Patterns of Nerve Conduction Abnormalities in Patients with Type 2 Diabetes Mellitus According to the Clinical Phenotype Determined by the Current Perception Threshold
Article information
Abstract
Background
Clinical manifestations of diabetic peripheral neuropathy (DPN) vary along the course of nerve damage. Nerve conduction studies (NCS) have been suggested as a way to confirm diagnoses of DPN, but the results have limited utility for evaluating clinical phenotypes. The current perception threshold (CPT) is a complementary method for diagnosing DPN and assessing DPN symptoms. We compared NCS variables according to clinical phenotypes determined by CPT measurements.
Methods
We retrospectively enrolled patients with type 2 diabetes mellitus who underwent both NCS and CPT tests using a neurometer. CPT grades were used to determine the clinical phenotypes of DPN: normoesthesia (0 to 1.66), hyperesthesia (1.67 to 6.62), and hypoesthesia/anesthesia (6.63 to 12.0). The Michigan Neuropathy Screening Instrument (MNSI) was used to determine a subjective symptom score. DPN was diagnosed based on both patient symptoms (MNSI score ≥3) and abnormal NCS results.
Results
A total of 202 patients (117 men and 85 women) were included in the final analysis. The average age was 62.6 years, and 71 patients (35.1%) were diagnosed with DPN. The CPT variables correlated with MNSI scores and NCS variables in patients with diabetes. Linear regression analyses indicated that hypoesthesia was associated with significantly lower summed velocities and sural amplitudes and velocities, and higher summed latencies, than normoesthesia. Sural amplitude was significantly lower in patients with hyperesthesia than in patients with normoesthesia.
Conclusion
NCS variables differed among patients with diabetes according to clinical phenotypes based on CPT and decreased sural nerve velocities was associated with hyperesthesia.
INTRODUCTION
Diabetic peripheral neuropathy (DPN) is the most common and early complication of diabetes [12]. Accumulating evidence suggests that half of patients with type 2 diabetes mellitus (T2DM) will eventually experience neuropathy and progressive injury of nerve fibers during the course of diabetes, and severe neuropathic symptoms result in poor quality of life [3]. Although it has been suggested that the diagnosis of DPN can be confirmed by electrophysiological nerve conduction studies (NCS) [1], NCS do not always detect early changes due to nerve damage. In addition, NCS tests are limited for evaluating the clinical characteristics of DPN in patients with various symptoms.
The perception threshold test (current perception threshold [CPT]) has been used to quantify sensory fiber function from the early asymptomatic to advanced severe neuropathic symptom stages by assessing the functions of all three major subgroups of sensory nerve fibers: Aβ, Aδ, and C-fibers [4]. This enables the qualification of clinical phenotypes according to an automated grading system: normoesthesia, hyperesthesia, and hypoesthesia [56]. However, it is not known how NCS measurements and clinical phenotypes differ in patients with diabetes. We investigated the changes in NCS variables in patients with diabetes based on the clinical phenotypes determined by CPT and attempted to identify the differences between patients with diabetes and those with hyperesthesia in terms of these variables.
METHODS
Subjects
Retrospective medical data were collected for patients with T2DM who were referred to the Diabetes Clinic of Inje University Sanggye Paik Hospital by a nearby clinic or hospital for further management of their hyperglycemia and evaluation of complications from January 2014 to December 2016; such management and evaluation addressed clinical, laboratory, and electrophysiological variables. The study enrolled patients with T2DM (n=265) examined on at least one previous occasion using both NCS and CPT within 3 weeks. Exclusion criteria were as follows: exposure history to drugs or toxins that can provoke peripheral neuropathy, presence of carpal tunnel syndrome or other causes of peripheral neuropathy (alcohol consumption, renal failure, hypothyroidism, and vasculitis), and typical musculoskeletal anomalies. Clinical data included demographic data, duration of diabetes mellitus, diabetic neuropathy control medicine, Michigan Neuropathy Screening Instrument (MNSI) questionnaire score (abnormal was defined as three or more positive responses) [7], CPT results, and NCS variables. Body mass index was calculated as weight in kilograms divided by the square of height in meters. Hypertension was defined as blood pressure ≥140/90 mm Hg or the use of anti-hypertensive medication. This study was approved by the Inje University Sanggye Paik Hospital Research Ethics Committee (2017–02–032–003).
