
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
Original Article
- Basic Research
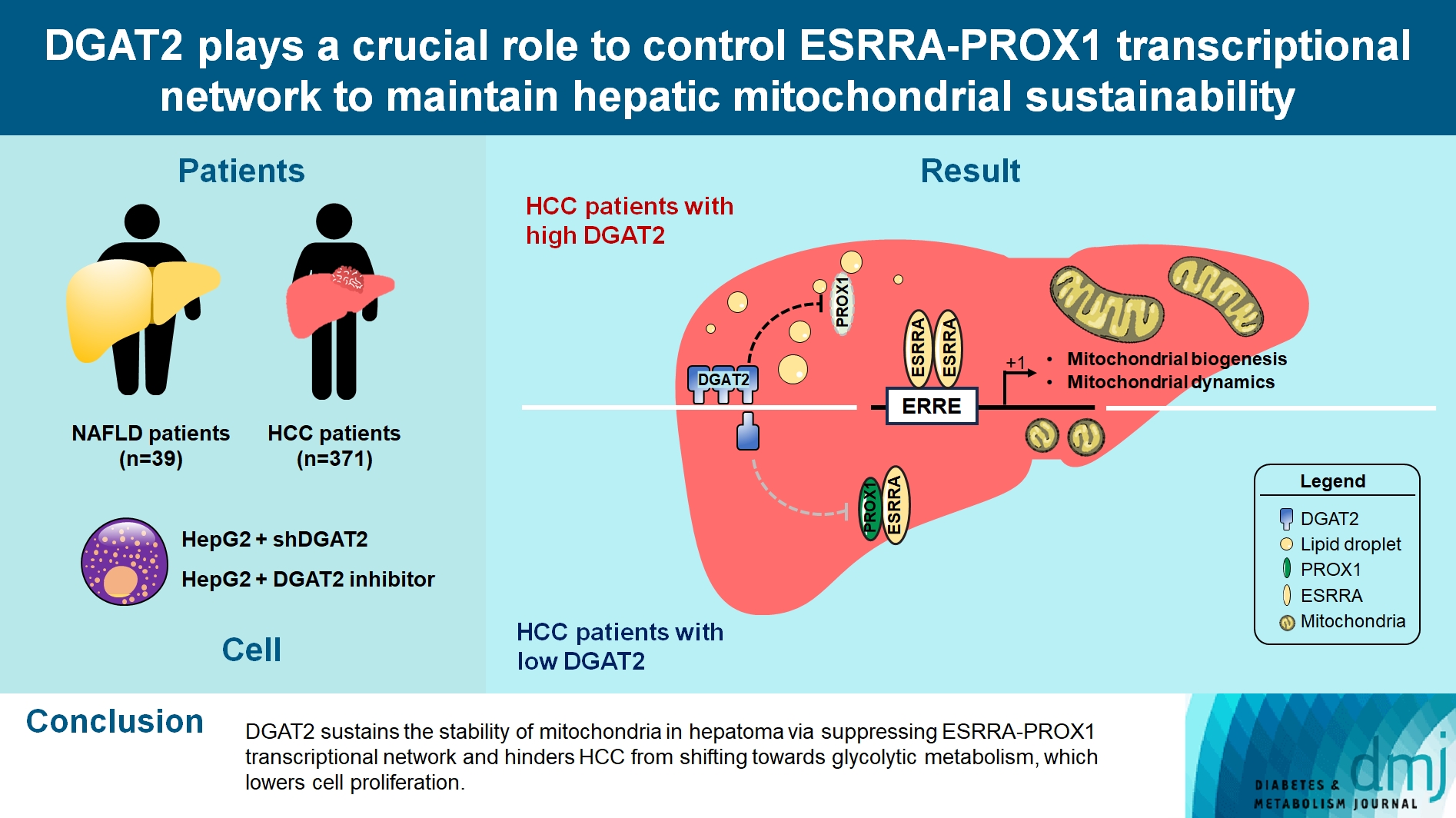
- DGAT2 Plays a Crucial Role to Control ESRRAPROX1 Transcriptional Network to Maintain Hepatic Mitochondrial Sustainability
- Yoseob Lee, Yeseong Hwang, Minki Kim, Hyeonuk Jeon, Seyeon Joo, Sungsoon Fang, Jae-Woo Kim
- Received October 13, 2023 Accepted December 11, 2023 Published online April 22, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0368 [Epub ahead of print]
- 355 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF - Background
Diacylglycerol O-acyltransferase 2 (DGAT2) synthesizes triacylglycerol (TG) from diacylglycerol; therefore, DGAT2 is considered as a therapeutic target for steatosis. However, the consequence of inhibiting DGAT2 is not fully investigated due to side effects including lethality and lipotoxicity. In this article, we observed the role of DGAT2 in hepatocarcinoma.
Methods
The role of DGAT2 is analyzed via loss-of-function assay. DGAT2 knockdown (KD) and inhibitor treatment on HepG2 cell line was analyzed. Cumulative analysis of cell metabolism with bioinformatic data were assessed, and further compared with different cohorts of liver cancer patients and non-alcoholic fatty liver disease (NAFLD) patients to elucidate how DGAT2 is regulating cancer metabolism.
Results
Mitochondrial function is suppressed in DGAT2 KD HepG2 cell along with the decreased lipid droplets. In the aspect of the cancer, DGAT2 KD upregulates cell proliferation. Analyzing transcriptome of NAFLD and hepatocellular carcinoma (HCC) patients highlights negatively correlating expression patterns of 73 lipid-associated genes including DGAT2. Cancer patients with the lower DGAT2 expression face lower survival rate. DGAT2 KD cell and patients’ transcriptome show downregulation in estrogen- related receptor alpha (ESRRA) via integrated system for motif activity response analysis (ISMARA), with increased dimerization with corepressor prospero homeobox 1 (PROX1).
Conclusion
DGAT2 sustains the stability of mitochondria in hepatoma via suppressing ESRRA-PROX1 transcriptional network and hinders HCC from shifting towards glycolytic metabolism, which lowers cell proliferation.
