Beneficial Effects of Aerobic Exercise Training Combined with Rosiglitazone on Glucose Metabolism in Otsuka Long Evans Tokushima Fatty Rats
Article information
Abstract
Background
Regular aerobic exercise is essential for the prevention and management of type 2 diabetes mellitus and may be particularly beneficial for those treated with thiazolidinediones, since it may prevent associated weight gain. This study aimed to evaluate the effect of combined exercise and rosiglitazone treatment on body composition and glucose metabolism in obese diabetes-prone animals.
Methods
We analyzed metabolic parameters, body composition, and islet profiles in Otsuka Long Evans Tokushima Fatty rats after 28 weeks of aerobic exercise, rosiglitazone treatment, and combined exercise and rosiglitazone treatment.
Results
Combined exercise with rosiglitazone showed significantly less increase in weight and epididymal fat compared to rosiglitazone treatment. Aerobic exercise alone and combined rosiglitazone and exercise treatment led to similar retention of lean body mass. All experimental groups showed a decrease in fasting glucose. However, the combined exercise and rosiglitazone therapy group showed prominent improvement in glucose tolerance compared to the other groups. Rescue of islet destruction was observed in all experimental groups, but was most prominent in the combined therapy group.
Conclusion
Regular aerobic exercise combined with rosiglitazone treatment can compensate for the adverse effect of rosiglitazone treatment and has benefit for islet preservation.
INTRODUCTION
Obesity is known to be a major predisposing risk factor for type 2 diabetes mellitus (T2DM) [1]. Obesity may lead to insulin resistance and β-cell dysfunction [23]. Major strategies for T2DM therapy focuses on the alleviation of insulin resistance, improvement of β-cell function, and weight loss for obese patients [4]. Rosiglitazone, a thiazolidinedione (TZD) class antidiabetic agent, acts through activating the nuclear transcription factor peroxisome proliferator-activated receptor γ [567]. In animal models of insulin resistance, rosiglitazone has been reported to decrease plasma glucose and insulin levels, improve insulin action in skeletal muscles, and prevent β-cell atrophy [8]. It also has been reported that rosiglitazone promotes β-cell survival and preserves β-cell mass in human islet amyloid polypeptide transgenic mice [89]. However, rosiglitazone causes weight gain as an adverse effect, leading to overweight and obesity, which may be a major limitation of its use [10].
Aerobic exercise has been traditionally prescribed for diabetes prevention and management. Aerobic exercise has beneficial effects on glucose metabolism, mainly through improvement of insulin sensitivity [11]. Aerobic exercise is an important component of lifestyle modification interventions for weight loss. Therefore, aerobic exercise that can lead to increased energy consumption may be beneficial in preventing the weight gain associated with the use of rosiglitazone [12]. Previous studies have shown the beneficial effects of combined exercise and rosiglitazone in improving cardiovascular risk factors in patients with T2DM [1314].
Beneficial effects of aerobic exercise on β-cell preservation has been suggested in animal and human studies [15]. However, the effect of combined regular aerobic exercise and rosiglitazone treatment on the pancreas islet in T2DM has not been evaluated. We hypothesized that the combination of aerobic exercise with rosiglitazone treatment can enhance positive effects by compensating for the adverse effect of weight gain caused by TZDs, as well as by accelerating the improvement of insulin resistance and β-cell preservation. In the present study, we investigated changes in body composition, glucose metabolism, insulin resistance, and islet morphology in a T2DM animal model after administrating aerobic exercise and rosiglitazone, alone and concomitantly.
METHODS
Animals
The Otsuka Long Evans Tokushima Fatty (OLETF) rat is a useful animal model for T2DM with obesity [16]. At 5 to 6 weeks, the OLETF rat develops obesity, while diabetic symptoms appear at about 24 weeks [17]. Male OLETF rats and age-matched male Long-Evans Tokushima Otsuka (LETO) rats were supplied by Tokushima Research Institute, Otsuka Pharmaceuticals (Tokushima, Japan). The rats were individually housed in a standard animal facility with access to standard food and water ad libitum and maintained on a 12-hour light-dark cycle (lights on at 7:00 AM) in a temperature- (22° to 25℃) and humidity- (50% to 60%) controlled colony room. All procedures were in accordance with institutional guidelines for animal research at Inha University in Incheon, the Republic of Korea.
