Soluble Dipeptidyl Peptidase-4 Levels Are Associated with Decreased Renal Function in Patients with Type 2 Diabetes Mellitus
Article information
Abstract
Background
Dipeptidyl peptidase-4 (DPP-4) is strongly expressed in the kidney, and soluble levels of this protein are used as a marker in various chronic inflammatory diseases, including diabetes, coronary artery disease, and cancer. This study examined the association between the serum soluble DPP-4 levels and renal function or cardiovascular risk in patients with type 2 diabetes mellitus.
Methods
In this retrospective analysis, soluble DPP-4 levels were measured in preserved sera from 140 patients with type 2 diabetes mellitus who had participated in our previous coronary artery calcium (CAC) score study.
Results
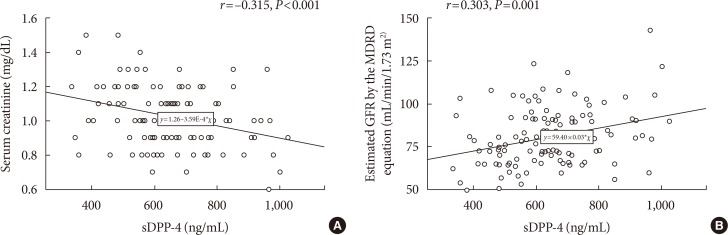
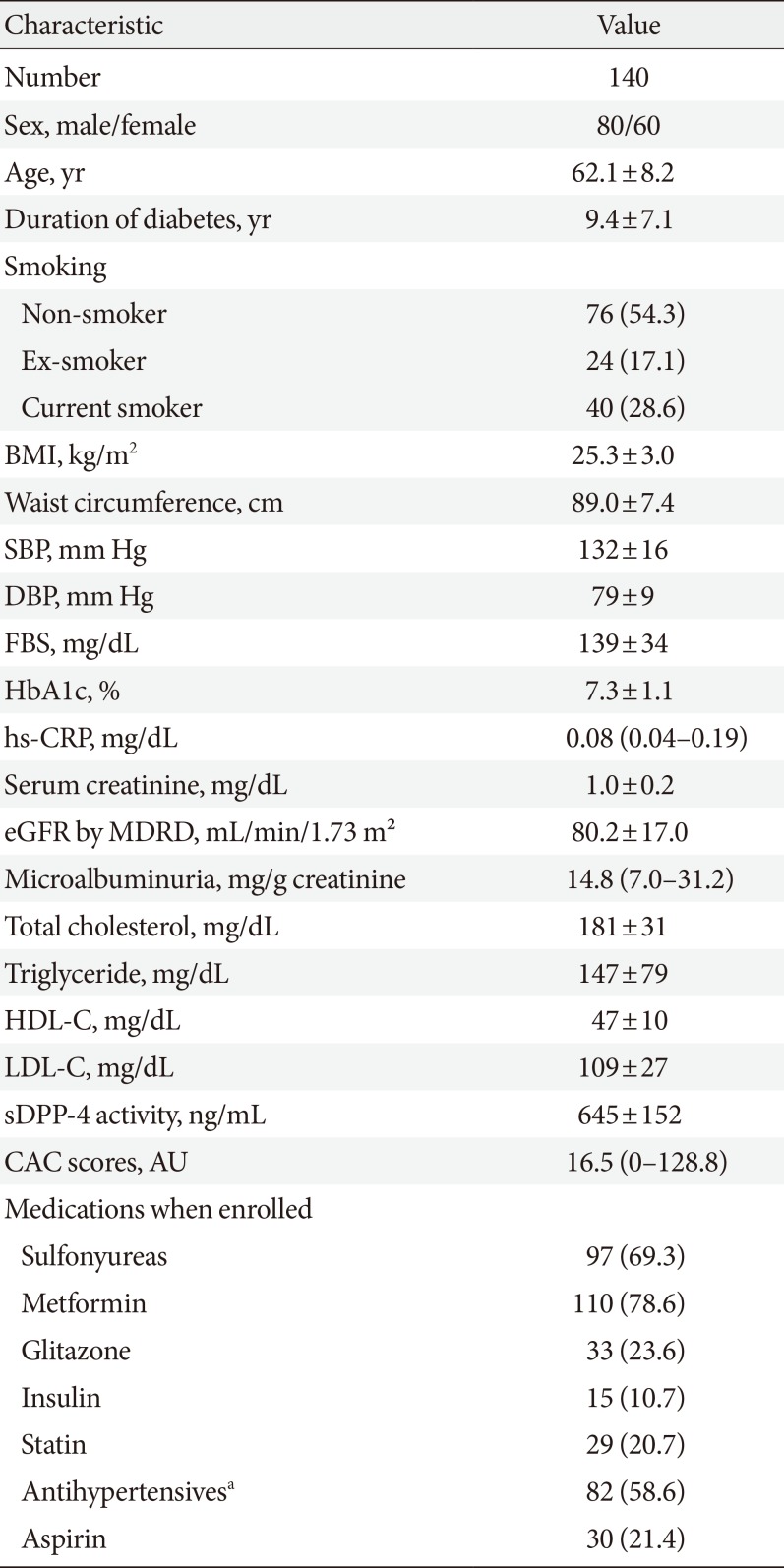
The mean±standard deviation soluble DPP-4 levels in our study sample were 645±152 ng/mL. Univariate analyses revealed significant correlations of soluble DPP-4 levels with the total cholesterol (r=0.214, P=0.019) and serum creatinine levels (r=−0.315, P<0.001) and the estimated glomerular filtration rate (eGFR; estimated using the modification of diet in renal disease equation) (r=0.303, P=0.001). The associations of soluble DPP-4 levels with serum creatinine and GFR remained significant after adjusting for age, body mass index, and duration of diabetes. However, no associations were observed between soluble DPP-4 levels and the body mass index, waist circumference, or CAC score.
Conclusion
These data suggest the potential use of serum soluble DPP-4 levels as a future biomarker of deteriorated renal function in patients with type 2 diabetes mellitus.
INTRODUCTION
Dipeptidyl peptidase-4 (DPP-4), a 110-kDa integral membrane glycoprotein with peptidase activity [1], was initially discovered by Hopsu-Havu and Glenner in 1966 and reported as CD26 [2]. The broad expression of DPP-4 in the kidney, small intestine, brain, lung, liver, and heart [34] suggests that this protein exhibits pleiotropic biological activities. DPP-4 mainly targets glucagon-like peptide, which regulates glucose metabolism and is particularly responsible for reducing glucose concentrations in the context of type 2 diabetes mellitus (T2DM) [1]. It also regulates stromal cell-derived factor-1 (SDF-1), known as C-X-C motif chemokine ligand 12 (CXCL12) [56], C-X-C chemokine 10 (known as interferon γ-induced protein-10) [6], peptide YY [7], and