic cardiomyopathy via antioxidant effect and normalization of glucose and lipid metabolism in the heart
Ge Yang, Qihe Zhang, Chao Dong, Guowen Hou, Jinjie Li, Xin Jiang, Ying Xin
Journal of Cellular Physiology.2024;[Epub] CrossRef - 3,4′,5-Trimethoxy-trans-stilbene ameliorates hepatic insulin resistance and oxidative stress in diabetic obese mice through insulin and Nrf2 signaling pathways
Yi Tan, Chunxiu Zhou, Lingchao Miao, Xutao Zhang, Haroon Khan, Baojun Xu, Wai San Cheang
Food & Function.2024; 15(6): 2996. CrossRef - Natural compounds protect against the pathogenesis of osteoarthritis by mediating the NRF2/ARE signaling
Zhenyu Wu, Zhouxin Yang, Luying Liu, Yong Xiao
Frontiers in Pharmacology.2023;[Epub] CrossRef - High fat diet exacerbates long-term metabolic, neuropathological, and behavioral derangements in an experimental mouse model of traumatic brain injury
Stanley Ibeh, Nour-Mounira Z. Bakkar, Fatima Ahmad, Judith Nwaiwu, Chloe Barsa, Sarine Mekhjian, Mohammad Amine Reslan, Ali H. Eid, Hayat Harati, Sanaa Nabha, Yehia Mechref, Ahmed F. El-Yazbi, Firas Kobeissy
Life Sciences.2023; 314: 121316. CrossRef - The Protective Effect of 11-Keto-β-Boswellic Acid against Diabetic Cardiomyopathy in Rats Entails Activation of AMPK
Jozaa Z. AlTamimi, Nora A. AlFaris, Ghedeir M. Alshammari, Reham I. Alagal, Dalal H. Aljabryn, Mohammed Abdo Yahya
Nutrients.2023; 15(7): 1660. CrossRef - Caffeic Acid Phenethyl Ester and Caffeamide Derivatives Suppress Oral Squamous Cell Carcinoma Cells
Yin-Hwa Shih, Chieh-Chieh Chen, Yueh-Hsiung Kuo, Lih-Jyh Fuh, Wan-Chen Lan, Tong-Hong Wang, Kuo-Chou Chiu, Thanh-Hien Vu Nguyen, Shih-Min Hsia, Tzong-Ming Shieh
International Journal of Molecular Sciences.2023; 24(12): 9819. CrossRef - The Role of Asiatic Acid in Preventing Dental Pulp Inflammation: An in-vivo Study
Arlina Nurhapsari, Risya Cilmiaty, Adi Prayitno, Bambang Purwanto, Soetrisno Soetrisno
Clinical, Cosmetic and Investigational Dentistry.2023; Volume 15: 109. CrossRef - Dexmedetomidine ameliorates diabetic cardiomyopathy by inhibiting ferroptosis through the Nrf2/GPX4 pathway
Fan Li, Zhenfei Hu, Yidan Huang, Haiting Zhan
Journal of Cardiothoracic Surgery.2023;[Epub] CrossRef - Metformin-mediated epigenetic modifications in diabetes and associated conditions: Biological and clinical relevance
Roberta Giordo, Anna Maria Posadino, Arduino Aleksander Mangoni, Gianfranco Pintus
Biochemical Pharmacology.2023; 215: 115732. CrossRef - Transient receptor potential vanilloid type 1: cardioprotective effects in diabetic models
Jiaqi Bao, Zhicheng Gao, Yilan Hu, Lifang Ye, Lihong Wang
Channels.2023;[Epub] CrossRef - Beyond Hepatoprotection—The Cardioprotective Effects of Bicyclol in Diabetes
Arun Samidurai, Rakesh C. Kukreja
Cardiovascular Drugs and Therapy.2023;[Epub] CrossRef - Sinapic acid ameliorates cardiac dysfunction and cardiomyopathy by modulating NF-κB and Nrf2/HO-1 signaling pathways in streptozocin induced diabetic rats
Mohammad Raish, Ajaz Ahmad, Yousef A. Bin Jardan, Mudassar Shahid, Khalid M. Alkharfy, Abdul Ahad, Mushtaq Ahmad Ansari, Ibrahim Abdelsalam Abdelrahman, Fahad I. Al-Jenoobi
Biomedicine & Pharmacotherapy.2022; 145: 112412. CrossRef - Recent Advances in KEAP1/NRF2-Targeting Strategies by Phytochemical Antioxidants, Nanoparticles, and Biocompatible Scaffolds for the Treatment of Diabetic Cardiovascular Complications
