
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
Original Articles
- Drug/Regimen
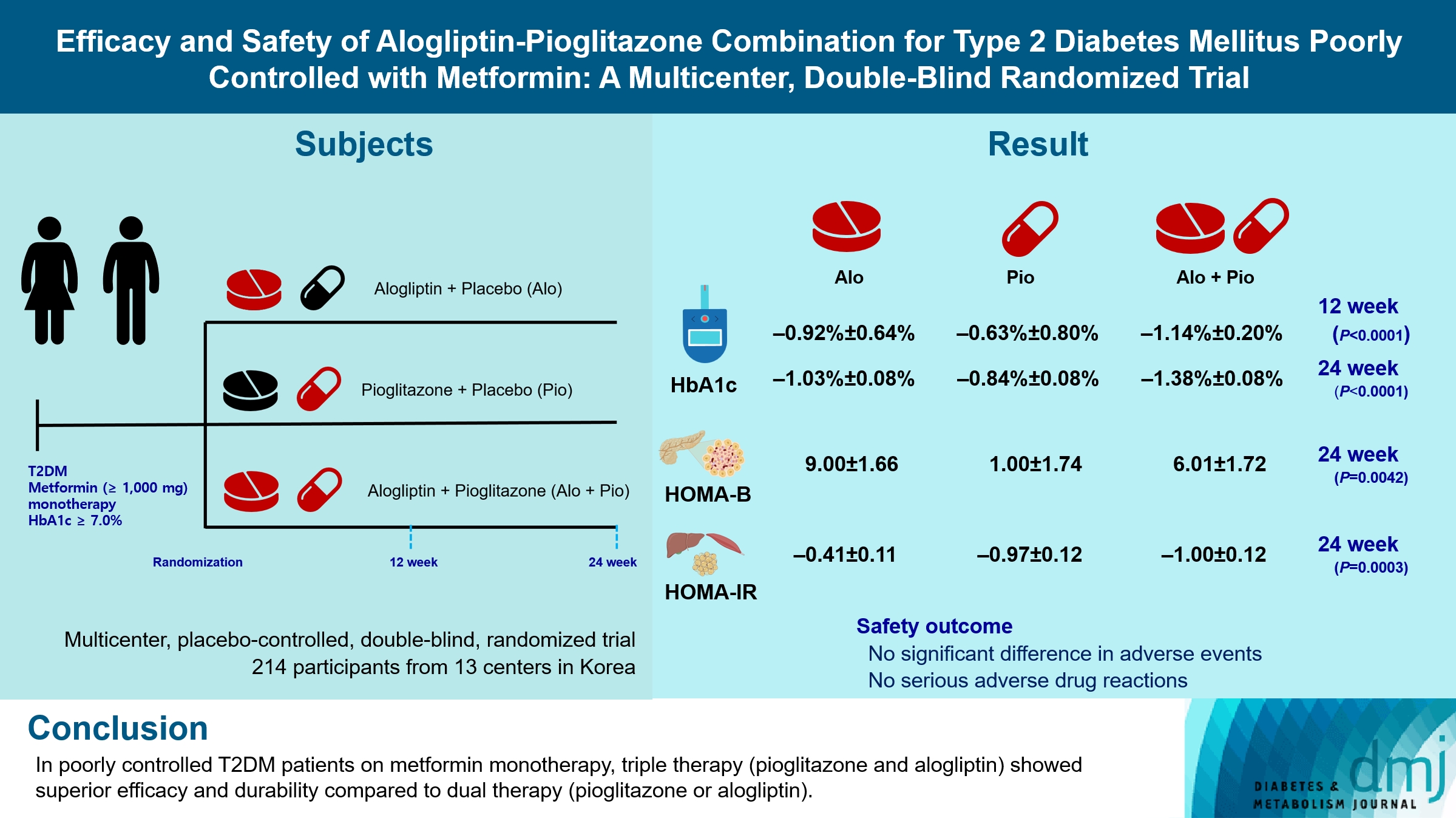
- Efficacy and Safety of Alogliptin-Pioglitazone Combination for Type 2 Diabetes Mellitus Poorly Controlled with Metformin: A Multicenter, Double-Blind Randomized Trial
- Ji-Yeon Park, Joonyub Lee, Yoon-Hee Choi, Kyung Wan Min, Kyung Ah Han, Kyu Jeung Ahn, Soo Lim, Young-Hyun Kim, Chul Woo Ahn, Kyung Mook Choi, Kun-Ho Yoon, the Practical Evidence of Antidiabetic Combination Therapy in Korea (PEAK) study investigators
- Received August 7, 2023 Accepted November 30, 2023 Published online April 23, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0259 [Epub ahead of print]
- 191 View
- 8 Download
-
 Abstract
Abstract
 PDF
PDF - Background
Guidelines for switching to triple combination therapy directly after monotherapy failure are limited. This study investigated the efficacy, long-term sustainability, and safety of either mono or dual add-on therapy using alogliptin and pioglitazone for patients with type 2 diabetes mellitus (T2DM) who did not achieve their target glycemic range with metformin monotherapy.
Methods
The Practical Evidence of Antidiabetic Combination Therapy in Korea (PEAK) was a multicenter, placebo-controlled, double-blind, randomized trial. A total of 214 participants were randomized to receive alogliptin+pioglitazone (Alo+Pio group, n=70), alogliptin (Alo group, n=75), or pioglitazone (Pio group, n=69). The primary outcome was the difference in glycosylated hemoglobin (HbA1c) levels between the three groups at baseline to 24 weeks. For durability, the achievement of HbA1c levels <7% and <6.5% was compared in each group. The number of adverse events was investigated for safety.
Results
After 24 weeks of treatment, the change of HbA1c in the Alo+Pio, Alo, and Pio groups were –1.38%±0.08%, –1.03%±0.08%, and –0.84%±0.08%, respectively. The Alo+Pio group had significantly lower HbA1c levels than the other groups (P=0.0063, P<0.0001) and had a higher proportion of patients with target HbA1c achievement. In addition, insulin sensitivity and β-cell function, lipid profiles, and other metabolic indicators were also improved. There were no significant safety issues in patients treated with triple combination therapy.
Conclusion
Early combination triple therapy showed better efficacy and durability than the single add-on (dual) therapy. Therefore, combination therapy with metformin, alogliptin, and pioglitazone is a valuable early treatment option for T2DM poorly controlled with metformin monotherapy.
- Metabolic Risk/Epidemiology
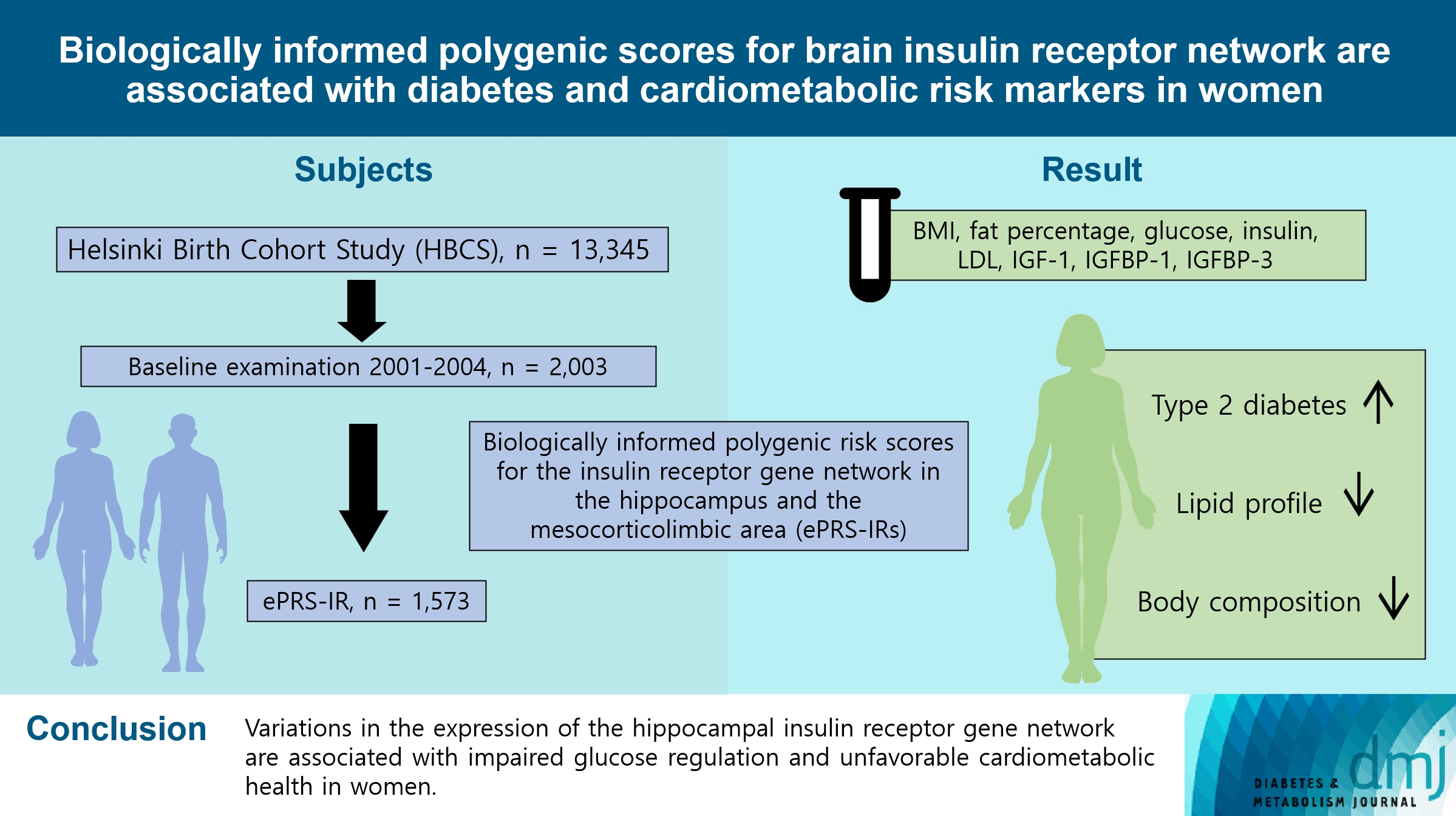
- Biologically Informed Polygenic Scores for Brain Insulin Receptor Network Are Associated with Cardiometabolic Risk Markers and Diabetes in Women
- Jannica S. Selenius, Patricia P. Silveira, Mikaela von Bonsdorff, Jari Lahti, Hannu Koistinen, Riitta Koistinen, Markku Seppälä, Johan G. Eriksson, Niko S. Wasenius
- Received February 10, 2023 Accepted November 25, 2023 Published online March 25, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0039 [Epub ahead of print]
- 694 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
To investigate associations between variations in the co-expression-based brain insulin receptor polygenic score and cardiometabolic risk factors and diabetes mellitus.
