Update on the Impact, Diagnosis and Management of Cardiovascular Autonomic Neuropathy in Diabetes: What Is Defined, What Is New, and What Is Unmet
Article information
Abstract
The burden of diabetic cardiovascular autonomic neuropathy (CAN) is expected to increase due to the diabetes epidemic and its early and widespread appearance. CAN has a definite prognostic role for mortality and cardiovascular morbidity. Putative mechanisms for this are tachycardia, QT interval prolongation, orthostatic hypotension, reverse dipping, and impaired heart rate variability, while emerging mechanisms like inflammation support the pervasiveness of autonomic dysfunction. Efforts to overcome CAN under-diagnosis are on the table: by promoting screening for symptoms and signs; by simplifying cardiovascular reflex tests; and by selecting the candidates for screening. CAN assessment allows for treatment of its manifestations, cardiovascular risk stratification, and tailoring therapeutic targets. Risk factors for CAN are mainly glycaemic control in type 1 diabetes mellitus (T1DM) and, in addition, hypertension, dyslipidaemia, and obesity in type 2 diabetes mellitus (T2DM), while preliminary data regard glycaemic variability, vitamin B12 and D changes, oxidative stress, inflammation, and genetic biomarkers. Glycaemic control prevents CAN in T1DM, whereas multifactorial intervention might be effective in T2DM. Lifestyle intervention improves autonomic function mostly in pre-diabetes. While there is no conclusive evidence for a disease-modifying therapy, treatment of CAN manifestations is available. The modulation of autonomic function by SGLT2i represents a promising research field with possible clinical relevance.
INTRODUCTION
This review will consider the dimensions and the clinical relevance of diabetic cardiovascular autonomic neuropathy (CAN), by providing the available information attesting to the fact that CAN is a common complication of diabetes, starts early, is associated with a number of old and new clinical correlates, and has an heavy impact on morbidity and prognosis. It will also consider how to detect CAN in clinical practice, candidates for screening and diagnosis, and the usefulness of diagnosis from a clinical perspective, and finally how to prevent it and manage its clinical consequences. The goal is to say what is defined and stated by scientific societies, what is new, and what is as yet unmet.
Some statements in this review are based on the conclusions of the Toronto consensus panel and on the general and specific reports on CAN [123]. Diabetic autonomic neuropathy was defined in the Toronto Consensus as “a disorder of the autonomic nervous system in the setting of diabetes or metabolic derangements of pre-diabetes after the exclusion of other causes.” While CAN was defined as “the impairment of autonomic control of the cardiovascular system.” Thus, one of the merits of the Toronto Consensus is the emphasis on the possible presence of an autonomic dysfunction already in pre-diabetes.
AUTONOMIC DYSFUNCTION IN PRE-DIABETES
As for diabetic polyneuropathy, a number of studies have considered the association between autonomic dysfunction and pre-diabetes (Table 1) [4567891011121314151617]. There has been a large heterogeneity among the studies with regard to categories of glucose abnormalities considered, sample size, and the modalities of CAN testing. Only four out of 14 studies have considered both impaired fasting glucose (IFG) and impaired glucose tolerance (IGT), one study included the category of combined IFG plus IGT [15], six studies were of a small sample (under 200 participants), while seven studies were population-based. CAN testing has varied largely from standard cardiovascular reflex tests (CARTs) to heart rate variability (HRV) measures, and standardized procedures and age-related reference values have not always been used [1314]. In most studies the percentage of abnormal tests has not been provided. Three studies did not find differences in subjects with pre-diabetes compared to those with normal glucose tolerance (NGT), whereas nine studies provided limited evidence of diminished HRV indices. In studies including both IFG and IGT, there was a trend toward worse or more widely impaired autonomic indices in IGT versus IFG [1117] or in combined IGT plus IFG versus isolated IFG and IGT [15].
Suggested meaning of association between pre-diabetes and CAN
The reduced HRV observed in IGT and/or IFG might indicate lower parasympathetic activity since time-domain measures of HRV and most frequency-domain indices of HRV are an expression of vagal activity [3]. Moreover, given the findings of reduced high-frequency (HF) power with a higher ratio between low-frequency (LF) and HF (LF:HF) in subjects with IGT (Table 1) [11], and of increased LF in normalised units (in particular during the night) in subjects with IFG and IGT compared to control subjects [9], some authors have suggested the presence of a sympathovagal imbalance with an abnormal sympathetic predominance in pre-diabetes [911].
The independent correlates of autonomic indices in pre-diabetes are age, body mass index (BMI), waist circumference, other components of metabolic syndrome, hypertension and antihypertensive drugs, fasting and 2-hour postload glucose level (Table 1). The role of postprandial hyperglycaemia has also been put forward as a pathogenetic factor or a risk marker for autonomic dysfunction [17].
In pre-diabetes and metabolic syndrome many factors can contribute to the presence of an autonomic dysfunction, which might appear as both parasympathetic depression and sympathetic overactivity or predominance. Sympathetic overactivity is documented in insulin resistant conditions, and attributed to an insulin-driven sympathetic activation through peripheral and central mechanisms as well as chemoreflex upregulation (Fig. 1) [1819]. Obstructive sleep apnoea syndrome (OSAS) is often associated with obesity and type 2 diabetes mellitus (T2DM) and induce chemoreflex upregulation, therefore further promoting sympathetic hyperactivity (Fig. 1) [19].
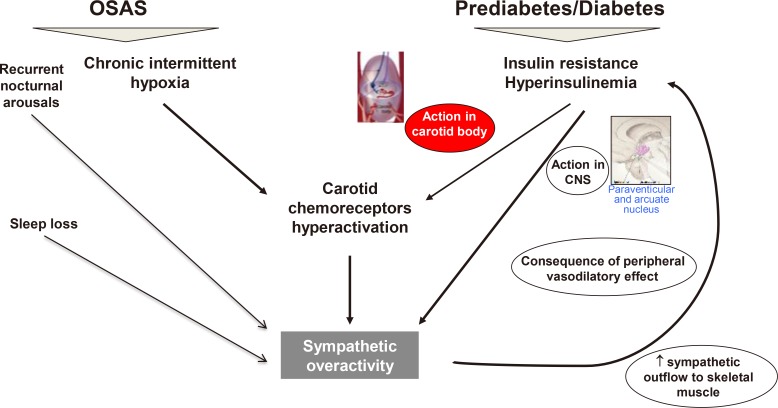
Mechanisms of sympathetic overactivity in insulin resistant conditions and obstructive sleep apnoea syndrome (OSAS). Sympathetic overactivity in insulin resistant conditions is attributed to an insulin-driven sympathetic activation through a peripheral mechanism at play in acute conditions (insulin causes endothelial-dependent vasodilatation resulting in baroreflex-mediated sympathetic activation), and a central mechanism mainly present in chronic conditions of hyperinsulinemia (insulin operates in the paraventricular nucleus of hypothalamus and the arcuate nucleus). Moreover, insulin-induced carotid body overactivity has been demonstrated in animal models of insulin resistance (insulin receptors have been found on carotid bodies) [18]. A role of carotid chemoreceptors in a long-term insulin-mediated increase in sympathetic activity in humans has been also suggested [19]. Comorbid OSAS leads to chemoreflex upregulation due to nocturnal chronic intermittent hypoxia and arousals, therefore fostering sympathetic activation. Modified from Greco et al. [19] with permission from Bentham Science Publishers. CNS, central nervous system.
In addition to OSAS [19], other factors in metabolic syndrome might be able to cause autonomic dysfunction, also with a bidirectional relationship [20212223], and then to exacerbate metabolic derangements at different levels (Fig. 2) [24].

Multiple factors in the relationship between metabolic syndrome and autonomic dysfunction. In addition to obstructive sleep apnoea syndrome (OSAS) with its consequences including microbiota perturbation [19], other factors in metabolic syndrome able to cause autonomic dysfunction are: obesity (also independently of dysglycemia) [24], liver steatosis [20], leptin (as a sympathetic activator) [19], and inflammation and neuroinflammation at hypothalamic level [2122]. Most of these components of metabolic syndrome have a bidirectional relationship with autonomic dysfunction, for example with respect to the autonomic regulation of the immune system and inflammation [23]. The end result of this complex system can be the exacerbation of metabolic derangements at different levels [1924], as well as of cardiovascular effects. IFG-IGT, impaired fasting glucose and/or impaired glucose tolerance; NAFLD, non-alcoholic fatty liver disease.
