Plasma CD36 and Incident Diabetes: A Case-Cohort Study in Danish Men and Women
Article information
Abstract
Background
Membrane CD36 is a fatty acid transporter implicated in the pathogenesis of metabolic disease. We aimed to evaluate the association between plasma CD36 levels and diabetes risk and to examine if the association was independent of adiposity among Danish population.
Methods
We conducted a case-cohort study nested within the Danish Diet, Cancer and Health study among participants free of cardiovascular disease, diabetes and cancer and with blood samples and anthropometric measurements (height, weight, waist circumference, and body fat percentage) at baseline (1993 to 1997). CD36 levels were measured in 647 incident diabetes cases that occurred before December 2011 and a total of 3,515 case-cohort participants (236 cases overlap).
Results
Higher plasma CD36 levels were associated with higher diabetes risk after adjusting for age, sex and other lifestyle factors. The hazard ratio (HR) comparing high versus low tertile of plasma CD36 levels was 1.36 (95% confidence interval [CI], 1.00 to 1.86). However, the association lost its significance after further adjustment for different adiposity indices such as body mass index (HR, 1.23; 95% CI, 0.87 to 1.73), waist circumference (HR, 1.21; 95% CI, 0.88 to 1.68) or body fat percentage (HR, 1.20; 95% CI, 0.86 to 1.66). Moreover, raised plasma CD36 levels were moderately associated with diabetes risk among lean participants, but the association was not present among overweight/obese individuals.
Conclusion
Higher plasma CD36 levels were associated with higher diabetes risk, but the association was not independent of adiposity. In this Danish population, the association of CD36 with diabetes risk could be either mediated or confounded by adiposity.
INTRODUCTION
The prevalence of type 2 diabetes mellitus has increased dramatically in recent decades and now affects 422 million individuals worldwide [1]. The upsurge has been paralleled by elevated rates of obesity, one of the strongest risk factors for developing type 2 diabetes mellitus [2]. The transmembrane glycoprotein CD36 is a multi-ligand class B scavenger receptor that may be involved in the development of diabetes through obesogenic mechanisms. CD36 promotes the uptake of long-chain fatty acids in liver and skeletal muscle [3], which potentially leads to metabolic conditions such as dyslipidemia, obesity, and insulin resistance [4]. Supporting a role of CD36 in the development of these conditions, genetic studies have shown the linkage between the chromosome location of the CD36 gene and components of the metabolic syndrome [567]. However, the association may be bi-directional, as membrane CD36 expression has also been shown to be up-regulated in adipose tissues and skeletal muscle of obese individuals and patients with type 2 diabetes mellitus [8910]. Therefore, adiposity could potentially confound or mediate the association between CD36 and diabetes.
Measurement of membrane CD36 requires fresh blood samples, which is typically unfeasible in large population-based epidemiological studies. To address this issue, Handberg et al. [11] recently developed a novel assay that measures circulating concentrations of CD36 in stored plasma samples, and a moderate correlation between plasma CD36 levels and membrane CD36 expression in liver tissue has been reported [12], suggesting that plasma CD36 levels may reflect the raised membrane CD36 expression and thus be an useful biomarker for diabetes. Several cross-sectional studies have shown plasma CD36 levels to be elevated in patients with prediabetes and prevalent type 2 diabetes mellitus [1113]; however, the temporal relations cannot be determined in these studies and reverse causality is a major concern. In the one prospective study conducted to date, higher plasma CD36 levels were positively associated with risk of diabetes in age- and sex-adjusted models, particularly in females [14], and persisted with adjustment for body mass index (BMI) categories [14]. However, as no other covariates were included simultaneously in that model, it is not clear to what extent residual confounding from other important lifestyle risk factors such as smoking, alcohol consumption, physical activity, and education, as well as superior measures of adiposity (such as continuous measures of visceral fat and fat distribution) could explain the association between plasma CD36 and incident type 2 diabetes mellitus.
