Effectiveness of Exercise Intervention in Reducing Body Weight and Glycosylated Hemoglobin Levels in Patients with Type 2 Diabetes Mellitus in Korea: A Systematic Review and Meta-Analysis
Article information
Abstract
Background
This study aimed to assess the effectiveness of exercise intervention in reducing body weight and glycosylated hemoglobin (HbA1c) level in patients with type 2 diabetes mellitus (T2DM) in Korea.
Methods
Cochrane, PubMed, Embase, KoreaMed, KMbase, NDSL, KCI, RISS, and DBpia databases were used to search randomized controlled trials and controlled clinical trials that compared exercise with non-exercise intervention among patients with non-insulin-treated T2DM in Korea. The effectiveness of exercise intervention was estimated by the mean difference in body weight changes and HbA1c level. Weighted mean difference (WMD) with its corresponding 95% confidence interval (CI) was used as the effect size. The pooled mean differences of outcomes were calculated using a random-effects model.
Results
We identified 7,692 studies through literature search and selected 23 articles (723 participants). Compared with the control group, exercise intervention (17 studies) was associated with a significant decline in HbA1c level (WMD, −0.58%; 95% CI, −0.89 to −0.27; I2=73%). Although no significant effectiveness on body weight was observed, eight aerobic training studies showed a significant reduction in body weight (WMD, −2.25 kg; 95% CI, −4.36 to −0.13; I2=17%) in the subgroup analysis.
Conclusion
Exercise significantly improves glycemic control; however, it does not significantly reduce body weight. Aerobic training can be beneficial for patients with non-insulin-treated T2DM in Korea.
INTRODUCTION
Diabetes mellitus (DM) is a pan-endemic disease, and its incidence and prevalence are rapidly increasing. Globally, approximately 108 million adults presented with DM in 1980. However, the number increased to around 422 million in 2014. The prevalence of DM has almost doubled since 1980 and has increased from 4.7% to 8.5% in the adult population globally. With the increasing risk for cardiovascular and other diseases, 2.2 million individuals have died due to higher-than-optimal blood glucose level [1]. The prevalence of DM in Korea has also increased from 10.1% to 12.9% in men and from 8.0% to 9.6% in women over the past 5 years (2012 to 2016) [2]. Majority of individuals have type 2 diabetes mellitus (T2DM), and this phenomenon is associated with an increase in the prevalence of the associated risk factors, such as obesity.
Because T2DM is a chronic condition and causes multiple complications, its treatment is challenging. Moreover, the goal for the management of T2DM is to prevent its complications by maintaining blood glucose and metabolic parameters at target levels. Of the important therapeutic approaches, lifestyle modification, including exercise and diet, as well as drug therapy are the primary recommended approaches. In addition, exercise and diet are emphasized as the fundamental approaches that prevent diabetes complications and reduce medical costs. Da Qing study [3], Finnish Diabetes Mellitus Prevention (FDPS) study [4], and Diabetes Prevention Program (DPP) [5] presented the effect of lifestyle modification in preventing T2DM. In Korea, education on diet, exercise, self-glucose monitoring, and consultations on patients' compliance on their lifestyle modification are generally performed as an initial non-pharmacological intervention for T2DM [6]. Particularly, exercise is recommended for patients with T2DM because it contributes to reducing the metabolic risk of developing diabetic complications [7]. Although some studies have shown the positive effects of exercise intervention on glucose metabolism [89], others have reported no significant difference in the glucose metabolism [1011] according to their study participants and designs.
Because the physical and genetic characteristics of the Asian population are different from those of the Western population, an ethnic-oriented therapeutic approach for Korean patients with T2DM must be used. Furthermore, in contrast to the publications of several meta-analyses that described multiple intervention studies conducted in Western countries, meta-analysis studies on patients with T2DM in Korea is still limited. In addition, no study on the effectiveness of exercise on glucose metabolism in the Korean population has been conducted, which is important in establishing exercise recommendations.
Thus, this study aimed to investigate the effectiveness of exercise intervention in reducing glycosylated hemoglobin (HbA1c) levels and weight loss by analyzing the effect size of previous studies in accordance with the Cochrane methodology among patients with T2DM in Korea.