Experimental protocol
At 12 weeks of age, a total of 56 OLETF rats were randomly allocated into four groups: (1) OLETF rats with no treatment (OC, n=14), (2) OLETF rats with rosiglitazone treatment only (OR, n=14), (3) OLETF rats with exercise only (OEx, n=14), and (4) OLETF rats with combined treatment of rosiglitazone and exercise (OREx, n=14). LETO rats were used as normal controls (LETO, n=14). Additional non-treated OLETF rats (n=7) and LETO rats (n=7) were sacrificed at baseline (12 weeks old) for pathologic examination. Rosiglitazone maleate (3 mg/kg/day, Avandia; GSK Inc., Philadelphia, PA, USA) was orally administered to OR and OREx once a day, 6 days/week from week 12 as previously described [18] for 12 or 28 weeks. The OEx and OREx groups were submitted to a 30 minutes exercise at 15 m/min speed (low-intensity) on a forced exercise wheel system (rat activity cage; JD-A-06; Jungdo B&P, Seoul, Korea) 5 days a week for 12 or 28 weeks. After 12 weeks (24 weeks old) and 28 weeks (40 weeks old) of treatment, intraperitoneal glucose tolerance test (IPGTT) was performed for the evaluation of glucose tolerance, and the animals were sacrificed for evaluation of the pancreatic islet cells and molecular analysis.
Analysis of blood samples
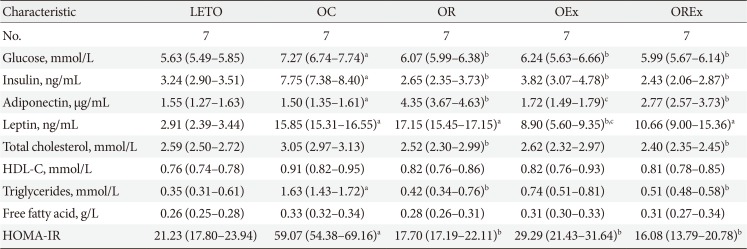
Blood samples were drawn after an overnight fast, and the serum samples were kept at −80℃ for subsequent assays. Insulin and leptin levels were determined with enzyme-linked immunosorbent assay (ELISA) kits (Linco Research Inc., St. Charles, MA, USA). Adiponectin levels were determined with an ELISA kit (ALPCO Diagnostics, Windham, NH, USA). Glucose, total cholesterol, high density lipoprotein cholesterol, low density lipoprotein cholesterol, triglycerides, and free fatty acids were assessed with an automated chemistry analyzer (ADVIA 1650; Bayer, Tokyo, Japan). A surrogate index of insulin sensitivity was calculated from fasting blood glucose and plasma insulin concentrations as follows: homeostasis model assessment of insulin resistance (HOMA-IR)=(G0×I0)/22.5, with glucose expressed as mmol/L and insulin expressed as µU/mL [19].
Glucose tolerance test
The IPGTT was performed as described previously [17]. Blood glucose level was analyzed using a glucometer (One Touch Instrument; Roche, Basel, Swiss). Area under the curve (AUC) for glucose was calculated according to the trapezoidal rule from the glucose measurements at baseline, 30, 60, 90, 120, and 180 minutes.
Dual energy X-ray absorptiometry
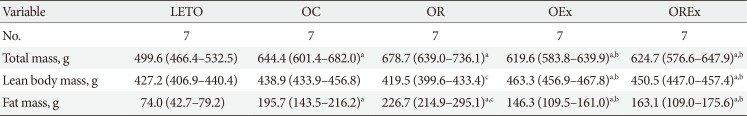
Body composition was measured at 30 weeks of age by dual-energy X-ray absorptiometry (DEXA). Before undergoing a whole-body scan, the rats were anaesthetized with Ketamine mixtures (Ketamine 35 mg/kg+Xylazine 3 mg/kg+Acetylpro-mazine 0.75 mg/kg), and then total body mass, bone mineral content, lean body mass, and fat mass were measured in vivo with a QDR 4500A DEXA using small animal analysis software (DEXA; Hologic Inc., Waltham, MA, USA).