Andrea Rampin, Michele Carrabba, Martina Mutoli, Charlotte L. Eman, Gianluca Testa, Paolo Madeddu, Gaia Spinetti
Antioxidants & Redox Signaling.2022; 36(10-12): 707. CrossRef - Exercise and Urtica Dioica extract ameliorate mitochondrial function and the expression of cardiac muscle Nuclear Respiratory Factor 2 and Peroxisome proliferator-activated receptor Gamma Coactivator 1-alpha in STZ-induced diabetic rats
Seyyedeh Masoumeh Seyydi, Asghar Tofighi, Masoud Rahmati, Javad Tolouei Azar
Gene.2022; 822: 146351. CrossRef - 3,3′,4,5′-Tetramethoxy-trans-stilbene Improves Insulin Resistance by Activating the IRS/PI3K/Akt Pathway and Inhibiting Oxidative Stress
Yi Tan, Lingchao Miao, Jianbo Xiao, Wai San Cheang
Current Issues in Molecular Biology.2022; 44(5): 2175. CrossRef - The Beneficial Effects of Chinese Herbal Monomers on Ameliorating Diabetic Cardiomyopathy via Nrf2 Signaling
Yiwei Gao, Wu Liu, Xin Su, Xinyi Li, Fangning Yu, Ning Zhang, Yuli Huang
Oxidative Medicine and Cellular Longevity.2022; 2022: 1. CrossRef - Cardioprotective Effects and in-silico Antioxidant Mechanism of L-Ergothioneine in Experimental Type-2 Diabetic Rats
Ayobami Dare, Ahmed A Elrashedy, Mahendra L. Channa, Anand Nadar
Cardiovascular & Hematological Agents in Medicinal Chemistry.2022; 20(2): 133. CrossRef - Molecular Mechanisms of Ferroptosis and Relevance to Cardiovascular Disease
Lai-Hua Xie, Nadezhda Fefelova, Sri Harika Pamarthi, Judith K. Gwathmey
Cells.2022; 11(17): 2726. CrossRef - Cardiac adaptation and cardioprotection against arrhythmias and ischemia-reperfusion injury in mammalian hibernators
Lai-Hua Xie, Judith K. Gwathmey, Zhenghang Zhao
Pflügers Archiv - European Journal of Physiology.2021; 473(3): 407. CrossRef - Cardioprotective Effect of Glycyrrhizin on Myocardial Remodeling in Diabetic Rats
Vikram Thakur, Narah Alcoreza, Monica Delgado, Binata Joddar, Munmun Chattopadhyay
Biomolecules.2021; 11(4): 569. CrossRef - Therapeutic potential of targeting oxidative stress in diabetic cardiomyopathy
Nikole J. Byrne, Namakkal S. Rajasekaran, E Dale Abel, Heiko Bugger
Free Radical Biology and Medicine.2021; 169: 317. CrossRef - Punicalagin Protects Against Diabetic Cardiomyopathy by Promoting Opa1-Mediated Mitochondrial Fusion via Regulating PTP1B-Stat3 Pathway
Feng Fu, Chaoyang Liu, Rui Shi, Man Li, Min Zhang, Yanyan Du, Qiaojuan Wang, Jun Li, Guoen Wang, Jianming Pei, Mingge Ding
Antioxidants & Redox Signaling.2021; 35(8): 618. CrossRef - Elevated type I interferon responses potentiate metabolic dysfunction, inflammation, and accelerated aging in mtDNA mutator mice
Yuanjiu Lei, Camila Guerra Martinez, Sylvia Torres-Odio, Samantha L. Bell, Christine E. Birdwell, Joshua D. Bryant, Carl W. Tong, Robert O. Watson, Laura Ciaccia West, A. Phillip West
Science Advances.2021;[Epub] CrossRef - The potential role of Keap1-Nrf2 pathway in the pathogenesis of Alzheimer’s disease, type 2 diabetes, and type 2 diabetes-related Alzheimer’s disease
Ling He, Yi Sun
Metabolic Brain Disease.2021; 36(7): 1469. CrossRef - The impact of mitochondria on cancer treatment resistance
Michelle van der Merwe, Gustav van Niekerk, Carla Fourie, Manisha du Plessis, Anna-Mart Engelbrecht