CPT assessment
CPT measurements were performed using a neurometer (Neurotron, Baltimore, MD, USA). This device, which generates a constant alternating current (AC) stimulus, was applied at two different test sites: the right index finger (C7 dermatome) and the right big toe (L4/5 dermatome) [4]. The CPT was measured by a well-trained technician from the Cardiovascular and Metabolic Disease Center of Inje University Sanggye Paik Hospital. When a site on the right side was not testable, the assessment was performed on the left side. In brief, after a pair of small (1 cm) electrodes was taped to the test site, a conductive gel was used to maintain contact with the skin. The electrical stimulus was increased slowly until the patients reported a sensation. Three different frequencies of AC were applied: 2,000, 250, and 5 Hz. The procedure took an average of 7 minutes per patient. The CPT values were graded using Neuval software, which is provided with the device (grade 0=no abnormal measures; grade 12=completely anesthetic). This enabled the quantification of the automated grading system, and the CPT grade at the L4/5 dermatome was used to classify patients by the clinical DPN phenotypes as follows: normoesthesia (grade range, 0 to 1.66), hyperesthesia (grade range, 1.67 to 6.62), and hypoesthesia/anesthesia (grade range, 6.63 to 12.0) [8].
Conventional electrophysiological study
One technician performed NCS using standard methods [9]. Electromyography (Sierra Wave EMG system; Cadwell Industries, Kennewick, WA, USA) with standard filter settings (2 Hz to 10 kHz) and a surface stimulator was used for NCS. Round disk electrodes with a diameter of 10 mm were used to record potentials. The test was performed on the upper and lower extremities with more pronounced neuropathic symptoms (if a study was not testable on one side, it was performed on the other). The parameters included: distal motor and sensory latency, compound muscle action potential (CMAP) amplitude measured from negative to the negative peak, sensory nerve action potential (SNAP) amplitude measured from the negative to positive peak, and the motor and sensory conduction velocity. The average value from 10 or more responses was obtained. The instrument automatically calculated latencies and amplitudes. The motor and sensory conduction velocity was calculated by dividing the measured distance by the onset latency. Measurements were considered abnormal if they were outside normal limits after adjusting for age and height. The measured nerves and normal values of parameters were distal latency <3.9, <3.0, <5.3, and <5.4 m/sec for the median, ulnar, peroneal, and tibial nerves, respectively; CMAP >5, >6, >1, and >6 mV for the median, ulnar, peroneal, and tibial nerves; SNAP >10, >5, and >10 µV for the median, ulnar, and sural nerves; and nerve velocity >50.5, >49.4, >40.5, >41.1, and >32.1 m/sec for the median, ulnar, peroneal, tibial, and sural nerves. Abnormal NCS results were defined as more than one abnormal attribute in two separate nerves [10]. NCS variables in the lower extremities were included in these analyses.
Diagnosis of DPN
The diagnosis of DPN was confirmed if both patient symptoms (MNSI score ≥3) [7] and abnormal results of NCS as recommended by the Toronto Diabetic Neuropathy Expert Group [11] were satisfied.
Statistical analyses
SPSS version 23 (IBM Co., Armonk, NY, USA) statistical software was used to carry out the analyses. All data are expressed as the mean±standard deviation (SD). Differences in mean values or the prevalence of disease between groups were assessed using the independent Student's t-test, chi-square test, or analysis of variance, as appropriate. Variables showing a non-normal distribution in the Kolmogorov-Smirnov test were logarithmically transformed before analyses. Binary logistic regression analyses were performed with DPN as a dichotomous dependent variable (0=no, 1=yes). The predictors used were age, sex, glycosylated hemoglobin (HbA1c) level, and duration of diabetes. The Nagelkerke coefficient of determination (R2) was applied. We calculated the area under the receiver operating characteristic (ROC) curve to examine the sensitivity and specificity of CPT grade to identify patients with DPN, and the Youden index J was used to determine the optimal cut-off CPT grade for the diagnosis of DPN using MedCalc Statistical Software version 18 (MedCalc Software, Ostend, Belgium; http://www.medcalc.org; 2018). Correlations between variables were assessed using Pearson's correlation coefficient analyses. Linear regression analyses were performed with the NCS variables as dependent variables. The CPT phenotype was used as a predictor transformed to dummy variables in models 1 (no adjustment) and 2 (adjusted for age, sex, duration of diabetes, and HbA1c). When performing a two-sided test, P<0.05 was considered statistically significant.