The β-cell and islet morphology
Immunocytochemistry was performed as described previously [20]. For insulin staining, tissue sections were incubated with anti-insulin antibody (1:1,000; Biogenex, San Ramon, CA, USA) for 24 hours at 4℃. In situ hybridization for insulin was carried out on tissue sections. After treating with proteinase K, the sections were incubated with alkaline phosphatase-conjugated anti-digoxigenin antibody. Hybridization signals were revealed by nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate toluidinium. For the double immunostaining, tissue sections were treated with mouse anti-rat insulin antibody (1:1,000; Biogenex) and rabbit anti-rat glucose transporter 2 (GLUT-2) antibody (1:1,000; Alpha Diagnostic International, San Antonio, TX, USA). After applying the secondary antibody, fluorescein isothiocyanate (FITC) conjugated anti-mouse and rhodamine conjugated anti-rabbit antibody (Jackson Immuno-Research Lab. Inc., West Grove, PA, USA) were added. The tissue sections were observed using a Radiance 2100 confocal microscope (Bio-Rad and Nikon, Tokyo, Japan).
For morphometric analysis, insulin-positive cells were counted in the entire area of the tissue sections, and β-cell number per unit area (1 µm2) was calculated as reported previously [21]. To assess islet preservation, the islet destruction ratio (%) was calculated as (the number of destructed islets)/(the total islet number evaluated)×100 (%). For each pancreas, two slides were reviewed at 10× magnification and 20 photos without overlap were randomly taken to count the number of islets. The corresponding islets were examined at 40× magnification to evaluate whether there were signs of destruction. Destructed islets were defined as having lymphocyte infiltration and a distorted outline disabling clear demarcation of the islet area. The mean of the islet destruction ratios of the two slides was calculated. Image files were analyzed using Quantity One (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Nonparametric tests were performed to compare the metabolic parameters, body weight, and body composition of the LETO, OC, OR, OEx, and OREx groups at each age point, due to the relatively small sample size of each group. The Mann-Whitney U test was used to compare the glucose tolerance levels and AUCs between LETO and OC groups at the 12-week age point. We compared body weight, body composition, glucose metabolic parameters, and AUCs among LETO, OC, OR, OEx, and OREx groups at 24 and 40 weeks using the Kruskal-Wallis test and Tukey method, using ranks for multiple comparisons. Bonferroni adjustments for multiple tests were additionally carried out to compare body and epididymal fat weight variables. SPSS version 14.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. A level of 5% was used for statistical significance.
RESULTS
Metabolic characteristics
Table 1 reports the metabolic parameters of the LETO and OLETF rats of each group at 40 weeks (after the 28-week intervention). The plasma glucose and insulin levels were higher in the OC group, but reduced in the OR, OEx, and OREx groups. Serum adiponectin concentration was elevated in the OR (P<0.01) and OREx groups (P<0.01). The serum leptin concentration was decreased in the OEx group (P<0.01). Regarding plasma lipid profiles, significant decreases in serum total cholesterol (P<0.05 and P<0.01, respectively) and triglyceride (P=0.02 and P=0.03, respectively) levels were observed in both the OR and OREx groups. HOMA-IR, a surrogate marker of insulin resistance, was significantly increased in the OC group compared to the LETO group. The OR, OEx, and OREx groups all showed improved insulin sensitivity compared to the OC group to a similar extent (Table 1).
Effects of rosiglitazone and exercise on glucose tolerance
The IPGTT was performed at 12, 24, and 40 weeks of age to assess glucose tolerance (Fig. 1). A greater increase in blood glucose levels in response to the intraperitoneal glucose load was seen in the OC group compared to LETO (P<0.01) (Fig. 2A–C). At 24 weeks, the OC group showed significantly higher fasting glucose levels compared to the LETO group, and the OREx group showed lower fasting glucose levels compared to the OC group (Fig. 2B). At 40 weeks, fasting plasma glucose was significantly higher in the OC group compared to LETO, and the OR, OEx, OREx groups all showed a lower fasting plasma glucose level compared to the OC group (Fig. 2C). Glucose tolerance was further impaired with aging for the OC group, but significant improvement in glucose tolerance was seen in the OR, OEx, and OREx groups at 24 and 40 weeks. The OREx group showed a further improvement compared to the OR and OEx groups (Fig. 2B and C). At 24 weeks of age, the glucose AUCs of the OREx group were significantly lower than those of the OC group, while no significant changes were found in the OR and OEx groups. At 40 weeks, the OR, OEx, and OREx groups all showed lower glucose AUCs compared to the OC group, and the OREx group showed further improvement compared to the OR and OEx groups (Fig. 2D).
