The Role of the Sweet Taste Receptor in Enteroendocrine Cells and Pancreatic β-Cells
Article information
Abstract
The sweet taste receptor is expressed in taste cells located in taste buds of the tongue. This receptor senses sweet substances in the oral cavity, activates taste cells, and transmits the taste signals to adjacent neurons. The sweet taste receptor is a heterodimer of two G protein-coupled receptors, T1R2 and T1R3. Recent studies have shown that this receptor is also expressed in the extragustatory system, including the gastrointestinal tract, pancreatic β-cells, and glucose-responsive neurons in the brain. In the intestine, the sweet taste receptor regulates secretion of incretin hormones and glucose uptake from the lumen. In β-cells, activation of the sweet taste receptor leads to stimulation of insulin secretion. Collectively, the sweet taste receptor plays an important role in recognition and metabolism of energy sources in the body.
SWEET TASTE RECEPTOR IN TASTE BUDS
Taste sensation is important for animals and even exists in worms, including the model nematode, C. elegans [1]. In the fruit fly, Drosophila melanogaster, taste is first detected by neuronal cells which express a family of G-protein-coupled receptors (GPCRs). These GPCRs are activated by taste substances [2]. In vertebrates, cells detecting taste originate from the epithelium rather than neurons, receiving taste signals and transmitting them to adjacent neurons. Taste cells are distributed either singly in the epithelium or form densely packed clusters, taste buds, which consist of up to 100 taste cells. In mammals, taste buds are distributed in the oral epithelium of the tongue, palate, and pharynx. Taste cells are bipolar cells whose microvilli are exposed to the oral cavity. Taste receptors are located in the microvilli and sense taste substances in the oral cavity.
There are five well-recognized taste sensations including sweet, bitter, umami, salty, and sour [3]. Detection of sweet and umami taste is especially important for recognition of food and nutrition. It is conceivable that recognition of a sweet sensation evolved to detect sugars, an important source of energy. Sweet sensation is activated by various types of sweet compounds, including amino acids, peptides, proteins, carbohydrates, and other types of organic chemicals, some of which have no nutritional benefit. Sweet sensation plays a critical role in animals in the recognition of food and detection of sugars in these foods.
The molecular nature of the sweet taste receptor was revealed in the beginning of the 21st century by molecular cloning [4]. The sweet taste receptor consists of a heterodimer of two GPCRs, T1R2, and T1R3 (Fig. 1). T1R2 and T1R3, together with a related molecule T1R1, are members of the T1R family, which belongs to the class C GPCR. Heterodimers of T1R1 and T1R3 function as a umami receptor. Receptors in this family resemble, in many respects, those of the metabotropic glutamate receptor 1 (mGluR1) and the calcium-sensing receptors [5]. They have a large extracellular domain, termed the venous flytrap domain (VFTD), in the N-terminal portion, and a cystein-rich domain, which connects the VFTD and transmembrane (TM) domains. Based on the 3-dimensional structure of mGluR1, it is proposed that two cavities in the VFTD accept sweet molecules, such as sugars, sweet amino acids, and a synthetic dipeptide, aspartame [6]. Binding of these molecules may induce conformational changes in the flytrap domain, leading to activation of the receptor. Consistent with this notion, the amino acid-binding residues in the VFTD of the T1R2-T1R3 are highly conserved compared with those of mGluR1. However, the sweet taste receptor is activated by a variety of molecules with completely different structures. These include sugars, amino acids, peptides, proteins, and various other types of organic compounds. This implies that these molecules may bind to alternate portions of the receptor. In agreement with this concept, it has been proposed that sweet proteins interact with an external portion of the active form of the receptor. The cystein-rich region of T1R3 is another candidate site for the binding of sweet proteins [7]. Furthermore, the artificial sweetener cyclamate may bind to the C-terminal TM region of T1R3 [8,9]. Collectively, the sweet taste receptor is activated by various compounds with diverse structures, with different types of compounds potentially binding to different portions of the receptor.
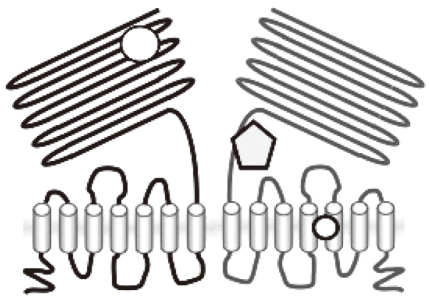
Structure of the sweet taste receptor. The sweet taste receptor consists of a heterodimer of the class C GPCRs, T1R2, and T1R3. Various sweet substances bind to different portions of the receptor.