The finding observed in the 1,882 participants of Offspring Cohort in the Framingham Heart Study is of interest, where both higher resting heart rate and lower HRV predicted the risk of high blood glucose, blood pressure (BP), triglycerides, and BMI, and low high-density lipoprotein (HDL) over 12 years, and the risk of incident diabetes, cardiovascular disease, and mortality over 20 years [25]. Thus, in a very early stage of dysglycemia, a reciprocal role of autonomic dysfunction and glucose metabolism abnormalities seems to occur with modalities that remain poorly exploited and deserve to be further elucidated.
EPIDEMIOLOGY OF AUTONOMIC NEUROPATHY/DYSFUNCTION IN DIABETES
CAN affects at least 20% of unselected patients, and up to 65% of those with increasing age and diabetes duration [2]. Data on the prevalence of autonomic neuropathy in pre-diabetes are very scarce; according to an epidemiological study with a reliable diagnostic approach (one of the issues with these studies is the use of non-established criteria) the prevalence in subjects with combined IFG and IGT was 11.4% [15]. This prevalence is not far from that of CAN at diagnosis in T2DM, which according to CARTs is reported to be around 7% and increases with diabetes duration by 4.6% to 6% per year [226].
In the Denmark branch of the Anglo-Danish-Dutch Study of Intensive Treatment in People With Screen-Detected Diabetes in Primary Care (ADDITION) study, 299 patients were evaluated for CAN at 6 and 13 years [27]. The prevalence of confirmed CAN (based on at least two abnormal CARTs) increased from 9.0% to 15.1% with an annual incidence of 1.8%, which is lower than previously documented [2]. However, the early screening-based diagnosis of T2DM, high intensity treatment from diagnosis, and overall good control of glycaemia and risk factors during the 13 years of follow-up might have mitigated CAN progression [27]. The specific context of this study might be representative of the present and future scenario of early and improved control in T2DM. On the other hand, Diabetes Control and Complications Trial (DCCT) and Epidemiology of Diabetes Interventions and Complications (EDIC) studies have shown that current strategies for optimizing glucose control in type 1 diabetes mellitus (T1DM) are insufficient to fully prevent or delay the development of CAN, as 29% of subjects in the former intensive group and 35% of subjects in the former control group had CAN by 14 years of EDIC follow-up [28].
Clinical correlates and predictors of CAN: old and new
In addition to age and diabetes duration, other established diabetes related clinical correlates or predictors of CAN are glycaemic control, the presence of retinopathy, nephropathy, and diabetic polyneuropathy [2], in particular in T1DM (in cross sectional studies) [2930]. The role of several cardiovascular risk factors has also been increasingly reported: BP or hypertension, smoking [29], low-density lipoprotein, HDL, triglycerides, weight, BMI, or obesity in T2DM [29], waist circumference, high insulin levels in T2DM, cardiovascular disease, and use of anti-hypertensive drugs [2]. Longitudinal studies in T2DM [2] have confirmed the independent predictive role of age, retinopathy, nephropathy, glycosylated hemoglobin (HbA1c) [27], BMI, and triglycerides [27].
New correlates of CAN
More recently, cross-sectional studies have documented the association of CAN with low vitamin B12 levels in T2DM [31], both low [323334] and high vitamin D levels in T1DM and T2DM [34], and with low bilirubin levels in T2DM, as observed in a large population from Korea [35].
Oxidative stress and inflammation biomarkers
New biomarkers related to putative pathogenetic mechanisms of diabetic neuropathy—i.e., oxidative stress and inflammation—have emerged very recently as predictors of a deterioration of CAN measures. In a longitudinal study from Germany, in 72 patients with diabetes, baseline superoxide anion predicted the decline at 6-year follow-up of autonomic indices of HRV (i.e., coefficient of variation at rest), as well as of sensory nerve function and even mortality [36]. In a cross-sectional study, the same authors showed that in patients with T2DM of a short duration, higher levels of serum interleukin (IL)-18, soluble intercellular adhesion molecule-1, and soluble E-selectin were independently associated with more impaired CARTs or lower HRV indices [21]. Moreover, in a large longitudinal study from the United Kingdom (UK; the Whitehall II cohort study), baseline inflammatory markers were independent predictors at 5-year follow-up of an increase in rest heart rate, like IL-1 receptor agonist, or of preservation of time- and frequency-domain indices of HRV, like adiponectin, but only in the T2DM subgroup of this cohort [22].
Ethnic differences
South Asians with diabetes have a higher prevalence of nephropathy and diabetic retinopathy than Caucasians, but a lower prevalence of diabetic polyneuropathy. Differently from diabetic polyneuropathy, the prevalence of CAN in South Asians is the same as in Caucasians accordingly to a recent review, where in small studies in South Asia from five secondary centres the prevalence was between 20% and 60%, and from three tertiary centres between 44% and 81% [37]. Considering South Asian people living in the UK compared to white Caucasians, one study [38] documented better results in the deep breathing test in South Asians, and another found a similar CAN prevalence as with white Caucasians [39].
Genetic susceptibility
Finally, the role of genetic predisposition is now gaining some attention, given the finding of associations between CAN and polymorphisms of genes encoding a few microRNAs, i.e., MIR146a, MIR27a, and MIR499 [4041]. MicroRNAs are regulators of gene expression at a posttranscriptional level, which are involved in many pathways and reported as dysregulated in diabetes and its complications. In an Italian cohort of patients with T2DM, the C allele of rs2910164 single nucleotide polymorphism (SNP) in MIR146A was associated with a lower risk of developing CAN, whereas the variant allele of rs895819 SNP in MIR27A was associated with a higher risk of developing early CAN [40]. Moreover, when analysing the rs3746444 SNP in the MIR499A gene, MIR499A GG genotype was found to contribute to early CAN together with disease duration and HbA1c, and GG genotype and disease duration were the main variables contributing to the CAN severity [41]. It is interesting to observe that microRNA-499 is preferentially expressed in the heart and areas of the central autonomic network (nucleus ambiguous), and involved in both cardiovascular disease and metabolic syndrome/diabetes, as well as MIR499 polymorphisms are in susceptibility to cardiovascular disease [41].
Glucose variability
Recently, glucose variability has been implicated in CAN in diabetes (and also in pre-diabetes). In T1DM, two studies in the DCCT failed to show any impact of glucose variability on the 5-year development of CAN, independently of HbA1c and mean blood glucose [4243], whereas another study showed an association between the severity of glucose variability on continuous glucose monitoring (CGM) and CAN [44]. In five out of seven studies on T2DM, CAN was independently associated with glucose variability (mainly measured using CGM) [45464748495051]. Among speculative mechanisms, oxidative stress promoted by acute glycaemic excursions [46] might mediate this association, through the increased formation of advanced glycation end-products and the activation of the nuclear factor κ-light-chain-enhancer of activated B cells and protein kinase C pathways, ultimately leading to increased vascular endothelial damage. The association between CAN and glycaemic variability, however, seems to be more supported in T2DM than in T1DM as with retinopathy and nephropathy; limited evidence exists to determine whether glucose variability is a risk factor of CAN independent of chronic hyperglycaemia, or a marker of disease severity, pre-existing complications, including autonomic dysfunction, and poor adherence to therapeutic strategy. In any case, the issue deserves to be the subject of further investigation.
Conclusion on epidemiology and clinical correlates of CAN
In the context of the diabetes epidemic, CAN is a common complication of diabetes, starts early in T2DM, and has multiple and new correlates. The patients at greater risk of CAN are those with a higher age, longer duration, poor and perhaps unstable glycaemic control, comorbid diabetic polyneuropathy, retinopathy and nephropathy, hypertension (on treatment), and other cardiovascular risk factors (in particular obesity and metabolic dyslipidaemia in T2DM). The role of additional emerging clinical correlates requires further research while the need to know CAN natural history in T2DM still remains unaddressed. In the ADDITION-Denmark study, a small number of subjects with confirmed CAN at 6 years from screening-based diagnosis of T2DM, changed their status to the absence of CAN at 13-year follow-up [27]. Whether this finding is a true challenge to the idea of irreversibility of confirmed CAN, or is somehow attributable to a limited ability of CARTs to define CAN stages in that specific population, requires further evaluation. This study might also suggest that an early diagnosis and intervention in T2DM might have a beneficial effect on CAN progression.