METHODS
Data sources and search strategies
Cochrane, PubMed, Embase, KoreaMed, KMbase, NDSL, KCI, RISS, and DBpia were searched from January 1965 to August 2017. The terms used for the literature search were “Type 2 diabetes,” “Self-glucose monitoring or Self-glycemic monitoring,” “Exercise, Physical activity,” “Education, Teaching,” and “Training, Coaching, Practice” in the databases for Korea. For Cochrane, PubMed, and Embase, we used the terms “Type 2 diabetes,” “Exercise, Physical activity,” and “Korea” to cover only Korean T2DM patients. In addition, languages were limited to Korean and English. The search terms were combined with “AND” for diabetes disease, exercise, education, counseling, training, self-monitoring, and research design, as well as with “OR” for exercise, physical activity, education, teaching, training, coaching, and practice. It is the literature-based descriptive study; therefore, approval by the Institutional Review Board or informed consent is not required.
Eligibility criteria
The inclusion criteria of the literature were as follows: studies on patients with T2DM who are not on insulin therapy (Patients, P) in Korea, those that described exercise intervention for the management of DM (Intervention, I), those that compared the non-intervention and control groups (Comparison. C), and those that described HbA1c levels and weight (Outcome, O) according to the research question (PICO). In addition, only studies that presented the mean and standard deviation of the results were selected.
We excluded studies on type 1 and gestational DM, those on patients with DM who are on insulin therapy (P), those that involved a combination of diet and exercise intervention (I), those that included a control group that was not a non-intervention group or that was under conventional care (C), and those with results other than the parameter of glucose metabolism, such as psychological parameter (O). The literature was independently selected by two researchers (J.E.J. and E.S.S.), and it was identified via a thorough discussion in cases of inconsistencies.
Data extraction
HbA1c level and body weight were used as variables for the effect of exercise intervention and were calculated using mean, standard deviation, number of participants, and baseline and final values. The contents of the literature were examined in detail, and the types of education, training, counseling, and self-monitoring blood glucose intervention were coded and presented. To analyze the effect of intervention on HbA1c levels and body weight according to the kind of exercise, we extracted variables for sub-analysis.
The use of treadmill, bike riding, walking (jogging), aerobic, stretching, and step box were coded as aerobic exercise. Muscular exercise, knee and abdominal strength exercise, back extension, chest exercise, and the use of elastic band or gym stick were coded as resistance exercise. If there is a numerical result due to the different kinds of exercise in the contents of one document, the data were extracted according to categories for the sub-analysis, and errors resulting from the duplication of the same documents were supplemented using statistical techniques.
Assessment of the study quality and risk of bias
The risk of bia (RoB) of the Cochrane Union was used as a bias risk assessment tool to assess the quality of randomized controlled or controlled clinical trials. Two researchers assessed the RoB according to seven estimation tools—random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias that threaten validity. The sample size of the experimental and control groups was also added for evaluation.
Eight items were evaluated as at high RoB, low RoB, and unclear. After assessing the RoB, two types of tables (RoB graph and summary) were generated and plotted using the Review Manager (RevMan) 5.3 program.
Statistical analysis
The RevMan 5.3 program was used to synthesize the effect sizes of the 23 selected documents. The weighted mean difference (WMD) between the baseline and final measurements of HbA1c level and weight according to the intervention was considered as the effect size, and the inverse variance method was used because the values were continuous variables. The random-effects model was chosen because of the diversity and heterogeneity of the intervention.
The heterogeneity of the results of the individual studies included in the meta-analysis was evaluated using the I2 test. Sensitivity analysis was performed when the number of individual studies was more than 2, and more than 60% of the individual studies were considered heterogeneous.
The sub-analysis was performed to compare the effect size differences in HbA1c level and weight between the groups in the included literature. The subtotal only method was used when a single literature provided data on multiple sub-groups. The results of the meta-analysis are presented in the table on the general characteristics for the inclusion documents, the forest plot showing the number of participants in the intervention and control groups, and the estimated value of the effect.