Methods
This cross-sectional study included 1,573 participants from the Helsinki Birth Cohort Study. Biologically informed expression-based polygenic risk scores for the insulin receptor gene network were calculated for the hippocampal (hePRS-IR) and the mesocorticolimbic (mePRS-IR) regions. Cardiometabolic markers included body composition, waist circumference, circulating lipids, insulin-like growth factor 1 (IGF-1), and insulin-like growth factor-binding protein 1 and 3 (IGFBP-1 and -3). Glucose and insulin levels were measured during a standardized 2-hour 75 g oral glucose tolerance test and impaired glucose regulation status was defined by the World Health Organization 2019 criteria. Analyzes were adjusted for population stratification, age, smoking, alcohol consumption, socioeconomic status, chronic diseases, birth weight, and leisure-time physical activity.
Results
Multinomial logistic regression indicated that one standard deviation increase in hePRS-IR was associated with increased risk of diabetes mellitus in all participants (adjusted relative risk ratio, 1.17; 95% confidence interval, 1.01 to 1.35). In women, higher hePRS-IR was associated with greater waist circumference and higher body fat percentage, levels of glucose, insulin, total cholesterol, low-density lipoprotein cholesterol, triglycerides, apolipoprotein B, insulin, and IGFBP-1 (all P≤0.02). The mePRS-IR was associated with decreased IGF-1 level in women (P=0.02). No associations were detected in men and studied outcomes.
Conclusion
hePRS-IR is associated with sex-specific differences in cardiometabolic risk factor profiles including impaired glucose regulation, abnormal metabolic markers, and unfavorable body composition in women.
- Type 1 Diabetes
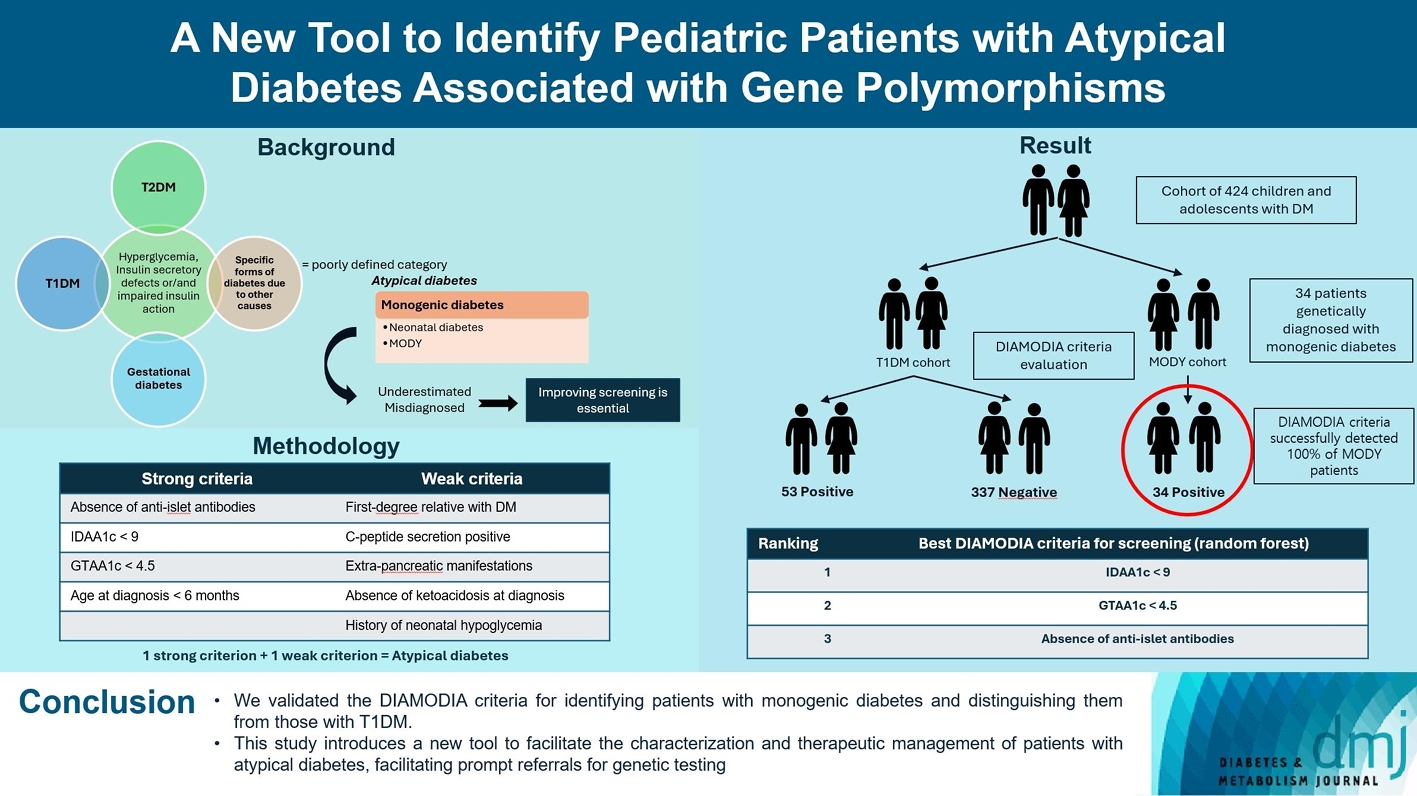
- A New Tool to Identify Pediatric Patients with Atypical Diabetes Associated with Gene Polymorphisms
- Sophie Welsch, Antoine Harvengt, Paola Gallo, Manon Martin, Dominique Beckers, Thierry Mouraux, Nicole Seret, Marie-Christine Lebrethon, Raphaël Helaers, Pascal Brouillard, Miikka Vikkula, Philippe A. Lysy
- Received May 26, 2023 Accepted November 25, 2023 Published online March 22, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0166 [Epub ahead of print]
- 779 View
- 48 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Recent diabetes subclassifications have improved the differentiation between patients with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus despite several overlapping features, yet without considering genetic forms of diabetes. We sought to facilitate the identification of monogenic diabetes by creating a new tool that we validated in a pediatric maturity-onset diabetes of the young (MODY) cohort.
Methods
We first created the DIAgnose MOnogenic DIAbetes (DIAMODIA) criteria based on the pre-existing, but incomplete, MODY calculator. This new score is composed of four strong and five weak criteria, with patients having to display at least one weak and one strong criterion.
Results
The effectiveness of the DIAMODIA criteria was evaluated in two patient cohorts, the first consisting of patients with confirmed MODY diabetes (n=34) and the second of patients with T1DM (n=390). These DIAMODIA criteria successfully detected 100% of MODY patients. Multiple correspondence analysis performed on the MODY and T1DM cohorts enabled us to differentiate MODY patients from T1DM. The three most relevant variables to distinguish a MODY from T1DM profile were: lower insulin-dose adjusted A1c score ≤9, glycemic target-adjusted A1c score ≤4.5, and absence of three anti-islet cell autoantibodies.
Conclusion
We validated the DIAMODIA criteria, as it effectively identified all monogenic diabetes patients (MODY cohort) and succeeded to differentiate T1DM from MODY patients. The creation of this new and effective tool is likely to facilitate the characterization and therapeutic management of patients with atypical diabetes, and promptly referring them for genetic testing which would markedly improve clinical care and counseling, as well.