PROGNOSTIC VALUE OF CAN
Apart from its symptomatic forms, the clinical impact of CAN relies on the strong evidence for its prognostic meaning. Diagnosis of confirmed CAN is associated with a relative risk of mortality of 3.65 in a meta-analysis of 15 longitudinal studies published until 2001 [52]. Subsequent studies have confirmed the predictive value of cardiac autonomic measures on all-cause and cardiovascular mortality with a relative risk between 1.5 to 7, with this risk being independent of multiple-diabetes correlates and cardiovascular risk factors [2]. These more recent studies have documented that in both T1DM, as in the European Diabetes (EURODIAB) Prospective Complications Study [53], and in T2DM, as in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study [54], the longitudinal studies with the highest sample size, standard CARTs or other measures of CAN were independent predictors of mortality for any and cardiovascular causes. Some of these studies [2] have indicated that combined indices are better predictors than individual ones.
Prediction of vascular morbidity
CAN has been also associated with vascular morbidities, first of all with silent myocardial ischemia—as documented in a meta-analysis [52] and confirmed by the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study [55]—with cardiovascular morbidity, with recurrent cardiovascular disease in patients with T2DM and a history of cardiovascular disease with an adjusted hazard ratio of 3 [56], and with stroke in T2DM [2]. A recent study described the predictive role of CAN present at DCCT closeout on the subsequent cardiovascular risk during EDIC study [57]. More subjects with CAN experienced cardiovascular events (fatal and non-fatal) compared to those without CAN (25% vs. 10%); however, the association between CAN and cardiovascular events was mitigated after adjusting for glucose control as a time-dependent covariate [57]. This may suggest that in this intervention study, where historic glycaemic exposure and metabolic memory were the principal determinants of long-term CAN, the interaction between glycaemic control and CAN were so close that the emergence of an independent prognostic role of CAN on cardiovascular morbidity might be hampered.
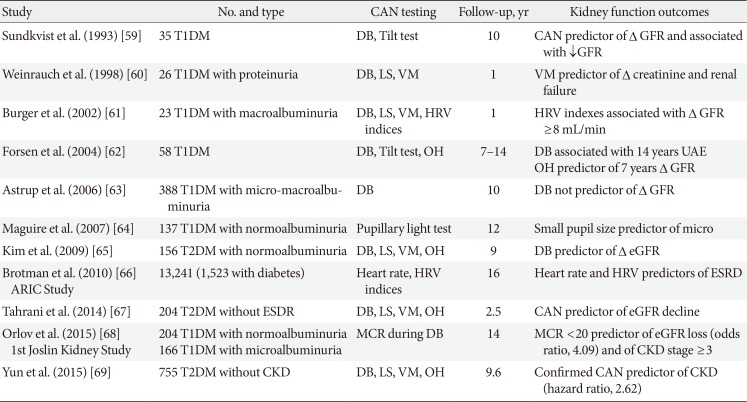
CAN has been also associated with perioperative instability during surgery, with a risk of severe hypoglycaemia [58], and has even been suggested as a progression promoter of diabetic nephropathy [2]. Table 2 shows 11 studies, seven on T1DM, which evaluated the role of autonomic neuropathy in the progression of albuminuria and/or decline in kidney function [5960616263646566676869]. Apart from one study [63], they have documented an independent association between CAN or autonomic function tests and nephropathy progression in both T1DM [68] and T2DM [6769]. A few mechanisms have been suggested as supporting the role of CAN in the progression of nephropathy [2] as kidney hemodynamic changes consequent to sympathetic denervation [59], CAN-associated erythropoietin deficiency anaemia [70], and CAN-driven altered circadian pattern of BP and albuminuria [2].
Conclusion on the prognostic impact of CAN in diabetes
There is definite evidence of the prognostic relevance of CAN for mortality. Some evidence supports CAN as a risk marker and as a likely risk factor for cardiovascular morbidity as well as a progression promoter of diabetic nephropathy. From a clinical perspective, a diabetic patient with CAN is at a higher risk of mortality, and of cardiovascular and renal complications.
Insights into the mechanisms of excess mortality associated to CAN
The mechanisms of this CAN-associated excess mortality and morbidity remain mostly unknown. A number of cardiovascular abnormalities have been found in association with CAN. They can represent (1) a form of morbidity in themselves, such as silent myocardial ischemia; (2) a recognized risk factor or marker for mortality or morbidity (resting tachycardia, postural hypotension, QT interval [QTi] prolongation, impairment of baroreflex sensitivity [BRS], nondipping, reduced HRV); (3) a potential pathogenetic link between CAN and mortality, such as an imbalance in sympathovagal activity, cardiac sympathetic dysinnervation, reduction in the sympathetically mediated vasodilatation of coronary vessels, dysregulation of cerebral circulation, new mechanisms like increased arterial stiffness and coronary calcium content, or inflammation [223]. Finally, peripheral vascular function abnormalities may exert a contributory role to diabetic foot complications. Some of these abnormalities are established risk markers for mortality and morbidity in diabetes [2].
Tachycardia
High resting heart rate is the least specific sign of CAN and can also reflect vagal impairment and/or sympathetic overactivity present in cardiac diseases, poor fitness, obesity, or anaemia. Tachycardia is of prognostic value in the general and diabetic population. A recent meta-analysis including 87 studies confirmed a relative risk per 10 bpm increase in resting heart rate of 1.15 for cardiovascular disease, and of 1.17 for all-cause mortality [71]. In the ADVANCE study of 11,140 patients with T2DM, a higher resting heart rate was associated with an increased risk of all-cause mortality (adjusted hazard ratio 1.15 per 10 bpm), and cardiovascular death [72]. However, as recognized by the authors, it remains unclear whether a higher heart rate directly conditions the increased risk or is a marker for other factors [72].
Orthostatic hypotension
Orthostatic hypotension (OH) is defined as a standing fall in systolic BP of 20 mm Hg or in diastolic BP of 10 mm Hg in normotensive subjects, and a fall in systolic BP of 30 mm Hg in hypertensive subjects [7374]. It is a common condition in the older general population, and in diabetic patients reaches prevalence similar to what is observed in elderly non-diabetic people (32%) [75].
A meta-analysis of 13 observational studies, including 121,913 subjects with and without diabetes and hypertension, with a median follow-up of 6 years, showed that prevalent OH was associated with an increased risk of all-cause death, incident coronary heart disease, heart failure, and stroke with a pooled estimate of relative risk for all-cause death of 1.78 for patients <65 years old, and 1.26 in the older subgroup [76]. Similarly, in patients with diabetes, OH raises the risk for mortality associated with parasympathetic neuropathy by between 30% and 100% [77].
In 4,266 participants in the ACCORD study (aged 40 to 79 years), OH was measured as the minimum standing (1, 2, 3 minutes after standing) minus the mean seated systolic and diastolic BP, at baseline, 12 and 48 months. OH occurred at ≥1 time point in 20% and was an independent marker for total mortality (hazard ratio, 1.62) and heart failure death or hospitalization (hazard ratio, 1.85) [78]. Postural BP swings (with an alternation of hypoperfusion and increased BP load in the end-organs), supine hypertension, and other consequences of the loss of autonomic cardiovascular control have been suggested as possible mechanisms of OH associated excess mortality [77].
Nondipping
Using ambulatory BP monitoring (ABPM), a decrease in BP from day to night <10% and <0% identifies nondipping and reverse dipping, respectively. Nondipping is more common in diabetes (39% to 62%) than in the general population, and reverse dipping is present in between 9% to 30% [77]. Reverse dipping is considered a sign of CAN. Nondipping occurs concomitantly with an impaired vagal increase during the night and a consequent sympathetic nocturnal predominance. More recently, further factors have emerged in the nondipping phenomenon in addition to the central role played by this autonomic derangement (Fig. 3) [77].