RESULTS
Search results and study characteristics
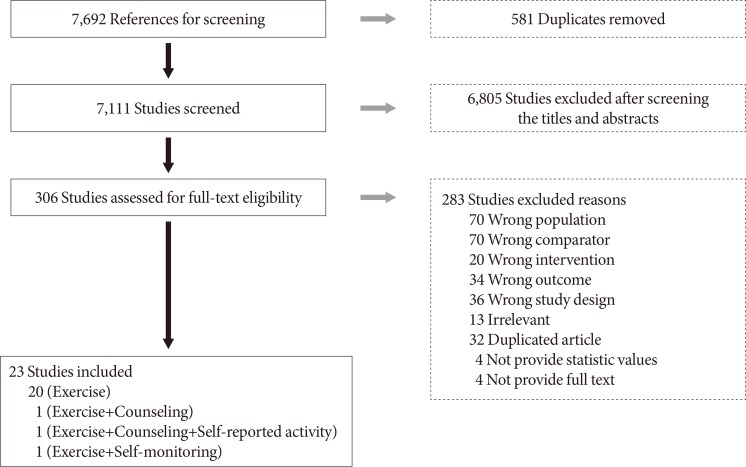
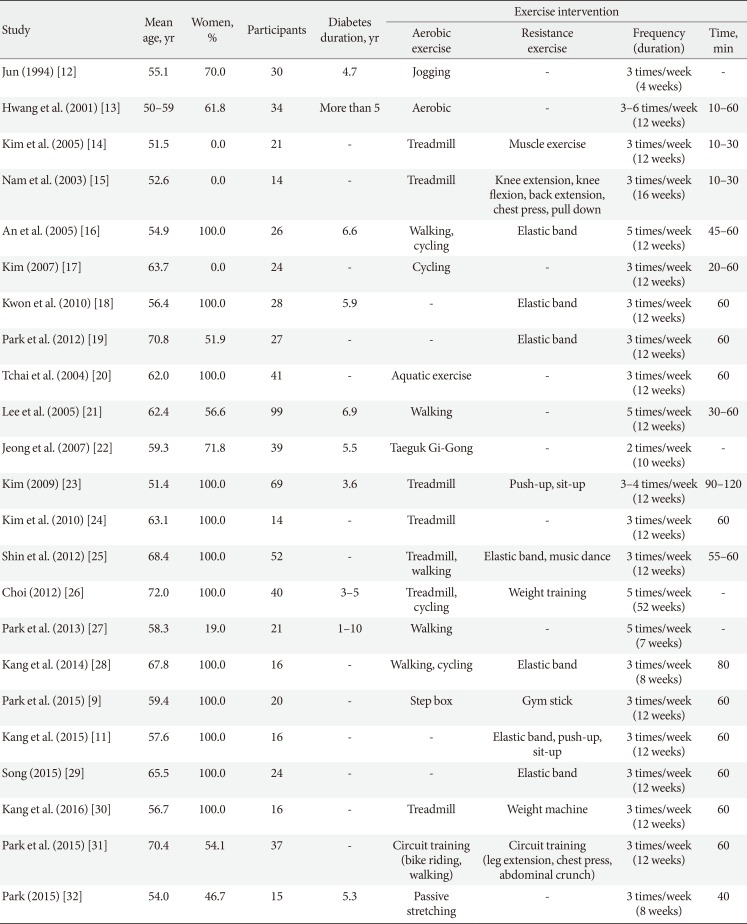
A total of 7,692 studies were selected according to our literature search method. After eliminating duplicate documents, the titles and abstracts of 7,111 studies were reviewed, and 6,805 papers that had no relevance with the original review according to the PICO were excluded. We selected 306 studies by reviewing the titles and abstracts, of which 23 were finally included in this study (Fig. 1). The total number of participants in the 23 studies [911121314151617181920212223242526272829303132] was 723 (432 in the intervention group and 291 in the control group; 190 [26.3%] men and 533 [73.7%] women), with a mean age of 60.4 years. The general characteristics of the included studies are summarized in Table 1.
