Efficacy and Safety of Pioglitazone versus Glimepiride after Metformin and Alogliptin Combination Therapy: A Randomized, Open-Label, Multicenter, Parallel-Controlled Study
Article information
Abstract
Background
There is limited information regarding the optimal third-line therapy for managing type 2 diabetes mellitus (T2DM) that is inadequately controlled using dual combination therapy. This study assessed the efficacy and safety of pioglitazone or glimepiride when added to metformin plus alogliptin treatment for T2DM.
Methods
This multicenter, randomized, active-controlled trial (ClinicalTrials.gov: NCT02426294) recruited 135 Korean patients with T2DM that was inadequately controlled using metformin plus alogliptin. The patients were then randomized to also receive pioglitazone (15 mg/day) or glimepiride (2 mg/day) for a 26-week period, with dose titration was permitted based on the investigator's judgement.
Results
Glycosylated hemoglobin levels exhibited similar significant decreases in both groups during the treatment period (pioglitazone: −0.81%, P<0.001; glimepiride: −1.05%, P<0.001). However, the pioglitazone-treated group exhibited significantly higher high density lipoprotein cholesterol levels (P<0.001) and significantly lower homeostatic model assessment of insulin resistance values (P<0.001). Relative to pioglitazone, adding glimepiride to metformin plus alogliptin markedly increased the risk of hypoglycemia (pioglitazone: 1/69 cases [1.45%], glimepiride: 14/66 cases [21.21%]; P<0.001).
Conclusion
Among patients with T2DM inadequately controlled using metformin plus alogliptin, the addition of pioglitazone provided comparable glycemic control and various benefits (improvements in lipid profiles, insulin resistance, and hypoglycemia risk) relative to the addition of glimepiride.
INTRODUCTION
When medical treatment is necessary for type 2 diabetes mellitus (T2DM), physicians and patients prefer oral antidiabetic drugs [1]. However, it can be difficult to achieve and maintain glycemic control using a single antidiabetic agent. Thus, to achieve glycemic goals and reduce the risk of chronic diabetic complications, combinations of antidiabetic drugs are required for most patients [234]. Nevertheless, the combination therapy must be carefully selected to achieve an additive effect on glycemic control, and this approach is guided by the drugs' mechanisms of action and side effects [5]. Furthermore, social factors and insurance coverage can strongly influence the drugs that are selected for combination therapy [6].
Metformin is widely used as the initial antidiabetic agent [23], and dipeptidyl peptidase-4 (DPP-4) inhibitors are becoming increasingly common as a second agent that is combined with metformin [789]. For example, the number of American patients receiving metformin plus DPP-4 inhibitors has increased sharply [7]. This combination therapy is also prescribed for the overwhelming majority of Korean patients with T2DM [89]. Unfortunately, many patients cannot achieve or maintain their glycemic goals using only metformin plus DPP-4 inhibitors [10], and it can be difficult to select an appropriate third-line therapy for these patients. In Korea, the current health insurance system covers sulfonylurea (SU) or thiazolidinedione (TZD) as common third-line agents [11].
In East Asia, β-cell dysfunction has traditionally been recognized as the main etiological factor for T2DM [12]. However, recent evidence supports the importance of insulin resistance as the main pathogenic mechanism for T2DM in Korea [1314]. Therefore, it would be useful to compare the efficacies of SU or TZD when combined with metformin plus DPP-4 inhibitors in the third-line setting. The present study evaluated the efficacy and safety of adding pioglitazone or glimepiride for patients with T2DM that was inadequately controlled using metformin plus alogliptin.
METHODS
Study design
This multicenter, randomized, open-label, parallel design, phase IV trial (ClinicalTrials.gov: NCT02426294) was conducted between March 2015 and April 2018 at eight Korean centers. The study consisted of a 2-week screening period, a 26-week treatment period, and a 4-week follow-up period. The study protocol and other related documents were reviewed and approved by the Institutional Review Board (IRB No.: 1412-001-037) at each center. All patients provided written informed consent before their enrolment. The study complied with the Declaration of Helsinki, the Guidelines for Good Clinical Practice, and the applicable local laws and regulations.
