Association between Changes in Anthropometric Indices and in Fasting Insulin Levels among Healthy Korean Adolescents: The JS High School Study
Article information
Abstract
Background
This study investigated the association between changes in anthropometric indices and fasting insulin levels among healthy adolescents and whether the association differed by baseline obesity status.
Methods
This analysis was based on data collected for the JS High School study; 884 healthy adolescents aged 15 to 16 years followed up for 24 to 30 months were included. Changes in anthropometric indices and fasting insulin levels were computed as the difference between baseline and follow-up values. Multivariate linear regression models were used to determine the association between changes in anthropometric indices and fasting insulin levels. Based on body mass index (BMI)-for-age and waist circumference (WC)-for-age percentiles, participants were classified as normal weight (<85th percentile), overweight (85th percentile to <95th percentile), or obese (≥95th percentile).
Results
Changes in BMI, WC, waist-hip ratio, and waist-height ratio were significantly associated with changes in fasting insulin levels in both sexes (P<0.05). In analyses stratified by baseline obesity status, the association between change in BMI and change in fasting insulin was significantly stronger in overweight (males: standardized β=1.136; females: standardized β=1.262) and obese (males: standardized β=1.817; females: standardized β=2.290) participants than in those with normal weight (males: standardized β=0.957; females: standardized β=0.976) at baseline. Results were similar for changes in WC.
Conclusion
Changes in anthropometric indices were positively associated with fasting insulin level increases. Moreover, those who were overweight or obese at baseline had a higher absolute increase in fasting insulin levels per one standard deviation unit increase in anthropometric indices than adolescents with normal weight.
INTRODUCTION
According to the World Health Organization, childhood overweight and obesity are among the most serious health issues that cause secondary health problems and lead to obesity in adulthood [1]. In the United States, the prevalence of obesity for children and adolescents was 16.9% in 2009 to 2010 [2]. In Korea, the prevalence of childhood and adolescent obesity has increased from 5.8% in 1997 to 9.7% in 2005, with the increase more pronounced in boys, particularly those aged 13 to 18 years [3]. In particular, Korean adolescents have less physical activities and increased sedentary behavior because of their daily schoolwork, making them obese [45].
The association between obesity and insulin resistance has been reported in observational studies [678910]. Adolescent obesity is associated with an increased risk of developing insulin resistance in adulthood [910]. Recent studies have demonstrated that high levels of glucose homeostasis variables, such as glucose, insulin, and homeostasis model assessment of insulin resistance (HOMA-IR), in childhood not only persist into adulthood but also predict pre-diabetes and type 2 diabetes mellitus and are related to cardiometabolic risk factors in adults [78]. There is also evidence that insulin resistance are not only the result of obesity, but also the possibility of contributing to the onset of obesity [11]. Despite extensive studies on insulin resistance and obesity, the association between changes in anthropometric indices during adolescents and changes in levels of glucose homeostasis variables remains unclear.
Therefore, we investigated the association between changes in anthropometric indices and change in fasting insulin levels over 2.4 years and further investigated whether the association differs according to obesity status at baseline.
METHODS
Study population
Our study was based on data collected for the JS High School study, which is a prospective cohort study of a Korean adolescent population. The target population of the JS High School study was first graders at a high school located in a rural area of South Korea. Baseline examinations were conducted on 1,071 participants in 2007, 2010, 2011, and 2012. Participants enrolled from 2007 through 2011 were re-examined after 30 months of follow-up, and those enrolled in 2012 were re-examined after 24 months of follow-up. We based our study on the 884 participants who participated in both the baseline investigations at first grade and the re-examination at third grade. The details of the study design and procedures have been previously described [12]. All participants had no previous diagnosis of diabetes mellitus or hypertension. Informed consent was obtained from each student as well as from his/her parent or guardian after full explanation of the purpose and process of the study. The study protocol and consent procedure were approved by the Institutional Review Board of Severance Hospital at Yonsei University College of Medicine (approval No. 4-20100169).