Quality of the included studies
Eight items were assessed using the RoB tool, which is a randomized controlled study or controlled clinical trials assessment tool of the Cochrane Collaboration that is used to assess the quality of selected literature. The results of the quality evaluation showed that random sequence generation and allocation concealment are at high RoB except one article. Blinding of participants and personnel and blinding of outcome assessment were all uncertain because they did not mention related contents in 23 documents. That is, in all four of the eight items, the RoB was evaluated as high or uncertain in 23 domestic documents, and the overall quality of the literature was low.
In the incomplete outcome data category, 22 studies (95.7%) were rated as low RoB. In one document, the RoB was evaluated as high because the reason for drop off in one participant was not described after the intervention. Twenty-one articles (91.3%) had less than 20 interventions and control participants, indicating that the studies were small-scale intervention studies.
Potential bias was high in 13 studies (56.5%). Only selective reporting was evaluated as low in 23 studies. All studies reported the results according to the study objects. The basis of the quality evaluation for each study is shown in Supplementary Figs. 1 and 2.
Effectiveness of exercise on body weight
A total of eight articles have reported the effects of exercise intervention on weight. The seven studies have performed exercise intervention only, and one of the studies performed exercise intervention, counseling, and education. All the details on the changes in body weight were presented in kilograms (kg). No difference was observed between the two groups in terms of body weight at baseline (WMD, −1.35 kg; 95% confidence interval [CI], −3.62 to 0.92; P=0.24). Exercise intervention decreased the weight of the participants by 1.90 kg (95% CI, −4.15 to 0.35; I2=0%). However, the result was not statistically significant (P=0.10) (Fig. 2).
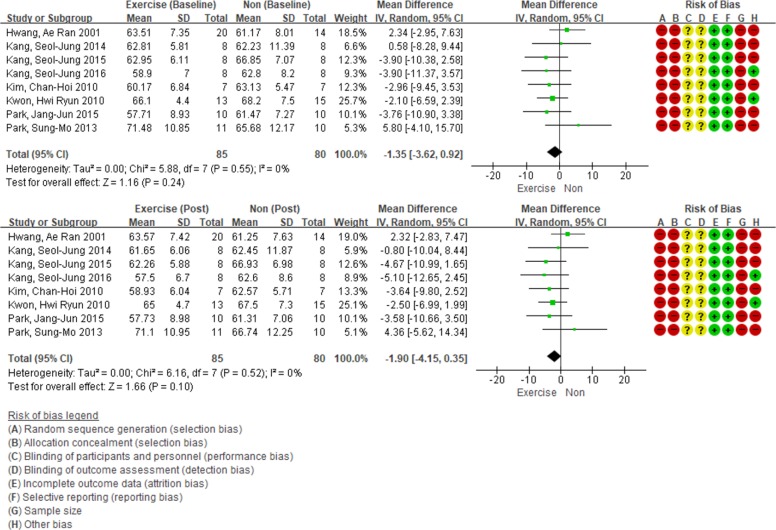
Effect of exercise intervention versus non-intervention on body weight (changes from baseline) in adults with type 2 diabetes mellitus. The squares indicate the study-specific outcome estimates, and the size of the squares corresponds to the study's weight in the meta-analysis. Horizontal lines denote the range of the 95% confidence interval (CI). The diamonds indicate pooled estimates. Weights are from random-effects analysis. SD, standard deviation; IV, inverse variance.
Effectiveness of exercise on glycemic control
A total of 17 studies have reported the effectiveness of exercise intervention in reducing HbA1c levels. Only 14 studies have performed exercise intervention; one study has conducted exercise intervention with counseling and education; another study has conducted exercise intervention with counseling and self-reports; and the remaining study conducted exercise intervention with self-blood glucose monitoring.
Although the baseline HbA1c level was statistically higher in the exercise intervention group (WMD, 0.21%; 95% CI, 0.07 to 0.35; P<0.01), the group's HbA1c level after intervention decreased to 0.58% (95% CI, −0.89 to −0.27; P<0.01) (Fig. 3). In addition, due to high heterogeneity (I2=73), sensitivity analysis was performed. Except in two articles [1415] that have shown asymmetric features in the funnel plot, the remaining 15 studies were analyzed. As a result, the heterogeneity was reduced to 35%. HbA1c levels declined by 0.35% (95% CI, −0.56 to −0.14), and the result was statistically significant (P<0.01).