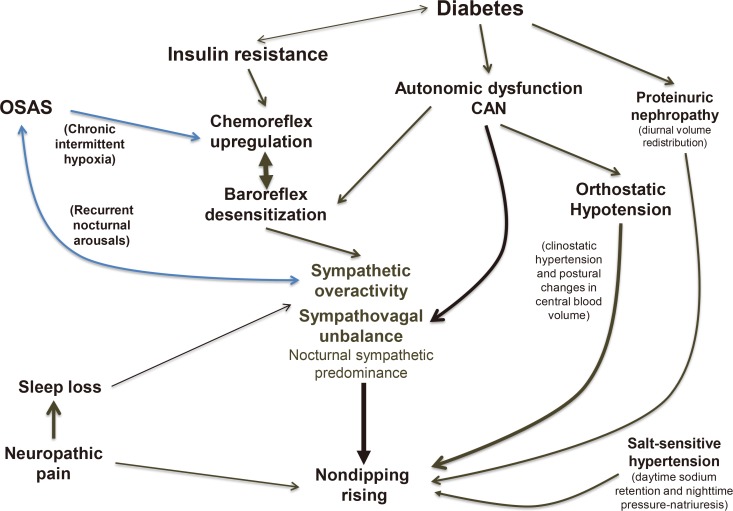
Multifactorial pathogenesis of nondipping in diabetes. In addition to the central role of autonomic derangement, insulin resistance in type 2 diabetes mellitus and diabetes-associated obstructive sleep apnoea syndrome (OSAS) can induce chemoreflex upregulation and baroreflex impairment, and reinforce sympathetic overactivity. In advanced cardiovascular autonomic neuropathy (CAN), orthostatic hypotension can favour nondipping through postural changes in blood volume and supine hypertension. Moreover, there is documentation that the fluid redistribution from the extra to the intravascular compartment in the presence of proteinuria, the mechanism of compensatory nocturnal pressure-natriuresis in salt-sensitive hypertension and in renal failure, the sleep loss so common in diabetes, and even the neuropathic pain may act as contributory factors. Adapted from Spallone [77], with permission from Springer Nature.
Four meta-analyses in the general hypertensive population confirm the predictive role of day-night variation in BP, nondipping and reverse dipping on all-cause mortality, and cardiovascular mortality and events, suggesting a stronger value for night-time BP and for reverse dipping with a doubled risk compared to dipping [79808182]. In diabetes the prognostic role of nondipping and reverse dipping is even stronger with between a 2-fold and an 8-fold increased risk of cardiovascular morbidity in six studies of an overall population of 880 patients, mainly with T2DM, and a mean follow-up of 4.4 years [77]. Furthermore, reverse dipping was found to be an independent predictor of the progression from overt nephropathy to renal failure or dialysis in patients with T2DM [77].
Conclusion on the mechanisms of CAN associated excess mortality
Clinical forms of CAN are established conditions of increased mortality risk in the diabetic population, with the causative pathways only partially known. Other expressions of altered autonomic cardiovascular control like HRV and BRS are associated with mortality with stronger evidence in the general population. New mechanisms are also emerging, like left ventricular dysfunction, arterial stiffness, arterial calcification, inflammation, which suggest a widespread potential influence of autonomic dysfunction in diabetic patients. From a clinical perspective, it is advisable to actively look for clinical forms of CAN that are associated with mortality, treat them and consider autonomic indices in cardiovascular risk stratification.
ASSESSMENT AND DIAGNOSIS OF CAN
Methods of CAN assessment in clinical practice include the assessment of symptoms and signs, CARTs, and ABPM. Methods like those assessing HRV and BRS can be used in clinical and research fields, whereas other techniques like muscle sympathetic nerve activity (MSNA) are strictly limited to research [23].
Autonomic symptoms
Diabetic autonomic neuropathy is multiform and may present with signs and symptoms regarding the several functions controlled by the autonomic nervous system: heart, vessels, gut, bladder, pupillary, erectile, and sudomotor function. Cardiovascular symptoms are tachycardia, exercise intolerance and orthostatic symptoms like light-headedness, dizziness, blurred vision, neck pain, and fainting when standing up [75].
Although scientific guidelines [283] consider autonomic symptoms as the first level of investigation in any diabetic patient for their potential impact on quality of life and in order to obtain a differential diagnosis, there is an assumption that due to their lack of specificity, they do not represent a useful tool for CAN diagnosis. However, this idea has been challenged by recent findings obtained through the Composite Autonomic Symptom Score 31 (COMPASS 31) questionnaire, developed from the more complex Autonomic Symptom Profile [84]. It was validated towards a CARTs-based CAN diagnosis and showed fair diagnostic accuracy, with an area under the receiver operating characteristic curve of 0.75, and a sensitivity of 75% and 70% for CAN and confirmed CAN, and a specificity of 65% and 67%, respectively [85]. This questionnaire might allow for a standardized and straightforward assessment of autonomic symptoms and also act as a diagnostic tool for CAN, as also suggested for the Survey of Autonomic Symptoms scale [86].
Cardiovascular signs
Tachycardia, OH, QTi prolongation and reverse dipping on ABPM are typical findings of CAN, can be easily detected during clinical evaluation or at a relatively widely-used ABPM, and may alert the physician to the presence of CAN. With the exception of tachycardia, they are specific—albeit insensitive—markers of CAN [878889], with sensitivity and specificity being 31% and 98% for OH, 28% and 86% for a QTi corrected for heart rate >441 msec, and 26% and 95% for reverse dipping. The Toronto Consensus recommended screening for orthostatic symptoms in any diabetic patient, a yearly OH test, in particular with patients over the age of 50 and hypertensive patients, referral to CARTs in the presence of unexplained tachycardia, QTi prolongation, and reverse dipping [2]. Despite the European Society of Cardiology/European Society of Hypertension guidelines for the management of arterial hypertension [90] indicating that lying and standing BP measurements should be considered in people with diabetes, the need remains to implement awareness, detection and management of this condition in clinical practice. Moreover, a seated-to-standing manoeuvre instead of the standard supine-to-upright test has been recently put forward as an easier alternative when the standard method is less feasible, with a suggested cut-off of 15 mm Hg for systolic and 7 mm Hg for diastolic BP drop [91]. However, a lower BP threshold requires even more careful BP measurements, misclassification is possible [92], and the unproven or unconfirmed diagnostic accuracy of the seated-to-standing manoeuvre in different populations [92], including hypertensive subjects, should restrict its application to situations where the use of the gold-standard modality is considered impossible.
Cardiovascular autonomic reflex tests
CARTs measure heart rate and BP response to provocative physiological manoeuvres, and still represent the gold standard in autonomic testing as stated by the Toronto Consensus [2] and recognized by neurological scientific societies [93].
The Toronto Consensus recommends that diagnosis of CAN be based on the use of CARTs, i.e., heart rate response to deep breathing, standing, Valsalva manoeuvre, and BP response to standing, and that more than one heart rate test and OH test are required [2]. Moreover, the performance of CARTs should be standardized, the influence of confounding variables minimized, and age-related normal ranges of heart rate tests strictly required [2]. For a review of confounding factors, requisites and instructions for performing CARTs, see the specific reference [94].
CARTs also allow for CAN staging, according to the number of test abnormalities: one abnormal cardiovagal test result identifies possible or early CAN, two abnormal cardiovagal results a definite or confirmed diagnosis of CAN, and the presence of OH identifies severe or advanced CAN [2]. The progressive stages of CAN are associated with an increasingly worse prognosis [2].