Measurements
Health-related lifestyle factors and disease history were evaluated using self-administered questionnaires. Smokers were defined as participants who smoked at least 100 cigarettes in their lifetime. Drinkers were defined as participants who consumed alcoholic beverages at least once a month over the last year. Regular exercise was defined as engaging in physical activity for at least 30 minutes once a week at moderate intensity, either indoor or outdoor. Height was measured to the nearest 0.1 cm using a stadiometer, while body weight was measured to the nearest 0.1 kg on a digital scale (Seca 763; SECA, Hamburg, Germany), with the subject wearing his/her school uniform. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2). Waist circumference (WC) was measured between the lower borders of the rib cage and the iliac crest with a measuring tape. Hip circumference was measured at the level of widest circumference over the greater trochanters. Waist-hip ratio (WHR) was calculated as WC divided by hip circumference. Waist-height ratio (WHtR) was calculated as WC divided by height.
Resting blood pressure was measured twice at 5-minute intervals using an automatic sphygmomanometer (Dinamap 1846 SX/P; GE, Chicago, IL, USA) with the participant in the sitting position. If the two readings differed by more than 10 mm Hg, additional measurements were obtained and the last two readings were averaged. Fasting blood samples were drawn after at least an 8-hour fasting. Serum concentrations of total cholesterol, high density lipoprotein cholesterol (HDL-C), and triglycerides were measured via enzymatic methods using an autoanalyzer (ADVIA 1800; Siemens Healthcare Diagnostics Inc., Deerfield, IL, USA). Fasting glucose level was measured via a glucose hexokinase method. The insulin level was measured via a radioimmunometric method. The HOMA-IR was calculated as the product of the fasting plasma insulin level (µIU/L) and the fasting plasma glucose level (mg/dL) divided by 405.
Statistical analysis
Data are presented either as mean±standard deviation or number with percent. Differences between baseline and follow-up were analyzed using a paired t-test. Correlations between variables were evaluated using Spearman's correlation coefficient. Multivariate linear regression models were used to examine the association between anthropometric indices and fasting insulin levels, adjusting for potential confounding variables, including age, smoking status, alcohol intake, and physical activity. To examine whether the association was dependent on baseline obesity status, we stratified the analyses according to the following baseline categories of BMI and WC. Sex-specific BMI-for-age and WC-for-age percentiles were calculated following the 2007 Korean National Growth Charts [13]. Based on BMI-for-age percentiles, participants were classified as normal weight (<85th percentile), overweight (85th percentile to <95th percentile), or obese (≥95th percentile). Based on WC-for-age percentiles, participants were classified as normal (<90th percentile) or obese (≥90th percentile). The changes in anthropometric indices and fasting insulin levels were computed as the difference between baseline and follow-up values. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA). Statistical significance was defined as a two-sided P value less than 0.05.
RESULTS
The clinical and biochemical characteristics at baseline and their changes over 2.4 years are presented in Table 1. As would be expected, anthropometric indices, blood pressure, total cholesterol, HDL-C, and fasting insulin levels were increased in both sexes. Meanwhile, triglycerides and fasting glucose significantly decreased in both sexes. Although BMI significantly increased in both sexes, BMI z-score decreased by 0.06 in males and by 0.19 in females on average. Fasting glucose decreased by 5.1 mg/dL in males and by 4.7 mg/dL in females on average. At follow-up, the proportion of hypertension, hyperglycemia, hypertriglyceridemia, and low HDL-C concentrations was observed in 2.8%, 1.5%, 3.9%, and 17.2% of males, respectively. In females, the proportion of hypertension, hyperglycemia, hypertriglyceridemia, and low HDL-C concentrations was observed in 0.7%, 0.7%, 3.5%, and 33.6%, respectively.