vascular endothelial growth factor (VEGF) [8]. Specifically, DPP-4 truncates many bioactive peptides of various medically important proteins and thus mediates important alterations in chemokine activities and receptor specificities [1]. The particularly strong expression of DPP-4 in the kidney [4], as well as in vascular endothelial cells and immune cells, suggests that this protein may play a role in renal and cardiovascular function [9].
The soluble form of DPP-4 is cleaved from the membranes of human adipocytes and smooth muscle cells and released to the plasma and other body fluids [11011]. In humans, approximately 95% of serum DPP-4 activity is associated with a relatively high serum concentration of soluble DPP-4 (sDPP-4) [1112], leading to the designation of this soluble bioactive molecule as a new adipokine [1013]. Other researchers have suggested the potential use of sDPP-4 as a diagnostic or prognostic marker of diabetes, coronary artery disease, depression, osteoporosis, and bronchial inflammation [1415161718]. For example, the development of diabetic nephropathy is associated with inflammatory cytokines such as interleukin 1 (IL-1), IL-6, tumor necrosis factor-α [192021], SDF-1 (CXCL12) [22], VEGF [23], and oxidative stress [21]. Various proinflammatory cytokines, SDF-1 (CXCL12), and VEGF contain DPP-4 truncation sites, suggesting a role for sDPP-4 in diabetic nephropathy.
DPP-4 also appears to play a role in normal renal function. Within the kidney, DPP-4 is expressed predominantly in the glomeruli and S1 to S3 segments of the proximal tubules and, to a lesser extent, in other segments [242526], where it facilitates the absorption of cleaved dipeptides and regulates the function of sodium/hydrogen exchanger-3 in the proximal tubules. Additionally, sDPP-4 exerts non-enzymatic functions via binding to newly recognized receptors such as the mannose-6-phosphate/insulin-like growth factor 2 receptor (M6P/IGF2-R) or protease-activated receptor 2 to initiate signal transduction [27]. Despite the evidence linking DPP-4 with renal function, only a few studies have investigated this role of sDPP-4 in the context of diabetes. Previous studies demonstrated a strong correlation of the serum sCD26 levels with circulating DPP-4 activity in humans [2829], suggesting that serum sCD26 may reflect DPP-4 activity. In this study, we investigated the endogenous association of serum sDPP-4 levels with renal function or cardiovascular risk in patients with T2DM.