Binding of sweet substances to the sweet receptor T1R2-T1R3 activates trimeric G protein(s) and generates second messengers in taste cells. Candidate G proteins include gustducin [10], a 'taste cell-specific' trimeric G protein resembling transducin and Gs Gustducin has been shown to be involved in the signal transduction pathway activated by bitter taste receptors. Given the fact that mice lacking the gustducin gene have impaired sweet sensation [11], it is thought that gustducin is also involved in the sweet receptor signaling system.
With regard to the second messengers generated by activation of the sweet taste receptor, both cyclic AMP (cAMP) and calcium may act as second messengers. Using membrane fractions isolated from the anterior surface of the rat tongue, Striem et al. [12] demonstrated that sucrose stimulates adenylate cyclase activity. This result suggests involvement of Gs in the signaling pathway activated by the sweet taste receptor. Also, sucralose elevates cAMP levels in taste buds through a calcium-independent mechanism [13]. The signaling pathway downstream of cAMP production is still uncertain, and there is a controversy as to whether or not protein kinase A is involved in the downstream signaling.
Another second messenger generated by the sweet taste receptor is calcium. Binding of a sweet tastant activates phospholipase C-β2 (PLC-β2) [14], presumably by a G protein-dependent mechanism. Activation of PLC-β2 leads to generation of inositol (1, 4, 5)-trisphosphate (Ins-P3) and diacylglycerol. Ins-P3 mobilizes calcium from the endoplasmic reticulum (ER) by activating the type III Ins-P3-receptor channel. One of the unique properties of sweet taste receptor signaling is that calcium released from the ER activates the TRPM5 channel in the plasma membrane [15]. TRPM5 is a calcium-activated cation channel, and activation of TRPM5 results in sodium entry, which depolarizes the plasma membrane and thereby induces calcium entry through the voltage-gated calcium channel. Consistent with this signaling cascade, knockout of the PLC-β2 or TRPM5 gene reduces sweet taste sensation [16,17].
Molecular identification of the sweet taste receptor and its signaling effectors enabled us to investigate the expression of this receptor in various organs and tissues other than the gustatory system. It is now evident that the expression of the functional sweet taste receptor system is not restricted to the taste buds but is also present in other tissues, including cells in the gastrointestinal epithelium [18-20], endocrine cells of the pancreas [21], and glucose-sensing neurons in the brain [22]. It is now thought that the sweet taste receptor has regulatory roles in these extra-gustatory cells.
SWEET TASTE RECEPTOR EXPRESSED IN GASTROINTESTINAL TRACT
The expression of the signaling component of the sweet taste receptor in the extra-gustatory system was first described by Hofer et al. [23]. They found that α-gustducin is expressed in rat stomach, duodenum, and pancreatic duct. More recently, expression of PLC-β2 and TRPM5 was reported in the gastrointestinal tract [14,24]. Although it is technically difficult to demonstrate coexpression of all components of the signaling system at the single cell level, these results suggest that the sweet taste receptor system is expressed in the gastrointestinal tract, especially the small intestine and colon. In accordance with these findings, Dyer et al. [25] noted expressions of T1R2, T1R3, and α-gustducin in mouse duodenum and small intestine at the mRNA and protein levels. The expressions of T1R2 and T1R3 are greater in the jejunum and duodenum than in the ileum, indicating that expression is more abundant in the upper small intestine. They also found that the enteroendocrine cell line STC-1 expresses T1R2, T1R3, and α-gustducin [25] and postulated that the sweet taste receptor may function as a luminal sugar sensor in the gut. Expressions of the sweet taste receptor and its signaling components in humans have also been reported by Bezencon et al. [26]. These authors demonstrated that T1R2, T1R3, gustducin, PLC-β2, and TRPM5 are expressed in the duodenum, jejunum, ileum, and colon. T1R1 and T1R3, components of the umami receptor, are also expressed in the stomach [26].
At least two types of cells in the epithelium of the gastrointestinal tract express sweet taste receptors. One is the brush cell, a putative chemosensory cell morphologically resembling taste cells with microvilli in the apical region. Another type of cell expressing the sweet taste receptor is the enteroendocrine cell. Indeed, enteroendocrine L-cells secreting glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) and K-cells secreting glucose-dependent insulinotropic peptide (GIP) are positive for the sweet taste receptor. In the stomach, T1R3, a common subunit of the sweet and umami taste receptors, is expressed in brush cells and ghrelin-producing endocrine cells [27].