RESULTS
Demographics and prevalence of DPN
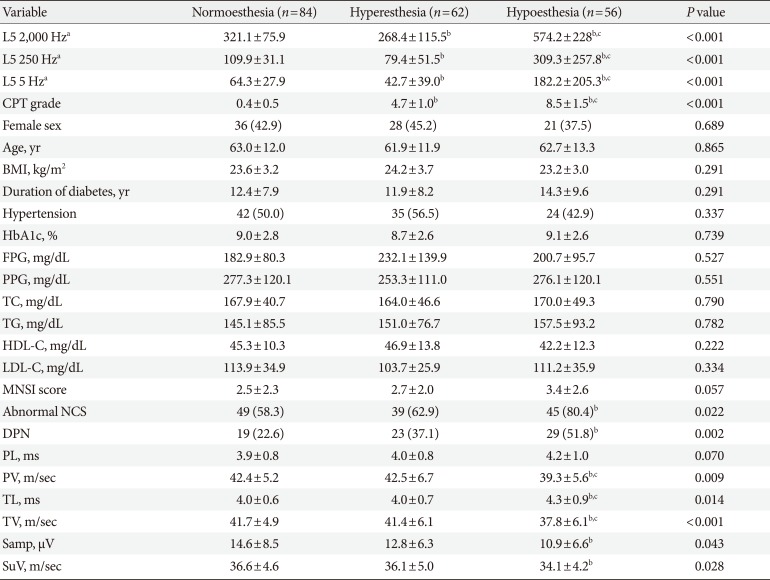
Of a total of 265 patients with T2DM, we excluded patients with an exposure history of drugs or toxins that can provoke peripheral neuropathy (n=18; 14 chemotherapy for malignancy, four anti-tuberculosis medication), presence of carpal tunnel syndrome (n=27) and other causes of peripheral neuropathy (n=18; six alcohol consumption, nine end-stage renal disease on dialysis, three hypothyroidism). Ultimately, a total of 202 patients (117 men and 85 women) were included. Demographic and clinical characteristics are presented in Table 1. The mean average age was 62.6 years. The mean HbA1c level was 8.9%±2.7%, and the mean duration of diabetes was 12.7±8.5 years. A total of 71 patients (35.1%) were diagnosed with DPN. A longer duration of diabetes and poorly controlled glycemic status (HbA1c and fasting plasma glucose) were observed in patients with DPN compared to those without DPN (P<0.05).
Relationships between CPT measures and MNSI and NCS variables
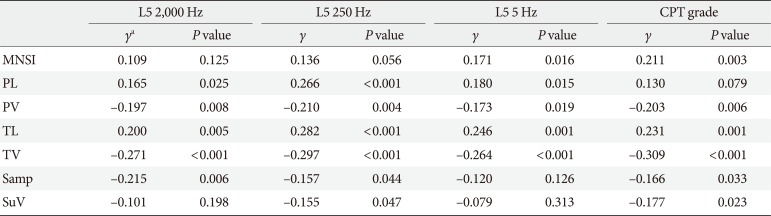
The relationships between measures of DPN (MNSI scores and NCS variables) and CPT variables were investigated by univariate correlation analyses (Table 2). The Pearson correlation (γ) between CPT grade and MNSI score was 0.211 (P=0.003). Each CPT variable (L5: 5, 250, and 2,000 Hz) was significantly correlated or inversely correlated with tibial and peroneal nerve conduction velocities and latencies, respectively. The CPT grade was significantly correlated with nerve conduction velocities in the lower extremities, and tibial nerve latency and sural nerve amplitude.
