Response: Dipeptidyl Peptidase-4 Inhibitors versus Other Antidiabetic Drugs Added to Metformin Monotherapy in Diabetic Retinopathy Progression: A Real World-Based Cohort Study (Diabetes Metab J 2019;43:640–8)
Article information
We appreciate the thoughtful comments by Dr. Moon on our recently published article, “Dipeptidyl peptidase-4 inhibitors versus other antidiabetic drugs added to metformin monotherapy in diabetic retinopathy progression: a real world-based cohort study” [1]. We also would like to thank the editor for the chance to further discuss our article.
Dr. Moon pointed out in his comment that the lack of critical risk factors such as glycemic control (glycosylated hemoglobin [HbA1c] level) and duration of diabetes requires careful interpretation, and also mentioned that the differences among dipeptidyl peptidase-4 inhibitors (DPP4is) need to be evaluated as there is no specific antidiabetic agent that protects against the progression of diabetes so far.
We agree that the lack of HbA1c data for glycemic control is a critical limitation of our study, as intensive treatment of hyperglycemia is known to reduce the rate of diabetic retinopathy (DR) progression [2]. This is why we had described it as a major limitation, although we tried to adjust fasting glucose levels instead in the subgroup analysis. This lack of HbA1c data would be a persistent weakness of future cohort studies using national database for DR-related investigations. We also agree that the differences among DPP4is need to be evaluated, as they differ widely in their chemistry, properties, and elimination pathways [3]. Subgroup analysis of different DPP4is would be of interest, while this should be conducted with a much larger sample size.
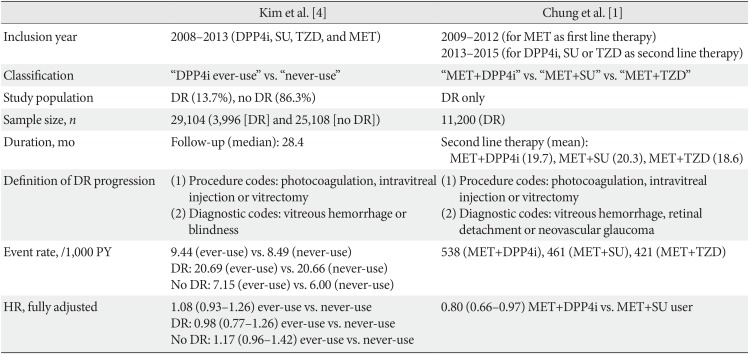
We would also like to take this opportunity for a further discussion with the study by Kim et al. [4] based on the same database provided by the National Health Insurance Service in Korea (NHIS). We summarized the differences between our study and that of Kim et al. [4], although the main conclusions were similar in that there was no definite aggravation of DR by DPP4is (Table 1). The major differences between the two studies were the definition of DR progression and the presence of DR at baseline. Patients with DR was 13.7% (n=3,996) in the study of Kim et al. [4]; the majority of patients were without DR at baseline. Our study included patients with preexisting DR and had a larger sample size (n=11,200) than that of Kim et al. [4], which is sufficient to investigate the effect of DPP4is in the progression of DR. Kim et al. [4] reported an increased risk of DR progression early in the treatment phase (<12 months), while this trend was limited to those without DR. Adjusted hazard ratio was below 1 for DPP4i ever-users in those with DR, which was similar with our study.

Comparison of study designs on DPP4is and DR progression based on National Health Insurance Service data
Further study with a larger sample size and longer duration of treatment would be helpful to clarify the effect of specific DPP4is. Thank you again for your interest in our article.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.