Review
- Metabolic Risk/Epidemiology
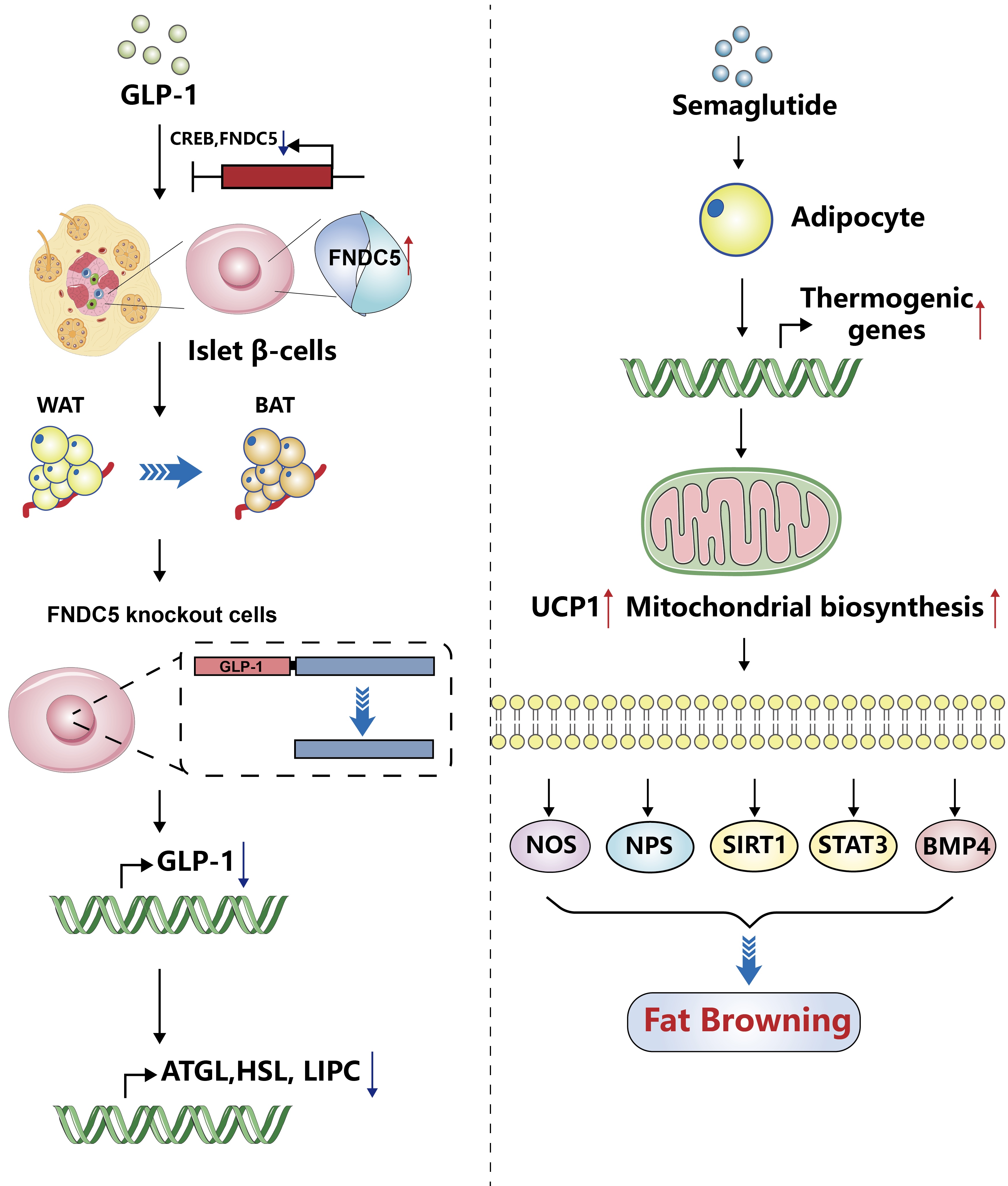
- Glucagon-Like Peptide-1: New Regulator in Lipid Metabolism
- Tong Bu, Ziyan Sun, Yi Pan, Xia Deng, Guoyue Yuan
- Received August 14, 2023 Accepted January 1, 2024 Published online April 1, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0277 [Epub ahead of print]
- 587 View
- 22 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Glucagon-like peptide-1 (GLP-1) is a 30-amino acid peptide hormone that is mainly expressed in the intestine and hypothalamus. In recent years, basic and clinical studies have shown that GLP-1 is closely related to lipid metabolism, and it can participate in lipid metabolism by inhibiting fat synthesis, promoting fat differentiation, enhancing cholesterol metabolism, and promoting adipose browning. GLP-1 plays a key role in the occurrence and development of metabolic diseases such as obesity, nonalcoholic fatty liver disease, and atherosclerosis by regulating lipid metabolism. It is expected to become a new target for the treatment of metabolic disorders. The effects of GLP-1 and dual agonists on lipid metabolism also provide a more complete treatment plan for metabolic diseases. This article reviews the recent research progress of GLP-1 in lipid metabolism.
Original Articles
- Metabolic Risk/Epidemiology
- 2023 Diabetic Kidney Disease Fact Sheet in Korea
- Nam Hoon Kim, Mi-Hae Seo, Jin Hyung Jung, Kyung Do Han, Mi Kyung Kim, Nan Hee Kim, on Behalf of Diabetic Kidney Disease Research Group of the Korean Diabetes Association
- Received July 30, 2023 Accepted January 26, 2024 Published online March 19, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0310 [Epub ahead of print]

- 758 View
- 58 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
To investigate the prevalence, incidence, comorbidities, and management status of diabetic kidney disease (DKD) and diabetes-related end-stage kidney disease (ESKD) in South Korea.
Methods
We used the Korea National Health and Nutrition Examination Survey data (2019 to 2021, n=2,665) for the evaluation of prevalence, comorbidities, control rate of glycemia and comorbidities in DKD, and the Korean Health Insurance Service-customized database (2008 to 2019, n=3,950,857) for the evaluation of trends in the incidence and prevalence rate of diabetes-related ESKD, renin-angiotensin system (RAS) blockers and sodium glucose cotransporter 2 (SGLT2) inhibitors use for DKD, and the risk of atherosclerotic cardiovascular disease (ASCVD) and mortality according to DKD stages. DKD was defined as albuminuria or low estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 in patients with diabetes mellitus.
Results
The prevalence of DKD was 25.4% (albuminuria, 22.0%; low eGFR, 6.73%) in patients with diabetes mellitus aged ≥30 years. Patients with DKD had a higher rate of comorbidities, including hypertension, dyslipidemia, and central obesity; however, their control rates were lower than those without DKD. Prescription rate of SGLT2 inhibitors with reduced eGFR increased steadily, reaching 5.94% in 2019. Approximately 70% of DKD patients were treated with RAS blockers. The prevalence rate of diabetesrelated ESKD has been steadily increasing, with a higher rate in older adults. ASCVD and mortality were significantly associated with an in increase in DKD stage.