Patients and eligibility
Patients with inadequately controlled T2DM (glycosylated hemoglobin [HbA1c] of 7.5% to <10%) were considered eligible if they had consistently received metformin plus alogliptin for ≥3 months before randomization, were 19 to 80 years old, and had a body mass index (BMI) of 18.5 to 35 kg/m2. The key exclusion criteria were type 1 diabetes mellitus, heart failure or history of heart failure (New York Heart Association Class III or IV), major cardiovascular disorders (e.g., myocardial infarction, cardiovascular intervention, stroke, and transient ischemic attack) during the last 6 months, renal or hepatic dysfunction (creatinine clearance <50 mL/min or elevated levels of aspartate aminotransferase [AST], alanine aminotransferase [ALT], alkaline phosphatase, or total bilirubin to ≥2.5×the upper normal limit), and pregnancy, breastfeeding, or unwillingness to use appropriate contraceptive measures (for women of reproductive age).
Data collection
The baseline and follow-up examinations included a physical examination, laboratory testing, medical review, and an in-person interview to collect information regarding medical conditions. Self-reported historical data (“yes” or “no”) regarding diabetic retinopathy and diabetic neuropathy were collected at the screening. The conventional method was used to calculate BMI (kg/m2). Serum specimens were typically collected after an 8-hour overnight fast and stored at −70℃ after processing.
Treatments
Participants were randomly assigned to receive either pioglitazone (15 mg/day) or glimepiride (2 mg/day) in addition to their current treatment using metformin plus alogliptin. After 12 weeks of treatment, the doses could be adjusted to 30 mg/day for pioglitazone or 4 mg/day for glimepiride, based on the investigator's decision.
Study outcome
The primary efficacy outcome was defined as the change in HbA1c levels from baseline to the end of treatment using pioglitazone or glimepiride. The secondary efficacy outcomes measures included the 26-week changes in lipid profiles, homeostatic model assessment of insulin resistance (HOMA-IR), and homeostatic model assessment β-cell function (HOMA-β), as well as the 12-week change in HbA1c levels (baseline to 12 weeks).
At each visit, all adverse events (AEs) were recorded and assessed for severity and possible relationship to the study medications. Participants performed self-monitored blood glucose (SMBG) testing at least once per day, or at any time when they experienced hypoglycemic symptoms. A hypoglycemic event was defined as confirmed hypoglycemia based on related symptoms and a SMBG result of <60 mg/dL, or any episode requiring intervention regardless of blood glucose levels. Other AEs were obtained via participant self-reporting.
Statistical analyses
The required sample size (154 patients divided evenly between the two groups) was calculated to achieve 90% power to detect a difference of 0.5% in HbA1c (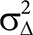 =1.71% and a two-sided α=0.05) between the baseline and 26-week measurements. The assumed drop-out rate was 20%.
=1.71% and a two-sided α=0.05) between the baseline and 26-week measurements. The assumed drop-out rate was 20%.
Analyses of the primary efficacy outcome and safety were performed based on the intention-to-treat (ITT) population, which was defined as participants who were exposed to at least one dose and then underwent at least one post-baseline assessment, and based on the per-protocol (PP) population, which was defined as participants who completed the study without any major protocol violation. The PP population was also used to analyze secondary efficacy outcomes and to compare efficacy outcomes between the two groups. The independent t-test was used to compare continuous variables between the two groups, while the paired t-test was used to compare the pre- and post-efficacies. The chi-square test or Fishe's exact test were used to compare categorical variables between the groups. All tests were two-tailed, and significance was set at P=0.05. All statistical analyses were performed using SAS software version 9.3 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Baseline characteristics
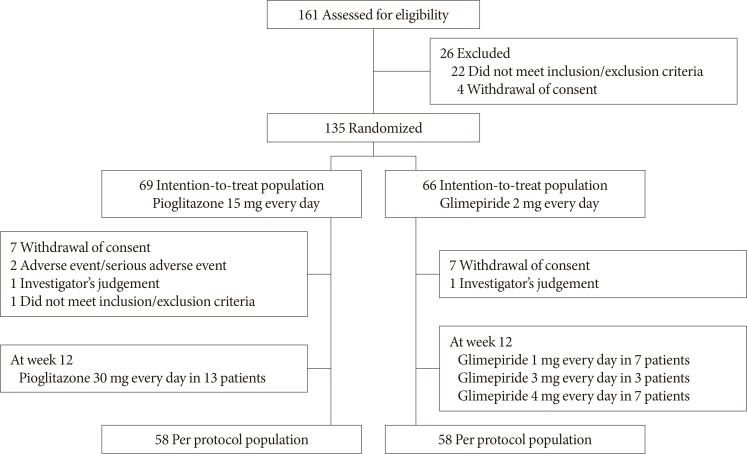
Fig. 1 shows the study flowchart. A total of 161 patients were screened for this study, and 135 patients (the ITT population) were randomized to receive pioglitazone (n=69) or glimepiride (n=66). After randomization, 19 patients dropped out and 116 patients (the PP population) completed the 26-week treatment using pioglitazone (n=58) or glimepiride (n=58). The most common reason for discontinuation was withdrawal of consent (seven patients in each group).