METHODS
Study subjects
For this retrospective study, we obtained data and sera collected from 140 patients with T2DM who visited the diabetes clinic at Kangwon National University Hospital [30] and participated in subset of a study performed from March to June 2007. DPP-4 inhibitors were introduced to the Korean market in 2008; accordingly, none of the study subjects had been exposed to these drugs.
The subject exclusion criterion was the presence of typical symptoms of ischemic heart disease or a history of previous coronary angiography or cardiovascular intervention. We also excluded patients with chronic illnesses such as chronic liver disease, chronic kidney disease (serum creatinine levels >1.4 mg/dL), and chronic obstructive pulmonary disease. This study was approved by the Institutional Review Board at Kangwon National University Hospital (IRB number 2012-08-001-001). Written informed consent was obtained from each individual.
Laboratory measurements
Blood samples were collected after an 8-hour fast and subjected to general chemistry, serum lipid, microalbuminuria, and high-sensitivity C-reactive protein (hs-CRP) analyses. For hs-CRP testing, we used a latex agglutination method that could detect levels as low as 0.01 mg/dL. Serum sDPP-4 levels in samples that had been frozen at −80℃ before use were measured using a human DPP-4/CD26 immunoassay kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. The intra-assay coefficient of variation (CV) for the DPP-4 control sample for the assay was 5.8% (mean, 8.97 absorbance units [AU]; standard deviation [SD], 0.549). The inter-assay CV for the DPP-4 control sample was 8.6% (mean, 8.95 AU; SD, 0.761).
The estimated glomerular filtration rate (eGFR) for each patient was calculated using the modification of diet in renal disease (MDRD) equation and given values. For patients affected by acute episodes, the laboratory results for at least 1 month following the complete resolution of the episode were excluded from the data collection.
Statistical analyses
Descriptive statistics for subject characteristics are presented as mean±SD for continuous variables or count (percentage) for categorical variables.
The chi-square test was used to compare categorical variables such as smoking history and medications used at enrollment. The serum sDPP-4 levels were a normally distributed variable, and Pearson correlation coefficients were calculated to analyze the relationships between serum sDPP-4 levels and clinical parameters. Statistical analyses were performed using SPSS version 21.0 (IBM Co., Armonk, NY, USA). All P values were two-tailed, and P<0.05 was considered statistically significant.
RESULTS
Participant characteristics
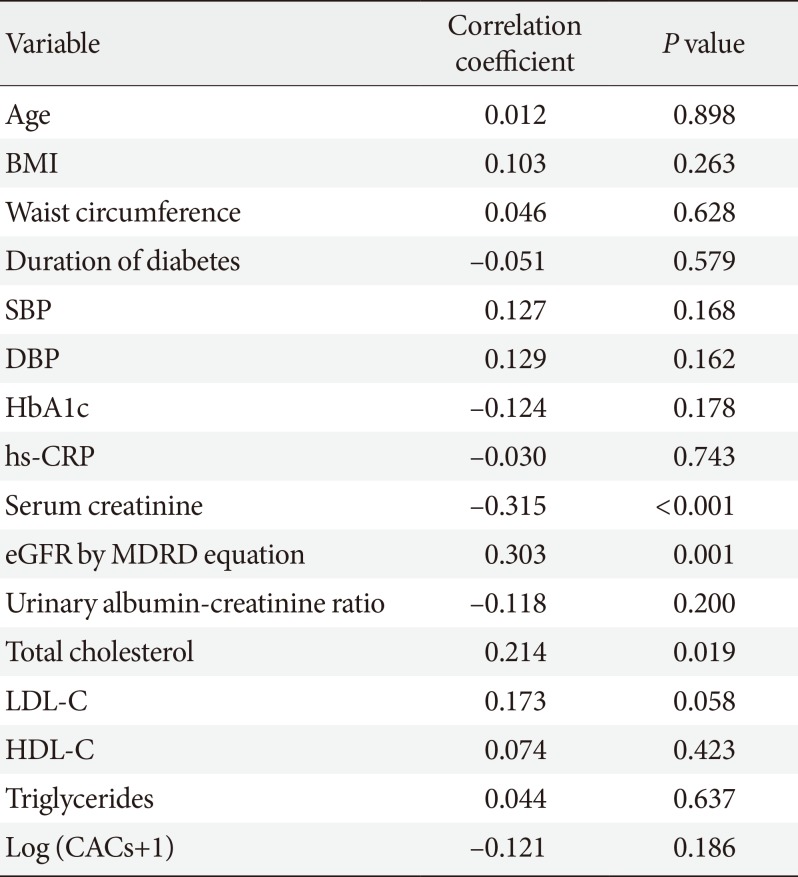
The study sample comprised 80 male and 60 female patients with a mean±SD age of 62.1±8.2 years and mean duration of diabetes of 9.4±7.1 years. The mean body mass index (BMI) was 25.3±3.0 kg/m2, and the mean waist circumference was 89.0± 7.4 cm. Regarding laboratory parameters, the mean glycosylated hemoglobin levels, mean serum creatinine levels, and mean eGFR were 7.3%±1.1%, 1.0±0.2 mg/dL, and 80.2±17.0 mL/min/1.73 m2, respectively. The mean sDPP-4 levels were 645±152 ng/mL. Detailed characteristics of the subjects are shown in Table 1.