Guidelines of scientific societies on screening and diagnosis of CAN
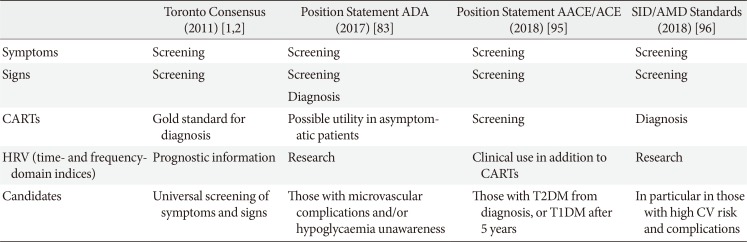
Table 3 shows for comparison indications on CAN screening and diagnosis of the Toronto Consensus, the American Diabetes Association (ADA), the American Association of Clinical Endocrinologists (AACE) with the American College of Endocrinology (ACE), and the Italian Society of Diabetology (SID) with the Italian Association of Clinical Diabetologists (AMD) [2839596]. There is a shared indication to assess autonomic symptoms and signs of CAN as the first level but slight differences in the recommendations on the use of CARTs, which are the gold standard for diagnosis for the Toronto Consensus, always needed for AACE/ACE and SID/AMD, and non strictly necessary in presence of symptoms and signs and possibly useful in asymptomatic patients for ADA.

Guidelines of scientific societies on screening and diagnosis of CAN in clinical practice, with regard to the indications of assessment modalities, and the candidates to screening
Attempts to address the challenge of numbers and sustainability
ADA's position statement expresses more concern on the burden and costs of CAN screening. It is not possible to ignore that there is a question of sustainability when considering the diabetic epidemic and the number of people with diabetes. With these huge numbers of potential candidates, it could be necessary to introduce selection. Thus, the Toronto Consensus indicates that at the very least screening for symptoms and signs should be universal, the ADA suggests this screening with those with microvascular complications (and hypoglycaemia unawareness), the AACE/ACE indicate almost universal screening, and Italian guidelines support CARTs in particular in the presence of a high cardiovascular risk or complications [2839596].
In this context, some scoring systems for CAN risk have been developed. In a Chinese population-based sample of 2,092 individuals (21% with diabetes), a CAN risk score based on age, BMI, hypertension and heart rate was identified in an exploratory analysis as a predictor of CAN (defined as two abnormal spectral indices of short-term HRV) with fair diagnostic accuracy (sensitivity of 75.4%, specificity of 62.5%) and a high negative predictive value (91.4%) [97]. Similarly, in the German population-based Kooperative Gesundheitsforschung in der Region Augsburg (KORA) S4 study of 1,332 participants (55 to 74 years) with NGT, IFG, IGT, and T2DM, a screening score including BMI, hypertension, smoking, heart rate, creatinine, and drugs with adverse effect on HRV displayed an area under the curve (AUC) of 0.86, sensitivity of 78%, specificity of 81% and a high negative predictive value (98%), for a diagnosis of CAN based on an abnormal time-domain index of short-term HRV (root mean squared successive difference) [15].
The limitations of the approach of these studies are (1) the validation of the proposed scoring system against a diagnosis of CAN based on measures of HRV and not CARTs, (2) the studied population including mostly non-diabetic subjects, and (3) basal heart rate as the only autonomic index in the score that is provided with a low specificity for CAN. Notwithstanding, these attempts have the merit of testing a risk stratification system for CAN, possibly allowing for the selection of patients with T2DM at a higher CAN risk and deserving subsequent referral to standard CARTs.
Attempts to address the need for CARTs' affordability
Moreover, other attempts have been made at answering the need for the accessibility of CARTs with a number of possible solutions: (1) a reduction in the number of repetitions of the Valsalva manoeuvre and deep breathing; (2) the use of a single breath in deep breathing test; (3) the use of a mini-battery of two to three CARTs; (4) the use of reference ranges derived from literature and obtained with a different methodology [98]; (5) the substitution of CARTs with HRV indices measured on short electrocardiography recordings (2 to 5 minutes); (6) the development of handheld devices and technical advancement (like telemetry and mobile derived approaches).
To simplify the approach to CARTs, researchers have investigated whether the full battery of CARTs could be reduced to one or two tests capable of including all the necessary information. However, there is no consensus on which tests are best-suited to this role, with the choice falling alternatively on deep breathing [99], lying to standing [100], or on the Valsalva manoeuvre [101]. Thus, the need for at least two heart rate tests plus OH would still appear to be the best compromise.
Short-term HRV indices might be additional resources, with them being easier, perhaps more sensitive, and patient-independent compared to CARTs. Although a number of studies have compared HRV spectral indices to CARTs mainly in diabetes, no conclusive data are available on their diagnostic accuracy compared to CARTs [3].
A cross-sectional study in a large dataset of a Shanghai population (2,092 subjects including 371 healthy subjects) evaluated the reference values for short-term HRV frequency-domain indices, and estimated their sensitivity and specificity using the Bayesian approach, in the absence of a gold standard, and compared in another independent dataset of 88 subjects the diagnostic performance of these indices and standard CARTs [102]. The study concludes that with regard to sensitivity and specificity for CAN, the HRV testing was not inferior to the traditional Ewing test (both with values between 75% and 85%) [102]. However, when analysing the results, HRV indices and Ewing tests do not seem to identify the same patients as having CAN, and when assuming CARTs as gold standard for CAN diagnosis the sensitivity of HRV indices is 49% and specificity 78%. Most probably, the two methods do not describe exactly the same functional abnormalities of cardiac autonomic control, and HRV indices should be considered as additional and not an alternative to CARTs in clinical practice, thus allowing for supplemental early and prognostic information to current CARTs [3].
Investigation for cardiovascular autonomic function in research
The most widely used and readily available diagnostic tests in clinical research are HRV in the time- and frequency-domain and BRS. They can provide information about the pathophysiology and the natural history of CAN, and may be used as sensitive and comprehensive endpoints in clinical trials together with MSNA [3]. For clinical trials, evaluating a specific intervention or prognostic implications, the ADA recommends the following CAN measures: CARTs, HRV indices, QTi, and resting heart rate [83]. The latter two, despite having been used in clinical trials as outcomes [103], might work better as prognostic predictors given their low sensitivity for CAN. On the other hand, BRS is considered by the ADA as a less accessible CAN measure [83].
Small fibre function tests as surrogate markers for CAN
Tests of sudomotor function have been advocated as surrogate measures for CAN with the additional advantage of assessing small nerve fibres in the lower limbs. The Neuropad plaster patch (TRIGOcare International, Wiehl, Germany) has shown an AUC of 0.71 and values of sensitivity between 59% and 89% and of specificity between 27% and 78% for CARTs-based CAN [104105]. Extending the observation period to 15 minutes might provide greater diagnostic usefulness [104]. Electrochemical skin conductance (ESC) measured at the feet by Sudoscan (Impeto Medical SAS, Paris, France; approved by the Food and Drug Administration [FDA]) has been proposed as a non-invasive, quick and easy test for sudomotor function with AUC for CAN confirmed (based on CARTs) of 0.66, and at a cut-off <70 µS a sensitivity of 60% and a specificity of 76% [106]. A 1-year longitudinal study in 37 young adults with T1DM did not find an association between changes in CARTs (no change) and ESC (deterioration), and the utility of ESC in the early assessment of CAN has been disputed [107]. On the other hand, in 70 obese patients with and without pre-diabetes or diabetes a significant improvement in feet ESC and HRV was observed at 24 weeks only in the patients with diabetes, with the improvement in ESC related to that in HbA1c [108]. Thus, there are no conclusive data and no unanimous position regarding the role of sudomotor function assessment for diabetic neuropathy [183], in particular of ESC measured using Sudoscan [109110].
Corneal confocal microscopy (CCM), which measures corneal small fibre innervation, in a single study has shown for its various parameters AUC of 0.89 to 0.91, sensitivity of 86% to 100% and specificity of 56% to 78% for CAN diagnosis [111]. However, in order to consider CCM as an alternative standard for diagnosis and staging of CAN, greater specificity, its pertinence to clinical forms of CAN and a predictive value for cardiovascular outcomes should be proven (the same is true for sudomotor function tests). Moreover, a possible role of CCM as an early biomarker for CAN requires larger studies, well matched groups, and a comparison with sensitive autonomic indices—like HRV and BRS—as well as a prospective design.
The vexed question: what is the clinical usefulness of CAN screening and diagnosis?
With regard to the legitimate and recurrent question about the effectiveness of CAN diagnosis in clinical practice, the answer is that CAN assessment in patients with clinical CAN allows for treatments targeting the clinical consequences of CAN and may also provide insight into general therapeutic strategy (Fig. 4) [2].