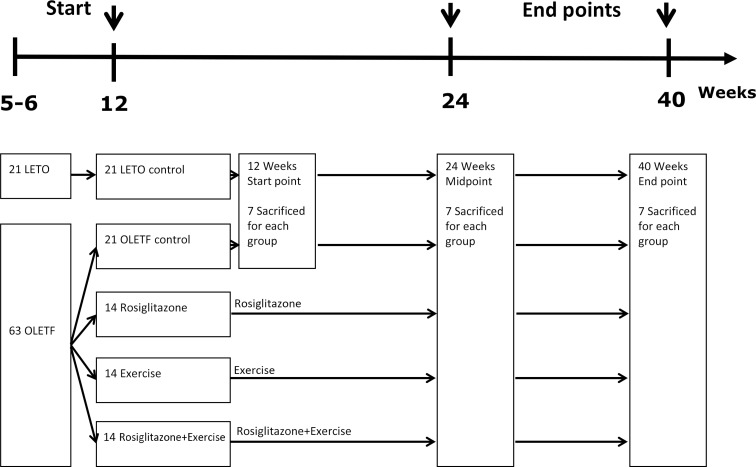
Experimental design. Flow chart of the experimental design is shown. LETO, Long-Evans Tokushima Otsuka; OLETF, Otsuka Long Evans Tokushima Fatty.
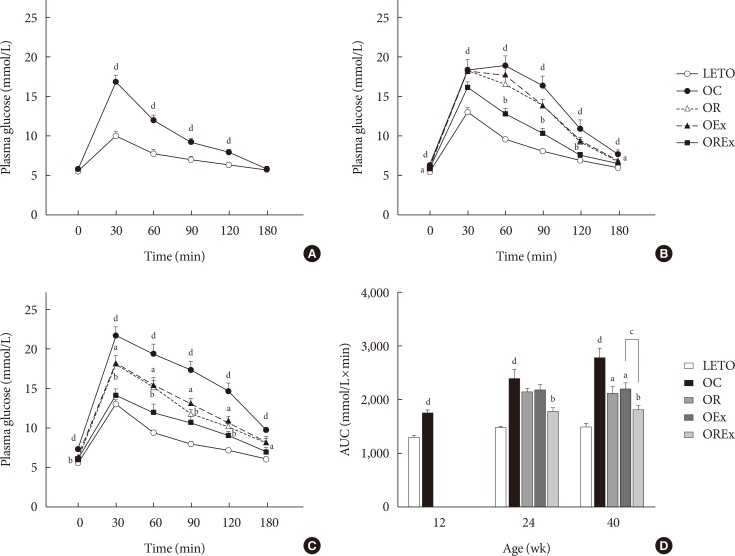
Effects of rosiglitazone and exercise treatments on glucose tolerance. Time course of blood glucose during the intraperitoneal glucose tolerance test is reported at (A) 12 weeks, (B) 24 weeks, and (C) 40 weeks of age. (D) The area under the curve (AUC) for glucose at 40 weeks were calculated. Values are presented as mean±standard error (n=7). LETO, Long-Evans Tokushima Otsuka; OC, Otsuka Long Evans Tokushima Fatty (OLETF) rats with no treatment; OR, OLETF rats with rosiglitazone treatment only; OEx, OLETF rats with exercise only; OREx, OLETF rats with combined treatment of rosiglitazone and exercise. aP<0.05 vs. OC, bP<0.01 vs. OC, cP<0.05, dP<0.01 vs. LETO.
Effects of rosiglitazone and exercise on body weight and composition
Changes of body weight and visceral fat are presented in Fig. 3. At 12 weeks of age, OLETF rats demonstrated significantly higher body weight than the LETO controls (393.8 [377.5 to 408.0] vs. 320.0 [307.5 to 328.75], P<0.01) (Fig. 3A). At 40 weeks of age (after 28 weeks of treatment), the OR group demonstrated a significantly higher weight gain (1.78 [1.70 to 1.93] fold) compared to the OC group (1.69 [1.64 to 1.76] fold), whereas weight gains were considerably lower in the OEx (1.62 [1.52 to 1.70] fold) and OREx (1.57 [1.49 to 1.60] fold) groups (Fig. 3B). This shows that rosiglitazone induced undesirable weight gain, and that this weight gain can be rescued by regular aerobic exercise. Epididymal fat in the experimental animals paralleled changes in body weight (Fig. 3C and D), implying that weight control by aerobic exercise is accompanied by suppressed visceral fat increase. These findings were closely related to the body composition evaluated by DEXA at 30 weeks of age (Table 2), showing a significant improvement in composition of both lean body mass and fat body mass in the OEx and OREX groups compared to the OR group. There was no significant difference in food intake among the groups, although there was a trend of increased intake in the OC group compared to the LETO group.