Associations of DPP-4 levels with clinical parameters
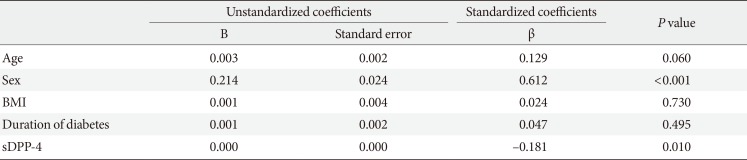
Univariate analyses revealed significant correlations of sDPP-4 levels with the total cholesterol (r=0.214, P=0.019) and serum creatinine levels (r= −0.315, P<0.001) and the eGFR (r=0.303, P=0.001), as shown in Table 2 and Fig. 1. We analyzed the association between baseline sDPP-4 levels and follow-up creatinine and eGFR with mean follow-up duration of 9.5 years (range, 2.8 to 11.0 years). There was negative correlation between baseline sDPP-4 levels and follow-up creatinine (r= −0.278, P=0.003) but there was no correlation between baseline sDPP-4 levels and follow-up eGFR (r=0.138, P=0.142).

Univariate correlation between soluble dipeptidyl peptidase-4 levels and clinical and biochemical variables
Associations of DPP-4 levels with renal function in patients with type 2 diabetes mellitus
In the enter-method multiple linear regression models, sex and sDPP-4 levels remained significantly associated with serum creatinine levels and eGFR (MDRD equation) after adjusting for age, BMI, and duration of diabetes (Tables 3 and 4). However, the urinary albumin-to-creatinine ratio was not found to correlate with the sDPP-4 levels.
Association of DPP-4 levels with cardiovascular risk factors in diabetes
No associations were observed between sDPP-4 levels and variables related to cardiovascular risk factors such as the BMI, waist circumference, or coronary artery calcium score.
DISCUSSION
With this study, we aimed to clarify the clinical role of serum sDPP-4 levels as markers of renal function or cardiovascular risk in patients with T2DM. In this era, however, DPP-4 inhibitors are widely prescribed to patients with diabetes. Therefore, we evaluated data and sera collected from a sample of subjects in 2007, before the introduction of DPP-4 inhibitors to the Korean market, to ensure that the subjects had not ever been exposed to these drugs. Our analysis revealed that the levels of endogenous serum sDPP-4 correlated inversely with the serum creatinine levels and positively with the eGFR in this sample of patients with diabetes.
Diabetic nephropathy is the most common cause of end-stage kidney disease requiring hemodialysis or kidney transplantation and is associated with increased mortality in patients with T2DM. Although albuminuria has been recognized as an early marker of diabetic nephropathy, significant glomerular injury occurs before the manifestation of this marker [31]. A previous report described a significant category of patients with diabetic nephropathy and advanced chronic kidney disease unaccompanied by albuminuria [32], and found that these patients had a higher prevalence of cardiovascular disease relative to those with a preserved eGFR [33]. Therefore, many investigators seek new biomarkers in an attempt to overcome the limitations of current detection and diagnostic tools for early diabetic nephropathy.
In this study, we observed a negative association of serum sDPP-4 levels with serum creatinine levels, which suggests the potential usefulness of sDPP-4 as an early diagnostic marker of renal dysfunction in patients with T2DM without chronic kidney disease. Moreover, our regression analysis demonstrated that this inverse association of sDPP-4 with serum creatinine levels was independent of age, BMI, and the duration of diabetes. These findings further support the relevance of the serum sDPP-4 levels as a new diagnostic marker of early renal dysfunction in diabetes.