Conclusion
DKD is prevalent among Korean patients with diabetes and is an independent risk factor for cardiovascular morbidity and mortality, which requiring intensive management of diabetes and comorbidities. The prevalence of diabetes-related ESKD has been increasing, especially in the older adults, during past decade. -
Citations
Citations to this article as recorded by- Endothelial NOX5 Obliterates the Reno-Protective Effect of Nox4 Deletion by Promoting Renal Fibrosis via Activation of EMT and ROS-Sensitive Pathways in Diabetes
Karin A. M. Jandeleit-Dahm, Haritha R. Kankanamalage, Aozhi Dai, Jaroslawna Meister, Sara Lopez-Trevino, Mark E. Cooper, Rhian M. Touyz, Christopher R. J. Kennedy, Jay C. Jha
Antioxidants.2024; 13(4): 396. CrossRef
- Endothelial NOX5 Obliterates the Reno-Protective Effect of Nox4 Deletion by Promoting Renal Fibrosis via Activation of EMT and ROS-Sensitive Pathways in Diabetes
- Metabolic Risk/Epidemiology
- Healthy Lifestyle and the Risk of Metabolic Dysfunction-Associated Fatty Liver Disease: A Large Prospective Cohort Study
- Qing Chang, Yixiao Zhang, Tingjing Zhang, Zuyun Liu, Limin Cao, Qing Zhang, Li Liu, Shaomei Sun, Xing Wang, Ming Zhou, Qiyu Jia, Kun Song, Yang Ding, Yuhong Zhao, Kaijun Niu, Yang Xia
- Received April 27, 2023 Accepted November 30, 2023 Published online March 19, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0133 [Epub ahead of print]

- 591 View
- 42 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The incidence density of metabolic dysfunction-associated fatty liver disease (MAFLD) and the effect of a healthy lifestyle on the risk of MAFLD remain unknown. We evaluated the prevalence and incidence density of MAFLD and investigated the association between healthy lifestyle and the risk of MAFLD.
Methods
A cross-sectional analysis was conducted on 37,422 participants to explore the prevalence of MAFLD. A cohort analysis of 18,964 individuals was conducted to identify the incidence of MAFLD, as well as the association between healthy lifestyle and MAFLD. Cox proportional hazards regression was used to calculate the hazard ratio (HR) and 95% confidence interval (CI) with adjustments for confounding factors.
Results
The prevalence of MAFLD, non-alcoholic fatty liver disease, and their comorbidities were 30.38%, 28.09%, and 26.13%, respectively. After approximately 70 thousand person-years of follow-up, the incidence densities of the three conditions were 61.03, 55.49, and 51.64 per 1,000 person-years, respectively. Adherence to an overall healthy lifestyle was associated with a 19% decreased risk of MAFLD (HR, 0.81; 95% CI, 0.72 to 0.92), and the effects were modified by baseline age, sex, and body mass index (BMI). Subgroup analyses revealed that younger participants, men, and those with a lower BMI experienced more significant beneficial effects from healthy lifestyle.
Conclusion
Our results highlight the beneficial effect of adherence to a healthy lifestyle on the prevention of MAFLD. Health management for improving dietary intake, physical activity, and smoking and drinking habits are critical to improving MAFLD.
- Complications
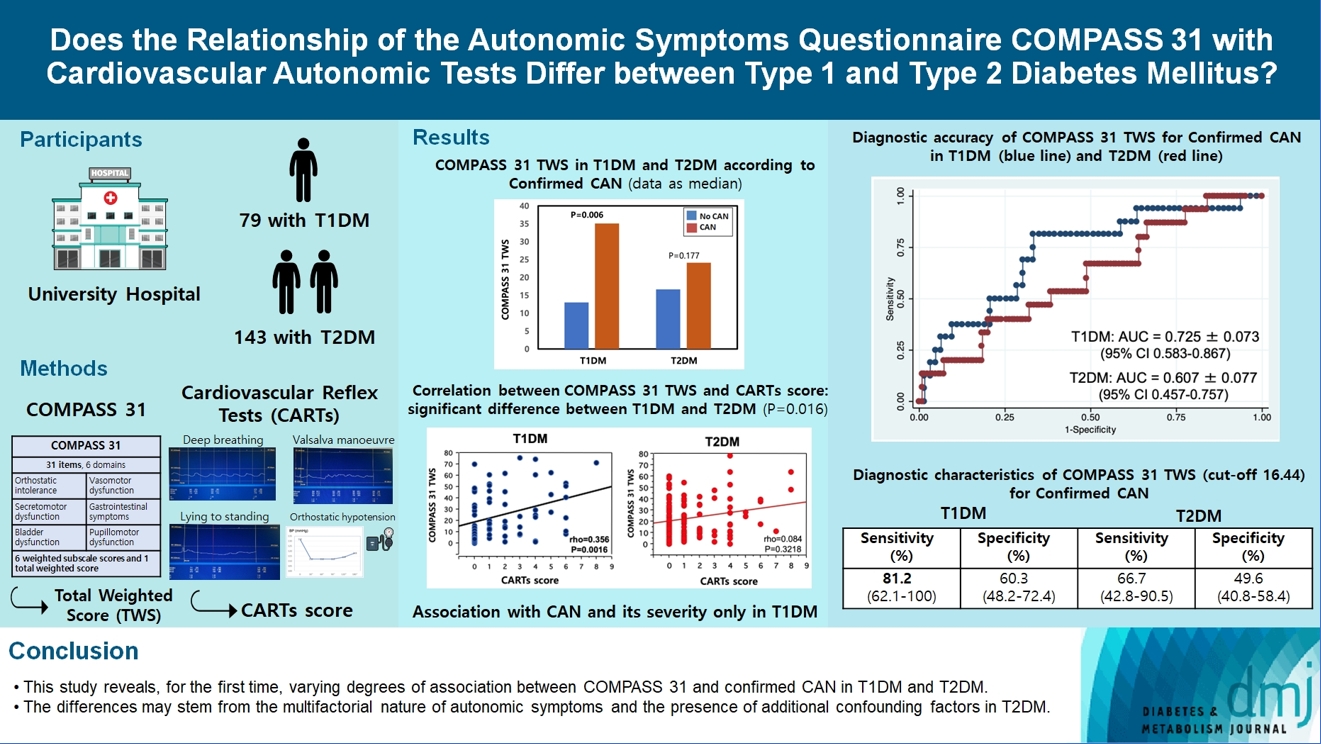
- Does the Relationship of the Autonomic Symptoms Questionnaire COMPASS 31 with Cardiovascular Autonomic Tests Differ between Type 1 and Type 2 Diabetes Mellitus?
- Ilenia D’Ippolito, Marika Menduni, Cinzia D’Amato, Aikaterini Andreadi, Davide Lauro, Vincenza Spallone
- Received August 28, 2023 Accepted November 22, 2023 Published online February 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0301 [Epub ahead of print]
- 624 View
- 44 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The aim was to investigate if autonomic symptoms questionnaire Composite Autonomic Symptom Score (COMPASS) 31 has different association with cardiovascular autonomic neuropathy (CAN) and diagnostic performance between type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM).
Methods
Seventy-nine participants with T1DM and 140 with T2DM completed COMPASS 31 before cardiovascular reflex tests (CARTs) for CAN, and assessment of symptoms, signs, vibration, and thermal perception thresholds for diabetic polyneuropathy (DPN) diagnosis.
Results
COMPASS 31 total weighted score (TWS) was similar in the two groups, but significantly associated with confirmed CAN only in T1DM (P=0.0056) and not T2DM group (P=0.1768) and correlated with CARTs score more strongly in T1DM (rho=0.356, P=0.0016) than in T2DM group (rho=0.084, P=0.3218) (P=0.016). Only in T1DM and not T2DM group, the area under the receiver operating characteristic curve (AUC) reached a fair diagnostic accuracy (>0.7) for confirmed CAN (0.73±0.07 vs. 0.61±0.08) and DPN (0.75±0.06 vs. 0.68±0.05), although without a significant difference. COMPASS 31 TWS (cut-off 16.44) reached acceptable diagnostic performance in T1DM, with sensitivity for confirmed CAN 81.2% and sensitivity and specificity for DPN 76.3% and 78%, compared to T2DM group (all <70%). AUC for DPN of orthostatic intolerance domain was higher in T1DM compared to T2DM group (0.73±0.05 vs. 0.58±0.04, P=0.027).