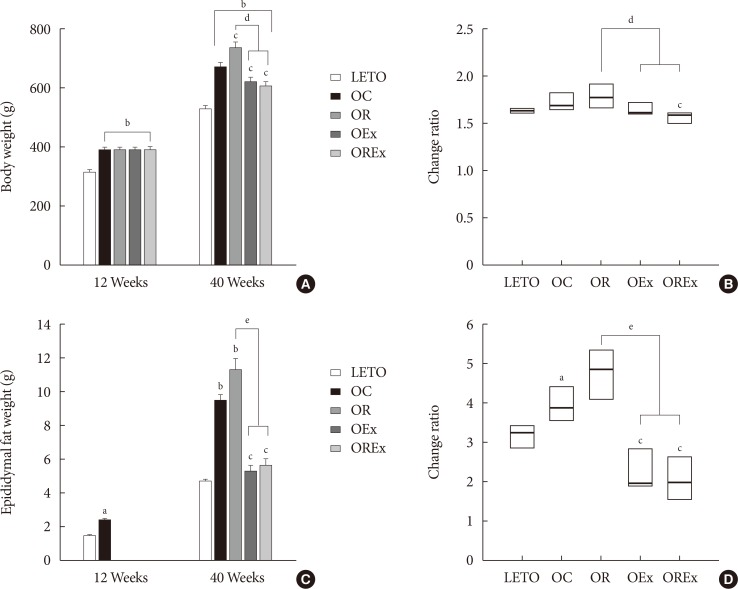
Effects of rosiglitazone and exercise on body weight and epididymal fat weight. (A) Body weight and (C) epididymal fat weight at 12 and 40 weeks are shown. Values are presented as mean±standard error (n=7). The change ratio of (B) body weight and (D) epididymal fat weight from 12 to 40 weeks were calculated. Each box (n=7) are presented as median (interquartile range). LETO, Long-Evans Tokushima Otsuka; OC, Otsuka Long Evans Tokushima Fatty (OLETF) rats with no treatment; OR, OLETF rats with rosiglitazone treatment only; OEx, OLETF rats with exercise only; OREx, OLETF rats with combined treatment of rosiglitazone and exercise. aP<0.05 vs. LETO, bP<0.01 vs. LETO, cP<0.01 vs. OC, dP<0.01, eP<0.001.
Effects of rosiglitazone and exercise on islet morphology
Fig. 3A shows chronological changes in islets in the LETO and OLETF rats. All experimental rats retained normal islet features with round or oval shape, compact cellular mass, and a distinct demarcation from the exocrine tissue at 12 weeks of age. Development of atypical islets were detected at 24 weeks of age in OLETF rats. The islets were somewhat inflated and started to lose outer demarcation, particularly in the OC group, whereas the islets of the LETO control group demonstrated no alteration at all. Alterations of the islet were aggravated at 40 weeks in the OC group, which showed a looser islet constitution owing to increased fiber substance as well as lymphocyte infiltration. The β-cells in the islets display relatively weak immunoreactivity for insulin (Fig. 4A). However, improvement in islet preservation was obtained by rosiglitazone treatment as well as by regular aerobic exercises. The islets of the OR and OEx groups revealed relatively well-organized islet features retaining a compact β-cell mass with distinguishable contours. Moreover, preservation of the islet was prominent in the OLETF rats treated with combined rosiglitazone and aerobic exercise (OREx) which showed a compact islet mass with strong insulin immunoreactive cells (Fig. 4A).