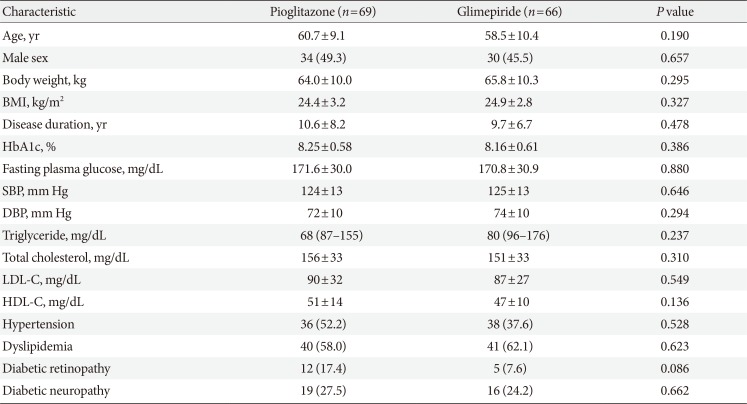
The baseline demographic and clinical characteristics were similar between the two groups in the ITT population (Table 1) and the PP population (data not shown). The mean duration of diabetes was approximately 10 years and the baseline HbA1c level was approximately 8.2%. The drug compliance rates during the study period were 95.5% for pioglitazone and 96.4% for glimepiride. At the 12-week evaluation, the treatment doses were adjusted for 13 patients in the pioglitazone group (to 30 mg/day) and for 17 patients in the glimepiride group (to 1 mg/day for seven patients, 3 mg/day for three patients, and 4 mg/day for seven patients).
Primary efficacy outcomes
Between baseline and the end of treatment, the HbA1c levels decreased significantly in both groups of the ITT population (P<0.001). The baseline HbA1c levels were 8.25%±0.58% in pioglitazone group and 8.16%±0.61% in glimepiride group. The corresponding changes in the HbA1c levels after 26 weeks were −0.81%±1.1% and −1.05%±0.87%, and no significant inter-group difference was observed (P=0.165) (Table 2).
In the PP population, the baseline HbA1c levels were not different between the pioglitazone group (8.21%±0.56%) and the glimepiride group (8.20%±0.63%). Similar to in the ITT population, the HbA1c levels in the PP population had decreased significantly by week 26 (P<0.001), although the inter-group difference was also not significant (pioglitazone: −1.04%±0.94%, glimepiride: −1.12%±0.87%; P=0.630). The proportion of participants achieving HbA1c <7.0% at the end of treatment was 48.3% with glimepiride (28/58) and 44.8% with pioglitazone (26/58) in the PP population (data not shown).
Secondary efficacy outcomes
The decreases in HbA1c levels between baseline and week 12 exhibited similar patterns to the changes between baseline and week 26. However, significant differences were observed between the pioglitazone and glimepiride groups in both the ITT population and the PP population (ITT: −0.64%±0.95% vs. −1.08%±0.73%, P=0.003; PP: −0.86%±0.76% vs. −1.16%±0.70%, P=0.029) (Fig. 2A).