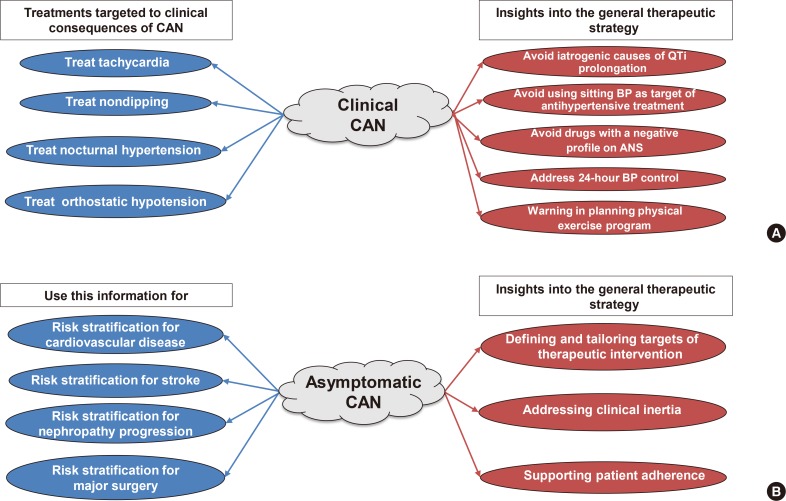
(A) Clinical effectiveness of cardiovascular autonomic neuropathy (CAN) diagnosis in clinical forms of CAN and (B) the awareness of CAN for the therapeutic strategy in asymptomatic forms of CAN. QTi, QT interval; BP, blood pressure; ANS, autonomic nervous system.
In asymptomatic patients, the detection of CAN provides useful information for risk stratification for diabetes-related complications, cardiovascular morbidity and mortality, for the defining and tailoring of the targets of diabetes therapy, to address clinical inertia and foster patient adherence (Fig. 4) [2]. In a Korean study in 894 participants with T2DM followed for 9.5 years, the percentage of episodes of severe hypoglycaemia increased from 5.4% to 17.2% and 22.7% in patients without CAN, with early and definite CAN; definite CAN was associated with an adjusted hazard ratio of 2.43 for the development of severe hypoglycaemia [58].
Conclusion on CAN assessment
CAN assessment is based on the evaluation of symptoms and signs with CARTs still being the gold standard for the diagnosis. Attempts are on the table at selecting candidates for CAN screening, at simplifying the diagnostic approach by reconciling affordability with accuracy, with clinical relevance and prognostic value for outcomes. To face the wide under-diagnosis of CAN, potential resources are the promotion of increased awareness of the usefulness of CAN assessment in clinical practice, and the identification of barriers to implementation (burden, costs, inertia, feeling of uselessness, and unaddressed educational needs). However, we have to admit that there is currently a lack of prospective studies assessing the cost-effectiveness of CAN testing.
PREVENTIVE AND THERAPEUTIC STRATEGIES
Lifestyle intervention targeting autonomic dysfunction
The first documentation of the beneficial effect of lifestyle intervention on measures of cardiovascular autonomic function was provided by the Diabetes Prevention Program trial with 2,980 participants with pre-diabetes, where lifestyle changes were able to improve heart rate, HRV, and QTi length, with a superiority on metformin on most of these indices [103]. Improvements in these indices were inversely associated with the development of diabetes, independently of weight change. However, in a small study of 25 non-diabetic subjects with metabolic syndrome, a 24-week lifestyle intervention (including supervised aerobic exercise and a Mediterranean diet) was able to significantly reduce all oxidative stress markers but did not change any CAN measures, i.e., CARTs, HRV indices, and [11C]meta-hydroxyephedrine ([11C]HED) positron-emission tomography (PET) imaging [112].
Effects of weight loss
A number of studies have demonstrated that even moderate calorie-restricted weight loss can improve cardiac autonomic modulation by increasing time and frequency-domain indices of HRV, ameliorating the sympathovagal balance and BRS, and lowering the sympathetic tone in normotensive individuals with obesity or in those with metabolic syndrome [24]. Five studies assessed the effects of weight loss in diabetes, including overall 100 obese and/or overweight subjects with T2DM [113114115116117], with some design limitations (with them being mostly uncontrolled and non-randomized), with a follow-up of 3 to 12 months, and weight loss obtained by bariatric surgery [113115116117] or caloric restriction diet [114117], with a weight loss of at least 10% effective [114117], and a very low calorie diet equivalent to a Roux-en-Y gastric bypass [117]. In these studies, weight loss was associated with an increase in parasympathetic indices of HRV and improved sympathovagal balance, and in one study also with an improvement in CARTs [115].
In the previously cited study, in 70 obese patients with NGT, pre-diabetes, or diabetes, bariatric surgery was associated with a significant improvement in heart rate in all groups, and in the HRV index in only the diabetic group, independently of changes in BMI or body fat [108].
In summary, given that obesity and early T2DM are associated with reduced HRV and sympathetic overactivity or predominance, available studies in mainly obese individuals without diabetes—but also small, uncontrolled studies on T2DM—document that weight loss, even moderate and however it was achieved, is associated with increased parasympathetic indices of HRV and reduced direct measures of sympathetic activity. A few unresolved questions remain like the efficacy of intervention in patients with CAN, the preventive ability of weight loss on the progression of autonomic dysfunction/neuropathy, and finally the protective effect of weight-loss-induced improvement in autonomic indices on cardiovascular outcomes (although extrapolation might be possible from other studies in the general population).
Effects of diet composition
With regard to the components of diet, a randomized 8-week pilot trial in 28 obese patients with T2DM compared a low-energy diet high in cereal fibre, free of red meat, and high in coffee with one low in fibre, high in red meat, and coffee free [118]. No striking differences between two diets were observed in weight loss or in the decrease in heart rate or increase in HRV, although the former diet seemed to impact a little better on sympathovagal balance. The change in HRV was associated with an increase in oxidative glucose utilization but not with changes in BMI, insulin sensitivity and inflammatory markers [118]. The possibility to modulate cardiac autonomic control through diet composition cannot be ruled out, since various manipulations of diet, mainly in the general population [119] but also in subjects with T2DM [120], including the introduction of a low-fat diet, Mediterranean diet, salmon diet, moderate-fat diet with pistachios, have been shown to benefit HRV acutely and in the longer term, also through their effects on sleep health [119].
Effects of physical exercise
Since the first randomized controlled study, showing in 50 men with T2DM that combined exercise training (aerobic and resistance) for 12 months was associated with improvement in BRS (but not in HRV measures) [121], a number of studies have evaluated the effects of physical activity on autonomic function in T2DM. Four reviews have considered 25 studies including six randomized controlled studies with an overall number of about 700 subjects [122123124125]. Most of them used time and frequency-domain HRV indices and not CARTs, included participants with T2DM and without CAN, and used aerobic and aerobic plus strength training. The studies mainly documented significant improvement in HRV and BRS compared to baseline and/or control group, with supervised exercise obviously being better than non-supervised; endurance exercise and intense combined exercise (resistance and aerobic training) were effective. A 45 to 75 minutes length of session, a frequency of more than 3 days/week, and duration of intervention of more than 3 to 4 months were needed in order for it to be effective [122123124125].
However, most studies were of low quality, of small sample size, with flaws in the study design, without a control group, and not blinded, and just four were randomised control trials of fair quality, with large heterogeneity in comparison groups, intervention modalities, and outcome measures. Studies in T1DM were lacking (only one study, on children), and only two studies were on patients with CAN [122123124125]. Thus, only low-grade evidence exists of a moderate beneficial effect on HRV indices in T2DM (without CAN or with early CAN), and there is a need for high-quality exercise interventions especially in T1DM, in order to obtain better evidence and clarify efficacy in different CAN stages and, therefore, the most appropriate exercise intervention.
A recent randomised study compared the effects of 4 months of aerobic and resistance training on HRV and BRS, in 30 subjects with T2DM without CAN, and found that aerobic and resistance training improved autonomic indices to a similar extent in both training modalities, but a significant effect on BRS was apparent only with aerobic training [126].