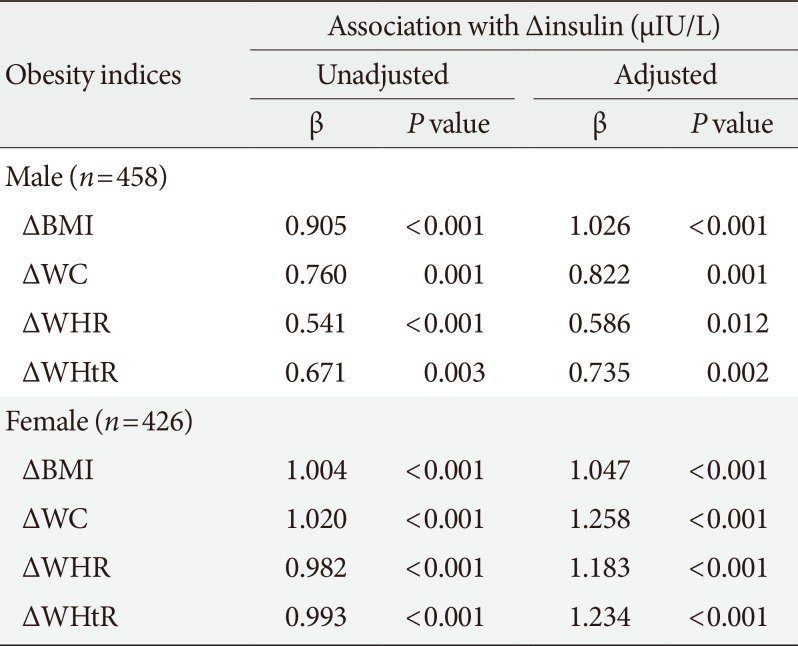
Results of multivariate linear regression analyses are presented in Table 2. In both sexes, increases in anthropometric indices were associated with increase in fasting insulin on follow-up in the unadjusted model. These associations remained significant even after adjusting for baseline age, BMI (or WC), smoking, physical activity, and alcohol intake. In males, the change in BMI was more strongly associated with change in fasting insulin (BMI: β=1.026, P<0.001) than indices of abdominal obesity (WC: β=0.822, P=0.001; WHR: β=0.586, P=0.012; WHtR: β=0.735, P=0.002). Meanwhile, in females, the change in indices of abdominal obesity was more strongly associated with change in fasting insulin (WC: β=1.258, P<0.001; WHR: β=1.183, P<0.001; WHtR: β=1.234, P<0.001) than BMI (β=1.047, P<0.001).
Results of subgroup analysis of obesity status at baseline are presented in Table 3. In analyses stratified by baseline BMI categories, the association between change in BMI and change in fasting insulin was stronger in the overweight group (males: β=1.136, P=0.028; females: β=1.262, P=0.389) and in the obese group (males: β=1.817, P=0.036; females: β=2.290, P= 0.040), than in the normal group (males: β=0.957, P=0.008; females: β=0.976, P<0.001) at baseline after adjusting for baseline age, WC, smoking, physical activity, and alcohol intake. Similarly, in analyses stratified by baseline WC categories, the association between change in WC and change in fasting insulin was stronger in the obese group (males: β=1.806, P= 0.060; females: β=2.080, P=0.017) than in the normal group (males: β=0.648, P=0.010; females: β=1.085, P<0.001) at baseline. There was a similar association between changes in obesity indices and change in HOMA-IR.
DISCUSSION
In this study, we demonstrated that the level of insulin increase was associated with changes in BMI, WC, WHR, and WHtR. Among the obesity indices, BMI and WC were more strongly associated with increase of fasting insulin levels than WHR and WHtR, and these associations were more pronounced among those who were overweight or obese at baseline compared to normal weight participants.
Our findings are consistent with other studies showing that obesity is associated with fasting insulin levels, and both BMI and WC were strongly associated with serum insulin levels compared to other anthropometric indices [141516]. In this study, fasting insulin levels are strongly associated with BMI in males, while they are associated with indicators of central obesity (WC, WHR, and WHtR) in females, presumably because of the difference in growth rate between males and females. Before women start menstruating, they have already reached close to their maximum height [17]. On the other hand, the growth rate of men peaks when they are approximately 14 years old, and the growth continues until about 18 to 20 years old [18]. Similarly, the average age of the participants in our study was 16 years old, which was when the growth spurts of the females was over, but the males continue to grow.