Cellular Oncology.2021; 44(5): 983. CrossRef - Therapeutic Approaches Targeting Proteostasis in Kidney Disease and Fibrosis
Jia-Huang Chen, Chia-Hsien Wu, Chih-Kang Chiang
International Journal of Molecular Sciences.2021; 22(16): 8674. CrossRef - Coronary Large Conductance Ca2+-Activated K+ Channel Dysfunction in Diabetes Mellitus
Tong Lu, Hon-Chi Lee
Frontiers in Physiology.2021;[Epub] CrossRef - Ferroptosis: New Dawn for Overcoming the Cardio-Cerebrovascular Diseases
Meng-Yi Luo, Jian-Hui Su, Shao-Xin Gong, Na Liang, Wen-Qian Huang, Wei Chen, Ai-Ping Wang, Ying Tian
Frontiers in Cell and Developmental Biology.2021;[Epub] CrossRef - Recombinant Human Growth Hormone Inhibits Lipotoxicity, Oxidative Stress, and Apoptosis in a Mouse Model of Diabetic Cardiomyopathy
Zuowei Pei, Xiang Wang, Chenguang Yang, Min Dong, Fang Wang, Juan Gambini
Oxidative Medicine and Cellular Longevity.2021; 2021: 1. CrossRef - Hydrogen sulfide mitigates myocardial inflammation by inhibiting nucleotide-binding oligomerization domain-like receptor protein 3 inflammasome activation in diabetic rats
Qiang Jia, Shomaila Mehmood, Xiaofen Liu, Shanfeng Ma, Rui Yang
Experimental Biology and Medicine.2020; 245(3): 221. CrossRef - Heme oxygenase-1 in protozoan infections: A tale of resistance and disease tolerance
Rafael C. M. C. Silva, Leonardo H. Travassos, Claudia N. Paiva, Marcelo T. Bozza, Marc-Jan Gubbels
PLOS Pathogens.2020; 16(7): e1008599. CrossRef - Distinct Approaches of Raloxifene: Its Far-Reaching Beneficial Effects Implicating the HO-System
Denise Börzsei, Renáta Szabó, Alexandra Hoffmann, Médea Veszelka, Imre Pávó, Zsolt Turcsán, Csaba Viczián, Krisztina Kupai, Csaba Varga, Anikó Pósa
Biomolecules.2020; 10(3): 375. CrossRef - The Mitochondria: A Target of Polyphenols in the Treatment of Diabetic Cardiomyopathy
Humna Bhagani, Suzanne A. Nasser, Ali Dakroub, Ahmed F. El-Yazbi, Assaad A. Eid, Firas Kobeissy, Gianfranco Pintus, Ali H. Eid
International Journal of Molecular Sciences.2020; 21(14): 4962. CrossRef - The Effects of Dietary Supplements that Overactivate the Nrf2/ARE System
Robert E. Smith
Current Medicinal Chemistry.2020; 27(13): 2077. CrossRef - Allopurinol reduces oxidative stress and activates Nrf2/p62 to attenuate diabetic cardiomyopathy in rats
Jierong Luo, Dan Yan, Sisi Li, Shiming Liu, Fei Zeng, Chi Wai Cheung, Hong Liu, Michael G. Irwin, Huansen Huang, Zhengyuan Xia
Journal of Cellular and Molecular Medicine.2020; 24(2): 1760. CrossRef - Rg1 protects H9C2 cells from high glucose‐/palmitate‐induced injury via activation of AKT/GSK‐3β/Nrf2 pathway
Haitao Yu, Juan Zhen, Yang Yang, Jian Du, Jiyan Leng, Qian Tong
Journal of Cellular and Molecular Medicine.2020; 24(14): 8194. CrossRef - Ranolazine protects against diabetic cardiomyopathy by activating the NOTCH1/NRG1 pathway
Xi Chen, Long Ren, Xing Liu, Xi Sun, Chaorun Dong, Yanan Jiang, Ying Qin, Huan Qu, Jinfeng Jiao, Shuo Wang, Yunlong Bai, Baofeng Yang
Life Sciences.2020; 261: 118306. CrossRef - Low Concentration of Withaferin a Inhibits Oxidative Stress-Mediated Migration and Invasion in Oral Cancer Cells
Tzu-Jung Yu, Jen-Yang Tang, Fu Ou-Yang, Yen-Yun Wang, Shyng-Shiou F. Yuan, Kevin Tseng, Li-Ching Lin, Hsueh-Wei Chang