Conclusion
COMPASS 31 is more weakly related to CAN in T2DM than in T1DM, with a fair diagnostic accuracy for confirmed CAN only in T1DM. This difference supports a multifactorial origin of symptoms and should be considered when using COMPASS 31.
- Cardiovascular risk/Epidemiology
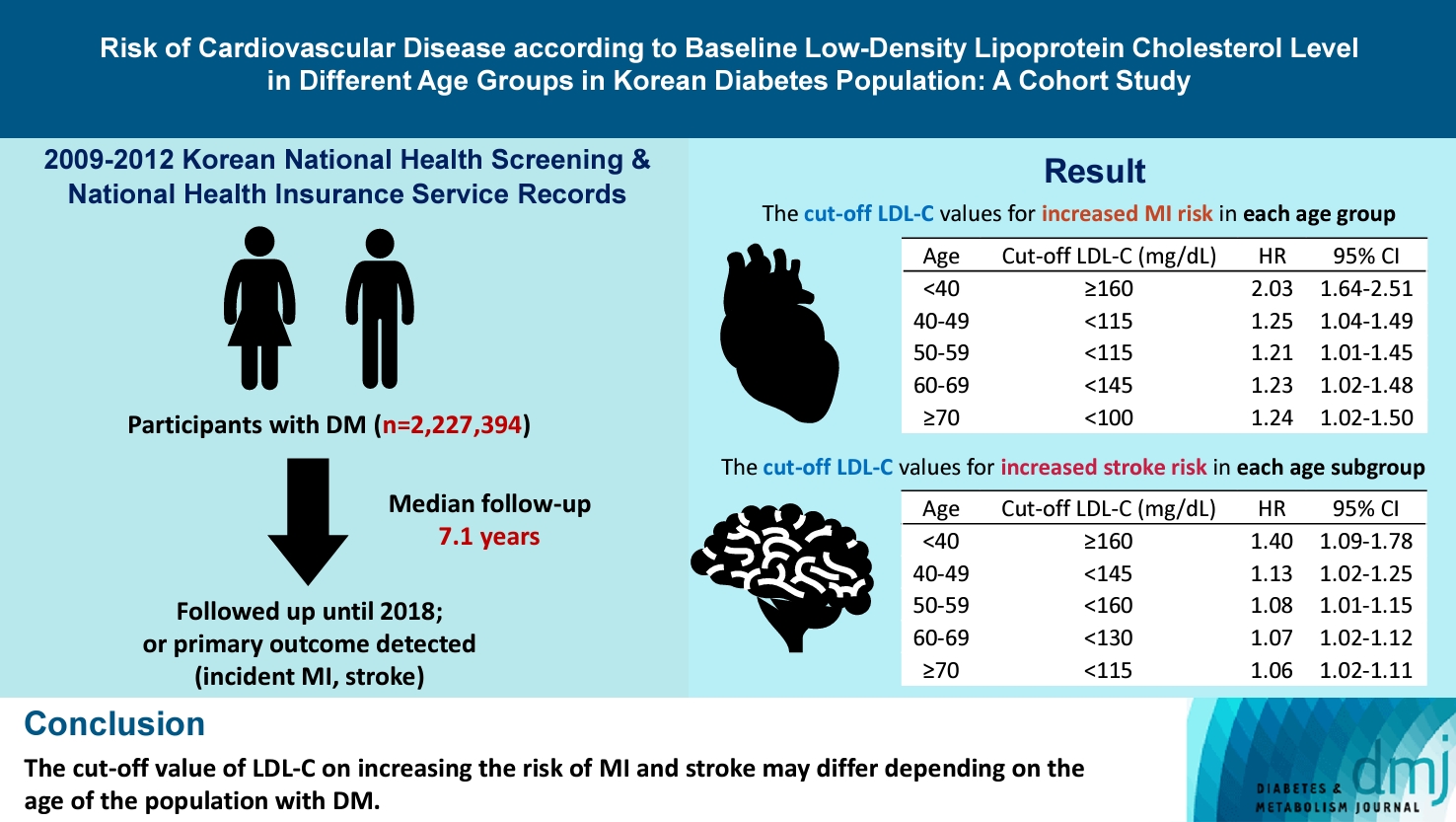
- Risk of Cardiovascular Disease according to Baseline Low-Density Lipoprotein Cholesterol Level in Different Age Groups in Korean Diabetes Population: A Cohort Study
- Tae Kyung Yoo, Kyung-Do Han, Eun-Jung Rhee, Won-Young Lee
- Diabetes Metab J. 2024;48(2):265-278. Published online February 26, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0443
- 750 View
- 149 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The association between low-density lipoprotein (LDL-C) levels and cardiovascular disease (CVD) risk in different age groups within the diabetes mellitus (DM) population remains unclear. The cohort study was conducted to investigate this relationship.
Methods
We assessed the 2009 to 2012 Korean National Health Screening and National Health Insurance Service records, with follow-up to the primary outcome (myocardial infarction [MI] or stroke) or December 2018. After excluding the participants with a history of MI or stroke, 2,227,394 participants with DM were included and categorized according to baseline LDL-C levels and age. Cox proportional hazards modeling was conducted. The CVD risk of age <40 years and LDL-C <70 mg/dL was set as the reference. In each age group, LDL-C <70 mg/dL was used as a reference for the subgroup analysis.
Results
The cut-off LDL-C value for increased MI risk in each age group varied (<40 years old, LDL-C ≥160 mg/dL: hazard ratios [HR], 2.03; 95% confidence interval [CI], 1.644 to 2.506) (40–49-year-old, LDL-C <115 mg/dL: HR, 1.245; 95% CI, 1.04 to 1.489) (50–59-year-old, LDL-C <115 mg/dL: HR, 1.21; 95% CI, 1.014 to 1.445) (60-69-year-old, LDL-C <145 mg/dL: HR, 1.229; 95% CI, 1.022 to 1.479) (≥70 years old group, LDL-C <100 mg/dL: HR, 1.238; 95% CI, 1.018 to 1.504). The cut-off LDL-C values for increased stroke risk varied in each age subgroup (<40 years old, LDL-C ≥160 mg/dL: HR, 1.395; 95% CI, 1.094 to 1.779) (40–49-year-old, LDL-C <145 mg/dL: HR, 1.13; 95% CI, 1.019 to 1.253) (50–59-year-old, LDL-C <160 mg/dL: HR, 1.079; 95% CI, 1.008 to 1.154) (60–69-year-old, LDL-C <130 mg/dL: HR, 1.07; 95% CI, 1.022 to 1.119) (≥70 years old, LDL-C <115 mg/dL: HR, 1.064; 95% CI, 1.019 to 1.112).