Review
- Metabolic Risk/Epidemiology
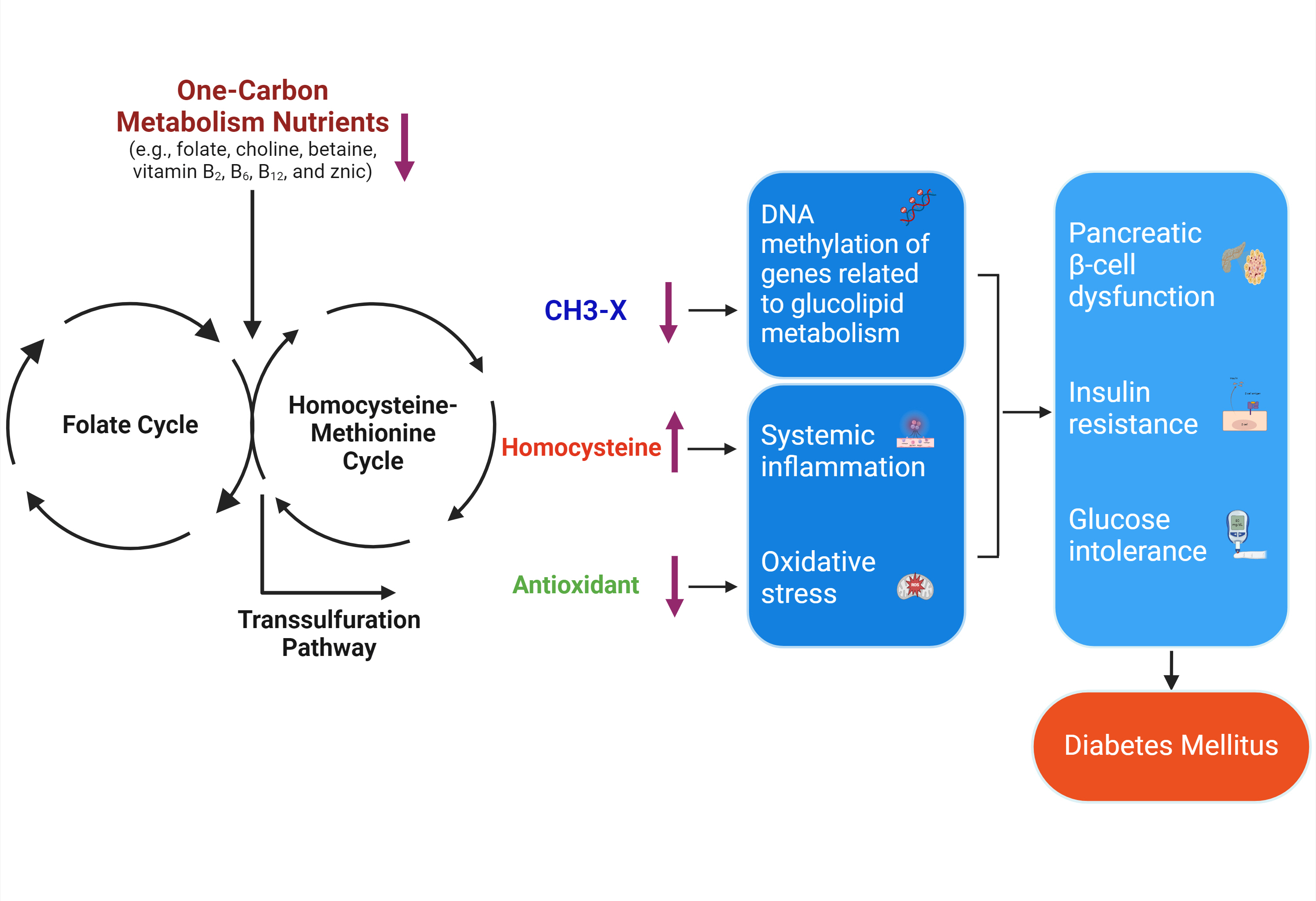
- One-Carbon Metabolism Nutrients, Genetic Variation, and Diabetes Mellitus
- Jie Zhu, Gunjana Saikia, Xiaotao Zhang, Xiaoxi Shen, Ka Kahe
- Diabetes Metab J. 2024;48(2):170-183. Published online March 12, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0272
- 1,130 View
- 153 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Diabetes mellitus (DM) affects about 9.3% of the population globally. Hyperhomocysteinemia (HHcy) has been implicated in the pathogenesis of DM, owing to its promotion of oxidative stress, β-cell dysfunction, and insulin resistance. HHcy can result from low status of one-carbon metabolism (OCM) nutrients (e.g., folate, choline, betaine, vitamin B6, B12), which work together to degrade homocysteine by methylation. The etiology of HHcy may also involve genetic variation encoding key enzymes in OCM. This review aimed to provide an overview of the existing literature assessing the link between OCM nutrients status, related genetic factors, and incident DM. We also discussed possible mechanisms underlying the role of OCM in DM development and provided recommendations for future research and practice. Even though the available evidence remains inconsistent, some studies support the potential beneficial effects of intakes or blood levels of OCM nutrients on DM development. Moreover, certain variants in OCM-related genes may influence metabolic handling of methyl-donors and presumably incidental DM. Future studies are warranted to establish the causal inference between OCM and DM and examine the interaction of OCM nutrients and genetic factors with DM development, which will inform the personalized recommendations for OCM nutrients intakes on DM prevention.
Original Articles
- Metabolic Risk/Epidemiology
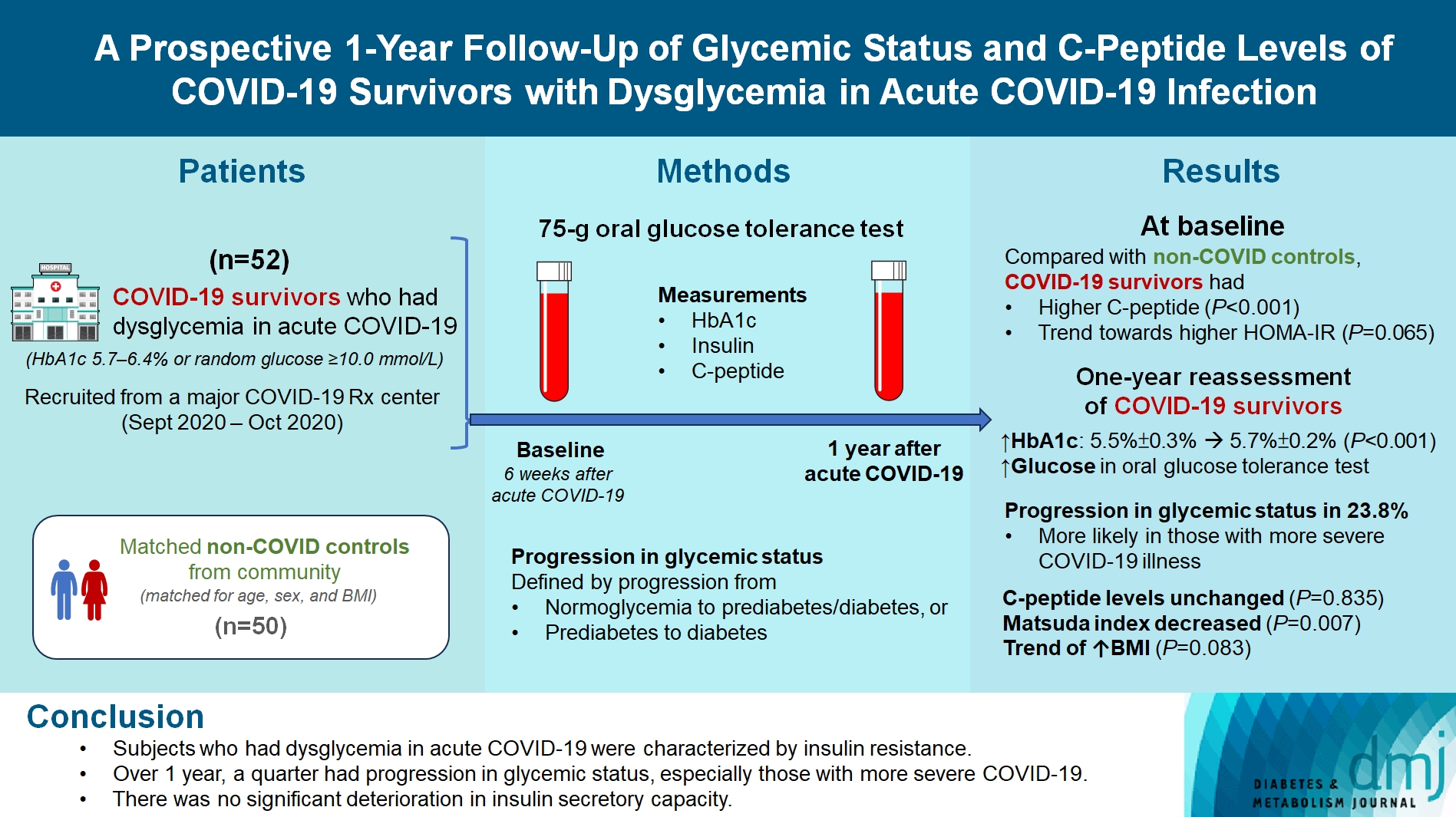
- A Prospective 1-Year Follow-Up of Glycemic Status and C-Peptide Levels of COVID-19 Survivors with Dysglycemia in Acute COVID-19 Infection
- David Tak Wai Lui, Chi Ho Lee, Ying Wong, Carol Ho Yi Fong, Kimberly Hang Tsoi, Yu Cho Woo, Kathryn Choon Beng Tan
- Received June 5, 2023 Accepted October 13, 2023 Published online March 11, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0175 [Epub ahead of print]
- 736 View
- 35 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
We evaluated changes in glycemic status, over 1 year, of coronavirus disease 2019 (COVID-19) survivors with dysglycemia in acute COVID-19.