Biomolecules.2020; 10(5): 777. CrossRef - Protective Effect of Simplicillium sp. Ethyl Acetate Extract against High Glucose-Induced Oxidative Stress in HUVECs
Ting-Ting Tian, Qi-Rui Li, Shi-Quan Gan, Chu-Rui Chang, Xiang-Chun Shen
Evidence-Based Complementary and Alternative Medicine.2020; 2020: 1. CrossRef - Deneysel Diyabet Oluşturulan Sıçanlarda Kalp ve İskelet Kası Nrf2 Yapımı ve Oksidatif Stres Üzerine Melatoninin Etkisinin İncelenmesi
Salim ÖZENOĞLU, İnci TURAN, Hale SAYAN ÖZAÇMAK, Veysel Haktan ÖZAÇMAK
Turkish Journal of Diabetes and Obesity.2020; 4(1): 46. CrossRef - Nicorandil alleviates apoptosis in diabetic cardiomyopathy through PI3K/Akt pathway
Xuyang Wang, Jinyu Pan, Dian Liu, Mingjun Zhang, Xiaowei Li, Jingjing Tian, Ming Liu, Tao Jin, Fengshuang An
Journal of Cellular and Molecular Medicine.2019; 23(8): 5349. CrossRef - Bailcalin Protects against Diabetic Cardiomyopathy through Keap1/Nrf2/AMPK-Mediated Antioxidative and Lipid-Lowering Effects
Ran Li, Yuan Liu, Ying-guang Shan, Lu Gao, Fang Wang, Chun-guang Qiu
Oxidative Medicine and Cellular Longevity.2019; 2019: 1. CrossRef - Omega 3 rich diet modulates energy metabolism via GPR120-Nrf2 crosstalk in a novel antioxidant mouse model
Deborah Amos, Carla Cook, Nalini Santanam
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids.2019; 1864(4): 466. CrossRef - Increased placental expressions of nuclear factor erythroid 2–related factor 2 and antioxidant enzymes in gestational diabetes: Protective mechanisms against the placental oxidative stress?
Balachandiran Manoharan, Zachariah Bobby, Gowri Dorairajan, Sajini Elizabeth Jacob, Victorraj Gladwin, Vickneshwaran Vinayagam, Rajaa Muthu Packirisamy
European Journal of Obstetrics & Gynecology and Reproductive Biology.2019; 238: 78. CrossRef - Another look at phenolic compounds in cancer therapy the effect of polyphenols on ubiquitin-proteasome system
Aleksandra Golonko, Tomasz Pienkowski, Renata Swislocka, Ryszard Lazny, Marek Roszko, Wlodzimierz Lewandowski
European Journal of Medicinal Chemistry.2019; 167: 291. CrossRef - Hydrogen sulfide-mediated regulation of cell death signaling ameliorates adverse cardiac remodeling and diabetic cardiomyopathy
Sumit Kar, Tyler N. Kambis, Paras K. Mishra
American Journal of Physiology-Heart and Circulatory Physiology.2019; 316(6): H1237. CrossRef - Genomic and Genetic Approaches to Deciphering Acute Respiratory Distress Syndrome Risk and Mortality
Heather Lynn, Xiaoguang Sun, Nancy Casanova, Manuel Gonzales-Garay, Christian Bime, Joe G.N. Garcia
Antioxidants & Redox Signaling.2019; 31(14): 1027. CrossRef - Enhanced Keap1-Nrf2 signaling protects the myocardium from isoproterenol-induced pathological remodeling in mice
Gobinath Shanmugam, Anil Kumar Challa, Silvio H. Litovsky, Asokan Devarajan, Ding Wang, Dean P. Jones, Victor M. Darley-Usmar, Namakkal Soorappan Rajasekaran
Redox Biology.2019; 27: 101212. CrossRef - Effects of PP2A/Nrf2 on experimental diabetes mellitus-related cardiomyopathy by regulation of autophagy and apoptosis through ROS dependent pathway
Yanhui Guan, Lichun Zhou, Yu Zhang, Huiqin Tian, Anqi Li, Xiuzhen Han
Cellular Signalling.2019; 62: 109339. CrossRef - An Aza resveratrol–chalcone derivative 6b protects mice against diabetic cardiomyopathy by alleviating inflammation and oxidative stress