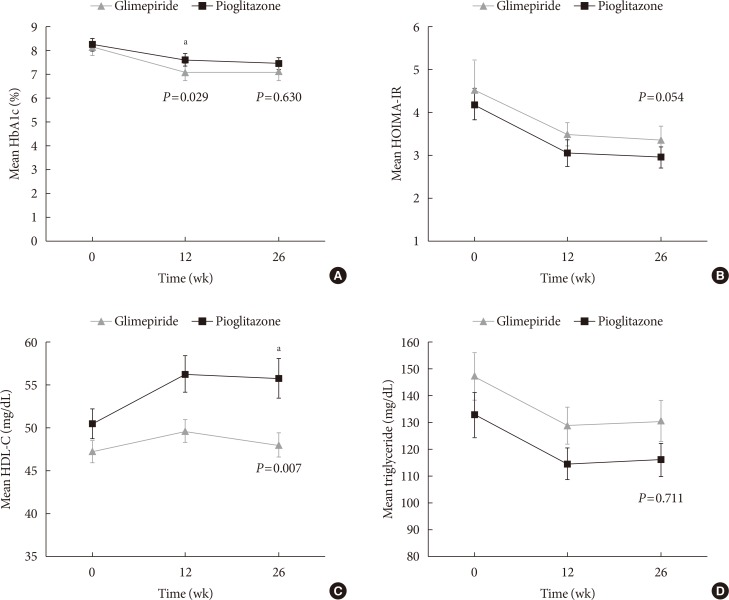
Changes in the measured variables at 12 weeks and 26 weeks. Mean±standard error values at baseline, 12 weeks, and 26 weeks were calculated for (A) glycosylated hemoglobin (HbA1c), (B) homeostatic model assessment of insulin resistance (HOMA-IR), (C) high density lipoprotein cholesterol (HDL-C), and (D) triglycerides during 26-week treatments using glimepiride (closed triangles) or pioglitazone (closed quadrangles) for patients with type 2 diabetes mellitus who were concurrently receiving combination therapy using alogliptin and metformin. aP<0.05.
The baseline HOMA-IR values were 4.10±3.03 in the pioglitazone group and 3.98±3.01 in the glimepiride group. At week 26, the HOMA-IR values had only decreased significantly in the pioglitazone group (2.58±1.56, P<0.001). However, there was no significant difference in the HOMA-IR changes of the two groups (pioglitazone: −1.53±2.74, glimepiride: −0.54±2.72; P=0.054) (Table 2, Fig. 2B). The absolute HOMA-β values, and the 26-week changes, were not significantly different between the two groups.
In the pioglitazone group of the PP population, the high density lipoprotein cholesterol (HDL-C) levels increased significantly from 49.19±14.20 mg/dL at baseline to 54.76±18.18 mg/dL at week 26 (P<0.001). However, no significant change in the HDL-C levels was observed in the glimepiride group (47.22±9.91 mg/dL at baseline and 48.16±11.29 mg/dL at week 26, P=0.403). There was a significant difference in the magnitude of the change in HDL-C levels when we compared the pioglitazone and glimepiride groups in the PP population (5.57±9.58 mg/dL vs. 0.94±8.47 mg/dL, P=0.007) (Table 2, Fig. 2C).
Triglyceride levels also decreased significantly in both groups (pioglitazone: 132.84±72.15 mg/dL to 113.02±51.47 mg/dL, P=0.034; glimepiride: 146.17±71.58 mg/dL to 130.72±67.03 mg/dL, P=0.043). However, there was no significant inter-group difference in the magnitude of these changes (−19.83±69.69 mg/dL vs. −15.45±56.76 mg/dL, P=0.711) (Table 2, Fig. 2D).