Intestinal neuroendocrine L-cells sense luminal glucose concentrations and secrete the incretin hormone GLP-1, which regulates insulin secretion from pancreatic β-cells. GLP-1 also modulates the appetite and motility of the gut. L-cells express T1R2 and T1R3 as well as gustducin, PLC-β2, and TRPM5 [28]. When glucose is administered to the stomach, secretion of GLP-1 is stimulated. This glucose-induced GLP-1 secretion is attenuated in α-gustducin knockout mice [28], and glucose-induced insulin secretion is delayed. Likewise, when glucose is directly administered to the duodenum, secretion of GLP-1 is induced. This effect is completely blocked in α-gustducin knockout mice [28]. When duodenal tissue is incubated in vitro, a high concentration of glucose induces a 3.5-fold increase in GLP-1-secretion. In duodenal tissue obtained from α-gustducin knockout mice, a high concentration of glucose induces only a 2-fold increase in GLP-1, significantly lower than that in normal mice [28]. These results indicate that in vivo luminal glucose-induced GLP-1 secretion is dependent on gustducin, and also there is a gustducin-independent action of glucose in duodenal cells. Using NCL-H716 cells, a human enteroendocrine L-cell line, Jang et al. [28] demonstrated that sucrose, glucose, and sucralose, an artificial sweetener, stimulate GLP-1 secretion, and that this stimulation is blocked by lactisole, an inhibitor of human T1R3. In humans, administration of glucose to the stomach or directly to the duodenum induces an elevation of plasma GLP-1 concentration. This GLP-1 response to glucose is markedly inhibited by lactisole, an inhibitor of the sweet taste receptor [29]. Interestingly, the inhibitory action of lactisole is greater when glucose is administered intragastrically. This raises the possibility that the sweet taste receptor may also be involved in the regulation of GLP-1 secretion in the stomach. As T1R2 is not detected in the stomach [26], a homodimer of T1R3 may function as a sweet taste receptor. Taken together, secretion of GLP-1 from enteroendocrine L-cells is induced by glucose, and this effect may be mediated, at least in part, by the sweet taste receptor [28].
Using mouse L-cells in primary culture, Reimann et al. [30] reported slightly different results. They isolated L-cells using a fluorescence-activated cell sorter from a transgenic mouse expressing a fluorescently tagged Venus protein under the control of the proglucagon promoter. Using these cells in culture, they found that the ATP-sensitive potassium channel is functional in these cells, and that the sodium-glucose cotransporter (SGLT1) is involved in glucose-induced GLP-1 secretion. They also suggested that involvement of the sweet taste receptor is unlikely in glucose-induced GLP-1 secretion. At present, the reason for the discrepancy is not certain. There are some points to be mentioned in regard to these disparate results. The L-cells obtained by a cell sorter may be a mixed population rather than a single population. In fact, in L-cells isolated from the colon, sucralose increases GLP-1 secretion, whereas L-cells obtained from upper small intestine do not respond to sucralose [30]. Consequently, the property of L-cells obtained by a cell sorter may differ depending upon their original location along the intestinal tract. It is also possible that L-cells obtained from a certain portion of the intestine are not a single population. If so, analysis of responsiveness in a single cell should be performed carefully. Culture of L-cells is rather difficult, and cells cultured for a relatively long period may be slightly different from those in vivo. As mentioned by Reinmann et al. [30], isolated L-cells survive in vitro only when cultured as a mixed population. Cultured L-cells may have slightly different properties compared to L-cells in vivo. It is also possible that responsiveness changes as a function of time when cultured in vitro. In this regard, assessment of GLP-1 secretion in knockout mice lacking T1R2 or T1R3 is needed to elucidate the definite role of the sweet taste receptor in GLP-1 secretion.
In addition to the regulation of incretins, the sweet taste receptor also controls glucose uptake from the intestine. Glucose uptake from the intestinal lumen by absorptive enterocytes is mediated by two different types of glucose transporters, SGLT-1 and the facilitative glucose transporter GLUT2 [31]. SGLT-1 has an apparent Km of 8 to 20 mM and is important at relatively low luminal concentrations of glucose. The expression of SGLT-1 in brush-border of enterocytes is regulated by a glucose sensor facing the luminal membrane.