Therefore, we examined the association between plasma CD36 levels and the risk of incident diabetes in a cohort of Danish men and women. Our comprehensive measures of adiposity and lifestyle factors allowed us to investigate whether the association was independent of, or modified by, adiposity.
METHODS
Study population
The Danish Diet, Cancer and Health (DCH) study is an ongoing prospective cohort study among 57,053 cancer-free participants aged 50 to 64 years of age living in the urban areas of Copenhagen and Aarhus [15]. At baseline from 1993 to 1997, participants filled in self-administered lifestyle and food frequency questionnaires, and technicians collected anthropometric measurements, as well as obtained a 30-mL non-fasting peripheral blood sample from each participant. Blood samples were separated into different components (e.g., plasma, serum, erythrocytes, buffy coat) and frozen at −150℃ for long-term storage. The detailed study design has been described previously [15]. The study protocol was approved by the National Committee on Health Research Ethics and the Danish Data Protection Agency (KF 01-116/96) and all procedures were complied with the Helsinki Declaration. All participants completed informed consent at the baseline interview.
Diabetes was identified via the National Diabetes Registry using the personal identification number assigned to all Danish citizens. The National Diabetes Registry links administrative records from the National Patient Registry, the National Health Service Registry, and the Danish National Prescription Registry, where the latter three registries kept records for hospital discharge, treatment and blood glucose measurements, and medication purchase, respectively. Patients were included in the National Diabetes Registry if they (1) were diagnosed of diabetes before hospital discharge; (2) received podiatry care for diabetes; (3) had five glucose measurements within 1 year or two measurements per year for 5 consecutive years; or (4) purchased oral glucose-lowering drugs or insulin. Diabetes diagnoses in the National Diabetes Registry are highly valid (positive predictive value=89%).
We evaluated the association between plasma CD36 and diabetes risk in a case-cohort study nested within the DCH, which was initially designed for analyzing incident non-fatal myocardial infarction and fatal coronary heart disease (CHD) [16]. We included all confirmed CHD cases between baseline and May 2008 (n=2,063), as well as a random sub-cohort of individual without CHD at baseline (n=1,824) in the case-cohort study (Supplementary Fig. 1). In the present analysis, we examined diabetes as the outcome and excluded participants with prevalent diabetes at baseline (n=151). Incident diabetes cases were identified as those who were diagnosed with diabetes between baseline and December 2011 in the remaining participants of the case-cohort. After excluding those with missing covariates, the case-cohort included 648 cases (236 within the reference sub-cohort) and 2,867 non-cases (total n=3,515).
Biochemical measurements
For the current analysis, ethylenediaminetetraacetic acid (EDTA) plasma samples from the baseline examination were sent to Aarhus University Hospital for measurement of plasma CD36 concentrations by Handberg's in-house enzyme-linked immunosorbent assay (ELISA) assay [11]. A pool of EDTA plasma was applied in increasing dilutions and used to produce a standard concentration curve, and phosphate-buffered saline served as background. Absorptions were calculated relative to the standard EDTA plasma pool and expressed as relative units. Internal controls consisting of the EDTA plasma pool and recombinant CD36 (generously donated by Randox, Laboratories, Antrim, UK) were run in duplicates and in four concentrations, respectively, on each plate. Analytical runs were accepted if one of the internal controls was within±1 standard deviation (SD) from mean provided that the other control was within±2 SD. The intra-assay coefficient of variation was 11% (plasma pool, mean 0.14 arbitrary units), and total day-to-day assay coefficient of variation was 25% (plasma pool) and 19% (recombinant CD36). We performed batch re-calibration by regressing plasma CD36 level on batch and age as well as sex, smoking status, alcohol intake, and education, variables associated with plasma CD36 level in our study population [17].