Diagnostic value of CPT measures for DPN
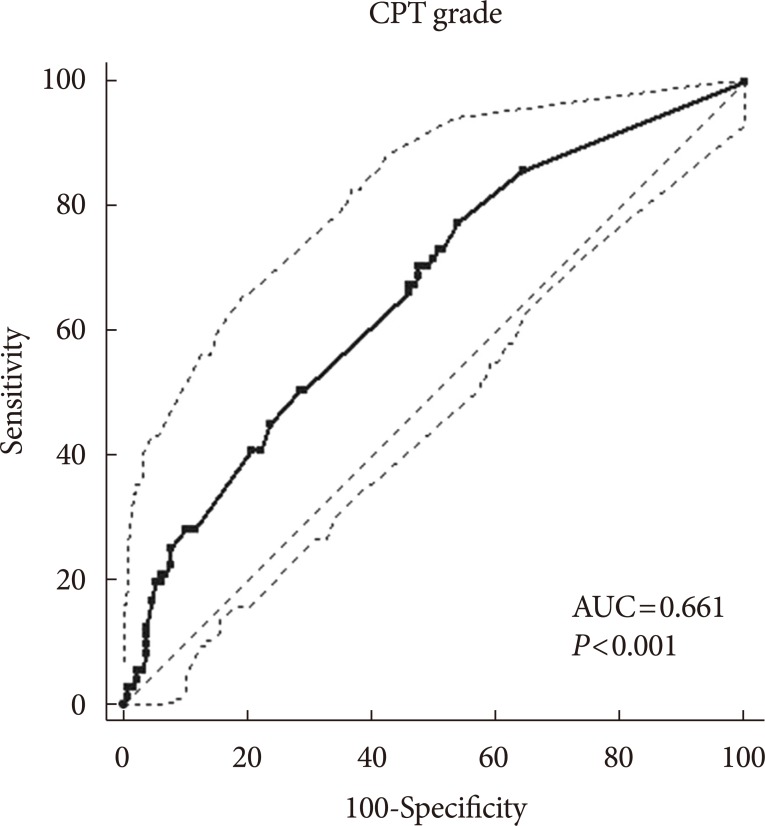
In bivariate logistic regression analyses in which the dependent variable was the presence or absence of DPN, the CPT grade was related to the presence of DPN (R2=18.4%; odds ratio [OR], 1.17; 95% confidence interval [CI], 1.07 to 1.28; P=0.001) after adjusting for age, sex, HbA1c, and duration of diabetes. ROC curve analyses found an area under the curve (AUC) of 0.661 (95% CI, 0.591 to 0.726; P<0.001). The sensitivity was 77.5%, the specificity was 46.2%, and the Youden index J was 0.24, with a CPT grade cut-off point greater than the associated criterion of 1.0 (Fig. 1).
Clinical and electrophysiological characteristics of patients by CPT phenotype: normoesthesia, hyperesthesia, and hypoesthesia
There were no significant differences in demographic and laboratory findings among the patients according to CPT phenotypes (normoesthesia, hyperesthesia, and hypoesthesia) (Table 3). Although several demographic (age and duration of diabetes) and clinical (HbA1c, postprandial glucose, total cholesterol, and high- and low-density lipoprotein cholesterol levels) variables differed between patients with hyperesthesia and those with normoesthesia or hypoesthesia, these differences did not reach levels of statistical significance. A chi-square test of independence was calculated to compare the frequency of DPN in patients with the three CPT phenotypes, and a significant interaction was found (χ2(2)=12.69, P=0.002). Patients with DPN were more likely to exhibit hypoesthesia. Most NCS variables differed between patients with normoesthesia and hypoesthesia. Where peroneal and tibial nerve velocities were lower in patients with hypoesthesia than in those with normoesthesia or hyperesthesia, the latencies in those nerves were higher.