Methods
COVID-19 survivors who had dysglycemia (defined by glycosylated hemoglobin [HbA1c] 5.7% to 6.4% or random glucose ≥10.0 mmol/L) in acute COVID-19 were recruited from a major COVID-19 treatment center from September to October 2020. Matched non-COVID controls were recruited from community. The 75-g oral glucose tolerance test (OGTT) were performed at baseline (6 weeks after acute COVID-19) and 1 year after acute COVID-19, with HbA1c, insulin and C-peptide measurements. Progression in glycemic status was defined by progression from normoglycemia to prediabetes/diabetes, or prediabetes to diabetes.
Results
Fifty-two COVID-19 survivors were recruited. Compared with non-COVID controls, they had higher C-peptide (P< 0.001) and trend towards higher homeostasis model assessment of insulin resistance (P=0.065). Forty-three COVID-19 survivors attended 1-year reassessment. HbA1c increased from 5.5%±0.3% to 5.7%±0.2% (P<0.001), with increases in glucose on OGTT at fasting (P=0.089), 30-minute (P=0.126), 1-hour (P=0.014), and 2-hour (P=0.165). At baseline, 19 subjects had normoglycemia, 23 had prediabetes, and one had diabetes. Over 1 year, 10 subjects (23.8%; of 42 non-diabetes subjects at baseline) had progression in glycemic status. C-peptide levels remained unchanged (P=0.835). Matsuda index decreased (P=0.007) and there was a trend of body mass index increase from 24.4±2.7 kg/m2 to 25.6±5.2 (P=0.083). Subjects with progression in glycemic status had more severe COVID-19 illness than non-progressors (P=0.030). Reassessment was not performed in the control group.
Conclusion
Subjects who had dysglycemia in acute COVID-19 were characterized by insulin resistance. Over 1 year, a quarter had progression in glycemic status, especially those with more severe COVID-19. Importantly, there was no significant deterioration in insulin secretory capacity.
- Type 1 Diabetes
- Optimal Coefficient of Variance Threshold to Minimize Hypoglycemia Risk in Individuals with Well-Controlled Type 1 Diabetes Mellitus
- Jee Hee Yoo, Seung Hee Yang, Sang-Man Jin, Jae Hyeon Kim
- Received March 14, 2023 Accepted August 12, 2023 Published online March 4, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0083 [Epub ahead of print]

- 510 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study investigated the optimal coefficient of variance (%CV) for preventing hypoglycemia based on real-time continuous glucose monitoring (rt-CGM) data in people with type 1 diabetes mellitus (T1DM) already achieving their mean glucose (MG) target.
Methods
Data from 172 subjects who underwent rt-CGM for at least 90 days and for whom 439 90-day glycemic profiles were available were analyzed. Receiver operator characteristic analysis was conducted to determine the cut-off value of %CV to achieve time below range (%TBR)<54 mg/dL <1 and =0.
Results
Overall mean glycosylated hemoglobin was 6.8% and median %TBR<54 mg/dL was 0.2%. MG was significantly higher and %CV significantly lower in profiles achieving %TBR<54 mg/dL <1 compared to %TBR<54 mg/dL ≥1 (all P<0.001). The cut-off value of %CV for achieving %TBR<54 mg/dL <1 was 37.5%, 37.3%, and 31.0%, in the whole population, MG >135 mg/dL, and ≤135 mg/dL, respectively. The cut-off value for %TBR<54 mg/dL=0% was 29.2% in MG ≤135 mg/dL. In profiles with MG ≤135 mg/dL, 94.2% of profiles with a %CV <31 achieved the target of %TBR<54 mg/dL <1, and 97.3% with a %CV <29.2 achieved the target of %TBR<54 mg/ dL=0%. When MG was >135 mg/dL, 99.4% of profiles with a %CV <37.3 achieved %TBR<54 mg/dL <1.
Conclusion
In well-controlled T1DM with MG ≤135 mg/dL, we suggest a %CV <31% to achieve the %TBR<54 mg/dL <1 target. Furthermore, we suggest a %CV <29.2% to achieve the target of %TBR<54 mg/dL =0 for people at high risk of hypoglycemia.
- Complications
- Switching from Conventional Fibrates to Pemafibrate Has Beneficial Effects on the Renal Function of Diabetic Subjects with Chronic Kidney Disease
- Rimi Izumihara, Hiroshi Nomoto, Kenichi Kito, Yuki Yamauchi, Kazuno Omori, Yui Shibayama, Shingo Yanagiya, Aika Miya, Hiraku Kameda, Kyu Yong Cho, So Nagai, Ichiro Sakuma, Akinobu Nakamura, Tatsuya Atsumi, on Behalf of the PARM-TD Study Group
- Received October 15, 2023 Accepted November 22, 2023 Published online February 29, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0370 [Epub ahead of print]
- 703 View
- 129 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Fibrates have renal toxicity limiting their use in subjects with chronic kidney disease (CKD). However, pemafibrate has fewer toxic effects on renal function. In the present analysis, we evaluated the effects of pemafibrate on the renal function of diabetic subjects with or without CKD in a real-world clinical setting.
Methods
We performed a sub-analysis of data collected during a multi-center, prospective, observational study of the effects of pemafibrate on lipid metabolism in subjects with type 2 diabetes mellitus complicated by hypertriglyceridemia (the PARM-T2D study). The participants were allocated to add pemafibrate to their existing regimen (ADD-ON), switch from their existing fibrate to pemafibrate (SWITCH), or continue conventional therapy (CTRL). The changes in estimated glomerular filtration rate (eGFR) over 52 weeks were compared among these groups as well as among subgroups created according to CKD status.
Results
Data for 520 participants (ADD-ON, n=166; SWITCH, n=96; CTRL, n=258) were analyzed. Of them, 56.7% had CKD. The eGFR increased only in the SWITCH group, and this trend was also present in the CKD subgroup (P<0.001). On the other hand, eGFR was not affected by switching in participants with severe renal dysfunction (G3b or G4) and/or macroalbuminuria. Multivariate analysis showed that being older and a switch from fenofibrate were associated with elevation in eGFR (both P<0.05).
Conclusion
A switch to pemafibrate may be associated with an elevation in eGFR, but to a lesser extent in patients with poor renal function.