A recent study identified the reno-protective effects of DPP-4 inhibitors against diabetic nephropathy [30] and suggested that these effects are mediated by a reduction in DPP-4 activity. In other recent studies, the sDPP-4 levels were suggested as a marker of metabolic syndrome [13], a predictor of new-onset hyperglycemia in Chinese patients [34], an indicator of new onset microalbuminuria in nondiabetic Chinese patients [35], and an early diagnostic marker of gastric cancer [36]. More specifically, Zheng et al. [37] suggested that an increase in plasma DPP-4 activity correlated with diabetic nephropathy in Chinese T2DM. However, our results oppose these earlier findings, as we observed an association of higher serum sDPP-4 levels with better renal function in our sample of diabetic patients. This discrepancy indicates that the mechanism by which increased sDPP-4 levels are associated with better renal function in patients with diabetes remains poorly understood. Actually, there are few papers dealing with the role of sDPP-4 levels or activity in diabetes and further studies to be required to evaluate the role of sDPP-4 levels and their relationship with usage of DPP-4 inhibitors in diabetes in the future.
In a previous study, diabetic patients with normoalbuminuria exhibited a significant increase in urine microvesiclebound DPP-4 activity when compared with controls, suggesting that urinary DPP-4 levels may serve as a biomarker of diabetic kidney disease [38]. We hypothesize that urinary DPP-4 secretion from tubular epithelial cells might increase following renal damage. Such damage is known to cause the leakage of endogenous endothelial DPP-4 from the kidney, which is responsible for the decreased sDPP-4 levels measured in patients with diabetes and deteriorated renal function. Furthermore, the DPP-4-mediated cleavage of regulatory peptides on lymphocytes, macrophages, or fibroblasts affects the biological inactivation of circulating hormones or chemokines and neuropeptides in the renal system [4]. Interestingly, a previous study observed significantly decreased plasma levels of DPP-4/CD26 in rheumatoid arthritis patients, compared to osteoarthritis patients, and noted that the negatively association of circulating DPP-4 levels with C-reactive protein levels suggested a role for the former as a negative acute phase protein [28]. Another study found an association of increased serum sDPP-4 levels with decreased disease activity in patients with systemic lupus erythematosus [29]. Moreover, people with normal renal function will fully express DPP-4 in their renal tissues. In a rat model, extraordinarily high concentrations of DPP-4 (up to 14,000 nmol/min/g) were detected in the kidney relative to other tissues [4]. We believe that people with impaired renal function may exhibit reduced DPP-4 activity consequent to a decreased renal functional mass. However, the importance of DPP-4-mediated chemokine cleavage for modified inflammatory responses in diabetic kidney disease is far less clearly established and remains a topic for future research.
The endogenous sDPP-4 levels observed in our study may not reflect the DPP-4 activities in the context of DPP-4 inhibitors or in tissues such as adipose or kidney tissue. Therefore, we cannot extrapolate our findings to determine the potential effects of DPP-4 inhibitors on renal function in diabetes. Previous studies observed significant associations of plasma endogenous DPP-4 activity with race [39] and DPP-4 polymorphisms in Malaysian subjects [40], suggesting that endogenous sDPP-4 levels may be affected by genetic background. Furthermore, it is important to establish main tissue sources of sDPP-4, other than bone marrow and endothelial cells, and to establish which circumstances or diseases can provoke abnormally elevated sDPP-4 concentrations if this protein is to be validated as a clinical marker or therapeutic target.
This study was designed to determine the relationship of serum sDPP-4 levels with renal function and cardiovascular risk factors in patients with T2DM, using an analysis in which potential confounders were well controlled. However, several important limitations of our study must also be considered. The patient sample was relatively small and recruited at a single center, and patients with advanced chronic kidney disease were excluded. Furthermore, the use of angiotensin II receptor blocker (ARB) or angiotensin-converting enzyme (ACE) inhibitors may have influenced the relationship between sDPP-4 levels and renal function. Serum levels of sDPP-4 were only measured at a single time point. Accordingly, it remains unknown whether changes in DPP-4 over time are associated with changes in renal function. Additionally, we did not compare serum sDPP-4 levels with total DPP-4 protein levels. Finally, we failed to demonstrate a mechanistic role of sDPP-4 in the pathogenesis of nephropathy.
In conclusion, we demonstrated associations of increased serum sDPP-4 levels with low serum creatinine levels and an increased glomerular filtration rate in subjects with T2DM. However, further studies are needed to clarify the regulatory role of sDPP-4/CD26 in the renal function of patients with diabetes.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.