A cohort study of 3,313 children and adolescents aged 5 to 17 years reported that elevated fasting insulin levels have been shown to persist over time in children [19]. In addition, another previous study showed that participants aged 12 to 23 years with persistently high levels of insulin over the 8-year period had adverse effects on the levels of BMI, blood pressure, total cholesterol, triglycerides, and lipoprotein cholesterols, and they were more likely to develop hypertension, dyslipidemia, and obesity in young adulthood [20]. In our study, increasing levels of obesity indicators in obese adolescents at baseline was more closely associated with increasing fasting insulin levels than in those with normal weight. Our results indicated that obesity may play an important role in the association of the increases of insulin levels. Chronic elevated blood insulin levels promote further insulin resistance through desensitization of target cells by reducing the number of receptors expressed at the cell surface [2122]. Based on these mechanisms, the increase in insulin levels during adolescence might be associated with the risk of early adulthood onset type 2 diabetes mellitus [23]. In addition, we also demonstrated that correlation between obesity and fasting blood glucose was weaker than insulin levels in adolescents (Supplementary Table 1). According to previous studies [2425], in adolescents, insulin resistance may be associated with normal fasting blood glucose due to pancreatic compensatory hyperinsulinemia. In young people without diabetes, although plasma glucose levels are maintained within a narrow range through puberty, have increased fasting insulin levels [2627]. In fact, according to our results, the blood glucose concentration decreased and the insulin concentration increased for 2 years. This may be a sign of insulin resistance, where insulin secretion increases with increasing adiposity to maintain normal glucose levels [28]. Adolescence is generally easy to think that healthy group with a low incidence rate of cardiovascular disease or mortality rate. However, even if the symptoms do not appear, most illnesses and many health problems already begin in adolescence. In the same vein, it can be overlooked that obese adolescents continue to have elevated levels of insulin to control their blood glucose.
This study has some limitations. First, most of the study participants were in the midst of puberty; thus, hormonal status may influence the indices of anthropometry, as well as glucose homeostasis variables. Moreover, insulin resistance during puberty cannot be distinguished from insulin resistance due to obesity. In addition, our results showed that insulin concentration and HOMA-IR increased, but triglyceride, a factor associated with insulin resistance, decreased. Although we measured fasting insulin and calculated HOMA-IR, these indicators may not reflect changes in insulin resistance in adolescence. Second, because of the lack of dietary intake data, this study did not address the role of diet in the regulation of glucose homeostasis and related risk of obesity. Finally, this study was conducted at a single high school and included one ethnic group; thus, the study findings cannot be generalized to other adolescent populations. In cross-sectional study of general Korean adolescents [29], insulin and HOMA-IR decreased by increasing age. When the same population was followed up as in our study, the results showed that insulin and HOMA-IR may increase by increasing age. Therefore, more follow-up studies are needed for adolescents.
In conclusion, this study suggests that increase in BMI, WC, WHR, and WHtR were positively associated with increase in fasting insulin levels. Among all anthropometric indices, BMI and WC were more associated with elevated fasting insulin levels. Furthermore, compared with healthy adolescents, overweight or obese adolescents at baseline may have higher insulin levels when anthropometric indices are increased. Although insulin measurements are not recommended in adolescents [30], our results suggest that changes in obesity indices in adolescence may lead to an increase in insulin concentrations and HOMA-IR. As in many areas of child health, increases in anthropometric indices during adolescence will affect the overall health during adulthood. Therefore, lifestyle practices that prevent or control obesity are important during adolescence, and these practices may help prevent hyperglycemia, insulin resistance, and even diabetes.
ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) grant funded by the Ministry of Education, Science and Technology (MEST) (No. 2010-0007860, No. 2015R1D1A-1A09057301).
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
SUPPLEMENTARY MATERIAL
Supplementary Table 1
Correlation between changes in obesity indices and changes in levels of glucose homeostasis variables