Conclusion
The effect of LDL-C on the risk of CVD differs depending on the age of the population with DM.
Review
- Pathophysiology
- Dysfunctional Mitochondria Clearance in Situ: Mitophagy in Obesity and Diabetes-Associated Cardiometabolic Diseases
- Songling Tang, Di Hao, Wen Ma, Lian Liu, Jiuyu Gao, Peng Yao, Haifang Yu, Lu Gan, Yu Cao
- Received July 4, 2023 Accepted October 29, 2023 Published online February 15, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0213 [Epub ahead of print]

- 1,051 View
- 64 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Several mitochondrial dysfunctions in obesity and diabetes include impaired mitochondrial membrane potential, excessive mitochondrial reactive oxygen species generation, reduced mitochondrial DNA, increased mitochondrial Ca2+ flux, and mitochondrial dynamics disorders. Mitophagy, specialized autophagy, is responsible for clearing dysfunctional mitochondria in physiological and pathological conditions. As a paradox, inhibition and activation of mitophagy have been observed in obesity and diabetes-related heart disorders, with both exerting bidirectional effects. Suppressed mitophagy is beneficial to mitochondrial homeostasis, also known as benign mitophagy. On the contrary, in most cases, excessive mitophagy is harmful to dysfunctional mitochondria elimination and thus is defined as detrimental mitophagy. In obesity and diabetes, two classical pathways appear to regulate mitophagy, including PTEN-induced putative kinase 1 (PINK1)/Parkin-dependent mitophagy and receptors/adapters-dependent mitophagy. After the pharmacologic interventions of mitophagy, mitochondrial morphology and function have been restored, and cell viability has been further improved. Herein, we summarize the mitochondrial dysfunction and mitophagy alterations in obesity and diabetes, as well as the underlying upstream mechanisms, in order to provide novel therapeutic strategies for the obesity and diabetes-related heart disorders.
Sulwon Lecture 2023
- Metabolic Risk/Epidemiology
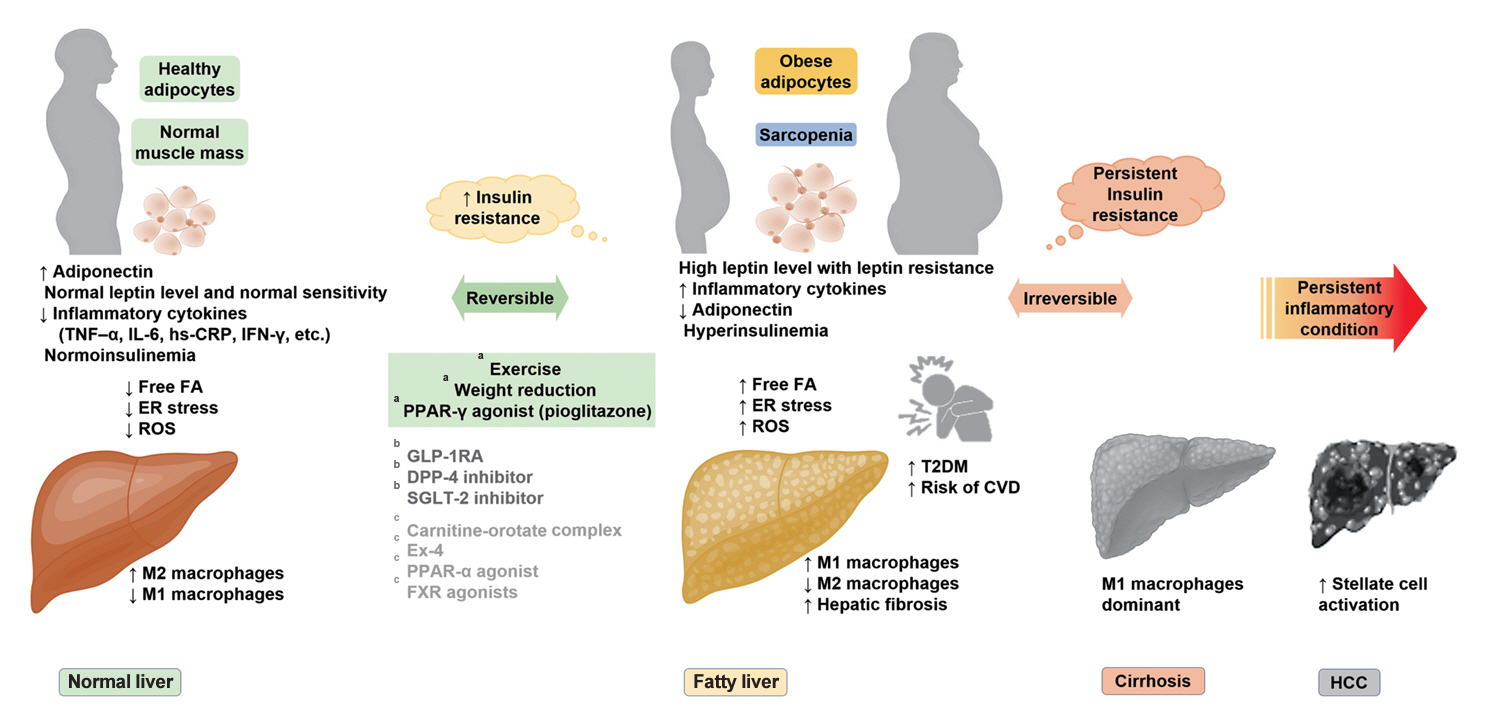
- Insulin Resistance, Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Clinical and Experimental Perspective
- Inha Jung, Dae-Jeong Koo, Won-Young Lee
- Received October 4, 2023 Accepted December 26, 2024 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0350 [Epub ahead of print]
- 1,005 View
- 60 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - It has been generally accepted that insulin resistance (IR) and reduced insulin secretory capacity are the basic pathogenesis of type 2 diabetes mellitus (T2DM). In addition to genetic factors, the persistence of systemic inflammation caused by obesity and the associated threat of lipotoxicity increase the risk of T2DM. In particular, the main cause of IR is obesity and subjects with T2DM have a higher body mass index (BMI) than normal subjects according to recent studies. The prevalence of T2DM with IR has increased with increasing BMI during the past three decades. According to recent studies, homeostatic model assessment of IR was increased compared to that of the 1990s. Rising prevalence of obesity in Korea have contributed to the development of IR, non-alcoholic fatty liver disease and T2DM and cutting this vicious cycle is important. My colleagues and I have investigated this pathogenic mechanism on this theme through clinical and experimental studies over 20 years and herein, I would like to summarize some of our studies with deep gratitude for receiving the prestigious 2023 Sulwon Award.