Margolskee et al. [20] reported that glucose absorption and the expression of SGLT-1 in enterocytes are regulated by dietary sugars and artificial sweeteners in normal mice. The effects of sugars and artificial sweeteners are attenuated in knockout mice lacking T1R3 or gustducin [20]. They also found that T1R2, T1R3, and gustducin are expressed in enteroendocrine cells [20]. Given that the sweet taste receptor regulates secretion of GLP-1 in enteroendocrine L-cells, they suggest that dietary sugars and artificial sweeteners act on the sweet taste receptor in L-cells, which stimulates GLP-1 secretion and, in turn, upregulates SGLT1 expression in enterocytes, thereby increasing glucose absorption [20].
The sweet taste receptor also regulates GLUT2 in enterocytes. GLUT2 is a facilitative glucose transporter with higher capacity but lower Km for glucose than SGLT1. This implies that, at high concentrations of luminal glucose, transport through GLUT2 is more important than transport through SGLT1. The sweet taste receptor is located in the brush border of enterocytes [32]. Activation of this receptor leads to insertion of GLUT2 into the brush border membrane, which facilitates glucose uptake via this transporter. For insertion of GLUT2, activation of protein kinase C-βII caused by the sweet receptor is critical [33]. Additionally, glucose uptake through SGLT1 causes depolarization of the plasma membrane since SGLT1 is a Na+-glucose cotransporter. Resultant depolarization induces calcium entry via the voltage-gated calcium channel Cav1.3, elevating cytoplasmic Ca2+ concentration. This also favors activation of protein kinase Cβ-II [32]. Taken together, the sweet taste receptor modulates the expressions and functions of SGLT1 and GLUT2 and upregulates glucose transport in intestinal epithelium.
SWEET TASTE RECEPTOR EXPRESSED IN PANCREATIC ISLET CELLS
Pancreatic endocrine cells originate from the endoderm and share many characteristics with enteroendocrine cells [34]. Pancreatic β-cells produce and secrete insulin, a principal hormone regulating fuel metabolism in the body. We have shown that the functional sweet taste receptor is expressed in pancreatic β-cells [21]. Thus, mRNA for T1R2 and T1R3, as well as that for gustducin, is expressed in mouse pancreatic islets. Immunohistochemistry using anti-T1R3 antibodies indicates that immunoreactivity of T1R3 is localized in insulin-producing β-cells. When the sweet taste receptor in islet cells is activated by sucralose, insulin secretion is augmented. The effect of sucralose is observed in the presence of a low concentration of glucose (2.8 mM). At higher concentrations of glucose, the effect of sucralose is more significant. The sweet taste receptor is also expressed in MIN6 cells, a glucose-responsive β-cell line. In these cells, artificial sweeteners such as sucralose, saccharin and acesulfame-K all induce insulin secretion. Again, the effects of sweeteners are observed in the presence of a low concentration of glucose and are much greater in the presence of high concentrations of glucose [21]. Sucralose significantly potentiates glucose-induced insulin secretion. Using MIN6 cells, it is now possible to monitor real-time changes in intracellular messengers in a single living cell. As shown in Fig. 2, sucralose induces an immediate and sustained elevation of cytoplasmic Ca2+ concentration ([Ca2+]c). The sucralose-induced elevation of [Ca2+]c is inhibited by gurmarin, an antagonist of the sweet taste receptor. In addition, calcium response to sucralose is not affected by an inhibitor of Gq, whereas the Gq inhibitor completely blocks carbachol-induced calcium response. Thus, results suggest that Gq is not involved in the action of sucralose. When extracellular calcium is removed, the sucralose-induced elevation of [Ca2+]c is markedly inhibited. A small initial peak is observed, but the sustained phase of [Ca2+]c elevation is abolished. Likewise, addition of nifedipine, an inhibitor of the L-type voltage-gated calcium channel abolishes a sustained elevation of [Ca2+]c. These results indicate that sucralose increases [Ca2+]c by causing release of calcium from an intracellular pool and also by stimulating calcium entry via voltage-gated calcium channels. Note that sucralose-induced calcium entry is completely dependent on extracellular Na+, suggesting that this sweetener stimulates Na+ entry, depolarizes the plasma membrane, and stimulates calcium entry via voltage-gated calcium channel. With regard to the effects of sucralose on cAMP, sucralose-induced elevation of [cAMP]c is not blocked by either removing extracellular calcium or by adding nifedipine. Hence, sucralose-induced changes in [cAMP]c may not be secondary to the changes in [Ca2+]c but are perhaps mediated by activation of Gs. In addition to elevations in [Ca2+]c and [cAMP]c, sucralose also rapidly activates PKC and induces phosphorylation of a PKC substrate (Fig. 2B). Collectively, the sweet taste receptor expressed in pancreatic β-cells is unique in the sense that it activates both calcium and cAMP messenger systems [21]. Presumably, the sweet taste receptor increases [cAMP]c by activating Gs and induces calcium signaling by activating a G protein different from Gq, possibly gustducin. A schematic presentation of the signal transduction pathway activated by the sweet taste receptor in β-cells is shown in Fig. 3.