- Drug/Regimen
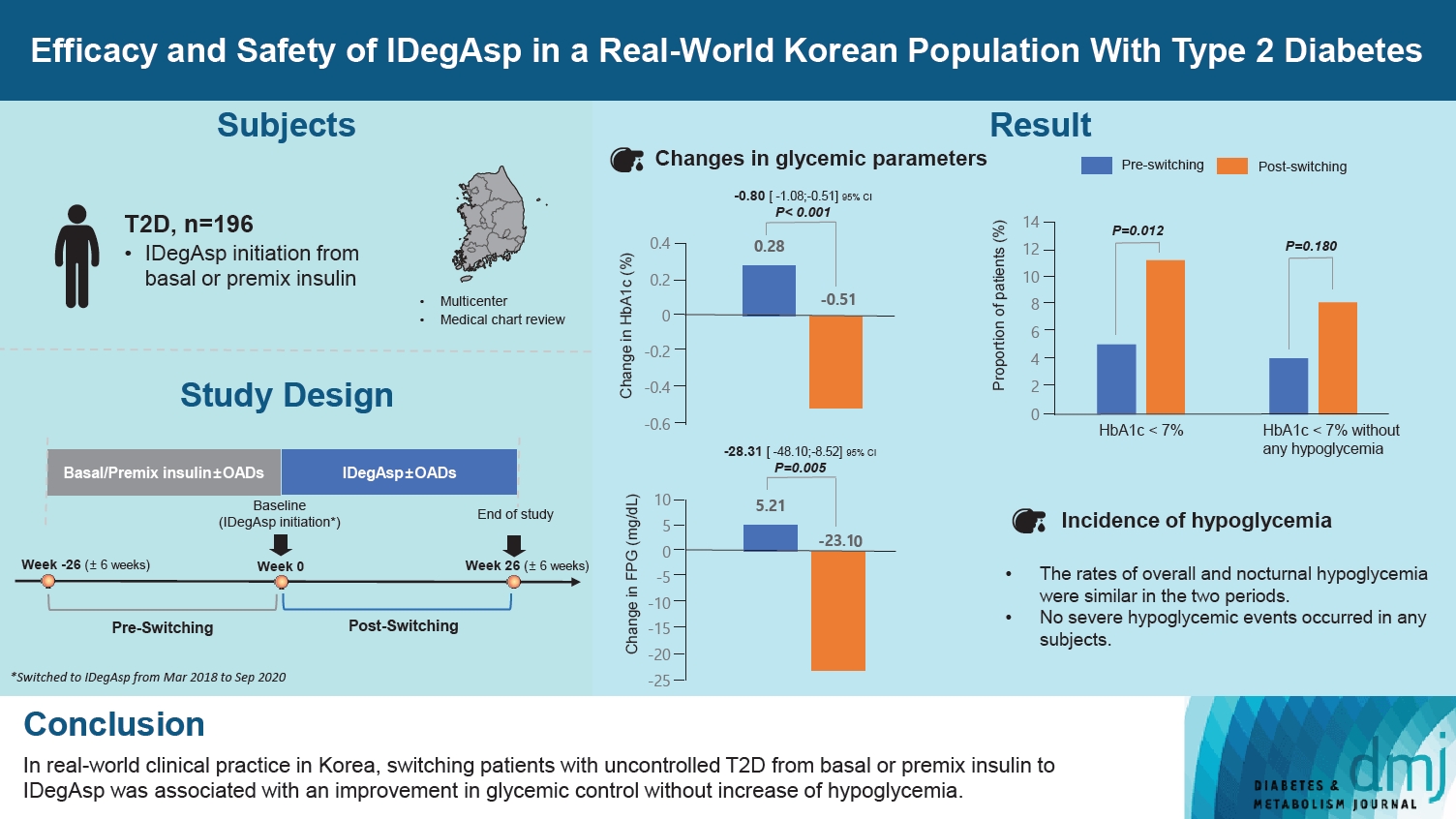
- Efficacy and Safety of IDegAsp in a Real-World Korean Population with Type 2 Diabetes Mellitus
- Shinae Kang, Yu-Bae Ahn, Tae Keun Oh, Won-Young Lee, Sung Wan Chun, Boram Bae, Amine Dahaoui, Jin Sook Jeong, Sungeun Jung, Hak Chul Jang
- Received August 24, 2023 Accepted November 22, 2023 Published online February 27, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0297 [Epub ahead of print]
- 644 View
- 42 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study investigated the real-world efficacy and safety of insulin degludec/insulin aspart (IDegAsp) in Korean adults with type 2 diabetes mellitus (T2DM), whose insulin treatment was switched to IDegAsp.
Methods
This was a multicenter, retrospective, observational study comprising two 26-week treatment periods, before and after switching to IDegAsp, respectively. Korean adults with uncontrolled T2DM treated with basal or premix insulin (±oral antidiabetic drugs) were enrolled. The primary objective was to compare the degree of glycosylated hemoglobin (HbA1c) change in each 26-week observation period. The analyses included changes in HbA1c, fasting plasma glucose (FPG), body weight, proportion of participants achieving HbA1c <7.0%, hypoglycemic events, and total daily insulin dose (ClinicalTrials.gov, number NCT04656106).
Results
In total, 196 adults (mean age, 65.95 years; mean T2DM duration, 18.99 years) were analyzed. The change in both HbA1c and FPG were significantly different between the pre-switching and the post-switching period (0.28% vs. –0.51%, P<0.001; 5.21 mg/dL vs. –23.10 mg/dL, P=0.005), respectively. After switching, the rate of achieving HbA1c <7.0% was significantly improved (5.10% at baseline vs. 11.22% with IDegAsp, P=0.012). No significant differences (before vs. after switching) were observed in body weight change, and total daily insulin dose. The rates of overall and severe hypoglycemia were similar in the two periods.
Conclusion
In real-world clinical practice in Korea, the change of insulin regimen to IDegAsp was associated with an improvement in glycemic control without increase of hypoglycemia, supporting the use of IDegAsp for patients with T2DM uncontrolled with basal or premix insulin.
- Complications
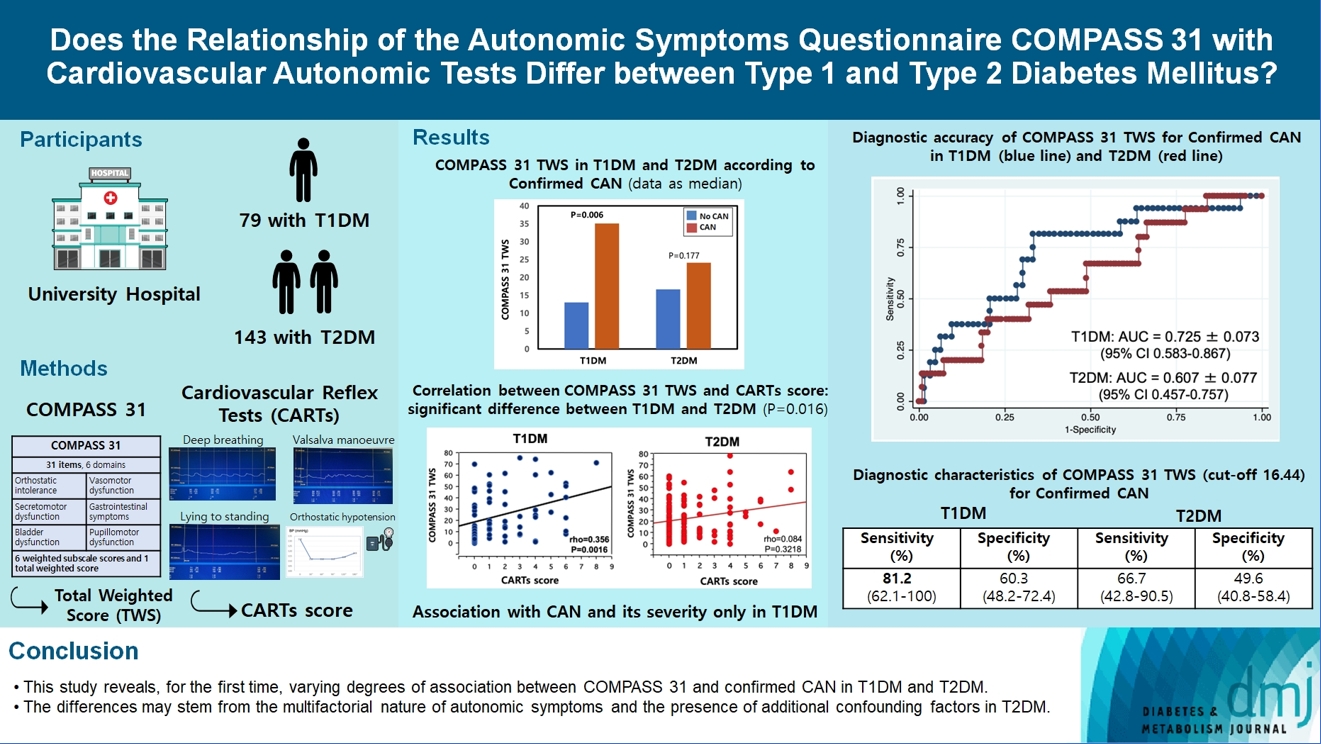
- Does the Relationship of the Autonomic Symptoms Questionnaire COMPASS 31 with Cardiovascular Autonomic Tests Differ between Type 1 and Type 2 Diabetes Mellitus?
- Ilenia D’Ippolito, Marika Menduni, Cinzia D’Amato, Aikaterini Andreadi, Davide Lauro, Vincenza Spallone
- Received August 28, 2023 Accepted November 22, 2023 Published online February 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0301 [Epub ahead of print]
- 601 View
- 43 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The aim was to investigate if autonomic symptoms questionnaire Composite Autonomic Symptom Score (COMPASS) 31 has different association with cardiovascular autonomic neuropathy (CAN) and diagnostic performance between type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM).
Methods
Seventy-nine participants with T1DM and 140 with T2DM completed COMPASS 31 before cardiovascular reflex tests (CARTs) for CAN, and assessment of symptoms, signs, vibration, and thermal perception thresholds for diabetic polyneuropathy (DPN) diagnosis.
Results
COMPASS 31 total weighted score (TWS) was similar in the two groups, but significantly associated with confirmed CAN only in T1DM (P=0.0056) and not T2DM group (P=0.1768) and correlated with CARTs score more strongly in T1DM (rho=0.356, P=0.0016) than in T2DM group (rho=0.084, P=0.3218) (P=0.016). Only in T1DM and not T2DM group, the area under the receiver operating characteristic curve (AUC) reached a fair diagnostic accuracy (>0.7) for confirmed CAN (0.73±0.07 vs. 0.61±0.08) and DPN (0.75±0.06 vs. 0.68±0.05), although without a significant difference. COMPASS 31 TWS (cut-off 16.44) reached acceptable diagnostic performance in T1DM, with sensitivity for confirmed CAN 81.2% and sensitivity and specificity for DPN 76.3% and 78%, compared to T2DM group (all <70%). AUC for DPN of orthostatic intolerance domain was higher in T1DM compared to T2DM group (0.73±0.05 vs. 0.58±0.04, P=0.027).