Shengban You, Jianchang Qian, Chuchu Sun, Hailing Zhang, Shiju Ye, Taiwei Chen, Zheng Xu, Jingying Wang, Weijian Huang, Guang Liang
Journal of Cellular and Molecular Medicine.2018; 22(3): 1931. CrossRef - Oxidative Stress in Mesenchymal Stem Cell Senescence: Regulation by Coding and Noncoding RNAs
Rosa Vono, Eva Jover Garcia, Gaia Spinetti, Paolo Madeddu
Antioxidants & Redox Signaling.2018; 29(9): 864. CrossRef - An overview of the emerging interface between cardiac metabolism, redox biology and the circadian clock
Rodrigo A. Peliciari-Garcia, Victor Darley-Usmar, Martin E. Young
Free Radical Biology and Medicine.2018; 119: 75. CrossRef - Klotho protects the heart from hyperglycemia-induced injury by inactivating ROS and NF-κB-mediated inflammation both in vitro and in vivo
Yue Guo, Xiaodong Zhuang, Zena Huang, Jing Zou, Daya Yang, Xun Hu, Zhimin Du, Lichun Wang, Xinxue Liao
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2018; 1864(1): 238. CrossRef - Paradoxical cardiotoxicity of intraperitoneally-injected epigallocatechin gallate preparation in diabetic mice
Nora O. Abdel Rasheed, Lamiaa A. Ahmed, Dalaal M. Abdallah, Bahia M. El-Sayeh
Scientific Reports.2018;[Epub] CrossRef - Oxidative stress-induced miR-27a targets the redox gene nuclear factor erythroid 2-related factor 2 in diabetic embryopathy
Yang Zhao, Daoyin Dong, E. Albert Reece, Ashley R. Wang, Peixin Yang
American Journal of Obstetrics and Gynecology.2018; 218(1): 136.e1. CrossRef - Regulation on SIRT1-PGC-1α/Nrf2 pathway together with selective inhibition of aldose reductase makes compound hr5F a potential agent for the treatment of diabetic complications
Zhihua Wang, Sheng Yuan, Yanbing Li, Zhe Zhang, Wei Xiao, Dan Tang, Kaihe Ye, Zhijun Liu, Congcong Wang, Yixiong Zheng, Hong Nie, Heru Chen
Biochemical Pharmacology.2018; 150: 54. CrossRef - Fibroblast growth factor-21 prevents diabetic cardiomyopathy via AMPK-mediated antioxidation and lipid-lowering effects in the heart
Hong Yang, Anyun Feng, Sundong Lin, Lechu Yu, Xiufei Lin, Xiaoqing Yan, Xuemian Lu, Chi Zhang
Cell Death & Disease.2018;[Epub] CrossRef - Salusin-β contributes to oxidative stress and inflammation in diabetic cardiomyopathy
Ming-Xia Zhao, Bing Zhou, Li Ling, Xiao-Qing Xiong, Feng Zhang, Qi Chen, Yue-Hua Li, Yu-Ming Kang, Guo-Qing Zhu
Cell Death & Disease.2017; 8(3): e2690. CrossRef - Role of Nrf2 Signaling in the Regulation of Vascular BK Channel β1 Subunit Expression and BK Channel Function in High-Fat Diet–Induced Diabetic Mice
Tong Lu, Xiaojing Sun, Yong Li, Qiang Chai, Xiao-Li Wang, Hon-Chi Lee
Diabetes.2017; 66(10): 2681. CrossRef - Regulation of vascular large-conductance calcium-activated potassium channels by Nrf2 signalling
Yong Li, Xiao-Li Wang, Xiaojing Sun, Qiang Chai, Jingchao Li, Benjamin Thompson, Win-Kuang Shen, Tong Lu, Hon-Chi Lee
Diabetes and Vascular Disease Research.2017; 14(4): 353. CrossRef - Metallothionein Is Downstream of Nrf2 and Partially Mediates Sulforaphane Prevention of Diabetic Cardiomyopathy
Junlian Gu, Yanli Cheng, Hao Wu, Lili Kong, Shudong Wang, Zheng Xu, Zhiguo Zhang, Yi Tan, Bradley B. Keller, Honglan Zhou, Yuehui Wang, Zhonggao Xu, Lu Cai