Original Articles
- Drug/Regimen
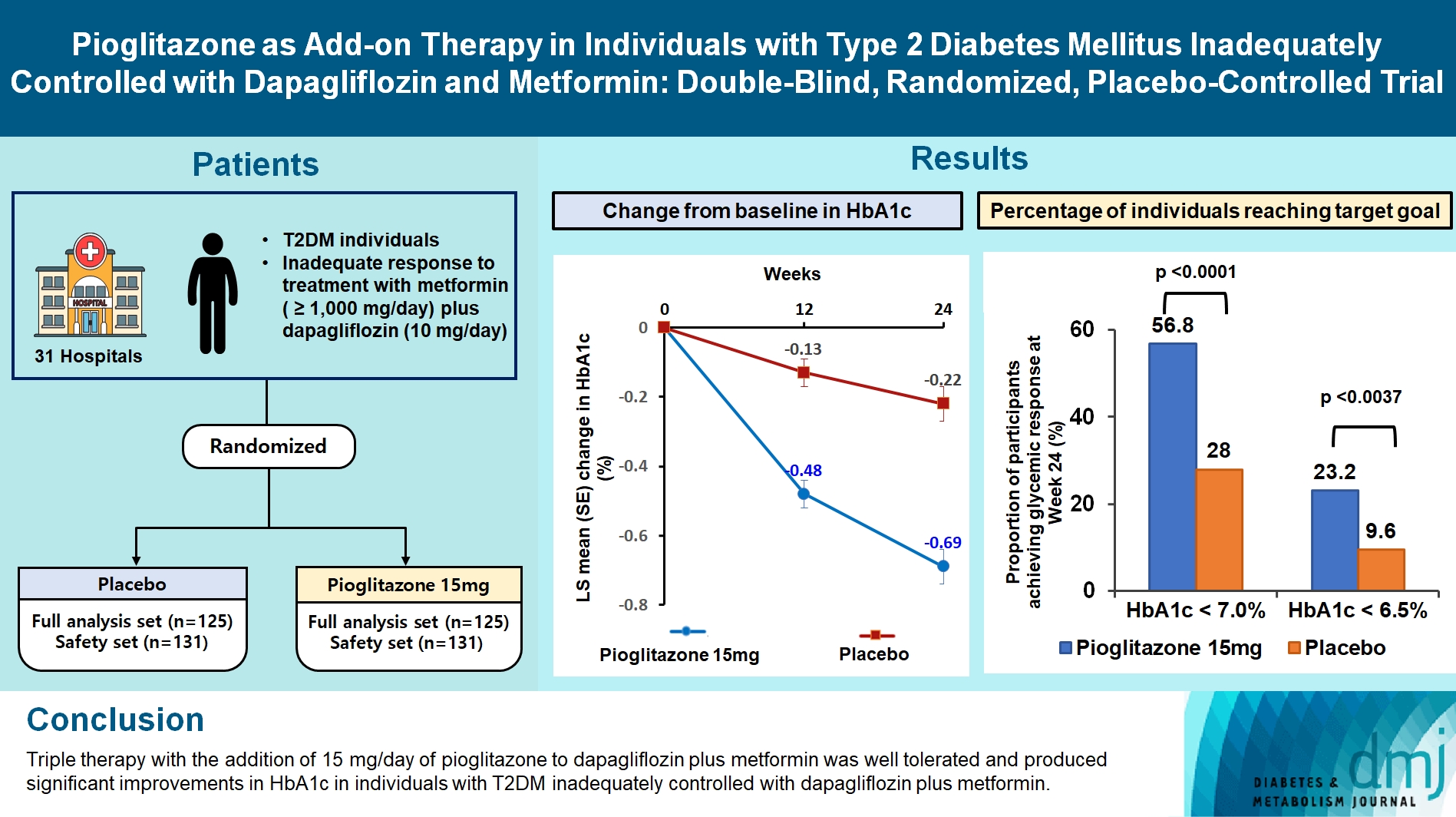
- Pioglitazone as Add-on THERAPY in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Dapagliflozin and Metformin: Double-Blind, Randomized, Placebo-Controlled Trial
- Ji Hye Heo, Kyung Ah Han, Jun Hwa Hong, Hyun-Ae Seo, Eun-Gyoung Hong, Jae Myung Yu, Hye Seung Jung, Bong-Soo Cha
- Received September 1, 2023 Accepted October 25, 2023 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0314 [Epub ahead of print]
- 1,246 View
- 127 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study assessed the efficacy and safety of triple therapy with pioglitazone 15 mg add-on versus placebo in patients with type 2 diabetes mellitus (T2DM) inadequately controlled with metformin and dapagliflozin.
Methods
In this multicenter, double-blind, randomized, phase 3 study, patients with T2DM with an inadequate response to treatment with metformin (≥1,000 mg/day) plus dapagliflozin (10 mg/day) were randomized to receive additional pioglitazone 15 mg/day (n=125) or placebo (n=125) for 24 weeks. The primary endpoint was the change in glycosylated hemoglobin (HbA1c) levels from baseline to week 24 (ClinicalTrials.gov identifier: NCT05101135).
Results
At week 24, the adjusted mean change from baseline in HbA1c level compared with placebo was significantly greater with pioglitazone treatment (–0.47%; 95% confidence interval, –0.61 to –0.33; P<0.0001). A greater proportion of patients achieved HbA1c <7% or <6.5% at week 24 with pioglitazone compared to placebo as add-on to 10 mg dapagliflozin and metformin (56.8% vs. 28% for HbA1c <7%, and 23.2% vs. 9.6% for HbA1c <6.5%; P<0.0001 for all). The addition of pioglitazone also significantly improved triglyceride, highdensity lipoprotein cholesterol levels, and homeostatic model assessment of insulin resistance levels, while placebo did not. The incidence of treatment-emergent adverse events was similar between the groups, and the incidence of fluid retention-related side effects by pioglitazone was low (1.5%).
Conclusion
Triple therapy with the addition of 15 mg/day of pioglitazone to dapagliflozin plus metformin was well tolerated and produced significant improvements in HbA1c in patients with T2DM inadequately controlled with dapagliflozin plus metformin.
- Metabolic Risk/Epidemiology
- Glycemic Control Is Associated with Histological Findings of Nonalcoholic Fatty Liver Disease
- Teruki Miyake, Shinya Furukawa, Bunzo Matsuura, Osamu Yoshida, Masumi Miyazaki, Akihito Shiomi, Ayumi Kanamoto, Hironobu Nakaguchi, Yoshiko Nakamura, Yusuke Imai, Mitsuhito Koizumi, Takao Watanabe, Yasunori Yamamoto, Yohei Koizumi, Yoshio Tokumoto, Masashi Hirooka, Teru Kumagi, Eiji Takesita, Yoshio Ikeda, Masanori Abe, Yoichi Hiasa
- Received June 24, 2023 Accepted September 21, 2023 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0200 [Epub ahead of print]

- 936 View
- 40 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Poor lifestyle habits may worsen nonalcoholic fatty liver disease (NAFLD), with progression to nonalcoholic steatohepatitis (NASH) and cirrhosis. This study investigated the association between glycemic control status and hepatic histological findings to elucidate the effect of glycemic control on NAFLD.