Logistic regression of NCS variables for CPT phenotypes
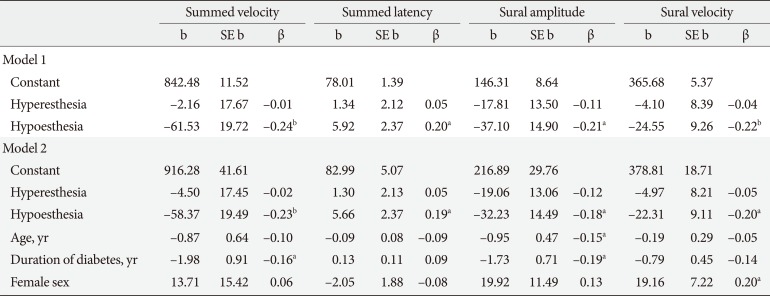
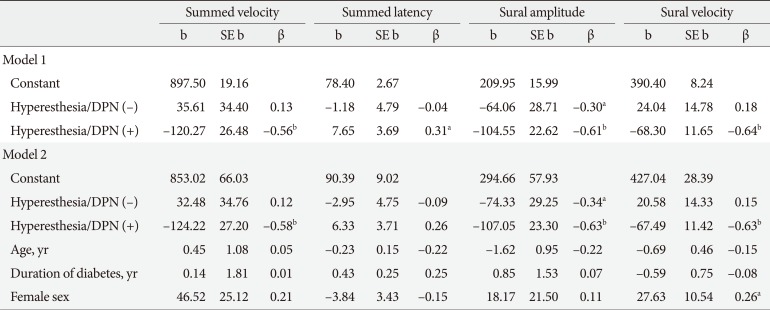
Linear regression analysis was used to assess whether the NCS variables differed significantly by CPT phenotype after adjusting for demographic factors and HbA1c levels. Hypoesthesia but not hyperesthesia was associated with significantly lower summed (tibial and peroneal nerve) velocities and sural amplitudes and velocities, but higher summed latencies compared to normoesthesia (Table 4). To explore the differences in NCS variables between normoesthesia and hyperesthesia over the clinical course of DPN, we performed the same analyses by transforming dummy variables as normoesthesia without DPN and hyperesthesia without and with DPN. Hyperesthesia both without and with DPN was associated with significant reductions in the sural nerve amplitude (adjusted R2=0.29, F(2, 46)=10.79, P<0.001; β=−0.30, P=0.031; and β=−0.61, P<0.001, respectively). These associations remained significant after adjusting for age, sex, duration of diabetes, and HbA1c levels (Table 5).

Linear regression evaluating the associations between clinical phenotypes and indices of nerve conduction studies in the whole population
DISCUSSION
CPT variables were correlated with MNSI scores and NCS variables in patients with diabetes, and those with a CPT grade indicating hypoesthesia were more likely to show deterioration in NCS results. NCS variables differed significantly by CPT phenotypes after adjusting for confounding factors. Hypoesthesia, but not hyperesthesia, was associated with significantly lower summed velocities (tibial and peroneal nerves) and amplitudes and velocities of the sural nerve, but higher summed latencies (tibial and peroneal nerves) compared to normoesthesia. Interestingly, multiple linear regression analyses indicated that the lower sural nerve amplitudes were associated with hyperesthesia regardless of DPN.
Both NCS and CPT are used to assess the severity of DPN, but they have been used differently depending on the purpose of the evaluation [12]. NCS are recommended as a gold standard for diagnosing DPN and should help in assessing large-fiber nerve damage [1]. The diagnostic role of NCS in the early detection of nerve damage in patients with diabetes has long been studied, but its use in the clinical evaluation of patients' symptoms and corresponding nerve damage is neither practical nor possible [1314]. Nevertheless, CPT may have merit in assessing the presence or absence of early DPN, because it is used as a quantitative sensory test to assess nerve fiber impairment [151617]; specifically, it evaluates sensory perception thresholds using different electrical currents: 5 Hz for unmyelinated C-fibers, 250 Hz for thin myelinated Aδ-fibers, and 2,000 Hz for large myelinated Aβ-fibers [18]. However, NCS variables have not previously been studied according to clinical phenotypes based on CPT grades in patients with diabetes.
In this study, each measure of CPT at different stimuli (5 to 2,000 Hz) was correlated with the NCS variables (velocities and latencies) for sensory nerves in the lower extremities. The CPT grades had stronger correlations with these variables and were related to the presence of DPN (OR, 1.17; 95% CI, 1.07 to 1.28; P=0.001) after adjusting for age, sex, HbA1c levels, and duration of diabetes. Therefore, we used the CPT grade rather than each frequency of CPT measurement, provided by the Neuval software accompanying the neurometer, to evaluate the functional integrity of peripheral nerve damage in patients with diabetes and to classify the clinical phenotypes for clinical purposes.