Slow breathing effects on BRS impairment in diabetes
Early impairment of BRS has been documented in T1DM [127] and it seems to have functional aspects because it is reversible during slow breathing, also in patients with long standing T1DM, and to a lower degree in those with early CAN [128]. Similarly, BRS impairment present in patients with T2DM is partially reversible during slow breathing even in the presence of chronic diabetic kidney disease [128].
The suggested scenario of this slow breathing driven beneficial effect on BRS is an abnormal cardio-respiratory reflex interaction, centred on baroreflex and chemoreflex and characterized by BRS attenuation and chemoreflex augmentation. Slow breathing transiently would be able to enhance BRS and reduce chemoreflex responses, while physical training would produce the same effects in the long-term [128].
Conclusion on the prevention strategy for CAN
An effective preventive strategy for CAN should include weight control, physical activity, smoking cessation, healthy dietary changes, glycaemic control, control of cardiovascular risk factors like BP and dyslipidemia, and a behavioural approach aimed at stress control, and last but not least, education [2]. In this regard, the Toronto Consensus concluded that lifestyle intervention may improve HRV in pre-diabetes and diabetes and recommended that it should be offered as a basic preventive measure [2]. Additional support for the favourable effect of lifestyle intervention on autonomic function has been obtained, whereas evidence is still lacking for confirmed CAN and for cardiovascular outcomes. The ADA recommends considering lifestyle modifications to improve CAN in patients with pre-diabetes [83].
Glycaemic control and CAN
There is clear evidence for the efficacy of intensive treatment of hyperglycaemia in T1DM with a prolonged benefit (EDIC study) [28], whereas efficacy is only apparent in the setting of a multifactorial strategy in T2DM and with prolonged benefits [129], albeit not confirmed in the ADDITION study [98]. However, in the ADDITION study [98] aimed at evaluating the effects on the prevalence of CAN at the 6-year follow-up of early detection with a screening-based diagnosis of T2DM and subsequent intensive treatment in primary care, no baseline assessment of CAN was done, and at follow-up the level of medications was also high in the routine care group.
The ADA recommends optimizing glucose control as early as possible to prevent or delay the development of CAN in T1DM, and also recommends considering a multifactorial approach targeting glycaemia and other risk factors to prevent CAN in T2DM [83].
New glucose-lowering medications and autonomic nervous system
Although a beneficial effect of metformin on the sympathovagal balance was documented in T2DM and mainly explained by the concomitant decrease in plasma free fatty acids and insulin resistance [130], an interesting point in the present era of new classes of agents for diabetes care is the relationship between new glucose-lowering medications and the autonomic nervous system.
Sodium glucose transporter 2 inhibitors
Starting with sodium glucose transporter 2 inhibitor (SGLT2i), the first consideration is that the target of SGLT2i is the same as with sympathetic nerves (i.e., renal tubular epithelial cells), where efferent sympathetic fibres promote tubular sodium reabsorption (Fig. 5) [131132]. In this direction, cross talk between the sympathetic nervous system and SGLT2 regulation has been conjectured on the basis of: (1) a pronounced increase in SGLT2 expression induced by sympathetic neurotransmitter noradrenaline in human renal proximal tubule cells [133]; and (2) the inhibiting action of SGLT2i dapaglifozin on the expression of tyrosine hydroxylase and noradrenaline in the kidney and the heart [133]. This observation opens up the new prospect of sympathetic upregulation of SGLT2 and a sympathoinibitory effect of SGLT2i with clinical relevance in T2DM and possibly also in other conditions of sympathetic overactivity. Moreover, BP reduction following 4 days of treatment with empaglifozin in 22 metformin-treated patients with T2DM did not lead to an observed increase in MSNA in response to diuretic effects as an expression of reflex sympathetic activation, suggesting at least a lowering modulation of sympathetic activity [134]. This lack of sympathetic activation corresponds to the absent change in heart rate.

Interaction between sodium glucose transporter 2 inhibitor (SGLT2i) and sympathetic nervous system. NE, norepinephrine; T2DM, type 2 diabetes mellitus; BP, blood pressure; MSNA, muscle sympathetic nerve activity.
It would be of interest to understand whether some of the positive effects on the cardiovascular system of these drugs are mediated by interaction with the autonomic nervous system in the kidney or directly in the central nervous system. However, clinical trials with SGLT2i using ABPM do not confirm a preferential lowering effect on nocturnal versus daytime systolic BP, despite the diuretic and natriuretic effect of these drugs and the dipping restoration found with SGLT2i in rat models of obesity and metabolic syndrome (Fig. 5) [135136].
Glucagon-like peptide 1 receptor agonists
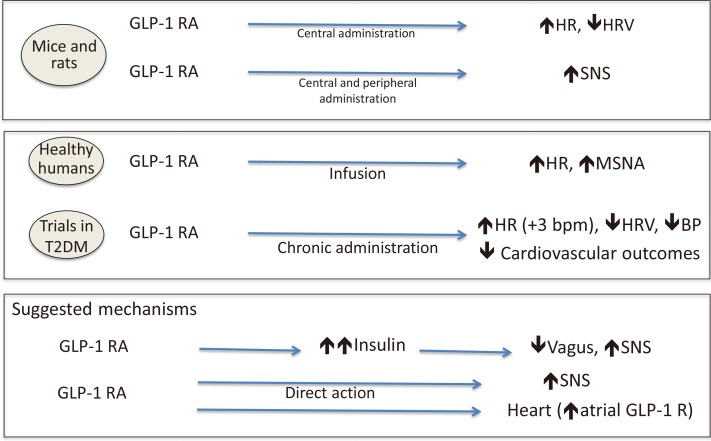
Experimental findings in mice and rats document that the central and peripheral administration of a glucagon-like peptide 1 receptor agonist (GLP1-RA) increased heart rate, reduced frequency-domain indices of HRV, and increased sympathetic activity (Fig. 6) [137138]. In healthy individuals, acute GLP1-RA infusion produced an increase in heart rate and in MSNA [139]. In clinical trials, GLP1-RAs increased heart rate by around 3 bpm [140] and lowered systolic BP as well as decreasing the cardiovascular risk, at least to some extent. A recent randomized, double-blind, placebo controlled 12+12-week crossover study with a 2-week washout period, included 39 overweight participants with newly diagnosed T2DM and coronary artery disease, treated with metformin, and randomized to liraglutide or placebo [141]. Liraglutide and not placebo determined an increase in heart rate and a decrease in time-domain indices of HRV (i.e., 24-hour standard deviation of NN intervals [SDNN]), persistent after multiple adjustments, despite weight loss and an improvement in metabolic parameters, suggesting for the authors an impairment in vagal activity after liraglutide treatment [141]. Different responses to liraglutide and lixisenatide were found in 60 patients with T2DM in a prospective, single-centre, randomized, open-label study [142], with an increase in 24-hour heart rate and in LF:HF ratio only in the liraglutide group. Moreover, in a real-world study of 28 patients with T2DM (on multiple antihyperglycaemic and antihypertensive treatment), chronic exenatide extended release (ER) once-weekly administration was associated with the expected increase in heart rate but an unexpected decrease in LF:HF after 3 and 6 months that was interpreted as the consequence of a compensatory mechanism [143]. The increase in heart rate is believed to have multiple reasons, among which: (1) an increase in sympathetic activity both directly [144] and mediated by the GLP1-driven increase in endogenous insulin [138]; and (2) an action on GLP1 receptors present on the cardiomyocytes (Fig. 6) [142145]. However, some discrepancies exist in the available preclinical and clinical findings suggesting possible species-specific patterns of GLP1 receptors in addition to differences between GLP1-RAs. Moreover, the GLP1-RA effects on heart rate and the autonomic nervous system need to be reconciled with the favourable cardiovascular outcomes in clinical trials of at least some GLP1-RAs (liraglutide, semaglutide, exenatide ER).