Methods
This observational study included 331 patients diagnosed with NAFLD by liver biopsy. Effects of the glycemic control status on histological findings of NAFLD were evaluated by comparing the following four glycemic status groups defined by the glycosylated hemoglobin (HbA1c) level at the time of NAFLD diagnosis: ≤5.4%, 5.5%–6.4%, 6.5%–7.4%, and ≥7.5%.
Results
Compared with the lowest HbA1c group (≤5.4%), the higher HbA1c groups (5.5%–6.4%, 6.5%–7.4%, and ≥7.5%) were associated with advanced liver fibrosis and high NAFLD activity score (NAS). On multivariate analysis, an HbA1c level of 6.5%– 7.4% group was significantly associated with advanced fibrosis compared with the lowest HbA1c group after adjusting for age, sex, hemoglobin, alanine aminotransferase, and creatinine levels. When further controlling for body mass index and uric acid, total cholesterol, and triglyceride levels, the higher HbA1c groups were significantly associated with advanced fibrosis compared with the lowest HbA1c group. On the other hand, compared with the lowest HbA1c group, the higher HbA1c groups were also associated with a high NAS in both multivariate analyses.
Conclusion
Glycemic control is associated with NAFLD exacerbation, with even a mild deterioration in glycemic control, especially a HbA1c level of 6.5%–7.4%, contributing to NAFLD progression. -
Citations
Citations to this article as recorded by- Combined effect of histological findings and diabetes mellitus on liver‐related events in patients with metabolic dysfunction‐associated steatotic liver disease
Akihito Shiomi, Teruki Miyake, Shinya Furukawa, Bunzo Matsuura, Osamu Yoshida, Takao Watanabe, Ayumi Kanamoto, Masumi Miyazaki, Hironobu Nakaguchi, Yoshio Tokumoto, Masashi Hirooka, Masanori Abe, Yoichi Hiasa
Hepatology Research.2024;[Epub] CrossRef
- Combined effect of histological findings and diabetes mellitus on liver‐related events in patients with metabolic dysfunction‐associated steatotic liver disease
- Basic Research
- Supplementation of Clostridium butyricum Alleviates Vascular Inflammation in Diabetic Mice
- Tian Zhou, Shuo Qiu, Liang Zhang, Yangni Li, Jing Zhang, Donghua Shen, Ping Zhao, Lijun Yuan, Lianbi Zhao, Yunyou Duan, Changyang Xing
- Received April 21, 2023 Accepted July 10, 2023 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0109 [Epub ahead of print]

- 596 View
- 85 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Gut microbiota is closely related to the occurrence and development of diabetes and affects the prognosis of diabetic complications, and the underlying mechanisms are only partially understood. We aimed to explore the possible link between the gut microbiota and vascular inflammation of diabetic mice.
Methods
The db/db diabetic and wild-type (WT) mice were used in this study. We profiled gut microbiota and examined the and vascular function in both db/db group and WT group. Gut microbiota was analyzed by 16s rRNA sequencing. Vascular function was examined by ultrasonographic hemodynamics and histological staining. Clostridium butyricum (CB) was orally administered to diabetic mice by intragastric gavage every 2 days for 2 consecutive months. Reactive oxygen species (ROS) and expression of nuclear factor erythroid-derived 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) were detected by fluorescence microscopy. The mRNA expression of inflammatory cytokines was tested by quantitative polymerase chain reaction.
Results
Compared with WT mice, CB abundance was significantly decreased in the gut of db/db mice, together with compromised vascular function and activated inflammation in the arterial tissue. Meanwhile, ROS in the vascular tissue of db/db mice was also significantly increased. Oral administration of CB restored the protective microbiota, and protected the vascular function in the db/db mice via activating the Nrf2/HO-1 pathway.
Conclusion
This study identified the potential link between decreased CB abundance in gut microbiota and vascular inflammation in diabetes. Therapeutic delivery of CB by gut transplantation alleviates the vascular lesions of diabetes mellitus by activating the Nrf2/HO-1 pathway.
- Metabolic Risk/Epidemiology
- Harnessing Metabolic Indices as a Predictive Tool for Cardiovascular Disease in a Korean Population without Known Major Cardiovascular Event
- Hyun-Jin Kim, Byung Sik Kim, Yonggu Lee, Sang Bong Ahn, Dong Wook Kim, Jeong-Hun Shin
- Received June 22, 2023 Accepted August 18, 2023 Published online February 1, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0197 [Epub ahead of print]

- 946 View
- 25 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study evaluated the usefulness of indices for metabolic syndrome, non-alcoholic fatty liver disease (NAFLD), and insulin resistance (IR), as predictive tools for cardiovascular disease in middle-aged Korean adults.
Methods
The prospective data obtained from the Ansan-Ansung cohort database, excluding patients with major adverse cardiac and cerebrovascular events (MACCE). The primary outcome was the incidence of MACCE during the follow-up period.
Results
A total of 9,337 patients were included in the analysis, of whom 1,130 (12.1%) experienced MACCE during a median follow-up period of 15.5 years. The metabolic syndrome severity Z-score, metabolic syndrome severity score, hepatic steatosis index, and NAFLD liver fat score were found to significantly predict MACCE at values above the cut-off point and in the second and third tertiles. Among these indices, the hazard ratios of the metabolic syndrome severity score and metabolic syndrome severity Z-score were the highest after adjusting for confounding factors. The area under the receiver operating characteristic curve (AUC) of the 10-year atherosclerotic cardiovascular disease (ASCVD) score for predicting MACCE was 0.716, and the metabolic syndrome severity Z-score had an AUC of 0.619.
Conclusion
The metabolic syndrome severity score is a highly reliable indicator and was closely associated with the 10-year ASCVD risk score in predicting MACCE in the general population. Given the specific characteristics and limitations of metabolic syndrome severity scores as well as the indices of NAFLD and IR, a more practical scoring system that considers these factors is essential to achieve greater accuracy in forecasting cardiovascular outcomes.
- Metabolic Risk/Epidemiology
- A Composite Blood Biomarker Including AKR1B10 and Cytokeratin 18 for Progressive Types of Nonalcoholic Fatty Liver Disease
- Seung Joon Choi, Sungjin Yoon, Kyoung-Kon Kim, Doojin Kim, Hye Eun Lee, Kwang Gi Kim, Seung Kak Shin, Ie Byung Park, Seong Min Kim, Dae Ho Lee
- Received June 18, 2023 Accepted August 16, 2023 Published online February 1, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0189 [Epub ahead of print]

- 780 View
- 50 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
We aimed to evaluate whether composite blood biomarkers including aldo-keto reductase family 1 member B10 (AKR1B10) and cytokeratin 18 (CK-18; a nonalcoholic steatohepatitis [NASH] marker) have clinically applicable performance for the diagnosis of NASH, advanced liver fibrosis, and high-risk NASH (NASH+significant fibrosis).