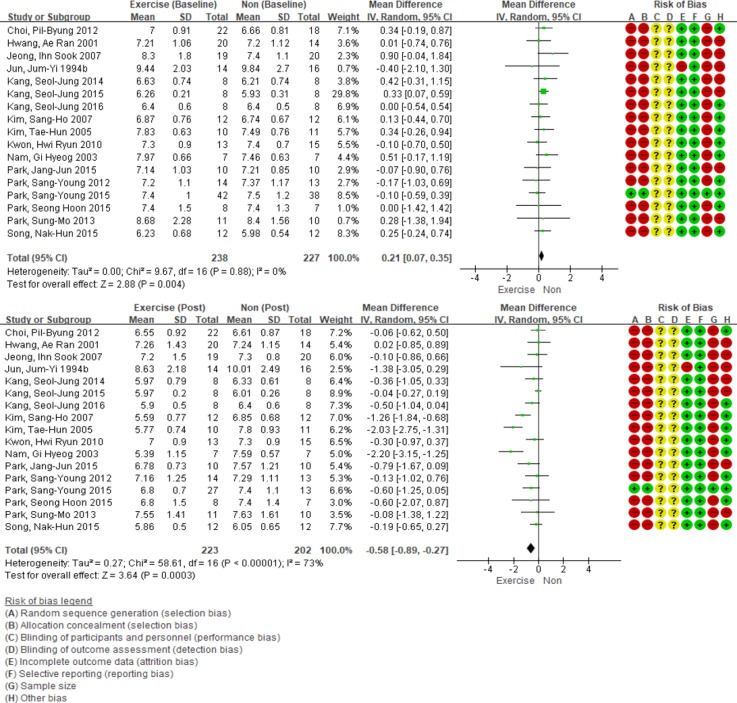
Effect of exercise intervention versus non-intervention on glycosylated hemoglobin level (changes from baseline) in adults with type 2 diabetes mellitus. SD, standard deviation; IV, inverse variance; CI, confidence interval.
We divided groups according to baseline HbA1c values before intervention and then performed subgroup analysis. In the group with a high baseline HbA1c level (≥7.5%), the exercise group showed the greatest reduction of HbA1c (WMD, −1.06%; 95% CI, −1.50 to −0.62; n=9; I2=78%). Similar results were obtained after sensitivity analysis for high heterogeneity (WMD, −0.68%; 95% CI, −1.09 to −0.28; n=6; I2=56%). Additionally, there was less, but significant, glucose lowering effect in groups with baseline HbA1c <7.5% and ≥7.0% (WMD, −0.44%; 95% CI, −0.61 to −0.27; n=11; I2=0%) and in groups below 7.0% (WMD, −0.30%; 95% CI, −0.59 to −0.01; n=7; I2=65%), with similar results after a sensitivity analysis (WMD, −0.11%; 95% CI, −0.27 to 0.05; n=6; I2=0%) (Supplementary Fig. 3).
Subgroup analysis according to the type of exercise intervention
A sub-analysis was conducted to determine whether the effects of exercise intervention differed according to exercise type. There were 27 articles that reported about the HbA1c levels according to the type of exercise, and 15 articles have reported weight changes. We synthesized the data of 15 articles reporting the weight results, and only aerobic exercise significantly decreased body weight (WMD, −2.25 kg; 95% CI, −4.36 to −0.13; n=8; I2=17%). Combined aerobic and resistance training (RT) reduced body weight by 3.38 kg (95% CI, −7.00 to 0.25; n=4) and RT alone reduced body weight by 2.80 kg (95% CI, −5.70 to 0.10; n=3), which were not statistically significant (Fig. 4).