Statistical analysis
Baseline demographic and lifestyle factors of subjected who developed diabetes during the follow-up and those from the random sub-cohort were presented as medians (5th to 95th) or percentages. BMI was calculated as weight (kg) divided by height (m) squared. Normal weight and overweight/obesity were defined as BMI <25 and ≥25 kg/m2. According to the WHO definition, abdominal obesity was defined as waist circumference >94 cm for men and >80 cm for women [18]. Body fat percentage was measured by bioelectrical impedance [19], and high body fat percentage was defined using sex-specific medians (>26.4% for men and >34.2% for women) in sub-cohort individuals. We estimated age- and sex-adjusted means for plasma CD36 in lower and higher categories of these adiposity indices. Plasma CD36 levels were categorized into tertiles based on distributions in sub-cohort participants. Cox proportional hazard regression using age as the underlying time-scale with standard inverse probability weights and robust variation was used to calculate the hazard ratio (HR) and 95% confidence interval (CI) for diabetes according to tertiles of plasma CD36. The weights were incorporated to account for the oversampling of CHD cases. A resampling procedure was used to appropriately estimate the variance [20]. Model 1 included age at baseline (continuous) and sex, and model 2 further adjusted for smoking (never, former, current <15, 15 to 24, ≥25 g of tobacco/day), length of school education (short <8, medium 8 to 10, long >10 years), alcohol intake (non-drinker, drinker <5, 5 to 9, 10 to 19, 20 to 39, ≥40 g of alcohol/day), and physical activity (continuous, metabolic equivalent of tasks/week). In subsequent models, adiposity indices (BMI, waist circumference, and body fat percentage) were included individually. In addition, we used restricted cubic spline regression with 4 knots at 5%, 35%, 65%, and 95% percentiles of plasma CD36 concentrations to evaluate the linearity of the relation between plasma CD36 and diabetes risk. Because no deviations from linearity were detected (P=0.77 for nonlinearity), we also calculated the diabetes risk per SD of plasma CD36. In addition, we tested for potential interaction of plasma CD36 (tertiles) with sex given the stronger associations observed for women in the previous prospective study [14]. Furthermore, we evaluated joint effects of plasma CD36 and adiposity measures, with the lowest tertile of plasma CD36 and the lower adiposity category used as the reference.
We used SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria) for all analyses, and considered two-sided P values of <0.05 to be statistically significant.
RESULTS
Population characteristics
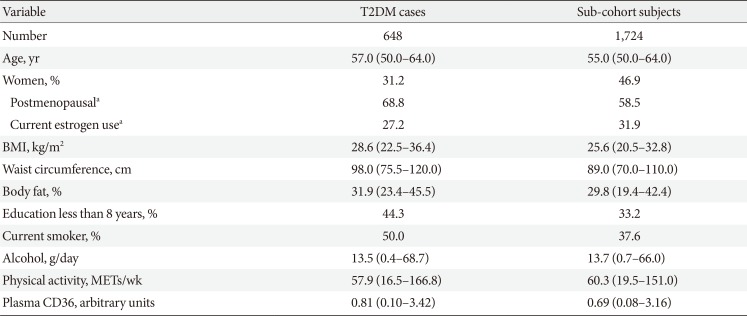
The characteristics of case-cohort participants are shown in Table 1. The median age at baseline was 57 years for participants who developed incident diabetes and 55 years for randomly selected sub-cohort individuals. In comparison to sub-cohort participants, cases were more likely to be male, current smokers, and have lower education. The spearman correlations between plasma CD36 and BMI, waist circumference and body fat percentage were 0.08, 0.09, and 0.06, respectively. In addition, cases had higher plasma CD36 levels, BMI, waist circumference, and body fat percentage. Within the sub-cohort, plasma CD36 levels were higher in participants with greater adiposity (P<0.05) (Fig. 1).