Conclusion
COMPASS 31 is more weakly related to CAN in T2DM than in T1DM, with a fair diagnostic accuracy for confirmed CAN only in T1DM. This difference supports a multifactorial origin of symptoms and should be considered when using COMPASS 31.
- Cardiovascular risk/Epidemiology
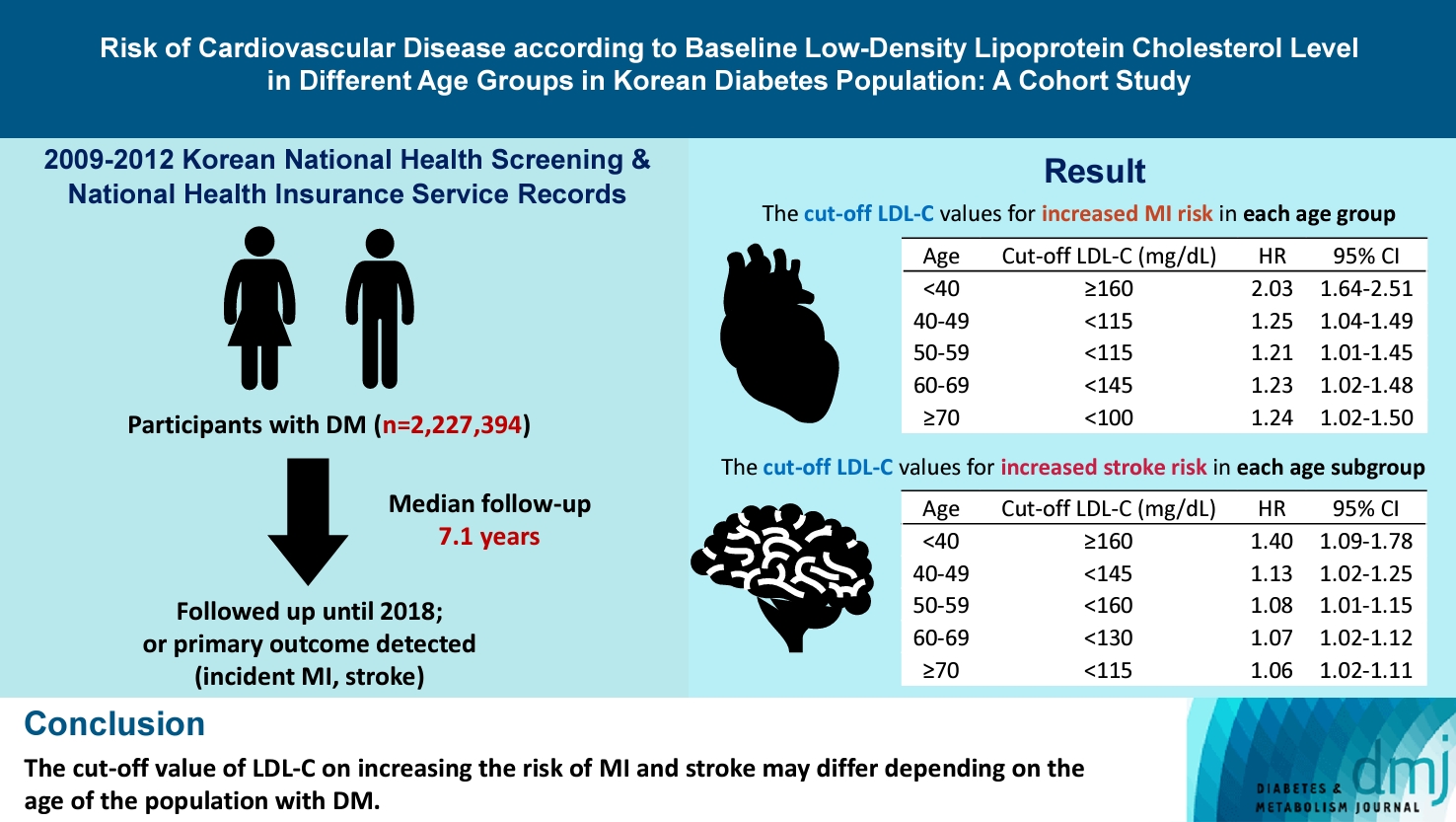
- Risk of Cardiovascular Disease according to Baseline Low-Density Lipoprotein Cholesterol Level in Different Age Groups in Korean Diabetes Population: A Cohort Study
- Tae Kyung Yoo, Kyung-Do Han, Eun-Jung Rhee, Won-Young Lee
- Diabetes Metab J. 2024;48(2):265-278. Published online February 26, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0443
- 705 View
- 137 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The association between low-density lipoprotein (LDL-C) levels and cardiovascular disease (CVD) risk in different age groups within the diabetes mellitus (DM) population remains unclear. The cohort study was conducted to investigate this relationship.
Methods
We assessed the 2009 to 2012 Korean National Health Screening and National Health Insurance Service records, with follow-up to the primary outcome (myocardial infarction [MI] or stroke) or December 2018. After excluding the participants with a history of MI or stroke, 2,227,394 participants with DM were included and categorized according to baseline LDL-C levels and age. Cox proportional hazards modeling was conducted. The CVD risk of age <40 years and LDL-C <70 mg/dL was set as the reference. In each age group, LDL-C <70 mg/dL was used as a reference for the subgroup analysis.
Results
The cut-off LDL-C value for increased MI risk in each age group varied (<40 years old, LDL-C ≥160 mg/dL: hazard ratios [HR], 2.03; 95% confidence interval [CI], 1.644 to 2.506) (40–49-year-old, LDL-C <115 mg/dL: HR, 1.245; 95% CI, 1.04 to 1.489) (50–59-year-old, LDL-C <115 mg/dL: HR, 1.21; 95% CI, 1.014 to 1.445) (60-69-year-old, LDL-C <145 mg/dL: HR, 1.229; 95% CI, 1.022 to 1.479) (≥70 years old group, LDL-C <100 mg/dL: HR, 1.238; 95% CI, 1.018 to 1.504). The cut-off LDL-C values for increased stroke risk varied in each age subgroup (<40 years old, LDL-C ≥160 mg/dL: HR, 1.395; 95% CI, 1.094 to 1.779) (40–49-year-old, LDL-C <145 mg/dL: HR, 1.13; 95% CI, 1.019 to 1.253) (50–59-year-old, LDL-C <160 mg/dL: HR, 1.079; 95% CI, 1.008 to 1.154) (60–69-year-old, LDL-C <130 mg/dL: HR, 1.07; 95% CI, 1.022 to 1.119) (≥70 years old, LDL-C <115 mg/dL: HR, 1.064; 95% CI, 1.019 to 1.112).
Conclusion
The effect of LDL-C on the risk of CVD differs depending on the age of the population with DM.
Review
- Pathophysiology
- Dysfunctional Mitochondria Clearance in Situ: Mitophagy in Obesity and Diabetes-Associated Cardiometabolic Diseases
- Songling Tang, Di Hao, Wen Ma, Lian Liu, Jiuyu Gao, Peng Yao, Haifang Yu, Lu Gan, Yu Cao
- Received July 4, 2023 Accepted October 29, 2023 Published online February 15, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0213 [Epub ahead of print]

- 984 View
- 61 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Several mitochondrial dysfunctions in obesity and diabetes include impaired mitochondrial membrane potential, excessive mitochondrial reactive oxygen species generation, reduced mitochondrial DNA, increased mitochondrial Ca2+ flux, and mitochondrial dynamics disorders. Mitophagy, specialized autophagy, is responsible for clearing dysfunctional mitochondria in physiological and pathological conditions. As a paradox, inhibition and activation of mitophagy have been observed in obesity and diabetes-related heart disorders, with both exerting bidirectional effects. Suppressed mitophagy is beneficial to mitochondrial homeostasis, also known as benign mitophagy. On the contrary, in most cases, excessive mitophagy is harmful to dysfunctional mitochondria elimination and thus is defined as detrimental mitophagy. In obesity and diabetes, two classical pathways appear to regulate mitophagy, including PTEN-induced putative kinase 1 (PINK1)/Parkin-dependent mitophagy and receptors/adapters-dependent mitophagy. After the pharmacologic interventions of mitophagy, mitochondrial morphology and function have been restored, and cell viability has been further improved. Herein, we summarize the mitochondrial dysfunction and mitophagy alterations in obesity and diabetes, as well as the underlying upstream mechanisms, in order to provide novel therapeutic strategies for the obesity and diabetes-related heart disorders.