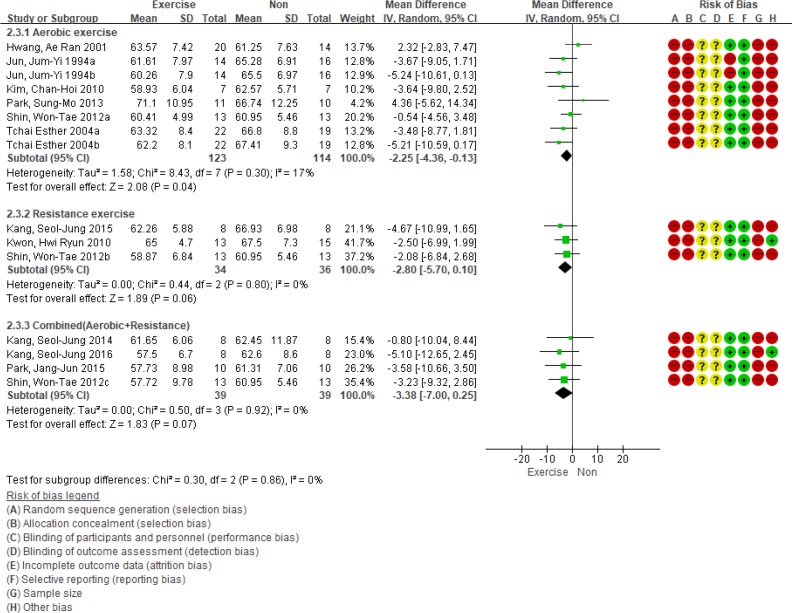
Subgroup analysis based on the type of exercise for body weight. SD, standard deviation; IV, inverse variance; CI, confidence interval.
The changes in HbA1c levels according to each exercise decreased in the order of aerobic exercise, aerobic and resistance exercise, and resistance exercise. There were significant differences between the groups (P<0.001). Compared with the non-intervention group, the aerobic exercise and combined exercise groups experienced 0.77% (95% CI, −1.07 to −0.46; n=11; I2=59%) and 0.67% (95% CI, −1.04 to −0.30; n=11; I2=77%) decreases in HbA1c levels, respectively, and the results were statistically significant. The resistance exercise decreased the HbA1c level by 0.15% (95% CI, −0.31 to 0.01; n=5; I2=0%). However, the result was not statistically significant (P=0.06) (Fig. 5). Sensitivity analysis was performed due to large heterogeneity in the combined exercise group. As a result, the effect size of HbA1c levels according to aerobic and resistance exercises decreased by 0.40% (95% CI, −0.62 to −0.18; n=9; I2=30%), although the result remained statistically significant (P<0.01).
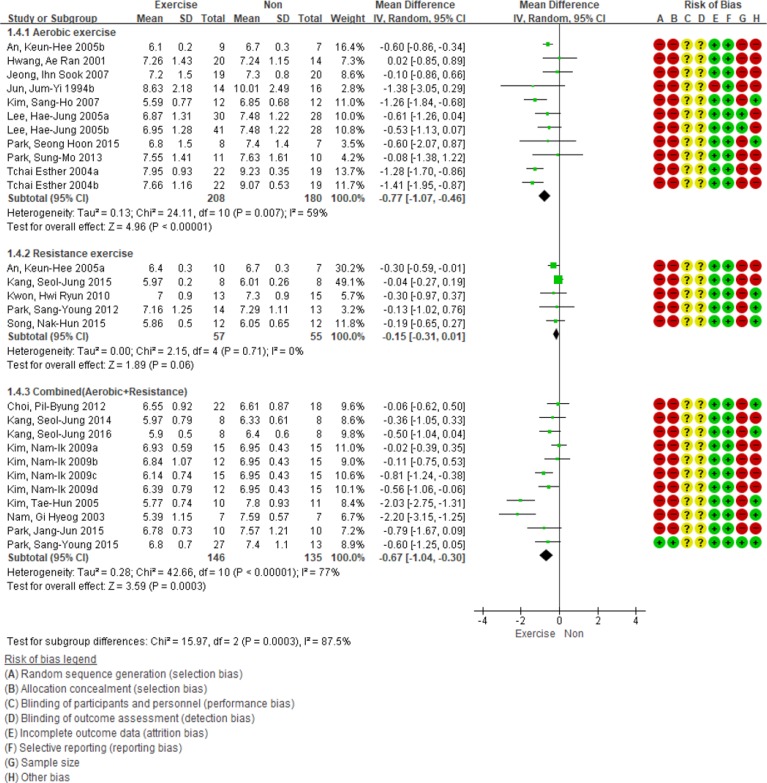
Subgroup analysis based on the type of exercise for glycosylated hemoglobin level. SD, standard deviation; IV, inverse variance; CI, confidence interval.