Baseline characteristics of participants who developed T2DM during follow-up and sub-cohort members in the Diet, Cancer and Health study
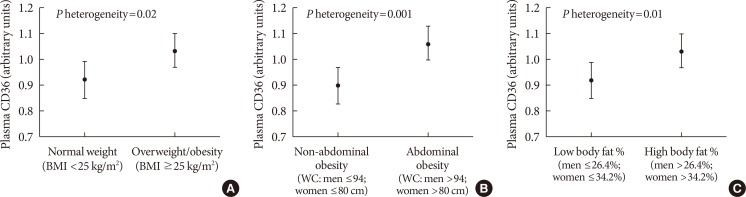
Adjusted means of plasma CD36 according to adiposity measures: (A) high vs. low levels of body mass index (BMI); (B) high vs. low levels of waist circumference (WC); (C) high vs. low levels of body fat percentage. Means were adjusted for age and sex. Error bars indicate standard deviations. P heterogeneity values were tested between the different mean values of plasma CD36.
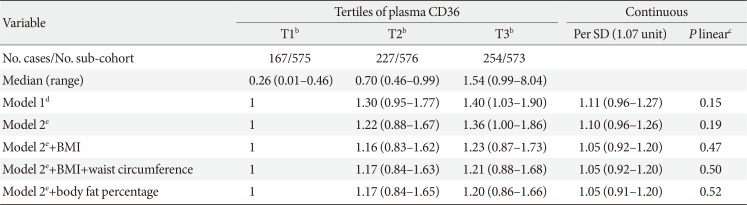
Association of plasma CD36 and type 2 diabetes mellitus risk
After adjustment for age, sex and lifestyle risk factors, higher plasma CD36 levels were associated with higher risk of type 2 diabetes mellitus (highest vs. lowest tertile of plasma CD36: HR, 1.36; 95% CI, 1.00 to 1.86) (Table 2). However, the association was attenuated and lost its significance after further adjustment for BMI (highest vs. lowest tertile: HR, 1.23; 95% CI, 0.87 to 1.73) or waist circumference (highest vs. lowest tertile: HR, 1.21; 95% CI, 0.88 to 1.68). Similar results were observed with adjustment for body fat percentage (highest vs. lowest tertile: HR, 1.20; 95% CI, 0.86 to 1.66). When modelling plasma CD36 as a continuous variable, the HR (95% CI) for type 2 diabetes mellitus per SD of plasma CD36 levels was 1.10 (95% CI, 0.96 to 1.26) in the multivariable-adjusted model, 1.05 (95% CI, 0.92 to 1.20) with further adjustment for BMI or waist circumference, and 1.05 (95% CI, 0.91 to 1.20) after adjusting for body fat percentage. We did not find any significant interaction between gender and plasma CD36 in the association with diabetes risk (P interaction=0.10; data not shown).
Joint analysis
The results for the joint analyses of CD36 and adiposity indices are shown in Fig. 2. The risk estimates associated with plasma CD36 levels in association with type 2 diabetes mellitus risk were stronger among people with lower levels of body fat percentage, BMI, and waist circumference. Among individuals with lower body fat percentage, a borderline positive association was observed between plasma CD36 levels and higher diabetes risk (per SD: HR, 1.31; 95% CI, 0.97 to 1.77; P trend=0.08). However, among people with higher body fat percentage, further elevation in plasma CD36 levels was not associated with higher diabetes risk (per SD: HR, 1.02; 95% CI, 0.89 to 1.18; P trend=0.75).

Hazard ratios (HRs) for joint analyses between plasma CD36 levels and adiposity indices on incident diabetes: (A) CD36 levels and high vs. low levels of body mass index (BMI) on diabetes; (B) CD36 levels and high vs. low levels of waist circumference on diabetes; (C) CD36 levels and high vs. low levels of body fat percentage on diabetes. Hazard ratios are adjusted for age, sex, alcohol, smoking, physical activity, and education. Error bars indicate 95% confidence intervals. P trend values were calculated in sub-groups separately. SD, standard deviation.
DISCUSSION
In this large, prospective case-cohort study among Danish men and women, we found a positive association between plasma levels of CD36 and risk of developing diabetes after adjusting for lifestyle factors such as age, sex, smoking status, alcohol consumption, physical activity, and education status. However, the association was attenuated to null after additional adjustment for adiposity indices such as BMI, waist circumference, and body fat percentage. Therefore, the positive association could be either mediated or confounded by adiposity.