Profiles of the pancreatic islets in rosiglitazone, exercise, and combined treatment. (A) Chronological changes of pancreatic islets in LETO rats and OLETF rats with and without anti-diabetic treatment are presented with insulin immunostaining at 12, 24, and 40 weeks. (B) Changes in islet alterations by percentage at 40 weeks. The number of the altered islets was counted in the pancreatic tissues and the ratio was calculated. Each box (n=7×2 sections) are presented as median (interquartile range). (C) β-Cell number of the total area of the pancreas was calculated at 40 weeks. The pancreatic islet area was measured using Quantity One (Bio-Rad). (D) In situ hybridization: insulin mRNA in LETO, OC, OR, OEx, and OREx groups at 40 weeks. LETO, Long-Evans Tokushima Otsuka; OC, Otsuka Long Evans Tokushima Fatty (OLETF) rats with no treatment; OR, OLETF rats with rosiglitazone treatment only; OEx, OLETF rats with exercise only; OREx, OLETF rats with combined treatment of rosiglitazone and exercise. aP<0.001 vs. LETO, bP<0.01 vs. OC, cP<0.001 vs. OC, dP<0.01.
To assess islet preservation, we assessed the islet destruction ratio as the percentage of islets showing an apparent morphological alteration (Fig. 4B). The β-cells mass was estimated by counting the number of β-cells per unit area (µm2) of the same tissue sections used for the determination of altered islets (Fig. 4B). Alteration and destruction of the islet was found to be severe in the OC group, especially at the age of 40 weeks (>80%). However, the islets of the OR and OEx groups demonstrated a significant reduction of this islet alteration and destruction. Furthermore, we found a remarkable improvement of islet preservation in experimental rats treated with the combination of rosiglitazone and aerobic exercise (OREx) compared to the OR and OE groups. Only a small number of altered islets (<5%) was detected in the OREx group. We also observed a significant increase of β-cell numbers in the OR, OEx, and OREx groups at 40 weeks of age (Fig. 4C).
Insulin expression in β-cells was confirmed at the insulin mRNA level by in situ hybridization, as an indication of insulin synthesis (Fig. 4D). Expression signal of insulin mRNA in the OC group was lower compared to the LETO controls, whereas the hybridization signals appeared to be improved in the OR, OEx and OREx groups.
Pancreatic GLUT-2 expression
To estimate glucose sensitivity of the β-cells, we examined GLUT-2 immunoreactivity of the β-cell membrane (Fig. 5). We found substantial loss of GLUT-2 immunolabeling in the β-cells of the OC rats compared to the LETO controls, but intact expression of GLUT-2 in the β-cells of the OR, OEx, and OREx rats.

Glucose transporter 2 (GLUT-2) expression in pancreatic islets with rosiglitazone, exercise, and combined treatment at 40 weeks. The pancreatic tissues were immunolabeled for insulin (green fluorescence in upper panel) and GLUT-2 (red fluorescence in middle panel). The immunofluorescent signals were merged and are shown in the lower panel. LETO, Long-Evans Tokushima Otsuka; OC, Otsuka Long Evans Tokushima Fatty (OLETF) rats with no treatment; OR, OLETF rats with rosiglitazone treatment only; OEx, OLETF rats with exercise only; OREx, OLETF rats with combined treatment of rosiglitazone and exercise.
DISCUSSION
Rosiglitazone is an antidiabetic agent used in patients with T2DM to improve insulin sensitivity and glucose metabolism [5622]. Rosiglitazone has superior durability when used for a long period of time compared to other antidiabetic agents, probably due to its beneficial effect on preserving β-cell function [23]. However, the adverse effect of body weight gain [10] may limit its clinical use. Therefore, an effort to limit unwanted weight gain will be necessary, and sufficient regular aerobic exercise may be beneficial in this clinical setting [24]. In this study, we evaluated the antidiabetic effects of aerobic exercise and its synergic advantages by combining it with TZD treatment. The combination of aerobic exercise and rosiglitazone treatment was able to further improve glucose tolerance, favorably affect body composition, and prevent destruction of pancreatic islets in OLETF rats as an additional benefit.
Several studies have demonstrated that sufficient amount of exercise can reduce body weight or prevent regain in both humans and rats [11222425]. We found that regular aerobic exercise effectively mitigates spontaneous obesity as well as rosiglitazone-induced weight gain in OLETF rats. Exercise is also of great benefit since it can reduce abdominal obesity, visceral fat, and cardiovascular risk factors, and increase both skeletal muscle mass and cardiorespiratory fitness, even without significant weight loss [26]. Indeed, aerobic exercise resulted in successful attenuation of total fat mass and visceral fat mass increases caused by rosiglitazone suggesting that the major obstacle of TZDs therapy can be eliminated by physical exercise.