Safety
Table 3 shows the safety outcomes for each treatment. A total of 122 patients (90.4%) reported at least one treatment-emergent AE (pioglitazone: 64/69 patients [92.75%], glimepiride: 58/66 patients [87.88%]; P=0.504). The most frequent AEs were upper respiratory tract infection (10/69, 14.49%) in the pioglitazone group and hypoglycemia (16/66, 24.24%) in the glimepiride group. The glimepiride group was more likely than the pioglitazone group to experience AEs that were rated as being possibly/probably/definitely related to the study drug (i.e., adverse drug reactions) (pioglitazone: eight patients [11.59%], glimepiride: 23 patients [34.85%]; P=0.003). The most frequently reported adverse drug reaction was hypoglycemia, which was significantly more common in the glimepiride group (pioglitazone: 1/69 [1.45%], glimepiride: 14/66 [21.21%]; P=0.001). Two patients in the pioglitazone group discontinued the study because of AEs. Two patients in the pioglitazone group reported experiencing trauma-related fractures (serious AEs), with one case involving a femoral fracture after a fall from second-floor stairs and the second case involving a toe fracture after unintentionally kicking a table leg. A third patient in the pioglitazone group experienced acute pyelonephritis as a serious AE. The investigators judged that these three events were not related to the study medication. The other AEs were generally considered mildly to moderately severe.
DISCUSSION
Among patients with inadequately controlled T2DM receiving metformin plus alogliptin, the addition of glimepiride or pioglitazone for 26 weeks significantly improved glycemic control. Relative to glimepiride, pioglitazone provided similar efficacy of glycemic control with fewer episodes of hypoglycemia. Furthermore, pioglitazone improved the patients' lipid profiles and HOMA-IR values. The addition of both drugs to metformin plus alogliptin provided a good safety profile with no previously unknown safety concerns.
The Thiazolidinediones Or Sulfonylureas Cardiovascular Accidents Intervention Trial (TOSCA.IT) aimed to compare the long-term effects of second-line pioglitazone or SUs, given in addition to metformin monotherapy, on cardiovascular events in patients with T2DM [15]. The results indicate that both SUs and pioglitazone had similar effects, when combined with metformin, on the incidence of total cardiovascular events. However, relative to metformin plus SUs, metformin plus pioglitazone provided better durability of glycemic control and a lower frequency of hypoglycemia. Similarly, our results indicate that adding pioglitazone to metformin plus alogliptin provided similar glycemic control but a lower risk of hypoglycemia compared to adding glimepiride. The TOSCA.IT trial was focused on second-line treatment for T2DM while our study suggests that pioglitazone was suitable alternatives as add-on treatment to metformin plus alogliptin combination treatment.
Various pathophysiological factors contribute to hyperglycemia, which inevitably requires combining antidiabetic agents with different mechanisms of action to successfully manage T2DM [234]. Metformin remains the first-line agent for treating T2DM, although recent recommendations have suggested patient-centered glycemic management, albeit without specific recommendations regarding the optimal second-line and third-line antidiabetic agents. In this context, insulin resistance is a major pathophysiological factor that influences T2DM, and improving insulin sensitivity is extremely important in the management of T2DM [1617]. Although metformin is usually classified as an insulin sensitizer [181920], its efficacy as an insulin sensitizer is very limited, while TZD has been recognized as a true insulin sensitizer [212223]. However, safety concerns were raised regarding the use of rosiglitazone, which has led to TZD being used infrequently [24].
The disadvantages of DPP-4 inhibitors include a modest glucose-lowering effect and an increased risk of hospitalization for heart failure [2526]. However, DPP-4 inhibitors are also associated with a markedly reduced risk of hypoglycemia [27], which has led to DPP-4 inhibitors typically being selected as the second-line agent after metformin monotherapy fails. Furthermore, there is an increasing number of patients who are receiving combination therapy using metformin plus a DPP-4 inhibitor [789], with >50% of Korean patients receiving this combination [9]. However, metformin plus a DPP-4 inhibitor provides unsatisfactory glycemic control, which inevitably leads to the addition of a third-line agent.
Pioglitazone is a well-recognized insulin sensitizer with similar glucose-lowering power to glimepiride, albeit with a slower response [28]. The findings of the present study also validate this point. For example, there was a significant difference between the pioglitazone and glimepiride groups at 12 weeks in terms of their changes in HbA1c levels, although this difference disappeared at the 26-week measurement. A slow-onset but durable glycemic control is a well-established characteristic of TZD treatment, and the rarity of hypoglycemia is another well-known benefit of pioglitazone treatment [29]. As expected, the addition of glimepiride was associated with more hypoglycemic events than after the addition of pioglitazone. Approximately 24% of patients in the glimepiride group experienced hypoglycemia, although severe hypoglycemic events were not observed in both treatment groups.