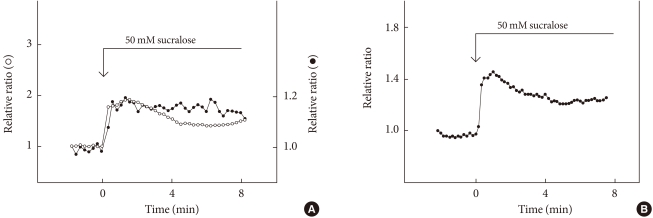
Effect of sucralose on [Ca2+]c, [cAMP]c, and PKC activation. (A) Epac-camp-expressing MIN6 cells loaded with fura-2 were incubated with 50 mM sucralose, and changes in [Ca2+]c (○) and [cAMP]c (•) were monitored. (B) MIN6 cells expressing MARCKS-GFP were incubated with 50 mM sucralose, and changes in the amount of MARCKS-GFP in the cytosol were monitored.
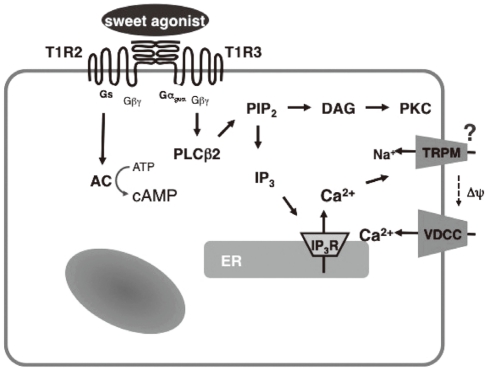
Signaling system activated by the sweet taste receptor in β-cells. PLCβ2, phospholipase C-β2; cAMP, cyclic AMP; ER, endoplasmic reticulum.
The physiologic significance of the sweet taste receptor in pancreatic β-cells is unclear at present. Unlike the sweet taste receptor in taste buds and gastrointestinal epithelium, the sweet taste receptor in β-cells is exposed to the bloodstream. Hence, sweet substance(s) in plasma may be a regulator of the receptor. An obvious candidate is glucose, a major energy source in the body. Since β-cells sensitively detect plasma glucose concentration, it would be interesting to clarify the role of the sweet taste receptor in the context of the glucose-sensing machinery of β-cells. As β-cells express the functional sweet receptor signaling system, and since this receptor is activated by glucose, the most straight-forward interpretation is that this receptor is part of the glucose-sensing machinery of β-cells. A critical question is to what extent the sweet taste receptor is involved in glucose-sensing in β-cells. In this regard, the sensitivity of this receptor to ambient glucose is not high, at least in a heterologous expression system [20]. Thus, it is uncertain whether or not the sweet receptor signaling system, as activated by ambient glucose, is reasonably detectable. This is an important issue and should be determined experimentally. Given that the sweet taste receptor is activated by various compounds with completely different chemical structures, it is also possible that some endogenous agonists apart from glucose exist, either in the extracellular fluid or cytosol. Such an endogenous compound, if any, would therefore modulate insulin secretion. This intriguing possibility should be examined experimentally. The sweet taste receptor expressed in β-cells has a unique signaling system in that it activates both the calcium and cAMP signaling systems. Since agents which increase cAMP production in β-cells would protect these cells from various stresses and apoptosis, the sweet taste receptor may be a potential molecular target for developing novel therapeutic agents to treat diabetes.
ACKNOWLEDGMENTS
The authors are grateful to Mayumi Odagiri for secretarial assistance during the preparation of the manuscript.
Notes
No potential conflict of interest relevant to this article was reported.