Disease modifying treatments
A number of beneficial effects have been attributed to α-lipoic acid, including an improvement in glucose homeostasis and lipid profile, an anti-inflammatory action and the ability to reduce oxidative stress, as well as an increase in nitric oxide production and in Na+/K+-ATPase activity and a reduction in protein glycosylation [146147148]. Most of these pathways are involved in diabetic neuropathy pathogenesis. Pharmacological interventions with α-lipoic acid achieved some limited effect in the Deutsche Kardiale Autonome Neuropathie (DEKAN) Study of 73 patients with T2DM through the oral administration of 800 mg for 4 months [149], a significant improvement in CARTs in an uncontrolled study of 49 participants with T1DM through 600 mg intravenous for 10 days followed by 600 mg oral for 50 days [150], and borderline efficacy in another study of 75 patients with T2DM on a HRV measure (i.e., SDNN) with an oral administration of 600 and 1,200 mg for 12 weeks each [151]. Moreover, the combination of α-lipoic acid 1,200 mg oral with nicotinamide 1,500 mg and allopurinol 300 mg failed to influence CAN measures in 31 patients with T1DM [152], pointing to possible negative metabolic interactions between the three antioxidants. On the other hand, in a post hoc analysis of the large Neurological Assessment of Thioctic Acid in Diabetic Neuropathy (NATHAN) 1 trial (480 patients with T1DM and T2DM), a 4-year improvement in the deep breathing test with the ALA compared to placebo was predicted by treatment at baseline with angiotensin converting enzyme inhibitors, oral antidiabetic agents and insulin, suggesting that a co-administration of α-lipoic acid and angiotensin converting enzyme inhibitors might exert an additional neurovascular beneficial effect [153]. No significant side effects were recorded in these trials.
In a meta-analysis, aldose reductase inhibitors (with the exception of ponalrestat) were somewhat effective on HRV measures and the 30:15 test, with a better control (HbA1c <8%) and a shorter diabetes duration (<9.5 years) being predictors of efficacy; they were safe [154].
Among other agents, isolated unconfirmed studies documented beneficial effects on CAN measures of C-peptide in T1DM [155] and of vitamin E in T2DM [156]. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers (quinapril, trandolapril, losartan) had inconsistent efficacy (effective in three out of six small studies) [157158159160161162], and β-blockers (metoprolol) was effective in one study [163]. All these findings, however, are insufficient to reach recommendations on these agents with the current guidelines.
Treatment of symptomatic OH
Treatments for clinical forms of CAN do however exist. With regard to OH, treatment is recommended only in symptomatic forms, with the objective of minimizing symptoms and increasing autonomy in daily life (not of normalizing standing BP). Non-pharmacological measures are often sufficient. The first step considered is the exclusion or dose reduction of drugs that can worsen OH, then the correction of volume depletion, and other measures like lower body strength training and moderate recumbent exercise, physical manoeuvres, and rapid drinking of 500 mL of water [164]. Appropriate education of patients is an essential element of this strategy. Pharmacotherapy of symptomatic OH includes midodrine and droxidopa (the only two FDA approved drugs for neurogenic OH, the latter with an orphan designation, based on the efficacy on symptoms in studies not including OH due to diabetic neuropathy, and not available on the market in all countries), fludrocortisone, a combination of drugs in non-responders, and other less established drugs, like pyridostigmine, acarbose, octeotride, desmopressin, and erythropoietin [275164].
Treating supine hypertension can be of particular difficulty because of the need to lower supine BP without worsening OH. A recent consensus redefined supine hypertension as the presence in patients with neurogenic OH of a systolic BP of ≥140 mm Hg and/or diastolic BP of ≥90 mm Hg, measured after at least 5 minutes of rest in a supine position [165], and its severity as mild, moderate or severe with values of systolic or diastolic BP of 140 to 159 or 90 to 99 mm Hg, 160 to 179 or 100 to 109 mm Hg, and ≥180 or ≥110 mm Hg, respectively [165]. Treatment is needed for severe forms and can utilize short-acting antihypertensive medications at bedtime like captopril, losartan and low-dose nitroglycerin patch [164].
Targeting nondipping and reverse dipping
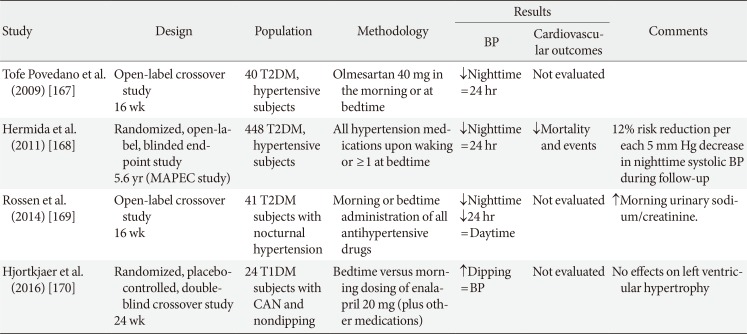
A 2011 Cochrane analysis including 21 RCTs in 1,993 patients with hypertension compared the effects of once-daily evening versus morning dosing regimen on BP levels with patients with primary hypertension [166]. It found that an evening administration obtained slightly better 24-hour BP control than the morning regimen, but the impact of this on death and adverse cardiovascular outcomes was not known [166]. Four studies on patients with diabetes explored the effects on BP levels and outcomes of bedtime administration of antihypertensive drugs (Table 4) [167168169170], among which the largest was the Spanish Monitorización Ambulatoria para Predicción de Eventos Cardiovasculares (MAPEC) study [168], and just one was in T1DM [170]. All the studies documented—despite differences in design—that nocturnal BP was lowered or nocturnal BP fall was increased with bedtime administration of at least one or all the antihypertensive medications, but only the MAPEC study was able to document significantly reduced cardiovascular morbidity and mortality, with less than 12% of events per each 5 mm Hg decrease in asleep systolic BP [168]. Although further long-term outcome trials are needed to evaluate the efficacy and safety of bedtime administration of antihypertensive treatment, night-time BP and nondipping might be an appropriate treatment target in the diabetic more than in the general population, also because reverse dipping might represent a surrogate marker for CAN.
Conclusion on therapeutic options for CAN
The role of intensive diabetes therapy in delaying the development of CAN in T1DM is confirmed, whereas only limited evidence exists for intensive multifactorial intervention in T2DM. Emerging data from the new class of SGLT2i introduces the possibility of a modulatory effect on cardiovascular autonomic function. No conclusive evidence for pharmacological disease modifying treatment exists (as with diabetic polyneuropathy). On the other hand, symptomatic treatment of clinical correlates of CAN like OH and nocturnal hypertension is both available and advisable.
CONCLUSIONS
In conclusion, CAN dimensions are still relevant. CAN starts very early: available data on autonomic dysfunction in pre-diabetes are increasing. Preliminary data might challenge the idea of CAN irreversibility, but the natural history of CAN is still undefined. CAN has bad, old and new companions: the new correlates might become new targets for prevention. There have been confirmatory data on the prognostic meaning of CAN and increasing evidence of its central role in cardiovascular health.
CAN detection can help both the clinician and the patient, although cost-effective studies are still lacking. CAN under-diagnosis can be addressed through a mix of education, flexibility, adaptability to local resources and to the individual's risk profile, in addition to searching for more affordable testing.
New data support the efficacy of CAN prevention at an early stage, but CAN still needs more effective prevention and disease modifying treatment. CAN's clinical forms are treatable. In conclusion, CAN still needs to be fully dealt with in practice, knowledge, education, as well as in research.
ACKNOWLEDGMENTS
This review article is based on the presentation by Vincenza Spallone at the 2018 International Congress of Diabetes and Metabolism (ICDM), 11–13 October 2018, Seoul, Korea, and entitled, “Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management.”
Notes
CONFLICTS OF INTEREST: Vincenza Spallone has received research grants from Biocure srl Italy and Boehringer Ingelheim Italy. She has received remunerations for lectures or consultations for AWP srl Italy, Boehringer Ingelheim Italy, Daiichi Sankyo Europe, Ely-Lilly Italy, IRIS Servier France, Laborest Italy, Pfizer Italy, Sanofi Aventis Italy, Schwarz Pharma Europe, Wörwag Pharma Germany. She has served on advisory boards for Angelini S.p.A Italy, TRIGOcare International Germany, and Wörwag Pharma GmbH & Co Germany.