Along with the CPT phenotypes, the proportion of patients with abnormal NCS increased significantly (χ2(2)=7.59, P=0.022). The hypoesthesia group was more likely to exhibit deterioration in the NCS variables compared with the normoesthesia or hyperesthesia group (Tables 3 and 4), which is consistent with many previous studies [56]. In our study, nearly half the patients with abnormal NCS (n=62/133) remained subclinical (MNSI score ≤3), which is also in agreement with previous reports [19]. Among these, 30 patients (48.4%) were stratified as normoesthetic. Therefore, the role of neurometry in the diagnosis of nerve injury in patients with subclinical DPN is limited, and the diagnostic value of neurometry was not sufficiently high to supplant NCS (specificity 46.2%, Youden index J 0.24). However, threshold for CPT at each frequency was significantly decreased in patients with hyperesthesia compared with those with normoesthesia, and they were increased in patients with hypoesthesia; however, all the NCV variables for the lower extremities and the mean MNSI score in patients with hyperesthesia did not differ from those in patients with normoesthesia. Because hyperesthesia precedes hypoesthesia in the progression of DPN [20], this likely reflects a continuum in the natural history of diabetic neuropathy from normal nerve function (normoesthesia) to excitable nerve damage (hyperesthesia), and then hypoesthesia or anesthesia [19]. Therefore, using neurometry for evaluating DPN in patients with T2DM could discriminate among the clinical phenotypes in patients with subclinical DPN. The detection of hyperesthesia based on CPT grade even before abnormal NCS findings develop could provide an opportunity to prevent diabetic foot problems [21]. Recently, neuropathic pain phenotyping has been suggested to predict the response to different mechanism-based medications [22]. Therefore, this complementary diagnostic approach may lead to more stratified treatment and, potentially, to personalized pain therapy.
Logistic regression analysis indicated that decreased sural nerve velocities were significantly associated with hyperesthesia compared with normoesthesia with normal NCS results regardless of DPN (Table 5). A lower conduction velocity or SNAP of the sural nerve has a high sensitivity in the diagnosis of peripheral polyneuropathy. One study observed sural nerve conduction abnormalities in 38% and 42% of patients with the subclinical stage of type 1 diabetes mellitus (T1DM) and T2DM, respectively, but no other peripheral nerve abnormalities were found [23]. Pastore et al. [24] suggested that both sural action potential amplitudes and sural conduction velocities are useful for detecting DPN in T1DM and T2DM patients. Recently, Zhang et al. [25] showed that sensory nerve abnormalities, particularly sural action potential amplitudes, were more obvious than were motor nerve abnormalities in symptomatic diabetes patients than in asymptomatic healthy controls. In agreement with previous studies [232425], all the NCS indices in our study showed compatible changes in patients with DPN: lower nerve conduction velocities and higher latencies of sensory and motor nerves; additionally, we suggest that decreased sural nerve velocities reflect nerve injuries in patients with hyperesthesia.
Several limitations of this study should be considered. First, this was a single-center study performed at a tertiary center, not in the community, with a relatively small number of patients. However, we calculated sample size with the Z-test in binary logistic regression analyses using G*Power (http://www.gpower.hhu.de/). The mean±SD CPT grade was 4.0±3.5 in the study population, and the prevalence of DPN below the mean CPT value was 24.7% (Pr[Y=1|X=1]H0), and at 1 SD above the mean it was 60.6% (Pr[Y=1|X=1]H1). The two-tailed α level was 0.05 and the power (1−β) was 0.80. Based on these data, the total sample size was 159 and the power was 0.80 [26]. Second, the study group was selected from patients who visited our clinic but were not hospitalized. Although glycemic status reflected a less pronounced difference among patients with different phenotypes of nerve damage by CPT grade, duration of diabetes was significantly associated with abnormal NCS results in bivariate logistic analyses after adjusting for age, sex, and HbA1c levels (OR, 1.09; 95% CI, 1.04 to 1.15; P=0.001). Therefore, the long-term effects of poor metabolic control and long duration of diabetes should be considered when interpreting the results, because patients in this study were recruited at a tertiary hospital after referral for management of their hyperglycemia; the comorbidities, mean age, and HbA1c levels were relatively high; and the duration of diabetes was long [2728]. Therefore, the results may not be generalizable to the whole population and must be interpreted with caution. Despite these limitations, we found that CPT is a useful instrument for detecting differences in NCS variables between clinical phenotypes determined by CPT grade.
NCS is an indispensable tool in the evaluation of peripheral nerve abnormalities to confirm DPN [9], but it is of limited use for the evaluation of various clinical phenotypes. In this study, we found that NCS variables differed across patients with diabetes according to clinical phenotype determined by CPT. Moreover, decreased sural nerve velocities were associated with changes in the NCS variables even in non-DPN patients with hyperesthesia. These results support a more stratified or even personalized treatment approach in the future.
ACKNOWLEDGMENTS
This work was supported by the grant from the Priority Research Centers Program and Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (NRF-2010-0020224, NRF-2015R1A2A1A13001900).
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.