Diabetes.2017; 66(2): 529. CrossRef - Kimchi methanol extracts attenuate hepatic steatosis induced by high cholesterol diet in low-density lipoprotein receptor knockout mice through inhibition of endoplasmic reticulum stress
Minji Woo, Mijeong Kim, Jeong Sook Noh, Yeong Ok Song
Journal of Functional Foods.2017; 32: 218. CrossRef - Aspalathin Protects the Heart against Hyperglycemia-Induced Oxidative Damage by Up-Regulating Nrf2 Expression
Phiwayinkosi Dludla, Christo Muller, Elizabeth Joubert, Johan Louw, M. Essop, Kwazi Gabuza, Samira Ghoor, Barbara Huisamen, Rabia Johnson
Molecules.2017; 22(1): 129. CrossRef - Intermittent hypoxia-induced cardiomyopathy and its prevention by Nrf2 and metallothionein
Shanshan Zhou, Xia Yin, Jingpeng Jin, Yi Tan, Daniel J. Conklin, Ying Xin, Zhiguo Zhang, Weixia Sun, Taixing Cui, Jun Cai, Yang Zheng, Lu Cai
Free Radical Biology and Medicine.2017; 112: 224. CrossRef - Inhibitory effects of rosmarinic acid on pterygium epithelial cells through redox imbalance and induction of extrinsic and intrinsic apoptosis
Ya-Yu Chen, Chia-Fang Tsai, Ming-Chu Tsai, Yu-Wen Hsu, Fung-Jou Lu
Experimental Eye Research.2017; 160: 96. CrossRef - Ischemia reperfusion injury, ischemic conditioning and diabetes mellitus
Anne Lejay, Fei Fang, Rohan John, Julie A.D. Van, Meredith Barr, Fabien Thaveau, Nabil Chakfe, Bernard Geny, James W. Scholey
Journal of Molecular and Cellular Cardiology.2016; 91: 11. CrossRef - The Role of the Nrf2/ARE Antioxidant System in Preventing Cardiovascular Diseases
Robert Smith, Kevin Tran, Cynthia Smith, Miranda McDonald, Pushkar Shejwalkar, Kenji Hara
Diseases.2016; 4(4): 34. CrossRef - Prohibitin overexpression improves myocardial function in diabetic cardiomyopathy
Wen-qian Dong, Min Chao, Qing-hua Lu, Wei-li Chai, Wei Zhang, Xue-ying Chen, Er-shun Liang, Ling-bo Wang, Hong-liang Tian, Yu-guo Chen, Ming-xiang Zhang
Oncotarget.2016; 7(1): 66. CrossRef - Restoration of Nrf2 Signaling Normalizes the Regenerative Niche
Marc A. Soares, Oriana D. Cohen, Yee Cheng Low, Rita A. Sartor, Trevor Ellison, Utkarsh Anil, Lavinia Anzai, Jessica B. Chang, Pierre B. Saadeh, Piul S. Rabbani, Daniel J. Ceradini
Diabetes.2016; 65(3): 633. CrossRef - Myocardial transcription factors in diastolic dysfunction: clues for model systems and disease
Alexander T. Mikhailov, Mario Torrado
Heart Failure Reviews.2016; 21(6): 783. CrossRef - Antioxidant Properties of Whole Body Periodic Acceleration (pGz)
Arkady Uryash, Jorge Bassuk, Paul Kurlansky, Francisco Altamirano, Jose R. Lopez, Jose A. Adams, Guillermo López Lluch
PLOS ONE.2015; 10(7): e0131392. CrossRef - Mulberry leaves (Morus alba L.) ameliorate obesity-induced hepatic lipogenesis, fibrosis, and oxidative stress in high-fat diet-fed mice
Ji-Young Ann, Hyeyoon Eo, Yunsook Lim
Genes & Nutrition.2015;[Epub] CrossRef - Oxidative Stress and Human Hypertension: Vascular Mechanisms, Biomarkers, and Novel Therapies
Augusto C. Montezano, Maria Dulak-Lis, Sofia Tsiropoulou, Adam Harvey, Ana M. Briones, Rhian M. Touyz
Canadian Journal of Cardiology.2015; 31(5): 631. CrossRef - Haeme oxygenase signalling pathway: implications for cardiovascular disease
Laura E. Fredenburgh, Allison A. Merz, Susan Cheng
European Heart Journal.2015; 36(24): 1512. CrossRef
- Natural Products and Health

 KDA
KDA
 First
First Prev
Prev