Sulwon Lecture 2023
- Metabolic Risk/Epidemiology
- Insulin Resistance, Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Clinical and Experimental Perspective
- Inha Jung, Dae-Jeong Koo, Won-Young Lee
- Received October 4, 2023 Accepted December 26, 2024 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0350 [Epub ahead of print]
- 965 View
- 59 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
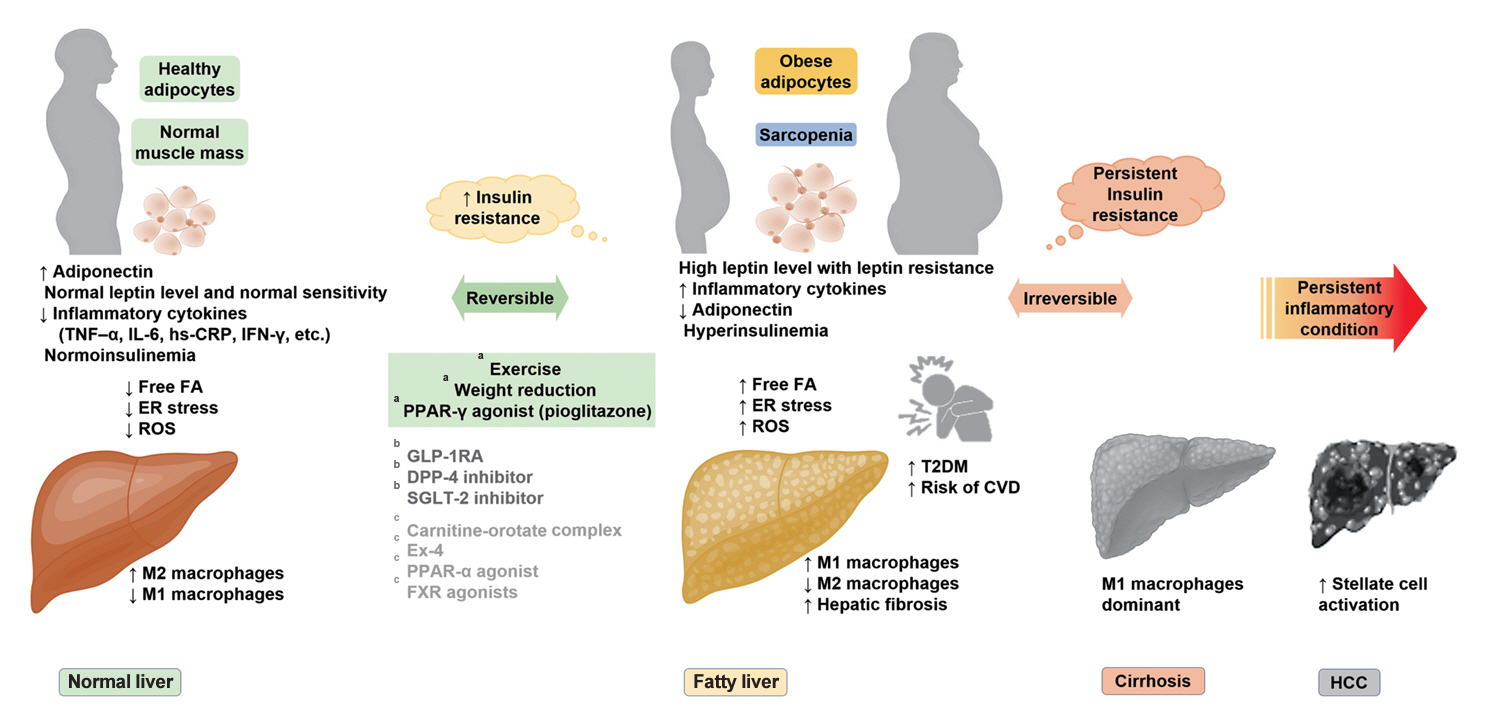
ePub - It has been generally accepted that insulin resistance (IR) and reduced insulin secretory capacity are the basic pathogenesis of type 2 diabetes mellitus (T2DM). In addition to genetic factors, the persistence of systemic inflammation caused by obesity and the associated threat of lipotoxicity increase the risk of T2DM. In particular, the main cause of IR is obesity and subjects with T2DM have a higher body mass index (BMI) than normal subjects according to recent studies. The prevalence of T2DM with IR has increased with increasing BMI during the past three decades. According to recent studies, homeostatic model assessment of IR was increased compared to that of the 1990s. Rising prevalence of obesity in Korea have contributed to the development of IR, non-alcoholic fatty liver disease and T2DM and cutting this vicious cycle is important. My colleagues and I have investigated this pathogenic mechanism on this theme through clinical and experimental studies over 20 years and herein, I would like to summarize some of our studies with deep gratitude for receiving the prestigious 2023 Sulwon Award.
Original Articles
- Drug/Regimen
- Pioglitazone as Add-on THERAPY in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Dapagliflozin and Metformin: Double-Blind, Randomized, Placebo-Controlled Trial
- Ji Hye Heo, Kyung Ah Han, Jun Hwa Hong, Hyun-Ae Seo, Eun-Gyoung Hong, Jae Myung Yu, Hye Seung Jung, Bong-Soo Cha
- Received September 1, 2023 Accepted October 25, 2023 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0314 [Epub ahead of print]
- 1,199 View
- 114 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
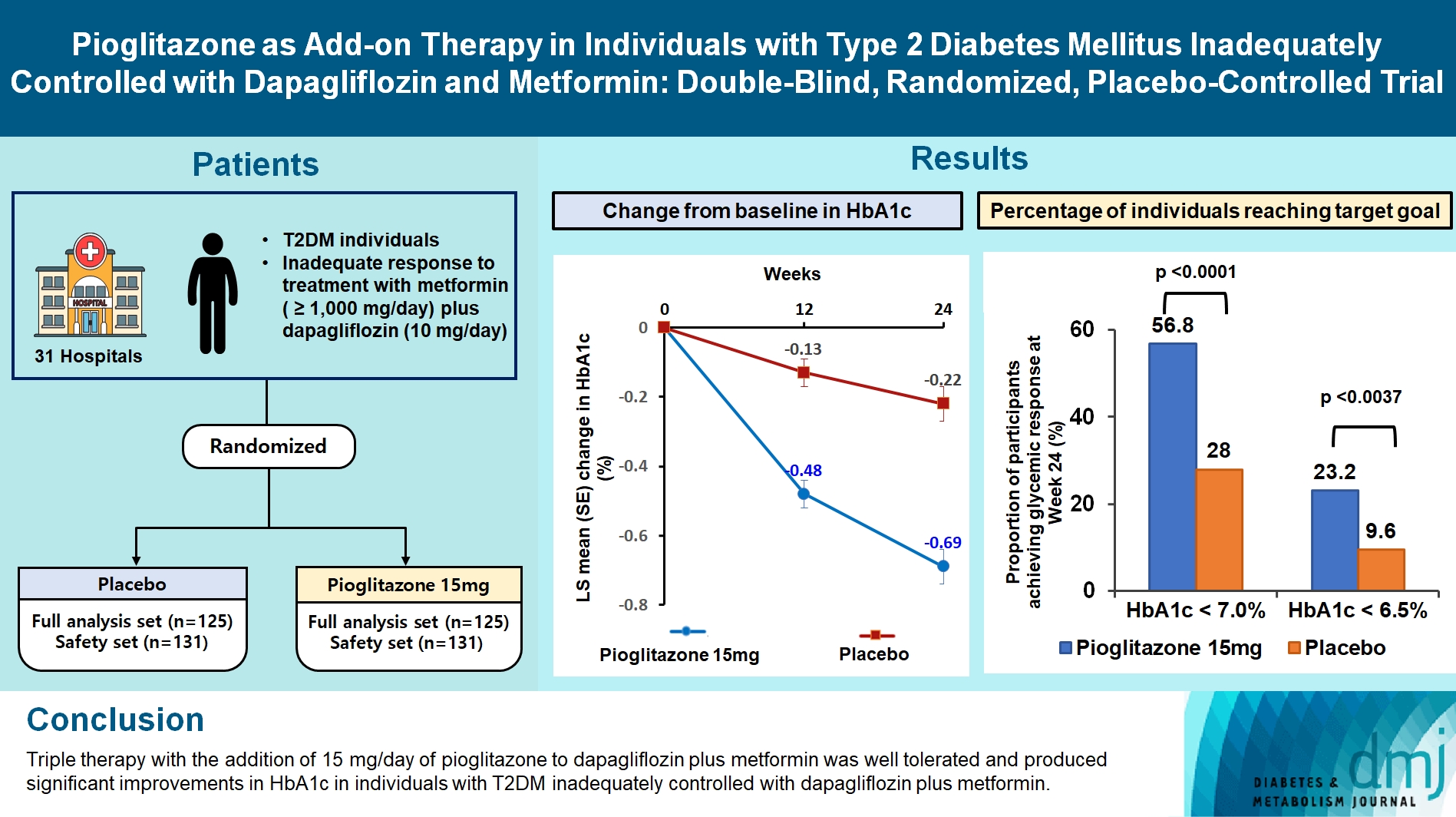
This study assessed the efficacy and safety of triple therapy with pioglitazone 15 mg add-on versus placebo in patients with type 2 diabetes mellitus (T2DM) inadequately controlled with metformin and dapagliflozin.