Pioglitazone improves the function of β-cells and adipose tissue [303132], with elevated production of adipokines (e.g., adiponectin and leptin) stimulating fatty acid uptake that helps protect non-adipose tissue from abnormal lipid accumulation [33]. However, the present study failed to detect significant increases in HOMA-β after the addition of glimepiride or pioglitazone to the patients' treatment. Nevertheless, the onset of diabetes is preceded by impaired insulin secretion, and we noted that most patients had an approximately 10-year history of diabetes, which may explain the lack of improvement in β-cell function. Furthermore, Korean patients with T2DM more commonly exhibit an insulin secretory defect leading to the development of diabetes, relative to Caucasian patients with a similar duration of diabetes [12]. Therefore, we conclude that glimepiride and pioglitazone were both unable to alter the patients' insulin secretory function in this study.
A recent study revealed that pioglitazone was safely used for 18 months in 101 patients with prediabetes or T2DM, as well as biopsy-proven non-alcoholic steatohepatitis, with 51% of the patients experiencing resolution of the non-alcoholic steatohepatitis [34]. In the present study, the pioglitazone group exhibited significant 26-week decreases in the values for HOMA-IR (−1.53±2.74, P<0.001), AST (−2.6±8.4 IU/L, P=0.012), and ALT (−6.4±11.8 IU/L, P<0.001). In addition, the magnitude of the decreases in the liver enzyme levels from baseline were significantly larger in the pioglitazone group than in the glimepiride group (P=0.011 for AST, P=0.01 for ALT). An increase in HDL-C and a decrease in triglyceride levels are well-known effects of pioglitazone treatment [3536], and the present study revealed similar findings. After 26 weeks of treatment, the pioglitazone group exhibited a significant increase in HDL-C, while both groups exhibited similar decreases in triglycerides, and neither group exhibited changes in low density lipoprotein cholesterol levels.
Weight gain and edema are frequently reported during TZD treatment [3738], although the present study revealed minimal incidences of these events, and none of the patients discontinued treatment because of these events. These findings might be explained by the fact that metformin has anorexic properties and is associated with an increase in gastrointestinal side effects [39]. Thus, any TZD-related weight gain might be minimized when it is added to a treatment regimen that includes metformin. An increased risk of fracture is a recently emerged adverse effect of TZD treatment [40], and we identified two fracture cases in the pioglitazone group. The first case involved a femoral fracture after a fall off from second-floor stairs and the second case involved a toe fracture after accidentally kicking a table leg. The investigators judged these fractures to not be related to the study medication, and we conclude that both pioglitazone and glimepiride were well tolerated throughout the study period.
In conclusion, the addition of pioglitazone to metformin plus alogliptin for patients with inadequately controlled T2DM resulted in a similar decrease in HbA1c levels to that induced by the addition of glimepiride. However, in addition to the comparable level of glycemic control, pioglitazone provided several better outcomes (improvements in lipid control, insulin resistance, and hypoglycemia risk). Therefore, pioglitazone can be used effectively and safely as a third-line agent for managing patients whose T2DM is not adequately controlled using metformin plus a DPP-4 inhibitor.
ACKNOWLEDGMENTS
None
Notes
CONFLICTS OF INTEREST: This study was funded by Takeda Pharmaceuticals Korea Co.
AUTHOR CONTRIBUTIONS:
Conception or design: S.S.K., I.J.K.
Acquisition, analysis, or interpretation of data: J.M.K., S.S.K., J.H.K., M.K.K., T.N.K., S.H.L., C.W.L., J.Y.P., E.S.K., K.J.L., Y.S.C., D.K.K., I.J.K.
Drafting the work or revising: J.M.K., S.S.K., I.J.K.
Final approval of the manuscript: J.M.K., S.S.K., J.H.K., M.K.K., T.N.K., S.H.L., C.W.L., J.Y.P., E.S.K., K.J.L., Y.S.C., D.K.K., I.J.K.