Exercise and rosiglitazone as a combination treatment was shown to have complementary and additional beneficial effects on glycemic indexes, insulin sensitivity, and cardiovascular risk factors compared to either exercise or rosiglitazone by itself in previous studies [131427282930]. In patients with T2DM, simultaneous treatment with rosiglitazone and exercise counteracted rosiglitazone induced weight gain, extended improvements of insulin sensitivity, glycemic control and fitness beyond those expected by their complementary actions in patients with T2DM after 8 months [13]. In obese Zucker fatty rats, exercise and troglitazone alone each had a beneficial effect on insulin sensitivity, while the combination of both treatments completely normalized insulin sensitivity to the level of lean control rats. Exercise and troglitazone displayed different mechanisms, and therefore the combined effect was able to have additional benefits [28]. The present study shows that at 40 weeks (after 28 weeks of treatment) groups treated with exercise alone or rosiglitazone alone showed improved glucose tolerance, and the combined rosiglitazone and exercise treatment group showed more prominent improvement in glucose tolerance, in accordance with previous studies. Adiponectin was significantly increased in the OR and OREx groups, while there was no significant change in the OEx group. This finding is consistent with previous studies in patients with T2DM [13]. The increase in plasma adiponectin level by rosiglitazone treatment, which is not seen in the exercise-alone group, would be an additional benefit of combining rosiglitazone to exercise, since adiponectin is known to have beneficial effects such as insulin sensitizing, anti-inflammatory, and anti-atherogenic properties [31]. Although we have not measured other cytokines, it is possible that other cytokines may have also played a role. In patients with T2DM, exercise, rosiglitazone, and combined treatment all suppressed interleukin 6 (IL-6), resistin, and IL-18, and increased IL-10 levels, while both combined treatment and rosiglitazone treatment alone decreased tumor necrosis factor α levels significantly [1314].
In addition to the known beneficial effects of the combined treatment of aerobic exercise and rosiglitazone, the current study shows the benefit of preservation of pancreas islet morphology in a T2DM rat model. This is the first study to evaluate pancreas morphology after concurrent treatment with exercise and a TZD. Since direct evaluation of the pancreas after treatment is not feasible in human studies, this animal data should be informative in providing knowledge on the effects of combined treatment in the pancreas. Diabetes is closely associated with defects of islet β-cells, and β-cell dysfunction is an early event leading to the development of T2DM [32]. In obese humans, there is an increase in β-cell mass compared to non-obese humans. Obese patients with impaired fasting glucose and T2DM had a significantly decreased β-cell mass compared to non-diabetic people [33]. This coincides with significant alterations of pancreatic islets as shown in non-treated diabetic OLETF rats. Both rosiglitazone [83234] and exercise [153536] have been reported to have beneficial effects on pancreatic islet preservation in animals. The present study further shows that aerobic exercise protects islet destruction particularly when combined with rosiglitazone treatment. Hyperglycemia, hyperlipidemia, and inflammation are thought to contribute to β-cell dysfunction [32]. Rosiglitazone has been shown to improve indices of β-cell function when assessed by the HOMA in patients with T2DM. Exercise also has been shown to improve β-cell function measured by the disposition index [1537]. The benefit of both exercise and rosiglitazone on the pancreas may be indirect, mainly by reducing the secretory demand, but there is evidence supporting direct beneficial effects on the pancreas, especially for rosiglitazone [3839].
In summary, the present study demonstrates that combined rosiglitazone with regular aerobic exercise can prominently improve glucose metabolism, and this is associated with protection from islet destruction in a T2DM prone-animal model. Combined regular aerobic exercise with rosiglitazone was also able to attenuate the increase in body weight and fat mass induced by rosiglitazone treatment. It is noticeable that aerobic exercise and rosiglitazone can complement each other and lead to additional benefits when combined, and can lead to better sustained glycemic control in accordance with its additional benefit on islet preservation. Therefore, participating in regular aerobic exercise should be strongly encouraged to patients with T2DM in conjunction with TZD prescription for better therapeutic effects, and to prevent the side effects of TZDs.
ACKNOWLEDGMENTS
This study was supported by a grant of the Korea Healthcare technology R&D Project Ministry of Health and Welfare, Republic of Korea (HI14C1062). Shan-Ji Piao received a grant supported by the Brain Korea 21 Project, Republic of Korea. This work was supported by the KOSEF (2006-05402, R01-2006-000-11386-0, and 2009-0091914) and NRF (2017R1D1A1B03034581), Republic of Korea.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.