Methods
In this multicenter, double-blind, randomized, phase 3 study, patients with T2DM with an inadequate response to treatment with metformin (≥1,000 mg/day) plus dapagliflozin (10 mg/day) were randomized to receive additional pioglitazone 15 mg/day (n=125) or placebo (n=125) for 24 weeks. The primary endpoint was the change in glycosylated hemoglobin (HbA1c) levels from baseline to week 24 (ClinicalTrials.gov identifier: NCT05101135).
Results
At week 24, the adjusted mean change from baseline in HbA1c level compared with placebo was significantly greater with pioglitazone treatment (–0.47%; 95% confidence interval, –0.61 to –0.33; P<0.0001). A greater proportion of patients achieved HbA1c <7% or <6.5% at week 24 with pioglitazone compared to placebo as add-on to 10 mg dapagliflozin and metformin (56.8% vs. 28% for HbA1c <7%, and 23.2% vs. 9.6% for HbA1c <6.5%; P<0.0001 for all). The addition of pioglitazone also significantly improved triglyceride, highdensity lipoprotein cholesterol levels, and homeostatic model assessment of insulin resistance levels, while placebo did not. The incidence of treatment-emergent adverse events was similar between the groups, and the incidence of fluid retention-related side effects by pioglitazone was low (1.5%).
Conclusion
Triple therapy with the addition of 15 mg/day of pioglitazone to dapagliflozin plus metformin was well tolerated and produced significant improvements in HbA1c in patients with T2DM inadequately controlled with dapagliflozin plus metformin.
- Metabolic Risk/Epidemiology
- Temporal Changes in Resting Heart Rate and Risk of Diabetes Mellitus
- Mi Kyoung Son, Kyoungho Lee, Hyun-Young Park
- Received August 29, 2023 Accepted November 13, 2023 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0305 [Epub ahead of print]

- 596 View
- 45 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
To investigate the association between the time-varying resting heart rate (RHR) and change in RHR (∆RHR) over time and the risk of diabetes mellitus (DM) by sex.
Methods
We assessed 8,392 participants without DM or atrial fibrillation/flutter from the Korean Genome and Epidemiology Study, a community-based prospective cohort study that was initiated in 2001 to 2002. The participants were followed up until December 31, 2018. Updating RHR with biennial in-study re-examinations, the time-varying ∆RHR was calculated by assessing the ∆RHR at the next follow-up visit.
Results
Over a median follow-up of 12.3 years, 1,345 participants (16.2%) had DM. As compared with RHR of 60 to 69 bpm, for RHR of ≥80 bpm, the incidence of DM was significantly increased for both male and female. A drop of ≥5 bpm in ∆RHR when compared with the stable ∆RHR group (–5< ∆RHR <5 bpm) was associated significantly with lower risk of DM in both male and female. However, an increase of ≥5 bpm in ∆RHR was significantly associated with higher risk of DM only in female, not in male (hazard ratio for male, 1.057 [95% confidence interval, 0.869 to 1.285]; and for female, 1.218 [95% confidence interval, 1.008 to 1.471]).
Conclusion
In this community-based longitudinal cohort study, a reduction in ∆RHR was associated with a decreased risk of DM, while an increase in ∆RHR was associated with an increased risk of DM only in female.
- Lifestyle
- Enhancing Diabetes Care through a Mobile Application: A Randomized Clinical Trial on Integrating Physical and Mental Health among Disadvantaged Individuals
- Jae Hyun Bae, Eun Hee Park, Hae Kyung Lee, Kun Ho Yoon, Kyu Chang Won, Hyun Mi Kim, Sin Gon Kim
- Received August 24, 2023 Accepted October 16, 2023 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0298 [Epub ahead of print]
- 688 View
- 95 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
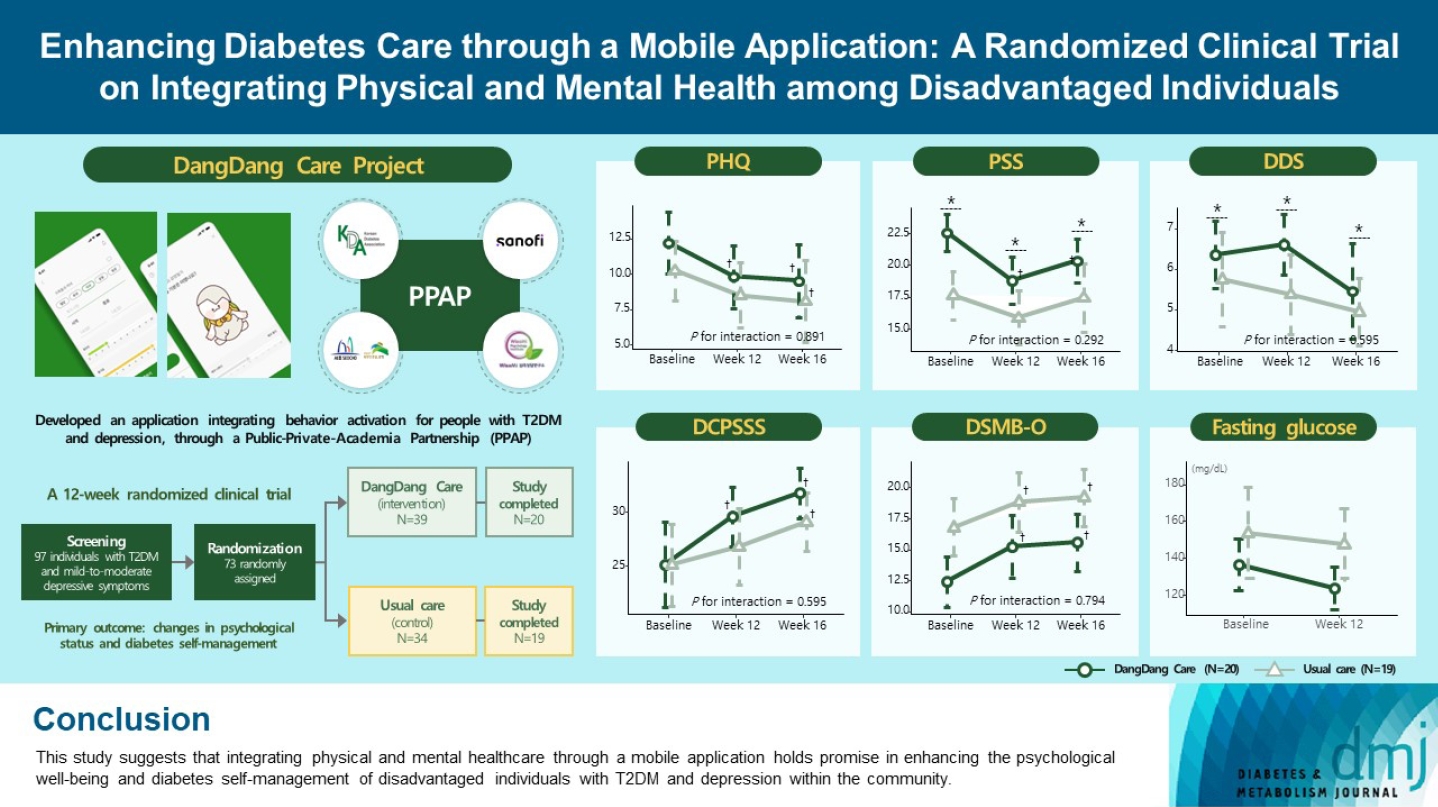
This study examines integrating physical and mental healthcare for disadvantaged persons with type 2 diabetes mellitus and mild-to-moderate depression in the community, using a mobile application within a public-private-academic partnership.
Methods
The Korean Diabetes Association has developed a mobile application combining behavioral activation for psychological well-being and diabetes self-management, with conventional medical therapy. Participants were randomly assigned to receive the application with usual care or only usual care. Primary outcomes measured changes in psychological status and diabetes selfmanagement through questionnaires at week 12 from the baseline. Secondary outcomes assessed glycemic and lipid control, with psychological assessments at week 16.
Results
Thirty-nine of 73 participants completed the study (20 and 19 in the intervention and control groups, respectively) and were included in the analysis. At week 12, the intervention group showed significant reductions in depression severity and perceived stress compared to the control group. Additionally, they reported increased perceived social support and demonstrated improved diabetes self-care behavior. These positive effects persisted through week 16, with the added benefit of reduced anxiety. While fasting glucose levels in the intervention group tended to improve, no other significant differences were observed in laboratory assessments between the groups.
Conclusion
This study provides compelling evidence for the potential efficacy of a mobile application that integrates physical and mental health components to address depressive symptoms and enhance diabetes self-management in disadvantaged individuals with type 2 diabetes mellitus and depression. Further research involving larger and more diverse populations is warranted to validate these findings and solidify their implications.

 KDA
KDA First
First Prev
Prev