Methods
A total of 116 subjects including healthy control subjects and patients with biopsy-proven nonalcoholic fatty liver disease (NAFLD) were analyzed to assess composite blood-based and imaging-based biomarkers either singly or in combination.
Results
A composite blood biomarker comprised of AKR1B10, CK-18, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) showed excellent performance for the diagnosis of, NASH, advanced fibrosis, and high-risk NASH, with area under the receiver operating characteristic curve values of 0.934 (95% confidence interval [CI], 0.888 to 0.981), 0.902 (95% CI, 0.832 to 0.971), and 0.918 (95% CI, 0.862 to 0.974), respectively. However, the performance of this blood composite biomarker was inferior to that various magnetic resonance (MR)-based composite biomarkers, such as proton density fat fraction/MR elastography- liver stiffness measurement (MRE-LSM)/ALT/AST for NASH, MRE-LSM+fibrosis-4 index for advanced fibrosis, and the known MR imaging-AST (MAST) score for high-risk NASH.
Conclusion
Our blood composite biomarker can be useful to distinguish progressive forms of NAFLD as an initial noninvasive test when MR-based tools are not available.
Brief Report
- Genetics
- Clinical Characteristics of Diabetes in People with Mitochondrial DNA 3243A>G Mutation in Korea
- Eun Hoo Rho, Sang Ik Baek, Heerah Lee, Moon-Woo Seong, Jong-Hee Chae, Kyong Soo Park, Soo Heon Kwak
- Received March 10, 2023 Accepted July 20, 2023 Published online February 1, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0078 [Epub ahead of print]

- 518 View
- 32 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Maternally inherited diabetes and deafness (MIDD) is a rare mitochondrial disorder primarily resulting from m.3243A>G mutation. The clinical characteristics of MIDD exhibit significant heterogeneity. Our study aims to delineate these characteristics and determine the potential correlation with m.3243A>G heteroplasmy levels. This retrospective, descriptive study encompassed patients with confirmed m.3243A>G mutation and diabetes mellitus at Seoul National University Hospital. Our cohort comprises 40 patients with MIDD, with a mean age at study enrollment of 33.3±12.9 years and an average % of heteroplasmy of 30.0%± 14.6% in the peripheral blood. The most prevalent comorbidity was hearing loss (90%), followed by albuminuria (61%), seizure (38%), and stroke (33%). We observed a significant negative correlation between % of heteroplasmy and age at diabetes diagnosis. These clinical features can aid in the suspicion of MIDD and further consideration of genetic testing for m.3243A>G mutation.
Review
- Metabolic Risk/Epidemiology
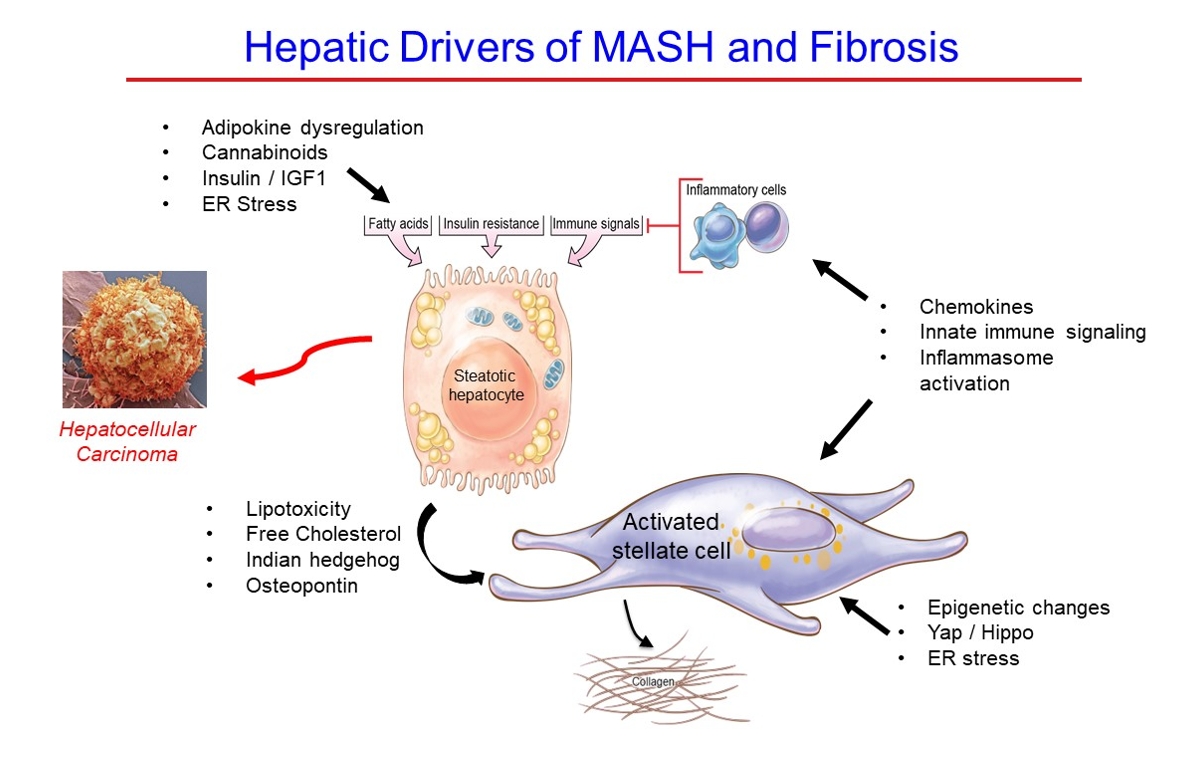
- Hepatic Fibrosis and Cancer: The Silent Threats of Metabolic Syndrome
- Scott L. Friedman
- Diabetes Metab J. 2024;48(2):161-169. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0240
- 2,375 View
- 290 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Metabolic dysfunction-associated steatotic (fatty) liver disease (MASLD), previously termed non-alcoholic fatty liver disease, is a worldwide epidemic that can lead to hepatic inflammation, fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). The disease is typically a component of the metabolic syndrome that accompanies obesity, and is often overlooked because the liver manifestations are clinically silent until late-stage disease is present (i.e., cirrhosis). Moreover, Asian populations, including Koreans, have a higher fraction of patients who are lean, yet their illness has the same prognosis or worse than those who are obese. Nonetheless, ongoing injury can lead to hepatic inflammation and ballooning of hepatocytes as classic features. Over time, fibrosis develops following activation of hepatic stellate cells, the liver’s main fibrogenic cell type. The disease is usually more advanced in patients with type 2 diabetes mellitus, indicating that all diabetic patients should be screened for liver disease. Although there has been substantial progress in clarifying pathways of injury and fibrosis, there no approved therapies yet, but current research seeks to uncover the pathways driving hepatic inflammation and fibrosis, in hopes of identifying new therapeutic targets. Emerging molecular methods, especially single cell sequencing technologies, are revolutionizing our ability to clarify mechanisms underlying MASLD-associated fibrosis and HCC.

 KDA
KDA
 First
First Prev
Prev