The frequency and duration of exercise were also different in each study, and we conducted a subgroup analysis for these. The frequency of exercise did not show a significant difference in HbA1c changes when divided into groups based on three times a week. Due to the limited number of references, it was difficult to determine the effect of exercise frequency on body weight. The difference in exercise duration did not show a significant effect on HbA1c and weight change (Supplementary Fig. 4).
DISCUSSION
Considering the discrepancy of exercise effectiveness on weight loss and glycemic control and the lack of evidence on the meta-analysis of the study participants with T2DM in Korea, we conducted a systematic search and selected 23 studies. Based on this meta-analysis, exercise was found to significantly improve glycemic control. However, it did not significantly reduce body weight. Notably, aerobic training can be a beneficial strategy in patients with non-insulin-treated T2DM in Korea.
With respect to the effectiveness of exercise in improving glucose homeostasis, the HbA1c level in the exercise intervention group was significantly lower (WMD, −0.58%; 95% CI, −0.89 to −0.27; n=17; I2=73%) than in the non-intervention group. Because of the high heterogeneity of the 17 articles, we additionally performed sensitivity analysis. After performing sensitivity analysis for high heterogeneity (I2=73%), the effect size declined by 0.35% (95% CI, −0.56 to −0.14; n=15; I2=35%; P<0.01), which was still statistically significant. Compared with those of four systematic review articles on the effectiveness of exercise in reducing HbA1c levels (0.37%), the results of our study were similar to those of a previous study. In the study by Boule et al. [33], the HbA1c level was 0.66% (95% CI, −0.98 to −0.34; n=14) lower in the exercise intervention group than in the non-exercise group. In addition, the HbA1c levels of the exercise intervention group were 0.25% (95% CI, −0.47 to −0.03; n=9), 0.34% (95% CI, −0.53 to −0.16; n=23), 0.25% (95% CI, −0.49 to −0.02; n=13) lower in the studies by Irvine and Taylor [34], Ishiguro et al. [35], and Zou et al. [36], respectively.
With regard to the beneficial role of exercise in improving glucose homeostasis, we performed a subgroup analysis on aerobic and resistance exercises. Results showed the dominant glycemic control effect of aerobic exercise. Furthermore, a similar glucose-lowering effect was observed when aerobic and resistant exercises were combined. However, there was no statistically significant difference in the blood glucose level when only resistant exercise was performed. The previous meta-analysis on the effectiveness of exercise on blood glucose level showed a consistent lowering effect regardless of the type and combination of exercise [1037]. However, these studies differed from the present study in that they only compared the before and after exercise, and they did not include a control group, different ratios of dietary intervention according to exercise type, and patients of various races.
With respect to weight reduction, there was no significant difference between the two groups. One possible reason is that exercise in patients with low physical activity may lead to an increase in lean body mass, particularly during resistant exercise. In addition, this study has analyzed previous studies that involved the presence or absence of exercise without diet control. Thus, it is possible that food intake increased to compensate for exercise. It is also important to consider the extent to which normal physical activity may be reduced in patients in the exercise group [33]. Aguiar et al. [38] have reported that the weight of the exercise group decreased by 3.79 kg (95% CI, −6.13 to −1.46; n=4) compared to that of the control group. However, this study population also performed diet intervention along with exercise. In another study, without diet intervention, the effect of exercise intervention on weight loss was not significant [10333940].