Previous literature suggests different functions of CD36 in different organs in the body. In skeletal muscle, CD36 has been shown to enhance insulin action to stimulate muscle glucose utilization [21], and in pancreatic cells, the activation of CD36 has shown to be improved by glucolipotoxic conditions [2223]. In terms of plasma CD36, to the best of our knowledge, so far only one prior study, a nested case-control study in Swedish men and women, has examined the association between plasma CD36 and diabetes risk. This study found a positive association between plasma CD36 and risk of diabetes, which persisted in a model that adjusted for age, sex, overweight, and obesity as defined by WHO cut-points (highest vs. lowest quartile: HR, 2.6; 95% CI, 1.2 to 5.9; P trend ≤0.02) [14]. The discrepancy between the Swedish study and ours may be due to the differences in participant characteristics and model adjustments. In comparison, our study included more incident diabetes cases (648 in our study vs. 173 in the Swedish study). In addition, to minimize residual confounding we explored various continuous adiposity measures in a model that simultaneously adjusted for several diabetes risk factors. Although BMI is the official standard to evaluate obesity, it has several flaws, such as its inability to differentiate between body fat and muscle weight, or its inability to capture visceral fat, which may play a greater role in contributing to insulin resistance than subcutaneous fat [2425]. Results were consistently attenuated after adjusting for each adiposity index, which suggested that the association between plasma CD36 and diabetes may be mediated by obesity. A recent study in China also reported a positive interaction between genetic CD36 variants and obesity on type 2 diabetes mellitus susceptibility; only obese people carrying the risk allele (rs7755) had significantly higher risk of developing type 2 diabetes mellitus [26].
Several lines of evidence support the mediating effect of obesity in the relationship between CD36 and diabetes development. Membrane CD36 transports long-chain fatty acids across the plasma membrane in liver, muscle and adipocytes [3]. Uptake of fatty acids in turn increased CD36 expression through a peroxisome proliferator-activated receptor γ-dependent mechanism, which leads to enhanced efficiency of fatty acid uptake [27]. Thus, membrane CD36 has been shown to play an important role from the earliest stage of ectopic fat accumulation in liver, skeletal muscle, and visceral tissue, which results in the development of overweight and obesity, and subsequently leads to insulin resistance and type 2 diabetes mellitus [4]. In other words, overweight/obesity may develop along the pathway between elevated CD36 and ultimate development of diabetes, and may therefore mediate the association between CD36 and diabetes development. In addition, membrane CD36 is a scavenger receptor on monocytes and macrophages that binds and internalizes oxidized low density lipoprotein and subsequently causes foam cell formation and vessel wall inflammation [28], which are involved in the pathophysiology of diabetes.
While elevated plasma CD36 levels may be a causal factor for the development of obesity and diabetes, it is also possible that plasma CD36 levels are elevated by one or several components of metabolic abnormalities (such as obesity) that ultimately lead to diabetes. Metabolic syndrome includes several components such as obesity, dyslipidemia (triglyceride/high density lipoprotein), increased blood pressure and hyperglycemia [29]. Among all the factors, obesity and accompanying insulin resistance, as well as hyperglycemia have been shown to be the strongest predictors of diabetes development [30], and all these conditions have been shown to upregulate CD36 expression [9103132]. In addition, although the exact mechanism of releasing plasma CD36 into the circulation is not clear yet, factors like hyperglycemia, insulin resistance, dyslipidemia and low-grade inflammation have been hypothesized to elevate plasma CD36 levels [33], and plasma CD36 concentrations have been shown to reflect the CD36 expression levels from tissues associated with the metabolic syndrome [28]. Furthermore, as reported in previous studies, plasma CD36 levels were positively correlated with adiposity, insulin resistance, and liver fat [121314]. Moreover, genetic studies have shown that the location of the CD36 gene on chromosome 7q has been linked to components of metabolic syndrome [567], and variants in the CD36 gene have been shown to influence the susceptibility to the metabolic syndrome [3435]. Therefore, increased plasma CD36 levels may be a marker of one or several metabolic abnormalities that eventually lead to diabetes. Thus, the association between plasma CD36 and diabetes may also be confounded by obesity. In the current study, we did not have information on other components of metabolic syndrome; therefore, we could not examine their impacts on the association between plasma CD36 and diabetes risk.