With regard to the beneficial mode of exercise on weight reduction, a 2.25 kg decrease in body weight was observed (95% CI, −4.36 to −0.13; n=8; I2=17%) when aerobic exercise is performed. However, the effectiveness of resistant exercise was limited. These differences may have been influenced by the exercise intensity. The energy consumption of resistant exercise is affected by exercise-related loads, such as set and repeat, break interval, and speed of movement [41], as well as the size of the muscles involved during exercise [42]. Therefore, resistance exercise, such as the use of elastic band, which was reviewed in this study, would not have been performed at high intensity to induce a significant increase in energy consumption [43]. In addition, although statistical significance was not shown, the papers included in the analysis noted a consistent weight loss pattern in the resistance exercise group. If a larger sample size was included, a significant effect on weight loss in resistance exercise might be expected. In relation to this, there is limited evidence suggesting that resistant exercise is not effective in reducing blood sugar level and weight.
In conclusion, exercise intervention among Koreans with diabetes had a positive role in reducing HbA1c levels and body weight. Therefore, exercise has a stronger cardioprotective effect, and it also has effects on glycemic control, body weight loss, as well as other factors which are beneficial [4445]. These results were consistent with those of a previous systematic review of literature, which proved that this evidence is applicable to patients with DM in Korea.
However, this study had some limitations. First, due to the significant difference in the HbA1c levels at baseline, there might be a bias in the interpretation. The differences in baseline values between the exercise and control groups were examined before the merging of the 17 articles. In addition, the baseline value of the exercise intervention group was 0.21% (95% CI, 0.07 to 0.35) higher than that of the control group. The HbA1c level after exercise may be underestimated because the experimental and control groups already had differences before the intervention. Second, there is a possibility of publication bias because the research literature is limited to the articles published in journals in Korea, and the intervention studies reported in graduation thesis and research reports were excluded. In general, studies with positive results are more likely to be published, which can overestimate the actual effect of exercise. In relation to this, we cannot exclude the possibility that the actual effect of exercise on HbA1c levels and body weight is lower than the results of the present study. Third, the results of this study cannot be generalized to all patients with T2DM. This study is a systematic review of literature that focused on specific clinical questions. Thus, it has limitations in applying the results to all T2DM patients. In addition, there is a possibility that the effect of exercise intervention may be overestimated or underestimated because the proportion of women was higher in this paper and there may be a difference in compliance with exercise according to gender [46]. Due to high heterogeneity, such as type, frequency and duration of exercise, and limited number of documents, the consensus and discussion of experts will be needed to clarify the effect of exercise in reducing body weight and HbA1c.
This study has key strengths. It is a systematic review of literature on patients with diabetes in Korea, which has not been performed previously. Furthermore, it clearly shows the positive effect of exercise intervention. This study used international systematic review methodology and made full use of its strengths, and it was conducted to analyze only refined studies with a control group, excluding those with dietary intervention, in a common culture and racial patients. Thus, we could clearly observe the effects of exercise-only therapy on blood glucose levels and body weight in Asian races. The results of this study can be used to prove the importance of non-medication interventions for patients with T2DM. Moreover, this study showed the need for large-scale clinical studies and analyzed the intervention studies for national diabetes management. In particular, a systematic review of literature can be applied irrespective of the area, and it can be useful to the clinical guideline developers, decision makers, and policy makers as a reliable basis for decision-making.
Through the systematic search of Korean studies, the effect size of exercise was identified by using the same criteria and synthesized using standardized statistical techniques. As a result, a significant decrease in HbA1c level and body weight according to the exercise intervention was observed. This result was consistent with that of previous studies, which shows the usefulness and validity of this study.
ACKNOWLEDGMENTS
None
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS:
Conception or design: J.E.J., B.W.L.
Acquisition, analysis, or interpretation of data: J.E.J., Y.C., B.W.L., E.S.S.
Drafting the work or revising: J.E.J., Y.C., S.H.L.
Final approval of the manuscript: J.E.J., Y.C., B.W.L.
References
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2018.0062.
Supplementary Fig. 1
Quality assessment of the included studies. Risk of bias graph.
Supplementary Fig. 2
Quality assessment of the included studies. Risk of bias summary.
Supplementary Fig. 3
Subgroup analysis based on baseline glycosylated hemoglobin level. SD, standard deviation; IV, inverse variance; CI, confidence interval.
Supplementary Fig. 4
(A–D). Subgroup analysis based on the frequency and duration of exercise. SD, standard deviation; IV, inverse variance; CI, confidence interval.