Our study has a number of important strengths. Our comprehensive measurements of adiposity allowed us to more fully assess its involvement in the association between plasma CD36 and diabetes. In addition, the prospective design minimized the potential for recall bias in exposure data (questionnaires, collected bio-specimen). Moreover, compared to the previous nested case-control study [14], our current study included more diabetes cases with a tripled sample size. In addition, we tried to minimize potential confounding from adiposity by adjusting for three markers (BMI, waist circumference, and body fat percentage), thus making our study more robust than the previous study that adjusted for BMI alone [14]. However, some limitations merit consideration. First, we identified diabetes cases via record linkage from the National Diabetes Registry, which doesn't contain information on type of diabetes. However, since the rare onset of type 1 diabetes mellitus in adults [36], we assume the cases included in our study represent type 2 diabetes mellitus. In addition, we measured plasma CD36 levels only once, and some measurement error was inevitable. Moreover, unmeasured confounding by other lifestyle factors and residual confounding by included covariates may exist. Furthermore, we did not have information on other intermediate variables for diabetes, such as ectopic fat measures, lipids, insulin, β-cell function surrogates, glucose or glycosylated hemoglobin, to further elucidate the underlying etiology between plasma CD36 and diabetes. The previous nested case-control study found that plasma CD36 levels were not a significant independent variable for diabetes risk when fasting triglycerides, plasma glucose and insulin were simultaneously included in the multivariable models [14]. Moreover, the present study was conducted among a Caucasian population living in Northern Europe, and may not be applicable to other ethnic groups.
In conclusion, we found a positive association between plasma CD36 levels and diabetes risk among Danish men and women, but the association was attenuated by adjustment for measures of adiposity. Therefore, the positive association could be either mediated or confounded by adiposity. From a pathophysiological perspective, plasma CD36 is a potential early risk marker for diabetes. Therefore, further longitudinal studies with repeated measurements of plasma CD36 and more detailed measures such as ectopic fat and other blood biomarkers are needed to further elucidate the underlying etiology and to validate the findings in other ethnic groups.
ACKNOWLEDGMENTS
We thank all the participants of the Danish Diet, Cancer and Health study. We also thank the technician Lone Larsen for dedicated measurements of CD36. The Diet, Cancer and Health study was funded by the Danish Cancer Society.
Notes
CONFLICTS OF INTEREST: Dr. Handberg and Aarhus University Hospital hold two patents for the measurement of CD36 in plasma: “Method of evaluation of the relative risk of developing atherosclerosis in patients” 2006, WO2005/116644 and “A method for diagnosing atherosclerotic plaques by measurement of CD36,” 2008, WO2008/095492.
Genentech provided unrestricted funding for the measurement of plasma CD36 in the samples.
AUTHOR CONTRIBUTIONS:
Conception or design: M.K.J.
Acquisition, analysis, or interpretation of data: J.Z., T.C., M.Y., A.H., M.K.J.
Drafting the work or revising: Y.W., J.Z., S.A., K.O., T.C., M.Y., A.T., A.H., M.K.J.
Final approval of the manuscript: Y.W., J.Z., S.A., K.O., T.C., M.Y., A.T., A.H., M.K.J.
References
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2018.0273.
Supplementary Fig. 1
Modified case-cohort design for analysis of plasma CD36 with incident diabetes in the Danish Diet, Cancer, and Health study. CHD, coronary heart disease. aParticipants who did not develop diabetes during follow-up.